Abstract
For an organism to be successful in an evolutionary sense, it and its offspring must survive. Such survival depends on satisfying a number of needs that are driven by motivated behaviors, such as eating, sleeping, and mating. An individual can usually only pursue one motivated behavior at a time. The circadian system provides temporal structure to the organism’s 24 hour day, partitioning specific behaviors to particular times of the day. The circadian system also allows anticipation of opportunities to engage in motivated behaviors that occur at predictable times of the day. Such anticipation enhances fitness by ensuring that the organism is physiologically ready to make use of a time-limited resource as soon as it becomes available. This could include activation of the sympathetic nervous system to transition from sleep to wake, or to engage in mating, or to activate of the parasympathetic nervous system to facilitate transitions to sleep, or to prepare the body to digest a meal. In addition to enabling temporal partitioning of motivated behaviors, the circadian system may also regulate the amplitude of the drive state motivating the behavior. For example, the circadian clock modulates not only when it is time to eat, but also how hungry we are. In this chapter we explore the physiology of our circadian clock and its involvement in a number of motivated behaviors such as sleeping, eating, exercise, sexual behavior, and maternal behavior. We also examine ways in which dysfunction of circadian timing can contribute to disease states, particularly in psychiatric conditions that include adherent motivational states.
‘Time’ he said, ‘is what keeps everything from happening at once’.
Ray Cummings, The Girl in the Golden Atom, 1922.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Rhythms
- Suprachiasmatic nucleus
- Anticipation
- Eating
- Exercise
- Sleep
- Sexual behavior
- Maternal behavior
- Amplitude
1 Circadian Considerations for Motivated Behaviors
One might reasonably ask why circadian rhythms are important for understanding motivated behaviors. In the context of evolution, the most important consideration is that motivated behaviors must satisfy biological needs that promote the survival of individuals and their offspring. These include eating and drinking , sleeping , mating, and parenting . Such motivated behaviors cannot be performed simultaneously. The circadian timing system provides temporal organization to motivated behaviors. A major function of the circadian clock is to regulate when during the day an animal will engage in specific behaviors, and when various biological functions are more or less likely to be expressed. When to satisfy these biological needs depends on a number of factors, both intrinsic and extrinsic to the organism. An important intrinsic factor is when the body, or a specific organ within the body, is ready to make use of the resource. Key among the extrinsic factors is the availability of the resource required to satisfy the need. Resources may only be available (or safely available) during a small temporal window. In such cases, the organism must structure its behavior to anticipate this availability. With respect to motivated behaviors, we suggest that the circadian timing system plays three roles. (1) Circadian rhythms provide the temporal structure that partition different goals to different parts of the day. (2) The circadian system orchestrates physiology and metabolism so that the body can most effectively satisfy the need underlying the goal. (3) The circadian system allows for anticipation of the availability of a temporally restricted goal.
1.1 Goal-Directed and Arousal Aspects of Motivation
There are two distinct, long-recognized components of motivated behavior, namely (1) a goal-directed, directional component and (2) an arousal , activational component (Hebb 1955; Duffy 1957; Salamone 1988)—and both are impacted by an internal circadian timing system. More specifically, Hebb (1955), in his beautifully worded paper on the “Conceptual Nervous System,” written in an era when “neurologizing” was verboten, argues that “Motivation” refers in a rather general sense to the energizing of behavior, and especially to the sources of energy in a particular set of responses that keep them temporarily dominant over others and account for continuity and direction in behavior. Duffy (1957) remains on the behavioral level of analysis, and argues that “all variations in behavior may be described as variations in either the direction of behavior or the intensity of behavior” and that “confusion of the direction of behavior with the intensity of behavior, resulting in their fortuitous combination in certain psychological concepts…” (p. 256, Duffy 1957). Restated: Duffy suggests that response amplitude and goal direction are confounded in behavioral analyses of motivation. Today, “neurologizing” has become mandatory, and there is an increased emphasis on the neurobiological basis of motivation in studies of both non-human and human mammals. While much research effort has advanced the understanding of the neural and neurochemical basis of motivated behaviors (Chap. Oxytocin, Vasopressin, and the Motivational Forces that Drive Social Behaviors of this volume) (Gore and Zweifel 2013; Trifilieff et al. 2013), there has been limited progress in dissociating the activational and directional components (Bailey et al. 2015). Studies of goal-directed action selection and general arousal tend to examine each of these aspects in isolation, as separate central nervous system entities, with the former focusing on reward circuits (e.g., Richard et al. 2013) and the latter on arousal pathways (e.g., Pfaff et al. 2012). Behavioral paradigms that are used to assess models, drugs, environmental conditions, etc., generally do not distinguish between directional and arousal components. Experiments designed to examine the neural circuitry of goal-directed action selection and general arousal tend to consider each behavior and circuit in isolation, without consideration for their interrelationships on either a neural or behavioral level. Understanding the neural basis of motivation is considered especially important in understanding the symptomatology of depression , schizophrenia , and other emotional and affective disorders that have among their symptoms alterations in motivation and in circadian timing. As is discussed below, the circadian timing system impacts both arousal and goal-directed components of behavior.
1.2 Circadian Rhythms Impact Goal-Directed Motivation
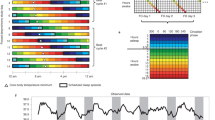
The circadian system regulates goal-directed components of motivation such that motivated behaviors are normally expressed in a coordinated, temporally appropriate fashion. This coordination of goal seeking means that specific motivated behaviors occur at species-characteristic times of day. For instance, sleeping and feeding each occur at specific times of day in most species, including humans (as in Fig. 1). Other motivated behaviors, such as mating and exercise, are partitioned around these behaviors.
The circadian clock gives temporal structure to our 24-h day. A typical North American might wake around dawn. They will engage in a number of motivated activities throughout their day, and the timing of these motivated behaviors tends to be consistent day-to-day. For instance, they will consume multiple meals at regular times, and hunger will increase over the regular mealtime until the meal is initiated (green-to-red gradient within the Eat box). The person may also engage in other motivated behaviors such as exercise or sex, and the timing of these behaviors may become habitual. Most people will have a particular phase angle of activity relative to dawn/dusk, with an average adult not initiating their major sleep bout until many hours after dusk, although this may be much earlier in “morning larks” and much later in “night-owls.” Some people may also have a minor sleep bout (nap) mid day. The motivation to sleep will increase near the person’s normal sleep time and continue to increase during their normal sleep period until sleep is initiated (green-to-red gradient in the sleep box). Many of these motivated behaviors are linked closely to neuroendocrine signals such as ghrelin which triggers hunger, cortisol which facilitates waking, and melatonin which is secreted during the night and facilitates sleep in diurnal species
1.3 Circadian Rhythms Impact Arousal Components of Motivation
Arousal level is related to motivation in that it impacts how vigorously a goal is pursued (amplitude of goal seeking). The circadian clock can also influence the amplitude of the motivated behavior (Fig. 2). As an example, the duration of sleep depends on circadian phase, not just on sleepiness (Czeisler et al. 1980). Thus, in a classic study, monkeys were sleep deprived for various lengths of time, and recovery sleep was monitored (Klerman et al. 1999). Deprivation ended at the normal wake time for the groups with the longest durations of sleep deprivation (and thus the most tired), yet the monkeys didn’t sleep! While the motivation to sleep should have been high due to homeostatic mechanisms, the circadian clock was able to override these homeostatic sleep mechanisms that were occurring at the wrong time of day, thus motivating the animal to be awake despite severe sleep loss. This illustrates the potency of the circadian clock in modifying motivation for a particular behavior. Circadian modulatory effects can also be seen in studies of cognitive performance following sleep deprivation. In a test of vigilance, subjects watch a red light and have to push a button when it turns on. Sleep deprivation beyond 17 h results in a dramatic increase in not only the latency to push the button, but also in complete misses of the light going on. Amazingly, these lapses in vigilance were under circadian control: After 8 days of sleep restricted to 4 h/day, subjects averaged about 8 more performance lapses at 0800 h than they had 10 h later at 1800 h (Mollicone et al. 2010).
An example of three behaviors (sleeping , eating , and exercise) from a hypothetical human. The desire to engage in different motivated behaviors changes over the course of the day (solid lines), and the timing of these behaviors can vary widely between different people. These rhythmic oscillations make the individual’s motivated behaviors more or less likely. Typically, when the amplitude climbs above a certain threshold (dashed lines), the motivated behavior is likely to occur, and when the motivation amplitude falls below this threshold, the behavior is less likely, even in cases when the person did not engage in that behavior
1.4 Distinguishing Circadian Arousal and Goal-Directed Components of Behavior
Goal-directed and amplitude effects of the circadian system can be experimentally distinguished. This is commonly attempted in studies of desynchrony , such as occur in studies of shift work or jet lag . As an example, in an early study of individual time series (Reinberg et al. 1988), circadian period and amplitude were evaluated in order to understand tolerance to shift work, effect of age, duration of shift work , speed of rotation, and type of industry. The measures used included sleeping, working, oral temperature, grip strength of each hand, peak expiratory flow, and heart rate. Here, the timing of these responses could be analyzed separately from their amplitude. The results indicate that intolerance to shift work is associated with both reduced circadian amplitude and internal desynchronization of responses, such that various responses no longer occurred at appropriate/typical times of day.
Circadian rhythmicity is a clock-like process which modulates sleep propensity and many other motivated physiological and behavioral responses. The circadian system modulates virtually all physiological and behavioral responses by generating an oscillatory signal every day. Sleep (and other goal-directed, motivated responses) is normally expressed at species characteristic, specific times of day. They remain consolidated and appropriately timed with respect to each other, if the amplitude of the circadian signal is sufficiently strong. In this sense, the circadian system determines both the timing of goal-directed motivation and the amplitude or strength of responses.
2 Cellular, Molecular, and Network Basis of SCN Circadian Timing
In mammals, the suprachiasmatic nucleus (SCN) of the hypothalamus functions as the master circadian clock produces daily rhythms in physiology and behavior and synchronizes them to the environmental day/night cycle (Antle and Silver 2005). In environments lacking all external cues to time, these daily oscillations in behavior and physiology persist, albeit with a period slight different than 24 h. These endogenously generated rhythms are said to be free-running rhythms, as the internal biological clock is running free of any external time cues. When the SCN is lesioned, all circadian rhythms in physiology and behavior are lost (Moore and Eichler 1972; Stephan and Zucker 1972). Remarkably, these rhythms can be restored by transplanting a fetal SCN graft (Lehman et al. 1987), and the period of the restored rhythm is that of the donor rather the host (Ralph et al. 1990). Such studies prove that the SCN is necessary and sufficient for daily oscillations in behavior and physiology.
