Abstract
Recent years have seen a resurgence of interest in motivational disturbances in schizophrenia . This is largely driven by the recognition that these disturbances are central to the “experiential” subdomain of negative symptoms and are particularly important determinants of functional disability. Research into the causes and treatment of experiential negative symptoms is therefore a high priority. This chapter reviews findings from experimental psychopathology and affective science relevant to understanding the neurobehavioral processes that underlie these negative symptoms. We focus on abnormalities in four processes that have received the most attention as likely contributors: anticipatory pleasure, reward learning, effort-based decision-making, and social motivation. We also review the research literature on pharmacological and psychosocial approaches to reduce functional deficits attributable to negative symptoms. Translational research is beginning to inform the development of new treatments specifically designed to target the experiential subdomain of negative symptoms.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Negative symptoms
- Motivation
- Schizophrenia
- Neurobehavioral
- Reward learning
- Effort-based decision-making
- Social motivation
1 Introduction
1.1 Historical Context
Motivation disturbances, predominantly considered in the context of negative symptoms, have long been acknowledged as a core clinical feature of schizophrenia (Meehl 1962). In Kraepelin’s original characterization of schizophrenia , he described avolition as a primary cause of the progressive deterioration he observed in this disorder (Kraepelin 1971). Similarly, Bleuler described schizophrenia as reflecting a breakdown in emotion and motivation, with affective indifference as the primary marker (Bleuler 1950). Recent years have seen a resurgence of interest in motivational disturbances in schizophrenia. This renewed interest is largely driven by the recognition that negative symptoms and associated motivational disturbances are key contributors to the profound functional disability that is a hallmark of schizophrenia. In addition, the emergence of concepts and methods from the burgeoning field of affective neuroscience has facilitated translational research into the neurobehavioral correlates of these disturbances. It is now understood that motivational disturbances are central to a particular subcomponent of negative symptoms.
1.2 Types of Negative Symptoms
Negative symptoms broadly refer to the absence or diminution of normal functions in the areas of emotion, sociality, productive goal-directed behavior, and communication. Although a number of clinical ratings scales have been used for several decades to assess negative symptoms, these scales were based on various definitions of negative symptoms and differed in the specific domains that were assessed. In 2006, an NIMH-sponsored conference was held with the goal of achieving consensus on the optimal assessment of negative symptoms and identifying the most impactful ways to advance novel treatment development for these disabling symptoms (Kirkpatrick, Fenton et al. 2006). Based on a comprehensive literature review and input from diverse stakeholders, five core consensus-based negative symptoms were defined. These include avolition, reflecting diminished engagement in intellectual, occupational, and social pursuits; anhedonia, a reduction in the range and intensity of pleasant emotions; asociality, social withdrawal or avoidance, and a lack of meaningful interpersonal connections; restricted affect, reduced emotional expression including diminished facial expressions, gestures, and vocal intonation; alogia, diminished verbal production and spontaneous speech.
The 2006 conference members evaluated the overall structure of negative symptoms after a comprehensive review of the literature on the factor structure of negative symptoms across a variety of clinical ratings scales. These studies provided consistent support for two separable factors: (1) an experiential dimension reflecting disturbances in motivation and pleasure that are central to avolition, anhedonia, and asociality; and (2) an expressivity dimension comprised of restricted affect and poverty of speech. It was also established that these factors were separable from other aspects of psychopathology, including cognitive impairment (which was included on some negative symptom scales) and positive symptoms (delusions, hallucinations, disorganization) (Blanchard and Cohen 2006). As discussed further below, the conference led to the development of two new state-of-the-art negative symptom assessment scales that are organized in terms of the experiential and expressive domains of negative symptoms (Kane 2013; Marder and Kirkpatrick 2014).
The recognition that negative symptoms consist of two components spurred interest in the question of whether they show similar or different relations to poor community outcome. Available research indicates that the experiential component shows stronger and more consistent relations to functioning, including the areas of work, independent living, and social networks. This pattern was demonstrated in studies using older negative scales (e.g., SANS) (e.g., Mueser 1994; Sayers et al. 1996; Milev et al. 2005; Siegel et al. 2006; Dowd and Barch 2010; Strauss et al. 2013). It has also been replicated in studies using the two more recently developed scales (Horan et al. 2011; Kirkpatrick et al. 2011; Strauss et al. 2012; Kring et al. 2013). Thus, the experiential negative symptoms , which are defined by motivational disturbances, comprise the subdomain that appears most strongly related to community functioning.
1.3 The Anhedonia Paradox
An important, and as yet unanswered, question is: Exactly what specific neurobehavioral process(es) contribute(s) to the motivational/emotion disturbances that manifest clinically as experiential negative symptoms ? Historically, it was believed that diminished engagement in rewarding and productive activities reflected a fundamental inability to experience pleasure. Indeed, the available clinical interview and self-report measures available up to the 1990s consistently indicated that patients reported experiencing diminished levels of pleasure in their daily lives. However, a major challenge to this assumption emerged in the early 1990s when laboratory studies using affective science methods were first applied to individuals with schizophrenia and revealed an apparently paradoxical set of findings. The studies showed that when individuals with schizophrenia are actually presented with evocative stimuli (e.g., pictures, food, film clips), they experience in-the-moment affective responses comparable to those of controls (for reviews see, Horan et al. 2006a; Strauss et al. 2014). Further, this intact “consummatory pleasure” has been verified using other research techniques including neuroimaging (Dowd and Barch 2010), physiological measures (Curtis et al. 1999; Volz et al. 2003; Horan et al. 2010), and implicit preference measures (Herbener 2009; Waltz et al. 2009).
