Abstract
Communication across the brain networks is dependent on neuronal oscillations. Detection of the synchronous activation of neurons can be used to determine the well-being of the connectivity in the human brain networks. Well-connected highly synchronous activity can be measured by MEG, EEG, fMRI, and PET and then analyzed with several types of mathematical algorithms. Coherence is one mathematical method that can detect how well 2 or more sensors or brain regions have similar oscillatory activity with each other. Phase synchrony can be used to determine if these oscillatory activities are in sync or out of sync with each other. Correlation is used to determine the strength of interaction between two locations or signals. Granger causality can be used to determine the direction of the information flow in the neuronal brain networks. Statistical analysis can be performed on the connectivity results to verify evidence of normal or abnormal network activity in a patient.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The interest in detecting and imaging the functional properties of the brain’s networks is driving the development of advance mathematical imaging techniques and analysis. This in turn guides the need to understand the different techniques for measuring and for analyzing the location and strengths of these functional brain networks (Cabral et al. 2014). Brain connectivity networks can be subdivided into 3 main categories: neuroanatomical, functional and effective connectivity (Friston et al. 1993; Sakkalis 2011; Greenblatt et al. 2012).
Neuroanatomical connectivity or structural connectivity is based on detection of the fiber tracts that physically connect the regions of the brain. Figure 1 is a representative image of how the fiber tracts in the human brain appear (Kubicki et al. 2005). These fiber tracts are detected using Diffusion magnetic resonance imaging (MRI). Diffusion MRI measures the water anisotropy in the white matter of the brain. Diffusion tensor imaging (DTI) (Le Bihan et al. 2001) estimates the direction and strength of anisotropic diffusion in each voxel while diffusion spectrum imaging (DSI) explores the strength of anisotropy in all directions, allowing the detection of the crossing of multiple fibers in a single voxel (Wedeen et al. 2008). To date, several DTI studies support the notion that frontal–temporal connectivity in the brain is likely disrupted in schizophrenia along with reduced organization of the cortico-cortical connections (Kubicki et al. 2005; Rotarska-Jagiela et al. 2008; White et al. 2011). DTI is a method to determine local fiber tract orientation which can be used to identify and analyze fiber tract pathways. The Human Connectome Project is compiling a neural connectivity database of diffusion MRI studies (Van Essen et al. 2013). More information can be found at their website: www.humanconnectomeproject.org.
Fiber traces from a human brain are colored such that fibers with similar endpoints are assigned similar colors. Slices from a T2-weighted volume add additional understanding of the anatomy. The view of the fiber traces on the left is coronal, while the view on the right is sagittal. (Kubicki et al. 2005)
Functional connectivity is established by identifying correlations of activity between multiple regions of the brain involved in basal brain/body function or higher order information processing that is required for sensory responses, motor responses, and intellectual or emotional processing. During cognitive and sensory processing, brain activity is characterized by bursts of information flow and correlated network activity. These bursts of regional brain activity are called nodes, and the links to other nodes are called edges (the fiber pathways). These regions (nodes) may only be active for a short period of time which emphasizes the dynamic fluctuation of information flowing around the brain during cognitive or sensory processing, or they may be active for minutes, hours, or even days as in the case of the epileptic network (Towle et al. 2007). Further, brain networks have frequency-dependent characteristics that differ with the scale of brain region that is measured. Recent studies of functional connectivity in patients with schizophrenia have shown beta- and gamma-band activity is abnormal (Hinkley et al. 2011). The dysfunctional oscillations in these frequencies may be due to abnormalities in the rhythm generating networks of GABA interneurons and cortico-cortical connections (Uhlhaas and Singer 2010). Coherence and phase synchrony are common mathematical methods for quantifying frequency-dependent coordination of brain activity. Figure 2 is an example of power distribution verses frequency graphs, frequency verses time as well as sensor space topography map of the phase synchrony differences between groups in the lower panel (Uhlhaas and Singer 2010). Functional connectivity does not determine the specific direction of information flow in the brain or an underlying structural model. It just shows that these regions are connected.