The SCN tissue is a multicellular oscillator in which many of its ~20,000 individual neurons function as cell-autonomous oscillators. These cells are networked together to produce a coherent tissue-level oscillation (Welsh et al. 2010). While numerous brain regions and body organs can exhibit circadian rhythmicity derived from the same molecular underpinnings (see below), they lack the network properties to maintain tissue-level oscillations in the long-term without organizing signals from the master circadian clock in the SCN, or from other exogenous rhythmic cues such as scheduled feeding . In contrast, the SCN continues to oscillate in the absence of phase-setting cues.
2.1 Cellular Oscillation
Circadian oscillation at the level of the individual cell emerges from interlocking positive and negative feedback loops in the transcription and translation of a number of circadian genes (Robinson and Reddy 2014). Briefly, the expression of clock genes is under control of E-box elements in their promoter regions. The E-boxes are activated by a dimer of CLOCK and BMAL1 proteins, leading to the translation of period and cryptochrome genes during the day. Translation into their corresponding proteins occurs with a time lag. The PERIOD and CRYPTOCHROME protein products of these genes then dimerize and return to the nucleus where they inhibit the activity of CLOCK and BMAL1, thus turning off their own expression. Levels of PERIOD and CRYPTOCHROME proteins fall steadily over the night, and when depleted, CLOCK and BMAL1 activity at E-boxes can resume, thus starting the cycle over again.
2.2 Amplitude of SCN Oscillation
The amplitude of SCN oscillation is key to achieving robust rhythmicity in physiology and behavior. A growing body of evidence suggests that coherent daily rhythms contribute to health, well-being, cognitive performance , and alertness (Ramkisoensing and Meijer 2015). In claiming that the circadian clock controls level of arousal, the suggestion is made that behavioral and physiological rhythms can have either high or low amplitude. A high-amplitude rhythm has a clear optimal peak time of expression and an equally clear amplitude trough (Fig. 3a). In contrast, the absence of a rhythm points to a behavior that is not optimally expressed at its peak. As an example, both sleep disruption and aging reduce the amplitude of circadian rhythms, while exercise increases amplitude (Fig. 3b). Disruptions can also be caused by social or environmental factors, such as shift work or jet lag , or by disease states that involve circadian disruption including Parkinson’s and Alzheimer’s disease (Karatsoreos 2014; Lim et al. 2014; Ondo 2014; Sterniczuk et al. 2014). As such, circadian problems are often a marker of a number of diseases. Furthermore, deterioration of the 24-h rhythm increases the risk for the development or exacerbation not only of neurodegenerative disease, but also some cancers , depression , obesity , cardiovascular disease , and sleep disorders (Baron and Reid 2014; Uth and Sleigh 2014; Alibhai et al. 2015; Saini et al. 2015). Altogether, it is clear that daily oscillations in physiology, behavior, and motivation are regulated by the circadian system. In a healthy organism, the amplitude and timing of behavioral and physiological responses are both regulated by the circadian system. Circadian disruptions are a component of many compromised states.
a Circadian systems are composed of multiple oscillatory units. This could be thought of as the cells that make up an oscillating tissue, or even the various oscillating organs within an organism. Oscillators must be synchronized to yield a coherent high-amplitude output. When they are desynchronized, the overall rhythmic output exhibits diminished amplitude. b A number of stimuli can improve synchrony among oscillators within and between tissues. Light is the dominant cue for setting the phase of the master oscillator, but both feeding and exercise also affect oscillator function. Engaging in these motivated behaviors at regularly scheduled times may improve overall clock function and health
The SCN is unique among the tissues of the body in that its clock cells form a network that is capable of sustained oscillation, even in vitro. Many of the factors determining amplitude of circadian oscillation at the cellular and system level of organization are understood. Basically, the amplitude of the SCN’s electrical rhythm is high when the individual neurons in the SCN are appropriately synchronized and low when the neurons are poorly synchronized (Pauls et al. 2014; Ramkisoensing and Meijer 2015). Among the external signals that support synchrony is photic input from the eye, delivered to the SCN via a specialized retinohypothalamic tract (Schmidt et al. 2011). Greater amplitude oscillation is seen in SCN slices harvested from animals that have been housed in a light:dark cycle compared to those harvested from animals housed in constant darkness. As noted above, circadian rhythm amplitude is reduced in aging . Remarkably, transplanting young SCN tissue into the ventricle of intact aged rats or hamsters improves behavioral circadian rhythmicity (Van Reeth et al. 1994; Hurd et al. 1995). Such transplants also improve the amplitude of the circadian clock itself. Older rats have low-amplitude behavioral rhythms, and these are mirrored by low-amplitude expression rhythms of the immediate early gene FOS in the SCN. When given a fetal SCN graft, the rhythms of FOS expression in the SCN are augmented in these older animals (Cai et al. 1997). These studies provide powerful evidence of the sufficiency of SCN outputs to support robust rhythmic responses even in an aged body. In summary, the individual SCN cells form networks that sustain oscillation. Synchronization of the phases among individual oscillator cells determines the amplitude of SCN oscillation. Amplitude of SCN oscillation determines coherence of behavioral activity/rest rhythms , and age-related disruption can be corrected by providing a young SCN.
2.3 SCN Afferents and Efferents
The SCN is well positioned to send and receive information about the internal and external environment, so as to optimally coordinate timing of physiological and behavioral responses. The major inputs to the SCN arise from the eye, the raphe nuclei in the brain stem, and the intergeniculate leaflet of the thalamus, although numerous other regions also send direct projections to the SCN (Krout et al. 2002). Information regarding the internal state of the body, such as those provided by testicular and ovarian hormones , acts directly on hormone receptors within the SCN (Vida et al. 2010; Model et al. 2015) which are themselves under circadian control. In turn, the SCN sends projections to a number of areas in the brain (Kriegsfeld et al. 2004), some of which may serve as nodes to distribute circadian signals widely (Vujovic et al. in press) or integrate circadian signals with other homeostatic and sensory signals (Saper et al. 2005a).
2.4 Circadian Organization of Motivation
We review evidence that the coordination of intrinsic and extrinsic factors, and the anticipation of each of these events, is achieved by the circadian timing system. Behaviors that are critical to the survival of the organism and the species (e.g., feeding , drinking , sleeping , mating , and parenting ) are highly motivated and highly motivating. In anticipation of performing these behaviors and associated physiological responses, the body prepares for their occurrence with a host of internal adjustments, coordinated by the circadian timing system. While these adjustments may not reach conscious awareness in humans, they nevertheless coordinate the expression of motivated behaviors and determine arousal levels. We therefore examine circadian modulation of a number of goal-directed, motivated behaviors, namely sleep/wake, exercise, feeding, mating, and maternal behavior . Disruption of circadian timing can produce motivational deficits and exacerbate or provoke emotional and affective disorders, and while the internal cues associated with these disruptions may not reach consciousness, their consequences do so.
3 Circadian Regulation of Sleep–Wake /Arousal Cycles
The window of time during which an organism is active, and thus the occurrence of motivated behaviors, is governed by the circadian clock, which appears to drive both sleep and wake times (Mistlberger 2005). Sleep itself is a motivated behavior that is homeostatically regulated. The drive to sleep increases with the duration of wakefulness, and animals that lose sleep will attempt to recover the loss when they are next able to sleep (Friedman et al. 1979). The interaction between the circadian and homeostatic drives to sleep has been described in the two-process model of sleep regulation (Borbély 1982). In this model, sleep and wake transition thresholds oscillate with a circadian rhythm such that the critical value of the homeostatic drive that will trigger the switch from wake to sleep (or vice-versa) varies across the 24-h day. Thus, there are times when it is very difficult to initiate sleep no matter how long you have been awake, and times when it is difficult to awaken even when you are well rested.
3.1 Homeostatic Regulation of Sleep: Adenosine
Accumulation of adenosine may drive the motivation to sleep (Porkka-Heiskanen et al. 2002; but see Blanco-Centurion et al. 2006). Adenosine inhibits wake-active neurons and allows sleep-active neurons to become active (Brown et al. 2012). Blocking adenosine receptor with drugs such as caffeine can transiently decrease the sleep drive and thus facilitate wakefulness. Histamine appears to play the opposite role, histamine agonists promote wakefulness (Brown et al. 2001, 2012), and histamine antagonists, such as those found in allergy medication and antiemetics, enhance the sleep drive (Krystal et al. 2013).
3.2 Circadian Modulation of Sleep
The motivation and drive to engage in sleep involves a large number of brain regions (Saper et al. 2005b; Antle 2015), many of which are regulated directly or indirectly by the circadian clock. Possibly, the circadian clock in the SCN regulates sleep and wake by regulating the activity of the subparaventricular zone (SPZ). The SPZ in turn innervates the dorsomedial hypothalamus (DMH) which provides major input to both hypocretin neurons and the ventrolateral preoptic area (VLPO, Saper et al. 2005b). A number of areas are active during wake, such as the noradrenergic locus coeruleus , serotonergic raphe , and histaminergic tuberomammillary nucleus . Other areas are active during sleep, notably the VLPO, which inhibits the activity of the wake-active areas. Lateral hypothalamic neurons containing hypocretin (also known as orexin ) appear to drive activity in the wake-active neurons. Their loss in people with narcolepsy leads to sleep attacks (intrusions of sleepiness into periods of normal wake).
While the circadian clock in the SCN may provide the master organizing circadian signal within an organism, brain areas involved in regulation of sleep and wake may be intrinsically rhythmic as well. The histaminergic cells in the tuberomammillary nucleus express BMAL1, a key component of the circadian transcription and translation feedback loops. BMAL1 appears to regulate both synthesis of histamine and the circadian activity of these neurons (Yu et al. 2014). Hypocretin neurons in the lateral hypothalamus and noradrenergic neurons in the locus coeruleus exhibit circadian oscillations of Per1 expression (Mahoney et al. 2013).