These findings converge to indicate that although individuals with schizophrenia show diminished engagement in rewarding and productive activities in their daily lives, this disengagement does not reflect a basic inability to experience pleasure. If individuals can and do experience normal levels of pleasure, why do not they seek out opportunities to engage in rewarding activities? Researchers have started to approach this question through a variety of conceptual frameworks and methods grounded in basic affective neuroscience. The following section reviews several of the main approaches that have been used.
2 Possible Factors that Contribute to Motivational Negative Symptoms
2.1 Abnormalities in Anticipatory Pleasure
An important insight from affective neuroscience is that “pleasure” is not a unitary construct. Instead, hedonic experience consists of multiple, distinct components. One key distinction that has been well established in basic animal research is between “liking” or consummatory, in-the-moment pleasure, and “wanting” or anticipatory motivation for future rewards. These involve dissociable neural substrates—liking involves serotoninergic/opioid systems in the nucleus accumbens shell, the ventral palladium, and the orbitofrontal cortex (Smith and Berridge 2007), whereas wanting involves the mesolimbic dopamine system and projections to ventral and dorsal striatal regions of the basal ganglia (Schultz et al. 1997; Barch and Dowd 2010). For a detailed review of the roles of wanting and liking in motivating behavior, see Robinson et al., in this volume. Although, as noted above, individuals with schizophrenia show relatively normal liking, that does not appear to be the case for anticipatory pleasure.
Experimental paradigms used in clinical research indicate neural and behavioral deficits in anticipatory pleasure in schizophrenia . One novel paradigm used an experimental approach to separately measure behavioral responses in a condition with an immediately available pleasant reward (repeated button presses could prolong exposure to rewarding pleasant images) versus a condition in which the pleasant reward is only imagined, or anticipated (Heerey and Gold 2007). There were no differences between patients and controls in the immediately available reward condition, but there were significant group differences in the anticipatory condition, such that patients responded at a lower rate (Heerey and Gold 2007).
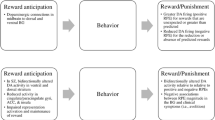
This separation between wanting and liking has also been measured in schizophrenia patients using experience sampling (Gard et al. 2007), neuroimaging (Dowd and Barch 2012), and event-related potentials (Wynn et al. 2010). For example, the ERP study by Wynn and colleagues used a cued, reaction-time contingent picture viewing task to assess two types of anticipatory ERP’s, one involving motor response preparation [contingent negative variation (CNV)] and one during anticipation of emotionally significant stimuli [not involving motor preparation; stimulus-preceding negativity (SPN)]. In this task, participants were instructed to make a button press as quickly as possible when a cue appeared on the computer screen (+, 0, or −) that signified the valence (pleasant, neutral, unpleasant) of a forthcoming picture; faster reaction times resulted in longer subsequent picture presentations, whereas slow reaction times resulted in shorter picture presentations. Patients and healthy controls demonstrated similar patterns of reaction time, as well as self-reported emotion while viewing the different types of pictures (Fig. 1). However, patients demonstrated generally lower CNV (prior to button pressing) and SPN (prior to picture viewing) amplitudes than controls across the picture conditions. These results further support a separation between wanting and liking processes in individuals with schizophrenia . Taken together, the experience sampling, neuroimaging, and electrophysiological findings converge to indicate intact responsiveness to immediately present rewards versus impaired responsiveness to future rewards, implicating a diminution of anticipatory pleasure that impedes motivated behavior. Further supporting the notion that the anticipatory deficit may have real implications for functional outcome are studies that have reported significant correlations between diminished anticipatory pleasure and clinically rated negative symptoms (Gold et al. 2012; Kring and Barch 2014).
Left panel: schematic of the trial structure for the cued emotion anticipation task. The CNV was measured as activity in the last 500 ms of the cue slide and the SPN as activity in the last 500 ms of the anticipatory period. Right panel: mean amplitudes (with standard error bars) for the CNV (top panel) and SPN (bottom panel) at electrodes Fz, FCz, Cz, CPz, and Pz for schizophrenia patients (red columns) and healthy controls (blue columns)
2.2 Alterations in Reward Learning
Another aspect of hedonic experience that has garnered significant attention in research aimed at understanding pathways to motivational deficits in schizophrenia is reward learning. Reward learning broadly involves the process of using feedback information to guide adaptive behavioral responding. A key aspect of reward learning involves the ability to adjust response tendencies in accordance with shifting reinforcement and punishment contingencies, which largely depends on functions associated with the ventral prefrontal cortex. A disturbance in the ability to correctly monitor and respond to positive or negative feedback signals would impede efforts to carry out goal-directed activities and could therefore contribute to motivational deficits in schizophrenia . Investigations into such deficits and their underlying neural mechanisms are grounded in well-established animal models, particularly those focusing on dopamine-mediated reward learning tasks. Reward learning deficits have been replicated across a range of paradigms in individuals with schizophrenia and have shown correlations with overall negative symptoms (Pantelis et al. 1999; Gold et al. 2008; Murray et al. 2008; Weiler et al. 2009; Waltz et al. 2013).