Neural oscillations and synchrony in schizophrenia. a Auditory steady state responses in patients with schizophrenia (ScZ) show lower power to stimulation at 40 Hz than control subjects. b Sensory evoked oscillations during a visual oddball task in patients with schizophrenia indicate the phase-locking factor of oscillations in the 20–100 Hz frequency range in the occipital cortex for healthy controls and patients with schizophrenia. c Dysfunctional phase synchrony during Gestalt perception in schizophrenia was significantly reduced relative to controls. In addition, patients with schizophrenia showed a desynchronization in the gamma band (30–55 Hz) in the 200–280 ms interval. The bottom panel shows differences in the topography of phase synchrony in the 20–30 Hz frequency range between groups. Red lines indicate less synchrony between two electrodes in patients with schizophrenia than in controls. Green lines indicate greater synchrony for patients with schizophrenia (Uhlhaas and Singer 2010)
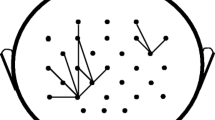
Effective connectivity takes functional connectivity one step further and determines the direct or indirect influence that one neural system may have over another, more specifically the direction of the dynamic information flow in the brain (Cabral et al. 2014; Horwitz 2003). Using mathematical techniques such as Granger causality, Hilbert transform, transfer entropy, and correlation, locations in the brain can be identified as a sender or receiver of the information flowing in the brain. Flow of information in the brain networks during a finger-tapping task is displayed in Fig. 3, where functional connectivity is seen in yellow and the effective connectivity is depicted by the arrows (Gross et al. 2002).
The map represents spatial distribution of coherence (in the 6–9 Hz range). Dynamic imaging of coherent sources were applied to MEG data. The dominant coupling direction is indicated by arrows. Note that left thalamus and right cerebellum are projected to the left surface for easier visualization (Gross et al. 2002)
Analytical techniques for estimating functional or effective connectivity of the brain to determine if or how 2 or more sensors or locations are connected/coupled fall under these main categories linear and nonlinear, bivariate, and multivariate. Bivariate techniques are mathematical algorithms that determine how activity at 2 brain locations or electrodes is related to each other based on the evaluation of the frequencies and patterns of neuronal oscillations. After performing the connectivity analysis, then the results can be put into graph or matrix format for further analysis. Figure 4 represents modes of brain connectivity in the macaque cortex along with their corresponding matrices (Honey et al. 2007). Weighted undirected functional connectivity forms a full symmetric matrix, with each of the elements encoding statistical dependence or proximity between two nodes (recording sites, regions). A threshold may be applied to yield binary undirected graphs. Effective connectivity yields a full non-symmetric matrix. Applying a threshold to these matrices yields binary directed graphs.
Modes of brain connectivity. Sketches at the top illustrate structural connectivity (fiber pathways), functional connectivity (correlations), and effective connectivity (information flow) among four brain regions in macaque cortex. Matrices at the bottom show binary structural connections (left), symmetric mutual information (middle), and non-symmetric transfer entropy (right) (Sporns 2007). Data were obtained from a large-scale simulation of cortical dynamics (Honey et al. 2007)
Brain signals are usually recorded by electroencephalography (EEG), magnetoencephalography (MEG), functional magnetic resonance imaging (fMRI), and positron emission tomography (PET). Recent developments have advanced the ability for connectivity to image directly into the specific regions of the brain (called source space, Fig. 3); in past years, connectivity was seen by connecting lines between electrodes or coils that had similar frequency profiles on the brain surface (called sensor space, bottom of Fig. 2). Figures 2 and 3 depict these 2 different types of images. In this review, we will discuss how brain connectivity can be detected and recorded and the different measures of connectivity used to display the brain networks. We will also include various studies of connectivity where the results may be clinically applicable.
2 Measures
When we investigate how the human brain functions, we tend to compare it to a computer’s circuit board. There are locations in the brain that perform certain tasks such as feeling, tasting, smelling, hearing, and seeing. These last two are similar to the computer’s ability to produce sounds and display images. Connectivity measures of the brain are performed to try to map out the communication networks (cortical circuits) needed for the brain to function. These networks are made up of neurons that function in unison to send signals to other parts of the brain. There are several properties of the neuron that play an important role in generating membrane potential oscillations that can be detected by neuroimaging devices. Neurons communicate with other neurons by releasing one of over 50 different types of neurotransmitters in the brain some of which are excitatory (stimulate the brain) and some are inhibitory (calm the brain) (Chana et al. 2013). Voltage-gated ion channels generate action potentials and periodic spiking membrane potentials which produces oscillatory activity and facilitates synchronous activity in neighboring neurons (Llinas 1988; Llinas et al. 1991). Coherent neuronal communications are based on neurotransmission dynamics dictated by major neurotransmitters such as the amino acids glutamate and GABA. Other important neurotransmitters include acetylcholine, dopamine, adrenaline, histamine, serotonin, and melatonin (Stephan et al. 2009; Haenschel and Linden 2011; Wang 2010). There is growing evidence that glutamatergic dysregulation may underlie schizophrenia and psychosis (Chana et al. 2013).