The problem of separating goal-directed and amplitude effects is well exemplified in studies of age-related sleep disruption. There is evidence that older people are more susceptible to the negative effects of circadian phase misalignment (such as occur with jet lag or shift work ) than young people (Harma et al. 1994; Juda et al. 2013). This impacts the timing and display of motivated behaviors. Changes in sleep timing and duration (i.e., performance of sleep vs. other motivated behaviors), from adolescence to old age, and between the sexes, have been amply described (Roenneberg et al. 2007). Hypothetically, these might be a consequence of a reduction in the amplitude of the circadian aspect of the sleep–wake rhythm, or a reduction in the need/motivation to sleep. That is, changes in sleep duration with age may reflect reduced sleep “need,” or may result from a reduced ability to sleep, due to unrelated causes. Separating the motivation/need to sleep from ability is important as our advice to an older person with short sleep will depend on whether we believe that sleep need declines with age.
3.3 Feedback of Sleep to Circadian System
Alterations in sleep may feedback and affect other motivational systems. In humans, sleep loss can lead to alterations in the activity of feeding-related areas of the brain (Greer et al. 2013). Additionally, the types of foods craved following sleep loss are different than when well rested. Specifically, after sleep loss, people eat more fats and carbohydrates (Brondel et al. 2010). In people, sleep restriction leads to an increase in the hunger hormone ghrelin and a decrease in the satiety hormone leptin (Spiegel et al. 2004). Furthermore, sleep deprivation leads to increased caloric intake in people (Brondel et al. 2010). Sleep loss can also affect the circadian system, with sleep deprivation phase shifting the hamster circadian clock (Antle and Mistlberger 2000) and impairing light-induced phase shifts of the circadian system (Mistlberger et al. 1997).
4 Exercise/Activity
4.1 Locomotor Activity is Rewarding
Many circadian studies employ the motivated behavior of wheel running to assess circadian phase in rodents. That said, there has been a concern that wheel running may be a laboratory artifact and represent a stereotypy rather than a natural behavior (Richter et al. 2014). In fact, wheel running is a strongly motivated behavior. Rodents will work to unlock or gain access to a wheel (reviewed in Sherwin 1998). Furthermore, when wheels are available in a natural setting, wild animals will not only use them upon discovery, but will return repeatedly to run in them (Meijer and Robbers 2014), arguing that wheel running is not an artifact of laboratory housing. There even appears to be some degree of homeostasis for exercise, as when rats are deprived of a wheel for 1, 3, or 10 h, there is rebound activity afterward, proportional to the lost activity (Mueller et al. 1999). There is circadian regulation of this motivated behavior.
While it is not surprising that nocturnal animals will engage in running at night when they have 24-h access to a wheel, animals will nonetheless run even if the wheels are available only during the day. However, rats with wheel access only during the night will increase their level of running to up to 4 times the baseline level progressively over time, while those with wheel access during the daytime (i.e., light phase of the day–night cycle) will remain at a baseline level of activity (Eikelboom and Lattanzio 2003).
4.2 Feedback of Exercise to Circadian System
There are also feedback effects on the circadian clock from such exercise seen by motivating an animal to engage in activity in a phase-dependent manner. Scheduled daily wheel access or exercise can entrain the circadian clock (Edgar and Dement 1991; Marchant and Mistlberger 1996). In animals housed in light:dark cycles, scheduled confinement to wheels for 3 h, which typically induces running, can alter the alignment between the circadian clock and the light:dark cycle (Sinclair and Mistlberger 1997). Confinement to a wheel for 1–3 h often elicits running behavior which leads to phase advances when confinement occurs during the day and phase delays when confinement occurs during the late night (Bobrzynska and Mrosovsky 1998). The exercise per se may not be the critical feature, as enforced arousal through gentle handling can also elicit these same changes in phase (Antle and Mistlberger 2000), and it is possible that wheel running is simply a strongly motivating behavior that produces sufficient wakefulness.
A question raised by the foregoing studies is whether the motivation/arousal feeds back to alter clock function. Not all hamsters, particularly older hamsters, are motivated to run when simply presented with a novel wheel. However, these animals can be motivated to run by augmenting the stimulus. For instance, when given a novel wheel in the presence of a sexually receptive but inaccessible female, older male hamsters will run vigorously and will exhibit phase shifts of their circadian clocks (Janik and Mrosovsky 1993; Mrosovsky and Biello 1994). Cold exposure can also be used to motivate exercise in both younger and older hamsters. In this case, one study reported that the animals will shift (Mistlberger et al. 1996), while another study failed to observe large phase shifts despite high activity (Janik and Mrosovsky 1993). This suggested that the motivational context for the arousal/exercise (accessing a female or keeping warm) might be critical to its ability to feedback to the circadian clock.
4.3 Activity Influences Other Motivated Behaviors
Scheduled wheel access and exercise can also alter other motivated behaviors. For example, rats will decrease food intake for about a week after gaining access to a running wheel (Looy and Eikelboom 1989; Lattanzio and Eikelboom 2003). Eating a high fat diet can alter circadian rhythms of eating and activity, but these effects are mitigated by being able to exercise on a wheel (Pendergast et al. 2014).
5 Eating
Eating is a motivated behavior that is highly rewarding and has clear adaptive value. While some species, such as grazers, eat around the clock, others feed at particular times of their 24-h day. Notably, Dr. Fred Stephan, who studied circadian control of feeding, was fond of pointing out that “when food competes with light, food usually wins” (p. 290, Stephan 2002). That is, even though rats are preferentially nocturnal, when food is only available during the day, rats will adjust their circadian rhythms to exploit this resource. To a hungry rat, the possibility of death due to predation is outweighed by the certainty of death due to starvation. In Americans, the timing of meals is a partially learned and culturally determined phenomenon, but is often broken into a number of meals with periodic snacking in between (U.S. Department of Agriculture, Agricultural Research Service 2014). For other species, feeding occurs at species-typical times of the day (Siegel 1961) but may be adjusted by external factors (Kersten et al. 1980).
Timing not only influences when animals are motivated to eat, but also influences what they are motivated to eat. Meal size often differs across the day. For instance, in North American cultures, breakfast is frequently the smallest meal of the day (Kramer et al. 1992). In First World societies where food is abundant and available in wide varieties, foods typically craved and consumed for breakfast often differ from those craved and consumed for the late-day meal (Birch et al. 1984). While these cravings differ among cultures, it has been suggested that cultural preferences might have developed on top of daily oscillations of circulating insulin, glucagon, and other signals that influence appetite and craving for particular nutrients (Birch et al. 1984). Finally, time of day may also influence feeding behavior. For instance, rats will eat next to their food source during the night, but will take food back to their nest box to consume it during the day (Strubbe et al. 1986). While this might be interpreted as a response to the light, which nocturnal rodents avoid, this is not the case. When the full photoperiod (12 h of light followed by 12 h of dark) is switched to a skeleton photoperiod (1 h of light at dawn and again at dusk), animals will still exhibit subjective day (when rodents behave as if it were day and sleep) and subjective night (when rodents behave as if it were night, and are awake). Under such conditions, the behavior of returning to the nest box to consume the food during the daytime hours persists, suggesting circadian control of feeding behavior (Strubbe et al. 1986).
5.1 Feeding Duration
The times when rats consumed food most quickly are at the start and end of the dark phase (Whishaw et al. 1992). Additionally, rats will take longer to eat a specific amount of food in the light than in the dark. While hunger increases the rate of eating, the circadian effect persists with mealtime during the light lasting longer (Whishaw et al. 1992). This is not simply inhibition of behavior by light, as turning off the lights slows eating even further, while turning the lights on during the dark phase does not change eating speed (Whishaw et al. 1992).
5.2 Peripheral Factors Determining Eating
Aside from eating behavior, circadian factors can influence digestion. Many functions of the gastrointestinal tract (GIT) exhibit circadian rhythmicity, such as gut motility, gastric acid secretion, turnover of the mucosal barriers along the GIT, production of digestive enzymes, cell proliferation, and nutrient transport in the small intestines (Konturek et al. 2011). Under ad libitum feeding conditions, the circadian rhythm in eating behavior dovetails nicely with the circadian rhythm in digestive processes so that the animal can easily digest what it has eaten. Given these factors, there are times of the day when, despite motivation, the gut may simply not be prepared to receive a meal. This might be the case when arriving in a new time zone and eating with the local population even though it is not your own mealtime.
5.3 Anticipatory Behavior Entails Circadian Timing
When food is not available ad libitum, but rather is regularly available at a specific time of day, animals will reorganize their activity (and physiology) so as to anticipate these scheduled meals (Stephan et al. 1979; Antle and Silver 2009). An example of regularly timed food availability in nature is seen in the rabbit doe and her pups. In nature, the mother rabbit nurses her pups with a circadian rhythm, returning to her nest for only about 3 min once each day (reviewed in González-Mariscal et al. 2015). During this brief window, the pups must consume all the milk that they will need to sustain them for the following 24 h. Rabbit pups anticipate the opportunity to nurse, as can be measured by increased movements just prior to feeding time (Jilge 1993).
As discussed above, the SCN is the master circadian oscillator, and it is synchronized to our environmental cycles primarily by light. However, anticipation of scheduled feeding is not regulated by the SCN, as this motivated behavior persists even in rats in which the SCN has been lesioned (Stephan et al. 1979; Mistlberger 1994). If an animal with an SCN lesion is deprived of food after exposure to a restricted feeding schedule, these animals will initially exhibit a bout of activity in anticipation of the normal mealtime. This activity disappears when it is clear that the meal has been missed. On the subsequent day, the anticipatory activity re-emerges at the correct time, clearly indicating a circadian clock phenomenon, despite the loss of the master circadian clock in the SCN. Such evidence points to the existence of a food-entrainable circadian oscillator(s) in other tissues.
5.4 Extra-SCN Circadian Oscillators Support Anticipatory Behaviors
Feeding can uncouple light- and food-entrained circadian timing. The phase of the SCN does not appear strongly influenced by scheduled feeding (Damiola et al. 2000; Stokkan et al. 2001; Challet et al. 2003). In contrast, the phases of other oscillators in the body, such as the liver (Stokkan et al. 2001) and stomach (LeSauter et al. 2009), are strongly affected by scheduled feeding.