Deficits in reward learning may be particularly evident when individuals are required to continually and flexibly adapt behavior. Reversal learning is a prime example of a situational paradigm that requires continual response adaptations. Reversal learning refers to tasks in which stimulus–response contingencies are initially learned with responses to “correct” stimuli reinforced more often than those to incorrect stimuli (e.g., 80 vs. 20 % reinforcement, respectively). Mental flexibility is required because the reward contingencies are reversed throughout the task after a certain number of correct responses, such that a different stimulus becomes the “correct” choice and previously correct choices intermittently become “incorrect.” Reversal learning tasks include an initial discrimination phase, in which subjects learn to detect correct versus incorrect stimuli, which is followed by a series of reversal phases, in which the response contingencies are inverted. Prediction errors are reflected in the dopaminergic neural signaling processes that underlie the initial learning and the relearning of reward contingencies following reversals. Prediction errors are termed “positive” when an unexpected reward triggers dopaminergic firing; prediction errors are “negative” when an expected reward is not delivered, and there is a decrease in or absence of dopaminergic firing. Functionally, prediction errors facilitate flexible reward learning of the alternating reward contingencies that occur throughout this type of task (Wise 2004). In both animal and human models, it is well established that reversal learning depends on dopamine-mediated striatal networks (e.g., Frank and Claus 2006; Pessiglione et al. 2006; Lee et al. 2007).
Reversal learning paradigms, as described above, are designed to sensitively test participants’ ability to quickly and flexibly change response patterns in the context of reversed reward contingencies. Individuals with schizophrenia consistently show impairments on these tasks (Waltz and Gold 2007; Murray et al. 2008; Prentice et al. 2008; Leeson et al. 2009; McKirdy et al. 2009; Waltz et al. 2013; Schlagenhauf et al. 2014). The common finding among the studies published to date is a significant deficit in ability to reverse learned contingencies, above and beyond difficulties learning initial reward–response relationships. Several mechanisms of action have been considered to explain this deficit, including problems with value representation and reduced reward sensitivity, more errors related to frequent response switching, and aberrant salience. Although there is no consensus on causal mechanism, it does appear that difficulties with flexibly adapting behavioral responses in accordance with new feedback precede illness onset, persist in periods of remission, are apparent in medicated and non-medicated samples, and are not solely caused by generalized cognitive deficits. Reversal learning deficits are likely an important contributor to motivational deficits . For a more detailed discussion on reward learning deficits in patients with schizophrenia , and how such deficits contribute to motivational impairment, see Waltz et al. in this volume.
2.3 Effort-Based Decision-Making
When considering whether to engage in activities that may be rewarding, we evaluate not only the potential benefits, but also the associated costs. Across non-human and human animals, the costs associated with the effort required to obtain a reward are a major factor in the decisional balance. Effort-based decision-making refers to an individual’s evaluation of the amount of effort required to obtain a given reward, and the subsequent decision of whether or not to engage in the effortful behavior. Animal studies show there is a general law of least effort in which organisms choose the least amount of effort expenditure necessary to obtain a given reward (Solomon 1948), but when a larger potential reward alternative is available, the decisional balance becomes more complex. Indeed, several factors contribute to the decision of how much effort to expend for a given reward. These factors include valuation of potential rewards, the perceived effort required to complete the associated task, and the likelihood that the reward will actually be received if the task is successfully completed. Thus, effort-based decision-making paradigms attempt to objectively assess the culmination of these processes—that is, motivated behavior defined as how much effort one is willing to exert for different levels of reward. Deficits in effort-based decision-making reflect impairments in the dopamine-rich regions of the prefrontal cortex, the anterior cingulate cortex, and the ventral striatum—this neural system mediates how the cost of effort is weighed against possible benefits (Kurniawan et al. 2010; Salamone and Correa 2012). Effort-based decision-making has been studied in different domains in animals and humans, including physical and cognitive effort, which appear to involve separable circuits (Hosking et al. 2014).
Abnormalities in effort-based decision-making have been examined in schizophrenia as potential contributors to negative symptoms . This has primarily been examined using tasks that measure physical effort. In the most commonly used type of task, participants must repeatedly press a button on a computer keyboard to earn various amounts of monetary reward. Participants make a series of choices between performing either low effort (few button presses)/low reward or higher effort (up to 100 button presses)/higher reward. A similar paradigm that involves choosing between low-effort/low-reward and higher effort/higher reward tasks using a handgrip device has also been used. In four out of six published studies of physical effort-based decision-making, individuals with schizophrenia were significantly less likely than healthy participants to select hard tasks as monetary reward, or probability of reward, increased, reflecting decreased willingness to exert physical effort for rewards (Fervaha, Graff-Guerrero et al. 2013; Gold et al. 2013; Barch et al. 2014; Hartmann et al. 2014; Docx et al. 2015; Treadway et al. 2015). All six studies examined associations between effort task performance and negative symptoms , and four found significant relations such that participants with more negative symptoms exerted less effort. An additional study used a progressive ratio break point task to examine cognitive effort-based decision-making (Wolf et al. 2014). In this task, participants chose when to cease exerting effort (in this case, making rapid numerical judgments) for additional increments of monetary reward—this cease juncture represents an effort “break point.” Individuals with schizophrenia had significantly lower break points than healthy controls, and lower break points reflecting less willingness to exert cognitive effort correlated with higher levels of experiential negative symptoms. Thus, there is emerging evidence that people with schizophrenia tend to avoid activities with higher perceived effort demands, which could contribute to diminished motivation to seek out and engage in many types of potentially rewarding activities.