Synchronized activity of large numbers of neurons can give rise to large magnetic field and electric field oscillations, which are detected by EEG/MEG (Hamalainen et al. 1993) and the secondary metabolic responses are detected by fMRI/PET (Ogawa et al. 1990). Coherent activity within the whole brain is evidence for a network of dynamic links (edges) between different brain regions (nodes) that distribute information (Varela et al. 2001). Detection of normal or abnormal networks can provide information on the underlying developmental and/or neurological disorder.
EEG uses electrodes pasted or glued to specific locations on the scalp that record the electric potential at the skin surface. MEG on the other hand uses coils suspended in a helmet placed around the head to detect the changing magnetic fields just outside the head. Both EEG and MEG signals come from the activation of neurons. EEG measures voltage potentials determined by electrical impedance boundaries of head structures that shape volume currents (return currents) where as MEG measures the magnetic field of primary or intracellular current flow. For a review of mathematical equations of connectivity measures used in EEG and MEG for neurologic disorders see Sakkalis (2011) or Greenblatt et al. (2012).
PET and FMRI measure the secondary or metabolic response from neuronal activation. They are both indirect measures of neuronal activation with low temporal resolution on the order of seconds. PET uses a radioactive-labeled tracer, tagged to glucose (blood sugar) that is injected into the subject to detect the parts of the brain that require energy to function (the glucose response). FMRI uses a strong magnetic field and radio waves to look at blood flow in the brain to detect areas of activity that need more oxygen to function (the hemodynamic response). The translation from the hemodynamic response or the glucose response back to the actual synaptic neuronal function is still not fully understood (Goense and Logothetis 2008; Logothetis and Pfeuffer 2004) For a review of connectivity measures of fMRI and PET for neurologic disorders see Rowe (2010).
Functional and effective connectivity techniques are dependent on calculating the communication of active neural signals that are oscillating over short and long periods of time. Techniques such as EEG and MEG, with their excellent temporal resolution, are optimal for calculating connectivity (Sakkalis 2011; Greenblatt et al. 2012). Traditionally to determine connectivity in the EEG or MEG, a frequency analysis was performed to convert the original EEG or MEG data into its frequency content; then, coherence analysis was used to obtain information about the temporal relationships of frequency components at different recording sites (electrode or coil). The results of the coherence analysis were typically displayed in sensor space using a template of the head with lines connecting the electrodes or coils that are coherent with each other. These results can now be displayed in source space due to improve analytical techniques.
MEG and EEG data are usually filtered and have noise artifacts removed prior to advanced analysis. In some cases, it maybe helpful to first decompose a signal in temporal and spatial modes using techniques such as principle component analysis (PCA) or independent component analysis (ICA). These techniques can be used to extract a particular signal of interest (i.e., an epileptic seizure) or an artifact signal such as cardiac activity to be removed from all the data channels.
3 Network Connectivity Measurement
Network connectivity measurements can be measured in the frequency domain with methods such as coherence and phase synchrony and in the time domain with methods such as correlation and Granger causality.
4 Coherence and Phase Synchrony
First, we look at the frequency domain methods for calculating neuronal networks; these tend to be symmetrical providing no information on directionality.