5.5 Oscillators Mediating Anticipation of Feeding
A number of organs and brain areas exhibit circadian rhythms in SCN-lesioned animals placed on a restricted feeding schedule. These include the DMH (Gooley et al. 2006; Mieda et al. 2006; Verwey et al. 2007, 2008), dorsal striatum and nucleus accumbens (Angeles-Castellanos et al. 2007; Verwey and Amir 2011), the cerebral cortex and hippocampus (Wakamatsu et al. 2001), the stomach (LeSauter et al. 2009), and the liver (Stokkan et al. 2001).
5.6 Brain Oscillators
In the brain, the DMH was an attractive area to serve as a node for regulating anticipation of daily meals. It receives input indirectly from the SCN and is responsive to a number of endocrine signals related to energy state (Chou et al. 2003). It also relays such signals to important sleep/wake areas such as the VLPO (Saper et al. 2005a). The circadian rhythm of expression of a number of genes is shifted in the DMH when animals are placed on a restricted feeding schedule or have ad libitum access to regular chow but, in addition, are given a “treat” or reward at the same time every day. Lesioning the DMH was initially reported to abolish anticipation of scheduled meals (Gooley et al. 2006); however, subsequent reports revealed that clear anticipation persisted in animals with unambiguous DMH lesions (Landry et al. 2006, 2007, 2011). While it is apparently not necessary and sufficient, the DMH may still participate in anticipation, as it can override daytime SCN signals that might drive sleep, thus permitting anticipatory activity at a time of day when wake and activity would not normally occur (Acosta-Galvan et al. 2011).
5.7 Peripheral Oscillators
In addition to oscillators in the brain, peripheral organs have circadian rhythms that can provide timing cues related to feeding. Daily signals participating in anticipation of scheduled daily meals may originate in the stomach’s parietal cells (i.e., oxyntic or delomorphous cells) that release ghrelin . Ghrelin is a potent orexigenic, stimulating feeding behavior when administered to rats, mice, and humans (Nakazato et al. 2001; Wren et al. 2001a, b; LeSauter et al. 2009). Ghrelin rises in people in anticipation of their regular mealtimes (Cummings et al. 2001). Ghrelin-containing cells in the stomach exhibit circadian rhythms in the levels of PER1 and PER2 proteins, integral components of the intracellular clock (LeSauter et al. 2009). This rhythmic expression can be synchronized to scheduled mealtimes (LeSauter et al. 2009). Anticipation of a daily meal is reduced, but not eliminated, in ghrelin receptor knockout animals (Blum et al. 2009; LeSauter et al. 2009).
5.8 Disrupted Circadian Control: Night Eating Syndrome
While eating time varies among cultures, in all cases eating is regulated in a homeostatic and a circadian fashion. People generally consume three meals each day, and these usually occur at regular times of the day. Typically, feeding behavior ends in the evening. However, in patients with night eating syndrome , at least 25 % of their daily calories are consumed after the major evening meal, or they may interrupt their sleep multiple times a week to consume food (Depner et al. 2014). These patients also typically reduce their calorie intake in the mornings. These symptoms are consistent with a phase-delayed circadian clock. As their sleep cycles are not shifted relative to the typical population (O’Reardon et al. 2004), this may represent a uncoupling of various clock systems. The phasing and amplitude of a number of physiological and hormonal rhythms are altered in night eating syndrome (Goel et al. 2009). Morning anorexia, a frequent symptom of night eating syndrome, is associated with poor glycemic control and higher body mass index in diabetic patients (Reutrakul et al. 2014). Eating during the normal sleep phase has been associated with greater weight gain in mice (Arble et al. 2009). This is not surprising as the circadian system and metabolism must be aligned for optimal energy balance (Waterhouse et al. 2005). Consuming calories at night when the body is storing energy may lead to greater weight gain (Arble et al. 2009). Interventions that realign the circadian system could treat night eating syndrome and improve health in these patients (Goel et al. 2009).
In summary, feeding appears to be the most important motivated behavior from a circadian perspective. Animals will leave their temporal niche and reorganize their daily behavioral rhythms when food is only available during the day (Stephan 2002). Feeding influences many of the oscillators of the body. While the master circadian clock in the SCN appears to remain relatively synchronized to the light:dark cycle, the phases of other oscillators appear to be more heavily influenced by scheduled feeding. Thus, the coupling between the SCN and extra-SCN oscillators is modified by scheduled feeding. While the location of the master circadian clock is known, evidence clearly demonstrates that the SCN is not the food clock. The location of the food-entrainable oscillator(s) is still unknown (Mistlberger and Antle 2011). Given that food is such a strong circadian signal, manipulations of mealtiming may be useful to expedite re-entrainment to new rotating shift work schedules, or following transmeridian travel. Shifting mealtimes to match up with a shift in the light:dark cycle can shorten the time needed to synchronize to a new time zone and overcome jet lag (Angeles-Castellanos et al. 2011). To do this, one can eat at the same time as the locals when arriving in a new time zone. If this is difficult as you may not feel hungry, it is recommended that you skip a meal to facilitate re-entrainment. However, given that your digestive system may not be prepared to digest a meal given the misalignment between your circadian clock and the local time, such meals should be smaller and should consist of foods that are easy to digest.
6 Mating/Sex
6.1 Sexual Behavior is Highly Motivated and is Under Temporal Control
Sexual behaviors in male and female animals are highly motivated, and various factors that influence sexual motivation are described in the chapter by Margarinos and Pfaff in this volume. The circadian system is a major regulator of sexual behavior. Given the differences in male and female sexual behavior, it should not be surprising that mating and reproduction are regulated by the circadian system in a sexually dimorphic manner.
Male sexual behavior exhibits a circadian rhythm. Under ad libitum access to sexually receptive females, male rats exhibit 80 % of their mounting, intromissions, and ejaculations during the night (Logan and Leavitt 1992). The timing of sexual behavior during the night may be species specific, with the peak of mating behavior occurring in the early (Logan and Leavitt 1992) or middle (Lisk 1969) portions of the night in rats, but late in the night in deer mice (Dewsbury 1981).
Motivated sexual behavior in female rodents is gated by two biological rhythms. It is most closely tied to the infradian estrus rhythm. Female rats are only sexually receptive once every 4–5 days. This sexual receptivity is gated by a luteinizing hormone (LH) surge that triggers ovulation and the transition from proestrus to estrus. However, timing of the LH surge is under control of the circadian clock (Williams and Kriegsfeld 2012). In free-running conditions where there are no external time cues, the LH surge tracks and precedes activity onset (Fitzgerald and Zucker 1976). As such, sexual receptivity in female rodents is greatest during their active phase (i.e., night in nocturnal rodents). If the temporal window during which the LH surge can occur is closed by treating the female with a barbiturate just prior to when the LH surge should occur, the surge and subsequent ovulation are both delayed until the gate opens at the same time the following day (Everett and Sawyer 1950; Alleva et al. 1971; Siegel et al. 1976; Stetson and Watson-Whitmyre 1977). However, when estradiol is held at a constantly high level, there does not appear to be a circadian rhythm in sexual receptivity, suggesting that the circadian clock controls the timing of the LH surge, while sexual receptivity is regulated by estradiol levels. While these are normally linked sequentially, the circadian clock does not appear to directly regulate sexual motivation in female rodents.
We have suggested that the circadian clock may independently regulate both specific goals and the associated arousal with respect to motivated behaviors. There is a clear interaction between the circadian cycle and the estrus cycle in terms of both timing and amplitude of motivated behaviors. Wheel running and intracranial self-stimulation both show highest levels during the night (Steiner et al. 1981). However, the levels of each of these behaviors are significantly higher on the night when female rats transition from diestrus to estrus (Steiner et al. 1981). This is not simply an increase in general arousal or activity, as other behaviors such as general locomotion, rearing, and grooming do not exhibit increases in their amplitude on the same night.
6.2 Interaction Between Sex and Other Motivated Behaviors
When the opportunity to engage in a variety of motivated behaviors is provided, some will take priority over others. When presented simultaneously with food and a receptive mate following long-term deprivation of both (6 days), male rats will choose to mate before eating (Sachs and Marsan 1972). Under situations where there is free access to a running wheel and sexually receptive females, males will reduce the amount and delay the onset of their wheel running and instead engage in sexual behavior (Logan and Leavitt 1992). This suggests that motivated behaviors are partitioned during the waking period and that pursuit of one goal (i.e., mating) occurs at the expense of another (i.e., wheel running and eating).
The circadian clock may not regulate sexual performance and sexual motivation to the same extent. Just as other example behaviors, anticipatory motivation and consummatory activity appear to involve distinct mechanisms. van Furth and van Ree (1994) suggest that sexual performance in male rats is tightly controlled by the circadian clock, with poor performance and longer refractory periods during their daytime rest phase. Motivation for sexual behavior can be measured using the bi-level mating chamber (Mendelson and Pfaus 1989). This chamber has two levels, one above the other, connected at each end by stairs, thus ensuring that the female can always choose to either approach or avoid the male. Once experienced with mating in this chamber, male rats alone in the chamber show behaviors that suggest searching the chamber for the female. This anticipatory behavior manifests as constant changing between the levels. The changing between levels is not observed in males that have not associated this chamber with mating. Using this task, there does not appear to be a circadian rhythm of sexual motivation in male rats, in that they show just as many anticipatory level changes during the day as during the night (van Furth and van Ree 1994). In females hamsters, proceptive behaviors (i.e., exploration/approach, as scored by time spent with intact vs. castrated males, and used as a metric of sexual motivation) and consummatory behaviors (i.e., lordosis) are regulated by distinct brain regions; exploratory/approach sexual behavior, but not lordosis, is reduced by infusion of gonadotropin-inhibitory hormone (Piekarski et al. 2013). Given that gonadotropin-inhibitory hormone is involved in circadian regulation of the LH surge (Gibson et al. 2008; Williams et al. 2011), this mechanism enables synchronizing of ovulation and regulation of exploratory/approach sexual behavior.
6.3 Anticipatory Activity
Male rats will anticipate a scheduled daily opportunity to mate (Landry et al. 2012). When the daily opportunity to mate occurs at night, robust anticipation is observed. Rats also exhibited a strong conditioned place preference to the mating chamber when scheduled mating happens at night (Landry et al. 2012). Anticipation is also observed when the scheduled mating occurs during the light phase, a time of day when rats are much less likely to engage in mating (Logan and Leavitt 1992). In this case, it is not clear if it is the mating itself that is affecting the clock. In the daytime scheduled mating paradigm, the male rats will engage in post-coital feeding and running. As discussed above, the circadian system can anticipate daily opportunities to feed, and the circadian clock is influenced by exercise. When opportunities to engage in these post-coital motivated behaviors (feeding and exercise) were prevented, male rats rarely anticipated the scheduled daytime mating opportunity (Landry et al. 2012), suggesting that the observed anticipation of daytime mating may have been related to the exercise and feeding after sex, rather than the mating itself. Similarly, the male rats show no conditioned place preference to the mating chamber when mating is scheduled during the day. These data suggest that while the animal can anticipate many motivated behaviors, when these motivated behaviors fall outside their normal ecological time, only some of these behavior (i.e., feeding), and not other (i.e., sexual behavior) have a strong effect on the circadian system.