2.4 Social Motivation (Approach/Avoidance)
Another construct that has been used to investigate neurobehavioral processes that contribute to experiential negative symptoms is social approach/avoidance motivation. Across several models of motivation, a basic distinction is made between behavioral approach and behavioral avoidance (Gray 1987; Gable and Gosnell 2013; Spielberg et al. 2013). Behavioral approach relies on a reward system (i.e., behavioral activation system; BAS) sensitive to appetitive stimuli and the termination of punishment. Behavioral avoidance (i.e., behavioral inhibition system; BIS), in contrast, is sensitive to aversive stimuli and activated by anxiety, novelty, and innate fear stimuli and is responsible for ceasing or inhibiting behavior. Neurophysiological research in animals implicates corticolimbic circuitry including amygdala, insula, and prefrontal cortex in avoidance responses and corticostriatal circuitry (including ventral striatum and nucleus accumbens) in approach motivation (Aupperle and Paulus 2010). Similar to basic motivation, social motivation depends on both approach and avoidance mechanisms and the resolution of the two opposing systems. In healthy adults, social motivation refers to achieving a sense of belonging by pursuing and maintaining healthy social ties with others. Such motivation is associated with well-being and has both a need for affiliation component and a need to avoid rejection aspect (Gable 2006).
Clinical studies in schizophrenia indicate that there may be different types of motivational drives that lead to social functioning deficits. Some patients show a profound disinterest in social interactions in the apparent absence of loneliness, while others are interested in social connections but avoid them because of social anxiety or concerns about the harmful intentions of others (Horan and Blanchard 2003; Horan et al. 2006b). A recently published study further supports the notion that social motivation disturbances can be caused by lack of approach or motivated avoidance in schizophrenia (Reddy et al. 2014). This study involved 151 individuals with schizophrenia who completed measures of approach and avoidance motivation. A cluster analysis identified four distinguishable subgroups, two of which had particularly poor social functioning. One was characterized by diminished interest in people and diminished drive to develop close interpersonal attachments. The other subgroup, with elevated avoidance motivation, was characterized by avoidance of social interactions and social aloofness attributable to anxiety and/or fear of rejection. These findings suggest that distinct types of social motivational drives can lead to asociality in schizophrenia and that considering different subtypes may help address the vexing issue of heterogeneity and guide efforts to make treatments for social dysfunction more personalized.
In summary, negative symptoms associated with motivational disturbances are major determinants of impaired functioning for many individuals with schizophrenia. We know people with schizophrenia do not lack the basic capacity to experience pleasure. Although individuals with schizophrenia can and do experience pleasure from rewarding stimuli, they report and demonstrate markedly decreased tendencies to seek out and engage in productive, rewarding activities in their daily lives. Translational neuroscience research points to four primary processes that likely contribute to motivational deficits : anticipatory pleasure, reward learning, effort-based decision-making, and social motivation. Several lines of translational research are currently providing new insights into neurobehavioral processes that underlie these motivational disturbances, for details see the chapter by Ward in this volume. Advances in this area hold considerable promise for facilitating the development of more effective functional recovery-oriented treatments. In the final section, we review the current status of pharmacological and psychosocial interventions that have been evaluated as potential treatments for negative symptoms.
3 Interventions for Motivational Negative Symptoms
As noted above, there is widespread agreement that negative symptoms are an unmet therapeutic need in a large proportion of individuals with schizophrenia (Kirkpatrick et al. 2006). The efficacy of various conventional pharmacological and psychosocial approaches has been evaluated in terms of their effects on negative symptoms, and results have, unfortunately, been generally modest. However, there have been a number of recent developments in clinical trial design and assessment methodology, as well as novel pharmacological and psychosocial treatments that are encouraging.
3.1 Conventional Approaches
Over the past several decades, negative symptoms were often assessed within studies of treatments that were primarily developed to manage the positive symptoms of schizophrenia . In this context, the impact of several classes of medications on negative symptoms has been examined. First- and second-generation antipsychotics, as well as antidepressants, have been evaluated in numerous clinical trials, but the majority of studies failed to produce clinically significant improvements in primary negative symptoms (Rummel et al. 2005; Singh et al. 2010). In addition, several key methodological and assessment issues complicate the interpretability of these studies. Studies often enrolled patients in the midst of acute psychotic symptom exacerbations and used various assessment measures based on different definitions of negative symptoms. Study designs also rarely addressed possible secondary sources of negative symptoms, such as depression, extrapyramidal side effects, and paranoia (Davis et al. 2014). Furthermore, expressive and experiential negative symptoms were not differentiated as treatment targets or in outcome assessments.