4.1 Coherence
Coherence is used to determine if different areas of the brain are generating signals that are significantly correlated (coherent) or not significantly correlated (not coherent), using a scale of 0–1. coherence is a measure of the synchronicity of the neuronal patterns of oscillator activity. The coherence analysis is a technique that can be applied to study functional relationships between spatially separated scalp electrodes or coils and to estimate the similarities of waveform components generated by the neurons in the underlying cortical regions (French and Beaumont 1984). Transients and oscillations of brain electric activity are found in MEG and EEG recordings of spontaneous brain activity. Coherence differs from correlation because the assessment of brain synchrony is done for very narrow frequency bands where the band activity is quantified by an amplitude and phase. These transient waveform oscillations can be quantified by first applying a time–frequency decomposition technique such as the fast Fourier transform (FFT), of a contiguous or slightly overlapping sequence of short data segments. This generates a sequence of amplitude/phase components for each narrow frequency bin of the FFT that spans the frequency content of the data. After transformation to a time–frequency representation, the strength of network interactions can be estimated by calculation of coherence, which is a measure of synchrony between signals from different brain regions for each FFT frequency component. This is the most common measure to describe how two or more time series are related. Strictly speaking, coherence is a statistic that is used to determine the relationship between 2 data sets. It is used to determine if the signal content of 2 inputs is the same or different. If the signals measured by 2 electrodes or coils are identical, then they have a coherence value of 1; depending on how dissimilar they are, the coherent value will approach 0. It is commonly used to estimate the spectral densities of two signals and so is equivalent to a correlation coefficient in the frequency domain. Unlike correlation, coherence has a range of 0–1, and since each FFT yields one pair of FFT components, multiple independent segments of data are needed for evaluation (Kelly et al. 1997).
As mentioned earlier, this technique can be applied to the MEG and EEG waveforms in sensor space, or it can be applied to the localized MEG solutions in source space. coherence has been widely used in studying epileptiform activity to determine seizure onset zones. In sensor space, Song et al. showed that EEG coherence can be used to characterize a pattern of strong coherence centered on temporal lobe structures in several patients with epilepsy (Song et al. 2013). In source space, Elisevich et al. showed that MEG coherence source imaging in the brain can provide targets for successful surgical resections in patients with epilepsy (Elisevich et al. 2011). Hinkley et al. used source space MEG to detect decreased and increased connectivity differences between patients with schizophrenia and control subjects that may prove to be important target areas for treatment (Hinkley et al. 2011).
Directionality of network interaction cannot be determined from coherence, and the exact amplitudes of the network interaction are not equal to region-to-region coherence amplitudes. Coherence does provide a global estimate of all important regions of network activity regardless of source amplitudes. Because of the need to minimize bias by increasing the number of data segments in calculations, coherence is not well suited for quantifying rapid temporal changes in synchronized activity. Rather, it is best when used for long time series of data to identify sources of brain network activity that persist for long durations. However, it is desirable that the individual FFT components follow temporal changes in network connectivity. Therefore, the length of segments of data used in the FFT transform should be selected in the same way as recommended for correlation calculations. For MEG data, we have found approximately 0.5 s of data to be near optimal for data filtered 3–50 Hz. When applied to very low-frequency band data, the FFT data segment length needs to be increased. An F-statistic can be used to test statistical significance relative to the hypothesis of true coherence (Kelly et al. 1997).
Coherence is best when used for long time series of data to identify sources of brain activity that are part of the same network. Coherence analysis supplies information on the degree of synchrony of brain activity at different locations for each frequency, independent of power. However, individual time points with large amplitudes are more highly weighted in the FFT transform and subsequently in coherence calculations. This is in contrast to phase synchrony which utilizes instantaneous measurements of only the phase differences between signals. As mentioned earlier, coherence analysis can be applied to the MEG and EEG waveforms in sensor space, or it can be applied to the localized MEG solutions in source space.
4.2 Phase Synchrony
The phase relationship is a way to estimate the synchrony of oscillations in EEG/MEG data. This is the process by which two or more cyclic signals tend to have oscillator activity that are at the same frequency (in phase) or out of synchrony (out of phase) by a relative phase angle. Phase synchrony is used to investigate whether two waveforms with the same narrow frequency band have relatively stable phase differences independent of their amplitude behavior. This is used to determine if the phases are coupled across the brain and to see if they are phase-locked to an external stimuli or event.
One example of phase synchronization of neuronal activity can be seen in the pattern of the oscillatory activity. If the oscillations are all in synchrony and positive at the same time, then the phase is 0°; if they were opposite (one positive and one negative) to each other, they would have a phase angle of 180°, See Fig. 5 (Uhlhaas and Singer 2010). Phase synchrony measures how stable the phase difference (small or large) varies over a short period of time. Phase relationships can be examined by testing the stability of the signals’ phase differences across trials (phase-locking) over a single electrode or between pairs of electrodes (Lachaux et al. 1999). This approach can yield estimates of the precision of local and long-range synchrony. Importantly, measures of phase-locking provide estimates of synchrony independent of the amplitude of oscillations. This is in contrast to measures of coherence where phase and amplitude are intertwined (Uhlhaas et al. 2009). Phase synchrony reflects the exact timing of communication between distant neural populations that are related functionally, the exchange of information between global and local neuronal networks, and the sequential temporal activity of neural processes in response to incoming sensory stimuli (Sauseng and Klimesch 2008).