6.4 Feedback of Sexual Behavior to the Circadian System
Sociosexual cues can also influence the circadian clock. Exposure to a receptive female during the sleep phase can accelerate a male hamsters’ re-entrainment to an advanced light:dark cycle (Honrado and Mrosovsky 1989) and can induce phase shifts (Mrosovsky 1988), but only if mating is prevented. It is likely that it is the associated enduring arousal (Antle and Mistlberger 2000) rather than the sociosexual cue itself that elicits the phase shift.
7 Maternal Behavior
Maternal care of offspring is a strongly motivated behavior in which the emergence of the motivation is closely tied to hormonal changes that accompany giving birth (Numan 2007). Maternal care in rodents includes nursing and licking/grooming of her pups. With respect to circadian rhythms, most attention has focused on nursing behavior. Dams engage in more nursing behavior during the day when they are inactive (Grota and Ader 1969; Pachón et al. 1995). Mice also crouch over their pups more during the day, but also show minor peaks in crouching behavior starting an hour after dark onset, and another peak beginning about midway through the dark phase. Prominent troughs in crouching behavior coincide with dawn and dusk (Hoshino et al. 2006).
7.1 Feedback of Maternal Behavior on the Circadian Clock
Nursing behavior can entrain the circadian clocks of neonatal mice, hamsters, and rabbits (Viswanathan and Chandrashekaran 1985; Viswanathan and Davis 1992; Jilge 1993; Viswanathan 1999; Caba and Gonzalez-Mariscal 2009). This phenomenon is most stunning in rabbits, where the doe only nurses her pups a single time each day for about 3–5 min. This nursing bout occurs at the same time each day, and the pups anticipate her arrival by increasing movements in the nest. They also show anticipatory increases in temperature, corticosterone secretion, and circulating ghrelin levels (Caba and Gonzalez-Mariscal 2009). During the brief feeding bout, they consume about 35 % of their body weight, leading to prominent distension of their stomach (Caba and Gonzalez-Mariscal 2009). All of this occurs in darkness in their burrow before pups are ever exposed to light.
7.2 Neural Basis of Anticipation of Maternal Care
This daily nursing can affect the brain of both the pups and the mothers. In female rabbits, there is a circadian rhythm of PER1 expression in dopaminergic neurons that regulate prolactin secretion. This rhythm peaks in the early night in non-nursing females, but shifts to align with the time of nursing in females that are nursing (Meza et al. 2011). While the circadian rhythm of PER1 protein in the SCN is not shifted in nursing does, the amplitude of the PER1 expression rhythm is lower relative to non-nursing does. In pups fed at the same time each day, clock gene expression (Per1, Per2 and Bmal1) is entrained. When feeding time is shifted, SCN clock gene expression rhythms exhibit a corresponding shift to follow feeding time (Caldelas et al. 2009). Entrainment appears to use both food and social cues. The circadian clock can be entrained by artificial feeding through a tube surgically implanted into the stomach (Morgado et al. 2011). However, olfactory cues from the dam are also critical, as when fed milk formula by gavage at the same time every day, the pups only anticipate this feeding when it is accompanied by natural or artificial maternal pheromones (Montúfar-Chaveznava et al. 2013).
7.3 Cellular Clock and Maternal Care
While a circadian clock organizes maternal nursing, it is also important for proper maternal care. Mice that carry the delta19 clock gene mutation exhibit alterations in maternal care. Both homozygous and heterozygous clock mutant moms have been studied. The occurrence of even a single mutant clock allele can compromise maternal behavior; heterozygous clock mothers are more active during the light phase, resulting in less nursing behavior during the light phase (Koizumi et al. 2013). Wild-type pups raised by homozygous clock mice grow less quickly (Hoshino et al. 2006). This is likely due to the fact that homozygous clock mothers have no rhythms in prolactin and produce less milk (Hoshino et al. 2006). Furthermore, this altered care appears to lead to alterations in adult behavior of pups raised by such mothers, even if they themselves have a normal clock gene. Specifically, wild-type mice reared by clock mutant moms appear more anxious as adults as assessed using the open field and elevated plus-maze (Koizumi et al. 2013).
8 Circadian Basis of Motivation Disorders
Circadian disruption due to jet lag , shift work , or disease can have numerous adverse health consequences (Foster and Kreitzman 2014). Circadian disruption may lead to dysregulation of the amplitude and direction/goal of motivated behaviors and physiological systems underlying motivated behaviors. The circadian disruption may result from extrinsic social or environmental factors, such as is the case for shift work, jet lag, or work/school start times out of phase with our internal clock. In other cases, the circadian disruption may be due to an internal disease state, such as depression (Karatsoreos 2014). When examining motivation and circadian disruption, there are three considerations. First, given that circadian rhythms dictate the phase of particular motivated behaviors (Fig. 1), when our circadian clock is out of phase with our environment, the wrong motivated behavior may be cued at a particular time, such as insomnia associated with jet lag. Second, imposed sleep and circadian disruption may alter motivational attributes for a particular behavior, such as the increased hunger and caloric intake associated with sleep deprivation (Brondel et al. 2010), even in cases where feeding time is properly phased. Finally, some diseases involve clear disruption of both circadian rhythms and overall motivation levels. It is possible that circadian disruption may worsen adherent motivation in various diseases. We explore three disease states where circadian disruption may be a key component of the altered motivational states that underlie the disease.
8.1 Depression and Schizophrenia
Depression and schizophrenia are both associated with diminished motivational levels. A key symptom of depression is anhedonia , or diminished experience of pleasure or reward. Patients with schizophrenia appear to suffer less from anhedonia than patients with depression (see Reddy et al. in this volume). However, both conditions are associated with a reduction in goal seeking and anticipatory motivation (Gard et al. 2014; Barch et al. in this volume). In both depression and schizophrenia, there are clear disruptions of the circadian system (Bunney and Potkin 2008; Wulff et al. 2012), and in many cases, disruption of sleep and circadian rhythm may exacerbate the symptoms of some psychiatric diseases (Gruber et al. 2011; Jagannath et al. 2013).
8.2 Attention Deficit Hyperactivity Disorder
Individuals with attention deficit hyperactivity disorder (ADHD) also exhibit motivational problems. Lack of engagement of the motivational and reward systems has been suggested to underlie ADHD (Wasserman and Wasserman 2015). Patients with ADHD constantly shift their focus; thus, behaviors are often not goal directed, or fail to maintain a goal. Similarly, hyperactivity might be considered pure motivational amplitude or drive without a goal. Up to 80 % of patients with ADHD may experience sleep and circadian disruptions (Van Veen et al. 2010; Kooij and Bijlenga 2013). Furthermore, these circadian changes can be exacerbated by some ADHD medications (Antle et al. 2012), and sleep problems can contribute to the major symptoms of ADHD, namely lack of attention and hyperactivity (Corkum et al. 2008).
8.3 Circadian Considerations for Treatment of Psychiatric Conditions
Many psychiatric disorders exhibit sleep and circadian problems, and treating the sleep problems does improve overall symptomatology (Jagannath et al. 2013; Karatsoreos 2014). Often, behavioral therapies for depression and insomnia include sleep hygiene components that include advice on when and when not to eat and exercise. Specifically, vigorous exercise , large meals, and alcohol consumption immediately before bedtime are contraindicated. That said engaging in motivated behaviors (i.e., eating and exercising) at the proper time of day may help diminish the symptoms of the disorder (Schroeder and Colwell 2013). For instance, remission from depression is much higher in patients that also engage in exercise (Belvederi Murri et al. 2015). Long-term exercise also helps improve insomnia (Passos et al. 2011). In this latter case, there was no difference between those who exercised in the morning versus the afternoon, suggesting that there may not be a best time of day to exercise, so long as you do not exercise right before sleep.
9 Conclusion
We have presented evidence that the circadian timing system is a critical feature of evolutionarily fundamental behaviors. Given that homeostatic behaviors that are critical to the survival of the organism and the species (e.g., feeding, drinking, sleeping, mating, and parenting) are highly pleasurable, it is interesting to consider the interface of the circadian system and limbic/prefrontal/striatal reward circuitry that regulates behavioral activation and effort-related functions (Robbins and Everitt 1996; Berridge and Kringelbach 2015).
The reward system of the brain participates in anticipation of regularly scheduled daily events, especially well documented for feeding behavior. For example, ghrelin has been implicated in full expression of anticipation of scheduled daily meals and appears to play a role in activating the reward system. Specifically, animals lacking ghrelin have reduced activation of cells (measured by FOS expression) in the ventral tegmental area and nucleus accumbens shell compared to wild-type animals when on feeding schedules (Lamont et al. 2012). Areas of the reward system (dorsal striatum and nucleus accumbens) rhythmically express clock genes (Wakamatsu et al. 2001), and regularly scheduled daytime meals can shift these rhythms (Angeles-Castellanos et al. 2007; Verwey and Amir 2011).
Perturbation of dopaminergic transmission alters the timing or amplitude of anticipatory activity (Liu et al. 2012; Smit et al. 2013). Loss of the D1 receptor attenuates anticipation of daily scheduled meals, even when the meal is palatable and high in fat (Gallardo et al. 2014). Viral replacement of D1 receptors to just the dorsal striatum restores anticipation of daily meals. Direct activation of D1 receptors in the dorsal striatum at the same time each day leads to anticipation of the injection time, as measured by high activity from a computer-scored video system (Gallardo et al. 2014). It appears that activation of D1 receptors in the dorsal striatum is both necessary and sufficient for anticipation of scheduled daily feeding, and possibly other rewarding situations. Consistent with these observations, daily rhythms in markers of dopaminergic activity have been observed in various mesolimbic and nigrostriatal target structures. “In rodents, rhythmic circadian clock gene expression has been observed in many reward-related brain regions, with the phase of peak expression depending both upon the gene and the brain structure examined” (see Table 3 in Webb et al. 2015). These studies suggest that dopamine signaling to D1R-expressing neurons in the dorsal striatum can synchronize circadian oscillators in the reward circuits, thereby modulating motivational processes and behavioral output.