Studies of conventional psychosocial approaches, while subject to the same study design and assessment limitations as pharmacological interventions, were somewhat more promising for negative symptoms . Social skills training (SST) was one of the first manualized interventions that targeted functional deficits (usually social communication). A large meta-analysis of SST revealed small-to-medium improvements in negative symptoms (effect size = 0.40) (Kurtz and Mueser 2008). Although gains in social communication skills are relevant to social motivation, SST does not directly address motivational impairments. Another intervention designed to improve functional outcomes, cognitive enhancement training (CET), combines cognitive remediation with SST and also showed significant improvements in negative symptoms at post-treatment and at follow-up (Eack et al. 2013). Finally, a number of studies of cognitive behavioral therapy (CBT) for psychosis have included assessments of negative symptoms as secondary outcomes. Some earlier studies reported significant benefits for negative symptoms at the end of treatment and/or at post-treatment follow-up (Wykes et al. 2008; Klingberg et al. 2011; Grant et al. 2012). However, a more recent meta-analytic review indicated that the overall effects were small and nonsignificant, suggesting that CBT studies focused on psychotic symptoms may not reduce negative symptom as well as previously thought (Velthorst et al. 2015). Importantly, neither SST, CET, nor CBT directly address any of the four processes hypothesized to contribute to motivational negative symptoms. The closest relation is SST and social motivation, but it is not a therapeutic target of the intervention. It has been recognized that motivation-enhancing techniques yield treatment-related improvements within cognitive remediation therapy. This work is reviewed by Saperstein & Medalia in this volume. In summary, conventional approaches inadequately address negative symptoms and novel treatment development that directly targets this symptom domain is sorely needed.
3.2 Recent Developments
Since the National Institute of Mental Health’s Consensus Development Conference on Negative Symptoms in 2006, it has become widely recognized that improved methodologies are required to convincingly demonstrate the efficacy of new treatments. The methodological issues fall in two categories: assessment tools and clinical trial design features. Regarding assessment tools, two new clinical interview assessment measures, the Brief Negative Symptom Scale (BNSS; Strauss et al. 2012) and the Clinical Assessment Interview for Negative Symptoms (CAINS; Horan et al. 2011), have recently been developed. The BNSS is a 13 items’ measure that yields six subscales: blunted affect, alogia, asociality, anhedonia, distress, and avolition. For the subscales assessing experiential symptoms, items are included to separately measure relevant subjective reports and objective assessments. The CAINS was created through a multisite, multiphase scale development process (Kring et al. 2013). The final version also includes 13 items, which are organized into separate motivation and pleasure [including avolition, anhedonia (current and expected pleasure), and asociality items] and expression (affective blunting, alogia) subscales. The motivation and pleasure items combine information about both subjective experience and actual level of engagement in relevant activities, rather than measuring these separately as in the BNSS. For both the BNSS and CAINS, evidence supporting their psychometric properties (inter-rater reliability, test-retest reliability) and two-factor structures has been documented. Furthermore, evidence supporting the external validity of both scales, particularly relations between experiential negative symptoms and community functioning, has been reported (Kring et al. 2013). For the CAINS, the scale, detailed manual, and training videos with gold-standard ratings are available on the Internet (http://www.med.upenn.edu/bbl/downloads/CAINSVideos.shtml) and the BNSS scale and manual are available from its developers. Both scales have been translated into several languages and distributed internationally.
Regarding clinical trial design issues, a recent international meeting established consensus on several design parameters for future pharmacological trials of novel compounds. These parameters include the following: (1) Study subjects should be under the age of 65; (2) subjects should be excluded for symptoms of depression that do not overlap with negative symptoms; (3) functional measures should not be required as a coprimary in negative symptom trials; (4) information from informants should be included for ratings when available; (5) Phase 2 negative symptom trials should be 12 weeks, and 26 weeks is preferred for Phase 3 trials; (6) prior to entry into a negative symptom study, subjects should demonstrate clinical stability for a period of 4–6 months by collection of retrospective information; and (7) prior to entry, the stability of negative and positive symptoms should be confirmed prospectively for 4 weeks or longer (Marder et al. 2013). With these consensus-based guidelines and the availability of new assessment measures, state-of-the-art methodological standards are now available to evaluate the efficiency of new intervention approaches.
Aside from these methodological advances, there has been considerable recent activity in the areas of psychopharmacological and psychosocial treatment development. For pharmacological treatments, a number of compounds have been considered with some showing greater promise than others. Agents showing the most promise, and in relatively late stages in pharmaceutical development, include those that target the glutamatergic system, the cholinergic system, and the hormone oxytocin (Davis et al. 2014). For example, regarding glutamate, the strongest evidence for decreasing negative symptoms comes from agents that increase NMDA glutamatergic receptor activity (e.g., glycine site agonists such as D-serine and the GlyT1 inhibitor sarcosine). For the cholinergic system, drugs that activate cholinergic receptors, including partial alpha 7-nicotinic agonists, currently show the most promise. Finally, exogenous oxytocin (delivered by intranasal spray) has been found to improve negative symptoms in two studies (Feifel 2012; Modabbernia et al. 2013). It should be noted, however, that the relevant studies typically used older trial design and assessment methods, and many of them targeted cognition or social cognition, rather than negative symptoms, as the primary treatment outcome.