Measuring neural synchrony in EEG/MEG signals. a Measuring phase synchrony in brain signals: The synchrony of oscillations in EEG/MEG data can be estimated by analyzing phase relationships. The top panel shows oscillatory brain signals recorded by two different groups of sensors (red and blue) placed in the positions shown in the bottom panel. The middle panel shows the difference in oscillatory phase between the red and blue signals. Phase difference values around zero indicate phase synchrony. The bottom panel illustrates patterns of synchrony between distant sensor sites at different time points. The black lines link synchronous sensors (Uhlhaas and Singer 2010). b Inter-electrode phase locking and single-electrode phase locking. The left panel shows brain signals recorded from two electrodes (i and j; black and red, respectively) across several trials. The electrodes display inter-electrode phase locking if their phase difference (distance between black and red vertical lines on top of the curves) remains relatively constant inside a time window (W) across the trials. This yields an estimate of functional coupling (long-range synchronisation) between two cortical areas. Note that it is not required that the phase of each electrode remains constant, only the difference between electrodes must be constant. The right panel shows recordings from a single electrode (k, green line) across several trials. The electrode shows single-electrode phase locking if, at a given time point after stimulus onset (black vertical line), the phase remains relatively constant across the trials. This is an index of stimulus dependent phase resetting but does not imply functional coupling between cortical areas. It is a concept very similar to the evoked potential but it depends only on the phase of the signal and does not depend on the local amplitude. Images courtesy of F. Roux and E. Rodriguez, Max Planck Institute for Brain Research, Frankfurt am Main, Germany
In the extensive study by Gaillard et al., significant long-distance phase synchrony in the beta range was observed after presented words; this increase was the only significant correlate with conscious access to the stimuli (Gaillard et al. 2009). In advance of the stimuli, attentional and working memory functions, in part, were correlated with phase-coupling of prefrontal and posterior brain areas. Gross et al. (2004) found frontal, parietal, and temporal beta coherence was relevant for the processing of stimuli in working memory. In the field of schizophrenia, Uhlhaas and Singer 2010 provided an in-depth review of abnormal neural oscillations and synchrony in this patient group. They review several studies that indicated that patients with schizophrenia have a reduced phase synchrony in the beta and gamma bands. (Uhlhaas and Singer 2010).
Phase synchrony is better used for short-duration events such as in an evoked event. Phase is used to determine how much the two locations (recording sites) are interacting within a very narrow time window (milliseconds). A great analogy for understanding the difference between when to use coherence or phase synchrony is soldiers marching in a parade: Phase synchrony is used to determine how synchronized their feet are marching in unison in a few steps, while coherence is used to see how synchronous their feet were marching in unison over the entire parade route. On a side note, it is possible to determine information flow with a Hilbert transform on the data (to obtain the instantaneous phases). Two measures can be computed from the phases. The first one is the synchronization index, which quantifies the degree of coupling of the phases of two signals [0 (no coupling) to 1 (strongest coupling)]. The second measure is a directionality index which quantifies the direction of coupling between two oscillators [−1 and 1 correspond to unidirectional coupling (away and toward the reference area, respectively), and 0 corresponds to symmetric bidirectional coupling]. The results from the analysis of phase synchronization can be used for the quantification of coupling strength and direction because these measures are more sensitive and robust than coherence and phase difference and independent of amplitude dynamics. This is usually applied to a narrow frequency band, usually less then 5 Hz as opposed to a larger frequency range that desynchronizes rapidly due to many varied frequencies mixed in.
5 Granger Causality and Correlation
Now, we turn to the time domain methods. Long-range connections between different brain structures involve time delays due to the finite conduction velocity of axons. Since most connections in the brain are reciprocal, they form feedback loops that support oscillatory activity. Neuronal activity recorded from multiple cortical areas around the brain can become synchronized and form a large-scale network. The effective connectivity (direction of information) of a brain network can be studied using a Granger causality measure (Brovelli et al. 2004; Gross et al. 2002), while the strength of the connection can be assessed using a correlation measure between two sites.