Taken together, the evidence points to robust links between circadian clock genes, dopaminergic neurotransmission, and highly motivated responses. Optimally utilizing knowledge of these temporal parameters is likely to be useful in optimizing performance in a very broad array of behaviors.
References
Acosta-Galvan G, Yi CX, van der Vliet J, Jhamandas JH, Panula P, Angeles-Castellanos M, Del Carmen Basualdo M, Escobar C, Buijs RM (2011) Interaction between hypothalamic dorsomedial nucleus and the suprachiasmatic nucleus determines intensity of food anticipatory behavior. Proc Natl Acad Sci U S A 108:5813–5818
Alibhai FJ, Tsimakouridze EV, Reitz CJ, Pyle WG, Martino TA (2015) Consequences of circadian and sleep disturbances for the cardiovascular system. Can J Cardiol 31:860–872
Alleva JJ, Waleski MV, Alleva FR (1971) A biological clock controlling the estrous cycle of the hamster. Endocrinology 88:1368–1379
Angeles-Castellanos M, Mendoza J, Escobar C (2007) Restricted feeding schedules phase shift daily rhythms of c-Fos and protein Per1 immunoreactivity in corticolimbic regions in rats. Neuroscience 144:344–355
Angeles-Castellanos M, Amaya JM, Salgado-Delgado R, Buijs RM, Escobar C (2011) Scheduled food hastens re-entrainment more than melatonin does after a 6-h phase advance of the light-dark cycle in rats. J Biol Rhythms 26:324–334
Antle MC (2015) Sleep: neural systems. In: Wright JD (ed) International encyclopedia of the social & behavioral sciences, 2nd edn. Elsevier, Oxford, pp 87–93
Antle MC, Mistlberger RE (2000) Circadian clock resetting by sleep deprivation without exercise in the Syrian hamster. J Neurosci 20:9326–9332
Antle MC, Silver R (2005) Orchestrating time: arrangements of the brain circadian clock. Trends Neurosci 28:145–151
Antle MC, Silver R (2009) Neural basis of timing and anticipatory behaviors. Eur J Neurosci 30:1643–1649
Antle MC, van Diepen HC, Deboer T, Pedram P, Pereira RR, Meijer JH (2012) Methylphenidate modifies the motion of the circadian clock. Neuropsychopharmacology 37:2446–2455
Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW (2009) Circadian timing of food intake contributes to weight gain. Obesity 17:2100–2102
Bailey MR, Jensen G, Taylor K, Mezias C, Williamson C, Silver R, Simpson EH, Balsam PD (2015) A novel strategy for dissecting goal-directed action and arousal components of motivated behavior with a progressive hold-down task. Behav Neurosci 129:269–280
Baron KG, Reid KJ (2014) Circadian misalignment and health. Int Rev Psychiatry 26:139–154
Belvederi Murri M, Amore M, Menchetti M, Toni G, Neviani F, Cerri M, Rocchi MB, Zocchi D, Bagnoli L, Tam E, Buffa A, Ferrara S, Neri M, Alexopoulos GS, Zanetidou S (2015) Physical exercise for late-life major depression. Br J Psychiatry. doi:10.1192/bjp.bp.114.150516
Berridge KC, Kringelbach ML (2015) Pleasure systems in the brain. Neuron 86:646–664
Birch LL, Billman J, Richards SS (1984) Time of day influences food acceptability. Appetite 5:109–116
Blanco-Centurion C, Xu M, Murillo-Rodriguez E, Gerashchenko D, Shiromani AM, Salin-Pascual RJ, Hof PR, Shiromani PJ (2006) Adenosine and sleep homeostasis in the basal forebrain. J Neurosci 26:8092–8100
Blum ID, Patterson Z, Khazall R, Lamont EW, Sleeman MW, Horvath TL, Abizaid A (2009) Reduced anticipatory locomotor responses to scheduled meals in ghrelin receptor deficient mice. Neuroscience 164:351–359
Bobrzynska KJ, Mrosovsky N (1998) Phase shifting by novelty-induced running: activity dose-response curves at different circadian times. J Comp Physiol A 182:251–258
Borbély AA (1982) A two process model of sleep regulation. Hum Neurobiol 1:195–204
Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D (2010) Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr 91:1550–1559
Brown RE, Stevens DR, Haas HL (2001) The physiology of brain histamine. Prog Neurobiol 63:637–672
Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW (2012) Control of sleep and wakefulness. Physiol Rev 92:1087–1187
Bunney JN, Potkin SG (2008) Circadian abnormalities, molecular clock genes and chronobiological treatments in depression. Br Med Bull 86:23–32
Caba M, Gonzalez-Mariscal G (2009) The rabbit pup, a natural model of nursing-anticipatory activity. Eur J Neurosci 30:1697–1706
Cai A, Lehman MN, Lloyd JM, Wise PM (1997) Transplantation of fetal suprachiasmatic nuclei into middle-aged rats restores diurnal Fos expression in host. Am J Physiol 272:R422–R428
Caldelas I, Gonzalez B, Montufar-Chaveznava R, Hudson R (2009) Endogenous clock gene expression in the suprachiasmatic nuclei of previsual newborn rabbits is entrained by nursing. Dev Neurobiol 69:47–59
Challet E, Caldelas I, Graff C, Pevet P (2003) Synchronization of the molecular clockwork by light- and food-related cues in mammals. Biol Chem 384:711–719
Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J (2003) Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J Neurosci 23:10691–10702
Corkum P, Panton R, Ironside S, Macpherson M, Williams T (2008) Acute impact of immediate release methylphenidate administered three times a day on sleep in children with attention-deficit/hyperactivity disorder. J Pediatr Psychol 33:368–379
Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS (2001) A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50:1714–1719
Czeisler CA, Weitzman E, Moore-Ede MC, Zimmerman JC, Knauer RS (1980) Human sleep: its duration and organization depend on its circadian phase. Science 210:1264–1267
Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U (2000) Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14:2950–2961
Depner CM, Stothard ER, Wright KP Jr (2014) Metabolic consequences of sleep and circadian disorders. Curr Diab Rep 14:507
Dewsbury DA (1981) Social dominance, copulatory behavior, and differential reproduction in deer mice (Peromyscus maniculatus). J Comp Physiol Psych 95:880–895
Duffy E (1957) The psychological significance of the concept of “arousal” or “activation”. Psychol Rev 64:265–275
Edgar DM, Dement WC (1991) Regularly scheduled voluntary exercise synchronizes the mouse circadian clock. Am J Physiol 261:R928–R933
Eikelboom R, Lattanzio SB (2003) Wheel access duration in rats: II. Day-night and within-session changes. Behav Neurosci 117:825–832
Everett JW, Sawyer CH (1950) A 24-hour periodicity in the “LH-release apparatus” of female rats, disclosed by barbiturate sedation. Endocrinology 47:198–218
Fitzgerald K, Zucker I (1976) Circadian organization of the estrous cycle of the golden hamster. Proc Natl Acad Sci U S A 73:2923–2927
Foster RG, Kreitzman L (2014) The rhythms of life: what your body clock means to you! Exp Physiol 99:599–606
Friedman L, Bergmann BM, Rechtschaffen A (1979) Effects of sleep deprivation on sleepiness, sleep intensity, and subsequent sleep in the rat. Sleep 1:369–391
Gallardo CM, Darvas M, Oviatt M, Chang CH, Michalik M, Huddy TF, Meyer EE, Shuster SA, Aguayo A, Hill EM, Kiani K, Ikpeazu J, Martinez JS, Purpura M, Smit AN, Patton DF, Mistlberger RE, Palmiter RD, Steele AD (2014) Dopamine receptor 1 neurons in the dorsal striatum regulate food anticipatory circadian activity rhythms in mice. Elife 3:e03781
Gard DE, Sanchez AH, Cooper K, Fisher M, Garrett C, Vinogradov S (2014) Do people with schizophrenia have difficulty anticipating pleasure, engaging in effortful behavior, or both? J Abnorm Psychol 123:771–782
Gibson EM, Humber SA, Jain S, Williams WP 3rd, Zhao S, Bentley GE, Tsutsui K, Kriegsfeld LJ (2008) Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology 149:4958–4969
Goel N, Stunkard AJ, Rogers NL, Van Dongen HP, Allison KC, O’Reardon JP, Ahima RS, Cummings DE, Heo M, Dinges DF (2009) Circadian rhythm profiles in women with night eating syndrome. J Biol Rhythms 24:85–94
González-Mariscal G, Caba M, Martinez-Gómez M, Bautista A, Hudson R (2015) Mothers and offspring: the rabbit as a model system in the study of mammalian maternal behavior and sibling interactions. Horm Behav, doi:10.1016/j.yhbeh.2015.05.011
Gooley JJ, Schomer A, Saper CB (2006) The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci 9:398–407
Gore BB, Zweifel LS (2013) Genetic reconstruction of dopamine D1 receptor signaling in the nucleus accumbens facilitates natural and drug reward responses. J Neurosci 33:8640–8649
Greer SM, Goldstein AN, Walker MP (2013) The impact of sleep deprivation on food desire in the human brain. Nat Commun 4:2259
Grota LJ, Ader R (1969) Continuous recording of maternal behaviour in Rattus norvegicus. Anim Behav 17:722–729
Gruber J, Miklowitz DJ, Harvey AG, Frank E, Kupfer D, Thase ME, Sachs GS, Ketter TA (2011) Sleep matters: sleep functioning and course of illness in bipolar disorder. J Affect Disord 134:416–420
Harma MI, Hakola T, Akerstedt T, Laitinen JT (1994) Age and adjustment to night work. Occup Environ Med 51:568–573
Hebb DO (1955) Drives and the C. N. S. (conceptual nervous system). Psychol Rev 62:243–254
Honrado G, Mrosovsky N (1989) Arousal by sexual stimuli accelerates the re-entrainment of hamsters to phase advanced light-dark cycles. Behav Ecol Sociobiol 25:57–63
Hoshino K, Wakatsuki Y, Iigo M, Shibata S (2006) Circadian clock mutation in dams disrupts nursing behavior and growth of pups. Endocrinology 147:1916–1923
Hurd MW, Zimmer KA, Lehman MN, Ralph MR (1995) Circadian locomotor rhythms in aged hamsters following suprachiasmatic transplant. Am J Physiol 269:R958–R968