There have also been a few promising psychosocial treatment developments. Progress in the area of cognitive therapy (CT) for negative symptoms has been particularly encouraging. This approach is grounded in Beck and colleagues’ recently proposed CT-based conceptualization of negative symptoms as stemming from certain dysfunctional attitudes (Grant and Beck 2009). For example, within this framework, defeatist performance beliefs (e.g., “Why bother trying, I always fail,” “It’s not worth the effort”; “If you can’t do something well, there’s little point in doing it at all”) about one’s self and ability to perform productive activities are posited to contribute to avolition and asociality. A handful of recent studies has attempted to target these types of dysfunctional beliefs through standard CT methods such as Socratic questioning, reality testing, and cognitive restructuring. Notably, these therapeutic techniques were originally developed for the treatment of depression (see Barch et al., this volume, for a review of similarities and differences in motivational deficits between schizophrenia and depression). These initial studies provide support for the efficacy of CT for improving experiential negative symptoms and functional deficits, with effect sizes of approximately 0.50 (Klingberg et al. 2011; Grant et al. 2012; Staring et al. 2013), although they used older symptom assessment measures.
Preliminary investigations have also started to explore some alternative methods for treating experiential negative symptoms . For example, a pilot study of six sessions of Loving Kindness Meditation, focused on mindfully directing compassion toward the self and others, led to improvements in motivational negative symptoms as measured by CAINS, and these benefits remained at a three-month follow-up (Johnson et al. 2011). The feasibility of a cognitive/pleasure skills training approach to enhance anticipatory pleasure has also been considered (Favrod et al. 2010), and improvements were found on a self-report measure of anticipatory pleasure (Gard et al. 2007) and a measure of daily activities. Thus, from a psychosocial treatment perspective, recent studies indicate that motivational deficits may be responsive to interventions that address defeatist beliefs, social connections, general compassion for oneself and others, and prospection.
4 Conclusions
Motivational deficits have long been understood to be a core component of schizophrenia, and the recent resurgence of interest in negative symptoms is elucidating the nature of these deficits. The experiential negative symptoms, avolition, anhedonia, and asociality, are driven by low motivation to engage in goal-oriented behaviors. Because these negative symptoms are consistently associated with poor functioning, research into their causes and treatment is a high priority. Translational research, based largely on animal models of motivation and reward processing, has allowed our field to make considerable strides in conceptualizing the likely causes of the deficits. This chapter reviewed the four factors that have received the most attention in clinical neuroscience research as likely contributors to motivation deficits in schizophrenia, which include anticipatory pleasure, reward learning, effort-based decision-making, and social motivation. This research is beginning to inform the development of new pharmacological and psychosocial treatments specifically designed to target negative symptoms. In line with recent advances in clinical assessment technology and clinical trial design guidelines, our field is poised to make important breakthroughs in treating the disabling motivational impairments associated with schizophrenia.
References
Aupperle RL, Paulus MP (2010) Neural systems underlying approach and avoidance in anxiety disorders. Dialogues Clin Neurosci 12(4):517–531
Barch DM, Dowd EC (2010) Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr Bull 36(5):919–934
Barch DM, Treadway MT et al (2014) Effort, anhedonia, and function in schizophrenia: reduced effort allocation predicts amotivation and functional impairment. J Abnorm Psychol 123(2):387–397
Blanchard JJ, Cohen AS (2006) The structure of negative symptoms within schizophrenia: implications for assessment. Schizophr Bull 32:238–245
Bleuler E (1950) Dementia praecox or the group of schizophrenias. International Universities Press, New York
Curtis CE, Lebow B et al (1999) Acoustic startle reflex in schizophrenia patients and their first-degree relatives: evidence of normal emotional modulation. Psychophysiology 36(4):469–475
Davis MC, Horan WP et al (2014) Psychopharmacology of the negative symptoms: current status and prospects for progress. Eur Neuropsychopharmacol 24(5):788–799
Docx L, de la Asuncion J et al (2015) Effort discounting and its association with negative symptoms in schizophrenia. Cogn Neuropsychiatry 20(2):172–185
Dowd EC, Barch DM (2010) Anhedonia and emotional experience in schizophrenia: neural and behavioral indicators. Biol Psychiatry 67(10):902–911
Dowd EC, Barch DM (2012) Pavlovian reward prediction and receipt in schizophrenia: relationship to anhedonia. PLoS ONE 7(5):e35622
Eack SM, Mesholam-Gately RI et al (2013) Negative symptom improvement during cognitive rehabilitation: results from a 2-year trial of Cognitive Enhancement Therapy. Psychiatry Res 209(1):21–26
Favrod J, Giuliani F et al (2010) Anticipatory pleasure skills training: a new intervention to reduce anhedonia in schizophrenia. Perspect Psychiatr Care 46(3):171–181
Feifel D (2012) Oxytocin as a potential therapeutic target for schizophrenia and other neuropsychiatric conditions. Neuropsychopharmacology 37(1):304–305
Fervaha G, Graff-Guerrero A et al (2013) Incentive motivation deficits in schizophrenia reflect effort computation impairments during cost-benefit decision-making. J Psychiatr Res 47(11):1590–1596
Frank MJ, Claus ED (2006) Anatomy of a decision: striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychol Rev 113(2):300–326
Gable SL (2006) Approach and avoidance social motives and goals. J Pers 74(1):175–222
Gable SL, Gosnell CL (2013) Approach and avoidance behavior in interpersonal relationships. Emot Rev 5(3):269–274
Gard DE, Kring AM et al (2007) Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res 93(1–3):253–260
Gold JM, Strauss GP et al (2013) Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol Psychiatry 74(2):130–136
Gold JM, Waltz JA et al (2012) Negative symptoms and the failure to represent the expected reward value of actions: behavioral and computational modeling evidence. Arch Gen Psychiatry 69(2):129–138
Gold JM, Waltz JA et al (2008) Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull 34(5):835–847
Grant PM, Beck AT (2009) Defeatist beliefs as a mediator of cognitive impairment, negative symptoms, and functioning in schizophrenia. Schizophr Bull 35(4):798–806
Grant PM, Huh GA et al (2012) Randomized trial to evaluate the efficacy of cognitive therapy for low-functioning patients with schizophrenia. Arch Gen Psychiatry 69(2):121–127
Gray JA (1987) The psychology of fear and stress. Cambridge University Press, Cambridge
Hartmann MN, Hager OM et al (2014) Apathy but not diminished expression in schizophrenia is associated with discounting of monetary rewards by physical effort. Schizophr Bull 41:503–512
Heerey EA, Gold JM (2007) Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. J Abnorm Psychol 116(2):268–278
Herbener ES (2009) Impairment in long-term retention of preference conditioning in schizophrenia. Biol Psychiatry 65:1086–1090
Horan WP, Blanchard JJ (2003) Emotional responses to psychosocial stress in schizophrenia: the role of individual differences in affective traits and coping. Schizophr Res 60(2–3):271–283
Horan WP, Green MF et al (2006a) Does anhedonia in schizophrenia reflect faulty memory for subjectively experienced emotions? J Abnorm Psychol 115(3):496–508
Horan WP, Kring AM et al (2006b) Anhedonia in schizophrenia: a review of assessment strategies. Schizophr Bull 32:259–273
Horan WP, Kring AM et al (2011) Development and psychometric validation of the clinical assessment interview for negative symptoms (CAINS). Schizophr Res 132(2–3):140–145
Horan WP, Wynn JK et al (2010) Electrophysiological correlates of emotional responding in schizophrenia. J Abnorm Psycholol 119(1):18–30
Hosking JG, Cocker PJ et al (2014) Dissociable contributions of anterior cingulate cortex and basolateral amygdala on a rodent cost/benefit decision-making task of cognitive effort. Neuropsychopharmacology 39(7):1558–1567
Johnson DP, Penn DL et al (2011) A pilot study of loving-kindness meditation for the negative symptoms of schizophrenia. Schizophr Res 129(2–3):137–140
Kane JM (2013) Tools to assess negative symptoms in schizophrenia. J Clin Psychiatry 74(6):e12
Kirkpatrick B, Fenton W et al (2006) The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull 32:296–303
Kirkpatrick B, Strauss GP et al (2011) The brief negative symptom scale: psychometric properties. Schizophr Bull 37(2):300–305
Klingberg S, Wolwer W et al (2011) Negative symptoms of schizophrenia as primary target of cognitive behavioral therapy: results of the randomized clinical TONES study. Schizophr Bull 37(2):S98–S110
Kraepelin E (1971) Dementia praecox and paraphrenia. Krieger Publishing Co., Inc., Huntington
Kring AM, Barch DM (2014) The motivation and pleasure dimension of negative symptoms: neural substrates and behavioral outputs. Eur Neuropsychopharmacol 24(5):725–36
Kring AM, Gur RE et al (2013) The clinical assessment interview for negative symptoms (CAINS): final development and validation. Am J Psychiatry 170:165–172
Kurniawan IT, Seymour B et al (2010) Choosing to make an effort: the role of striatum in signaling physical effort of a chosen action. J Neurophysiol 104(1):313–321
Kurtz MM, Mueser KT (2008) A meta-analysis of controlled research on social skills training for schizophrenia. J Consult Clin Psychol 76(3):491–504
Lee B, Groman S et al (2007) Dopamine D2/D3 receptors play a specific role in the reversal of a learned visual discrimination in monkeys. Neuropsychopharmacology 32(10):2125–2134
Leeson VC, Robbins TW et al (2009) Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biol Psychiatry 66(6):586–593
Marder SR, Alphs L et al (2013) Issues and perspectives in designing clinical trials for negative symptoms in schizophrenia. Schizophr Res 150(2–3):328–333
Marder SR, Kirkpatrick B (2014) Defining and measuring negative symptoms of schizophrenia in clinical trials. Eur Neuropsychopharmacol 24(5):737–743
McKirdy J, Sussmann JED et al (2009) Set shifting and reversal learning in patients with bipolar disorder or schizophrenia. Psychol Med 39(08):1289–1293
Meehl P (1962) Schizotaxia, schizotypy, schizophrenia. Am Psychol 17:827–838