Jagannath A, Peirson SN, Foster RG (2013) Sleep and circadian rhythm disruption in neuropsychiatric illness. Curr Opin Neurobiol 23:888–894
Janik D, Mrosovsky N (1993) Nonphotically induced phase shifts of circadian rhythms in the golden hamster: activity-response curves at different ambient temperatures. Physiol Behav 53:431–436
Jilge B (1993) The ontogeny of circadian rhythms in the rabbit. J Biol Rhythms 8:247–260
Juda M, Vetter C, Roenneberg T (2013) Chronotype modulates sleep duration, sleep quality, and social jet lag in shift-workers. J Biol Rhythms 28:141–151
Karatsoreos IN (2014) Links between circadian rhythms and psychiatric disease. Front Behav Neurosci 8:162
Kersten A, Strubbe JH, Spiteri NJ (1980) Meal patterning of rats with changes in day length and food availability. Physiol Behav 25:953–958
Klerman EB, Boulos Z, Edgar DM, Mistlberger RE, Moore-Ede MC (1999) Circadian and homeostatic influences on sleep in the squirrel monkey: sleep after sleep deprivation. Sleep 22:45–59
Koizumi H, Kurabayashi N, Watanabe Y, Sanada K (2013) Increased anxiety in offspring reared by circadian clock mutant mice. PLoS ONE 8:e66021
Konturek PC, Brzozowski T, Konturek SJ (2011) Gut clock: implication of circadian rhythms in the gastrointestinal tract. J Physiol Pharmacol 62:139–150
Kooij JJ, Bijlenga D (2013) The circadian rhythm in adult attention-deficit/hyperactivity disorder: current state of affairs. Expert Rev Neurother 13:1107–1116
Kramer FM, Rock K, Engell D (1992) Effects of time of day and appropriateness on food intake and hedonic ratings at morning and midday. Appetite 18:1–13
Kriegsfeld LJ, Leak RK, Yackulic CB, LeSauter J, Silver R (2004) Organization of suprachiasmatic nucleus projections in Syrian hamsters (Mesocricetus auratus): an anterograde and retrograde analysis. J Comp Neurol 468:361–379
Krout KE, Kawano J, Mettenleiter TC, Loewy AD (2002) CNS inputs to the suprachiasmatic nucleus of the rat. Neuroscience 110:73–92
Krystal AD, Richelson E, Roth T (2013) Review of the histamine system and the clinical effects of H1 antagonists: basis for a new model for understanding the effects of insomnia medications. Sleep Med Rev 17:263–272
Lamont EW, Patterson Z, Rodrigues T, Vallejos O, Blum ID, Abizaid A (2012) Ghrelin-deficient mice have fewer orexin cells and reduced cFOS expression in the mesolimbic dopamine pathway under a restricted feeding paradigm. Neuroscience 218:12–19
Landry GJ, Simon MM, Webb IC, Mistlberger RE (2006) Persistence of a behavioral food-anticipatory circadian rhythm following dorsomedial hypothalamic ablation in rats. Am J Physiol Regul Integr Comp Physiol 290:R1527–R1534
Landry GJ, Yamakawa GR, Webb IC, Mear RJ, Mistlberger RE (2007) The dorsomedial hypothalamic nucleus is not necessary for the expression of circadian food-anticipatory activity in rats. J Biol Rhythms 22:467–478
Landry GJ, Kent BA, Patton DF, Jaholkowski M, Marchant EG, Mistlberger RE (2011) Evidence for time-of-day dependent effect of neurotoxic dorsomedial hypothalamic lesions on food anticipatory circadian rhythms in rats. PLoS ONE 6:e24187
Landry GJ, Opiol H, Marchant EG, Pavlovski I, Mear RJ, Hamson DK, Mistlberger RE (2012) Scheduled daily mating induces circadian anticipatory activity rhythms in the male rat. PLoS ONE 7:e40895
Lattanzio SB, Eikelboom R (2003) Wheel access duration in rats: I. Effects on feeding and running. Behav Neurosci 117:496–504
Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL (1987) Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J Neurosci 7:1626–1638
LeSauter J, Hoque N, Weintraub M, Pfaff DW, Silver R (2009) Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proc Natl Acad Sci U S A 106:13582–13587
Lim MM, Gerstner JR, Holtzman DM (2014) The sleep-wake cycle and Alzheimer’s disease: what do we know? Neurodegener Dis Manag 4:351–362
Lisk RD (1969) Cyclic fluctuations in sexual responsiveness in the male rat. J Exp Zool 171:313–319
Liu YY, Liu TY, Qu WM, Hong ZY, Urade Y, Huang ZL (2012) Dopamine is involved in food-anticipatory activity in mice. J Biol Rhythms 27:398–409
Logan FA, Leavitt F (1992) Sexual free behavior in male rats (Rattus norvegicus). J Comp Psychol 106:37–42
Looy H, Eikelboom R (1989) Wheel running, food intake, and body weight in male rats. Physiol Behav 45:403–405
Mahoney CE, Brewer JM, Bittman EL (2013) Central control of circadian phase in arousal-promoting neurons. PLoS ONE 8:e67173
Marchant EG, Mistlberger RE (1996) Entrainment and phase shifting of circadian rhythms in mice by forced treadmill running. Physiol Behav 60:657–663
Meijer JH, Robbers Y (2014) Wheel running in the wild. Proc Biol Sci 281
Mendelson SD, Pfaus JG (1989) Level searching: a new assay of sexual motivation in the male rat. Physiol Behav 45:337–341
Meza E, Waliszewski SM, Caba M (2011) Circadian nursing induces PER1 protein in neuroendocrine tyrosine hydroxylase neurones in the rabbit doe. J Neuroendocrinol 23:472–480
Mieda M, Williams SC, Richardson JA, Tanaka K, Yanagisawa M (2006) The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker. Proc Natl Acad Sci U S A 103:12150–12155
Mistlberger RE (1994) Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci Biobehav Rev 18:171–195
Mistlberger RE (2005) Circadian regulation of sleep in mammals: role of the suprachiasmatic nucleus. Brain Res Brain Res Rev 49:429–454
Mistlberger RE, Antle MC (2011) Entrainment of circadian clocks in mammals by arousal and food. Essays Biochem 49:119–136
Mistlberger RE, Marchant EG, Sinclair SV (1996) Nonphotic phase-shifting and the motivation to run: cold exposure reexamined. J Biol Rhythms 11:208–215
Mistlberger RE, Landry GJ, Marchant EG (1997) Sleep deprivation can attenuate light-induced phase shifts of circadian rhythms in hamsters. Neurosci Lett 238:5–8
Model Z, Butler MP, LeSauter J, Silver R (2015) Suprachiasmatic nucleus as the site of androgen action on circadian rhythms. Horm Behav 73:1–7
Mollicone DJ, Van Dongen HP, Rogers NL, Banks S, Dinges DF (2010) Time of day effects on neurobehavioral performance during chronic sleep restriction. Aviat Space Environ Med 81:735–744
Montúfar-Chaveznava R, Trejo-Muñoz L, Hernández-Campos O, Navarrete E, Caldelas I (2013) Maternal olfactory cues synchronize the circadian system of artificially raised newborn rabbits. PLoS ONE 8:e74048
Moore RY, Eichler VB (1972) Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res 42:201–206
Morgado E, Juarez C, Melo AI, Dominguez B, Lehman MN, Escobar C, Meza E, Caba M (2011) Artificial feeding synchronizes behavioral, hormonal, metabolic and neural parameters in mother-deprived neonatal rabbit pups. Eur J Neurosci 34:1807–1816
Mrosovsky N (1988) Phase response curves for social entrainment. J Comp Physiol A 162:35–46
Mrosovsky N, Biello SM (1994) Nonphotic phase shifting in the old and the cold. Chronobiol Int 11:232–252
Mueller DT, Herman G, Eikelboom R (1999) Effects of short- and long-term wheel deprivation on running. Physiol Behav 66:101–107
Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S (2001) A role for ghrelin in the central regulation of feeding. Nature 409:194–198
Numan M (2007) Motivational systems and the neural circuitry of maternal behavior in the rat. Dev Psychobiol 49:12–21
Ondo WG (2014) Sleep/wake problems in Parkinson’s disease: pathophysiology and clinicopathologic correlations. J Neural Transm 121(Suppl 1):S3–13
O’Reardon JP, Ringel BL, Dinges DF, Allison KC, Rogers NL, Martino NS, Stunkard AJ (2004) Circadian eating and sleeping patterns in the night eating syndrome. Obes Res 12:1789–1796
Pachón H, McGuire MK, Rasmussen KM (1995) Nutritional status and behavior during lactation. Physiol Behav 58:393–400
Passos GS, Poyares D, Santana MG, D’Aurea CV, Youngstedt SD, Tufik S, de Mello MT (2011) Effects of moderate aerobic exercise training on chronic primary insomnia. Sleep Med 12:1018–1027
Pauls S, Foley NC, Foley DK, LeSauter J, Hastings MH, Maywood ES, Silver R (2014) Differential contributions of intra-cellular and inter-cellular mechanisms to the spatial and temporal architecture of the suprachiasmatic nucleus circadian circuitry in wild-type, cryptochrome-null and vasoactive intestinal peptide receptor 2-null mutant mice. Eur J Neurosci 40:2528–2540
Pendergast JS, Branecky KL, Huang R, Niswender KD, Yamazaki S (2014) Wheel-running activity modulates circadian organization and the daily rhythm of eating behavior. Front Psychol 5:177
Pfaff DW, Martin EM, Faber D (2012) Origins of arousal: roles for medullary reticular neurons. Trends Neurosci 35:468–476
Piekarski DJ, Zhao S, Jennings KJ, Iwasa T, Legan SJ, Mikkelsen JD, Tsutsui K, Kriegsfeld LJ (2013) Gonadotropin-inhibitory hormone reduces sexual motivation but not lordosis behavior in female Syrian hamsters (Mesocricetus auratus). Horm Behav 64:501–510
Porkka-Heiskanen T, Alanko L, Kalinchuk A, Stenberg D (2002) Adenosine and sleep. Sleep Med Rev 6:321–332
Ralph MR, Foster RG, Davis FC, Menaker M (1990) Transplanted suprachiasmatic nucleus determines circadian period. Science 247:975–978
Ramkisoensing A, Meijer JH (2015) Synchronization of biological clock neurons by light and peripheral feedback systems promotes circadian rhythms and health. Front Neurol 6:128