Milev P, Ho BC et al (2005) Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry 162(3):495–506
Modabbernia A, Rezaei F et al (2013) Intranasal oxytocin as an adjunct to risperidone in patients with schizophrenia. CNS Drugs 27(1):57–65
Mueser KT, Sayers SL et al (1994) A multisite investigation of the reliability of the scale for the assessment of negative symptoms. Am J Psychiatry 151(10):1453–1462
Murray GK, Cheng F et al (2008) Reinforcement and reversal learning in first-episode psychosis. Schizophr Bull 34(5):848–855
Pantelis C, Barber FZ et al (1999) Comparison of set-shifting ability in patients with chronic schizophrenia and frontal lobe damage. Schizophr Res 37(3):251–270
Pessiglione M, Seymour B et al (2006) Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature 442(7106):1042–1045
Prentice KJ, Gold JM et al (2008) The Wisconsin Card Sorting impairment in schizophrenia is evident in the first four trials. Schizophr Res 106(1):81–87
Reddy LF, Green MF et al (2014) Behavioral inhibition and activation systems in schizophrenia: an evaluation of motivational profiles. Schizophr Res 159(1):164–170
Rummel C, Kissling W et al (2005) Antidepressants as add-on treatment to antipsychotics for people with schizophrenia and pronounced negative symptoms: a systematic review of randomized trials. Schizophr Res 80(1):85–97
Salamone JD, Correa M (2012) The mysterious motivational functions of mesolimbic dopamine. Neuron 76(3):470–485
Sayers SL, Curran PJ et al (1996) Factor structure and construct validity of the Scale for the Assessment of Negative Symptoms. Psychol Assess 8:269–280
Schlagenhauf F, Huys QJM et al (2014) Striatal dysfunction during reversal learning in unmedicated schizophrenia patients. NeuroImage 89:171–180
Schultz W, Dayan P et al (1997) A neural substrate of prediction and reward. Science 275(5306):1593–1599
Siegel SJ, Irani F et al (2006) Prognostic variables at intake and long-term level of function in schizophrenia. Am J Psychiatry 163(3):433–441
Singh SP, Singh V et al (2010) Efficacy of antidepressants in treating the negative symptoms of chronic schizophrenia: meta-analysis. Br J Psychiatry 197(3):174–179
Smith KS, Berridge KC (2007) Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci 27(7):1594–1605
Solomon RL (1948) The influence of work on behavior. Psychol Bull 45(1):1–40
Spielberg JM, Heller W et al (2013) Hierarchical brain networks active in approach and avoidance goal pursuit. Front Hum Neurosci 7(284)
Staring ABP, ter Huurne M-AB et al (2013) Cognitive behavioral therapy for negative symptoms (CBT-n) in psychotic disorders: a pilot study. J Behav Ther Exp Psychiatry 44(3):300–306
Strauss GP, Hong LE et al (2012) Factor structure of the brief negative symptom scale. Schizophr Res 142(1–3):96–98
Strauss GP, Horan WP et al (2013) Deconstructing negative symptoms of schizophrenia: avolition-apathy and diminished expression clusters predict clinical presentation and functional outcome. J Psychiatr Res 47(6):783–790
Strauss GP, Waltz JA et al (2014) A review of reward processing and motivational impairment in schizophrenia. Schizophr Bull 40(Suppl 2):S107–S116. doi:10.1093/schbul/sbt197 Epub 2013 Dec 27
Treadway MT, Peterman JS et al (2015) Impaired effort allocation in patients with schizophrenia. Schizophr Res 161(2–3):382–385
Velthorst E, Koeter M et al (2015) Adapted cognitive–behavioural therapy required for targeting negative symptoms in schizophrenia: meta-analysis and meta-regression. Psychol Med 45(03):453–465. doi:10.1017/S0033291714001147
Volz M, Hamm AO et al (2003) Temporal course of emotional startle modulation in schizophrenia patients. Int J Psychophysiol 49(2):123–137
Waltz JA, Gold JM (2007) Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res 93(1–3):296–303
Waltz JA, Kasanova Z et al (2013) The roles of reward, default, and executive control networks in set-shifting impairments in schizophrenia. PLoS ONE 8(2):e57257
Waltz JA, Schweitzer JB et al (2009) Patients with schizophrenia have a reduced neural response to both unpredictable and predictable primary reinforcers. Neuropsychopharmacology 34(6):1567–1577
Weiler JA, Bellebaum C et al (2009) Impairment of probabilistic reward-based learning in schizophrenia. Neuropsychology 23(5):571–580
Wise RA (2004) Dopamine, learning and motivation. Nat Rev Neurosci 5(6):483–494
Wolf DH, Satterthwaite TD et al (2014) Amotivation in schizophrenia: integrated assessment with behavioral, clinical, and imaging measures. Schizophr Bull 40(6):1328–1337
Wykes T, Steel C et al (2008) Cognitive behavior therapy for schizophrenia: effect sizes, clinical models, and methodological rigor. Schizophr Bull 34(3):523–537
Wynn JK, Horan WP et al (2010) Impaired anticipatory event-related potentials in schizophrenia. Int J Psychophysiol 77(2):141–149
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Reddy, L.F., Horan, W.P., Green, M.F. (2015). Motivational Deficits and Negative Symptoms in Schizophrenia: Concepts and Assessments. In: Simpson, E., Balsam, P. (eds) Behavioral Neuroscience of Motivation. Current Topics in Behavioral Neurosciences, vol 27. Springer, Cham. https://doi.org/10.1007/7854_2015_379
Download citation
DOI: https://doi.org/10.1007/7854_2015_379
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-26933-7
Online ISBN: 978-3-319-26935-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)