Reinberg A, Motohashi Y, Bourdeleau P, Andlauer P, Levi F, Bicakova-Rocher A (1988) Alteration of period and amplitude of circadian rhythms in shift workers. With special reference to temperature, right and left hand grip strength. Eur J Appl Physiol Occup Physiol 57:15–25
Reutrakul S, Hood MM, Crowley SJ, Morgan MK, Teodori M, Knutson KL (2014) The relationship between breakfast skipping, chronotype, and glycemic control in type 2 diabetes. Chronobiol Int 31:64–71
Richard JM, Castro DC, Difeliceantonio AG, Robinson MJ, Berridge KC (2013) Mapping brain circuits of reward and motivation: in the footsteps of Ann Kelley. Neurosci Biobehav Rev 37:1919–1931
Richter SH, Gass P, Fuss J (2014) Resting is rusting: a critical view on rodent wheel-running behavior. Neuroscientist 20:313–325
Robbins TW, Everitt BJ (1996) Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol 6:228–236
Robinson I, Reddy AB (2014) Molecular mechanisms of the circadian clockwork in mammals. FEBS Lett 588:2477–2483
Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn M, Merrow M (2007) Epidemiology of the human circadian clock. Sleep Med Rev 11:429–438
Sachs B, Marsan E (1972) Male rats prefer sex to food after 6 days of food deprivation. Psychon Sci 28:47–49
Saini C, Brown SA, Dibner C (2015) Human peripheral clocks: applications for studying circadian phenotypes in physiology and pathophysiology. Front Neurol 6:95
Salamone J (1988) Dopaminergic involvement in activational aspects of motivation: Effects of haloperidol on schedule-induced activity, feeding, and foraging in rats. Psychobiology 16:196–206
Saper CB, Lu J, Chou TC, Gooley J (2005a) The hypothalamic integrator for circadian rhythms. Trends Neurosci 28:152–157
Saper CB, Scammell TE, Lu J (2005b) Hypothalamic regulation of sleep and circadian rhythms. Nature 437:1257–1263
Schmidt TM, Chen SK, Hattar S (2011) Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci 34:572–580
Schroeder AM, Colwell CS (2013) How to fix a broken clock. Trends Pharmacol Sci 34:605–619
Sherwin CM (1998) Voluntary wheel running: a review and novel interpretation. Anim Behav 56:11–27
Siegel PS (1961) Food intake in the rat in relation to the dark-light cycle. J Comp Physiol Psych 54:294–301
Siegel HI, Bast JD, Greenwald GS (1976) The effects of phenobarbital and gonadal steroids on periovulatory serum levels of luteinizing hormone and follicle-stimulating hormone in the hamster. Endocrinology 98:48–55
Sinclair SV, Mistlberger RE (1997) Scheduled activity reorganizes circadian phase of Syrian hamsters under full and skeleton photoperiods. Behav Brain Res 87:127–137
Smit AN, Patton DF, Michalik M, Opiol H, Mistlberger RE (2013) Dopaminergic regulation of circadian food anticipatory activity rhythms in the rat. PLoS ONE 8:e82381
Spiegel K, Tasali E, Penev P, Van Cauter E (2004) Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 141:846–850
Steiner M, Katz RJ, Baldrighi G, Carroll BJ (1981) Motivated behavior and the estrous cycle in rats. Psychoneuroendocrinology 6:81–90
Stephan FK (2002) The “other” circadian system: food as a Zeitgeber. J Biol Rhythms 17:284–292
Stephan FK, Zucker I (1972) Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A 69:1583–1586
Stephan FK, Swann JM, Sisk CL (1979) Anticipation of 24-hr feeding schedules in rats with lesions of the suprachiasmatic nucleus. Behav Neural Biol 25:346–363
Sterniczuk R, Rusak B, Rockwood K (2014) Sleep disturbance in older ICU patients. Clin Interv Aging 9:969–977
Stetson MH, Watson-Whitmyre M (1977) The neural clock regulating estrous cyclicity in hamsters: gonadotropin release following barbiturate blockade. Biol Reprod 16:536–542
Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M (2001) Entrainment of the circadian clock in the liver by feeding. Science 291:490–493
Strubbe JH, Spiteri NJ, Alingh Prins AJ (1986) Effect of skeleton photoperiod and food availability on the circadian pattern of feeding and drinking in rats. Physiol Behav 36:647–651
Trifilieff P, Feng B, Urizar E, Winiger V, Ward RD, Taylor KM, Martinez D, Moore H, Balsam PD, Simpson EH, Javitch JA (2013) Increasing dopamine D2 receptor expression in the adult nucleus accumbens enhances motivation. Mol Psychiatry 18:1025–1033
U.S. Department of Agriculture, Agricultural Research Service (2014) Meals and snacks: distribution of meal patterns and snack occasions, by gender and age. In: What We Eat in America, NHANES 2011–2012
Uth K, Sleigh R (2014) Deregulation of the circadian clock constitutes a significant factor in tumorigenesis: a clockwork cancer. Part II. studies. Biotechnol Biotechnol Equip 28:379–386
van Furth WR, van Ree JM (1994) Endogenous opioids and sexual motivation and performance during the light phase of the diurnal cycle. Brain Res 636:175–179
Van Reeth O, Zhang Y, Zee PC, Turek FW (1994) Grafting fetal suprachiasmatic nuclei in the hypothalamus of old hamsters restores responsiveness of the circadian clock to a phase shifting stimulus. Brain Res 643:338–342
Van Veen MM, Kooij JJ, Boonstra AM, Gordijn MC, Van Someren EJ (2010) Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biol Psychiatry 67:1091–1096
Verwey M, Amir S (2011) Nucleus-specific effects of meal duration on daily profiles of Period1 and Period2 protein expression in rats housed under restricted feeding. Neuroscience 192:304–311
Verwey M, Khoja Z, Stewart J, Amir S (2007) Differential regulation of the expression of Period2 protein in the limbic forebrain and dorsomedial hypothalamus by daily limited access to highly palatable food in food-deprived and free-fed rats. Neuroscience 147:277–285
Verwey M, Khoja Z, Stewart J, Amir S (2008) Region-specific modulation of PER2 expression in the limbic forebrain and hypothalamus by nighttime restricted feeding in rats. Neurosci Lett 440:54–58
Vida B, Deli L, Hrabovszky E, Kalamatianos T, Caraty A, Coen CW, Liposits Z, Kalló I (2010) Evidence for suprachiasmatic vasopressin neurones innervating kisspeptin neurones in the rostral periventricular area of the mouse brain: regulation by oestrogen. J Neuroendocrinol 22:1032–1039
Viswanathan N (1999) Maternal entrainment in the circadian activity rhythm of laboratory mouse (C57BL/6J). Physiol Behav 68:157–162
Viswanathan N, Chandrashekaran MK (1985) Cycles of presence and absence of mother mouse entrain the circadian clock of pups. Nature 317:530–531
Viswanathan N, Davis FC (1992) Maternal entrainment of tau mutant hamsters. J Biol Rhythms 7:65–74
Vujovic N, Gooley JJ, Jhou TC, Saper CB (in press) Projections from the subparaventricular zone define four channels of output from the circadian timing system. J Comp Neurol. doi:10.1002/cne.23812
Wakamatsu H, Yoshinobu Y, Aida R, Moriya T, Akiyama M, Shibata S (2001) Restricted-feeding-induced anticipatory activity rhythm is associated with a phase-shift of the expression of mPer1 and mPer2 mRNA in the cerebral cortex and hippocampus but not in the suprachiasmatic nucleus of mice. Eur J Neurosci 13:1190–1196
Wasserman T, Wasserman LD (2015) The misnomer of attention-deficit hyperactivity disorder. Appl Neuropsychol Child 4:116–122
Waterhouse J, Kao S, Edwards B, Weinert D, Atkinson G, Reilly T (2005) Transient changes in the pattern of food intake following a simulated time-zone transition to the east across eight time zones. Chronobiol Int 22:299–319
Webb IC, Lehman MN, Coolen LM (2015) Diurnal and circadian regulation of reward-related neurophysiology and behavior. Physiol Behav 143:58–69
Welsh DK, Takahashi JS, Kay SA (2010) Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol 72:551–577
Whishaw IQ, Dringenberg HC, Comery TA (1992) Rats (Rattus norvegicus) modulate eating speed and vigilance to optimize food consumption: effects of cover, circadian rhythm, food deprivation, and individual differences. J Comp Psychol 106:411–419
Williams WP 3rd, Kriegsfeld LJ (2012) Circadian control of neuroendocrine circuits regulating female reproductive function. Front Endocrinol (Lausanne) 3:60
Williams WP 3rd, Jarjisian SG, Mikkelsen JD, Kriegsfeld LJ (2011) Circadian control of kisspeptin and a gated GnRH response mediate the preovulatory luteinizing hormone surge. Endocrinology 152:595–606
Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR (2001a) Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 86:5992
Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, Batterham RL, Taheri S, Stanley SA, Ghatei MA, Bloom SR (2001b) Ghrelin causes hyperphagia and obesity in rats. Diabetes 50:2540–2547
Wulff K, Dijk DJ, Middleton B, Foster RG, Joyce EM (2012) Sleep and circadian rhythm disruption in schizophrenia. Br J Psychiatry 200:308–316
Yu X, Zecharia A, Zhang Z, Yang Q, Yustos R, Jager P, Vyssotski AL, Maywood ES, Chesham JE, Ma Y, Brickley SG, Hastings MH, Franks NP, Wisden W (2014) Circadian factor BMAL1 in histaminergic neurons regulates sleep architecture. Curr Biol 24:2838–2844
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Antle, M.C., Silver, R. (2015). Circadian Insights into Motivated Behavior. In: Simpson, E., Balsam, P. (eds) Behavioral Neuroscience of Motivation. Current Topics in Behavioral Neurosciences, vol 27. Springer, Cham. https://doi.org/10.1007/7854_2015_384
Download citation
DOI: https://doi.org/10.1007/7854_2015_384
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-26933-7
Online ISBN: 978-3-319-26935-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)