5.1 Correlation
Correlations is a mathematical technique to measure the similarity of 2 signals on a scale of −1 to +1. Correlation is one of the most commonly used methods to determine the strength of an interaction between two locations or signals. When two areas of the brain are active at the same time, they are most likely talking or communicating with each other. A correlation between one specific region in the brain and the entire brain can be analyzed; this can also be extended to all possible correlations within the brain. Correlations can be determined in several different ways: The Pearson product moment correlation, the Spearman rank order correlation, the Kendall rank order correlation, or by mutual information methods. The Pearson product moment correlation quantifies the linear correlations between two signals or locations, where the Spearman rank order correlation is a nonparametric measure of correlation between two signals based on the rank (i.e., the similarity of the orderings of the data when ranked by each individual quantity) and the Kendall tau (τ) coefficient which is a non parametric test that uses the relative ordering of ranks. The mutual information of two time series is a measure of their mutual dependence where the unit of measure is a bit. These measures have been widely used to quantify correlations between EEG or MEG recordings from healthy participants (Bonita et al. 2014) or patients with neurologic disorders such as traumatic brain injury (Castellanos et al. 2011), Alzheimer’s (Stam et al. 2007), epilepsy(Ponten et al. 2007), and with schizophrenia (Rubinov et al. 2009).
One advantage of computing correlation matrices is that these correlations can be further studied using graph theory to evaluate the topological properties of the functional networks or wavelet analysis applied to the temporal signals to compute frequency-dependent correlation matrices. Graph theory is used to create models of the complex functional brain networks that can be further studied (Stam et al. 2007; Stam and Reijneveld 2007; Bassett and Bullmore 2006). Graphs are composed of vertices (corresponding to neurons or brain regions) and edges (corresponding to synapses or pathways, or statistical dependencies between neural elements). Graphs of brain networks can be quantitatively examined for vertex degrees and strengths, degree correlations, clustering coefficients, path lengths (distances), and vertex and edge centrality, among other characteristics. Graph theory provides models of complex networks in the brain and allows one to better understand the relations between network structure and the processes taking place on those networks.
Despite its usefulness for detecting linear statistical dependencies, the correlation analysis has certain limitations. The most important relies on the fact that some networks are not spatially independent and can overlap. In other words, the same cortical region can belong to more than one active network (i.e., rest and memory). Therefore, the activation pattern of that region may turn out to be a sum of several simultaneously active networks, limiting the ability to capture the one functional network using correlation-based approaches (Smith et al. 2012).
5.2 Granger Causality
Granger causality as well as other methods such as directed transfer function (DTF) and partial directed coherence (PDC) are designed to determine causality of network activities (Sakkalis 2011). The basis for these techniques is a multiple linear regression model of future activity at a given site, determined by past activity at sites and times within the network. Granger causality is a statistical method for determining the direction of information flow in a brain network. By looking at the interaction between time series, information can be provided on how one signal may affect another signal. There is no a priori information used. Granger causality assumes that a cause precedes an event. Therefore, a signal can be predicated based on the past information of a second signal. Simply stated X causes Y if X provides information that predicts the future of Y better then any information already known about Y.
Granger causality has been used to study the effective connectivity in patients with schizophrenia using fMRI (Demirci et al. 2009) more so than with EEG or MEG. When used with FMRI data, an independent component analysis (ICA) is initially used to extract the time courses of spatially independent components, and then, a Granger causality test is used to investigate causal relationships between brain activation networks. This study found evidence that distributed networks are organized in a fashion suggestive of hubs of activity within specific circuits that directly or indirectly influence other neural function in normal controls but that this connectivity is abnormal in patients with schizophrenia (Demirci et al. 2009).
Granger causality analysis for studying effective connectivity in neural systems has a few limitations; one is that it relies heavily on sensor space analyses when source space analysis may provide more information, also there is the use of an unrealistically small sets of factors that make analyses vulnerable to spurious correlation, and finally the failure to address the complex and chaotic nature of neural processes.
Both directed transfer function (DTF) (Kaminski and Blinowska 1991) and partial directed coherence (PDC) (Sameshima and Baccala 1999) have been developed based on network model approaches such as Granger causality. DTF extends Granger causality to multichannel MEG and EEG. It has been used to estimate functional connectivity in neurological disorders such as epilepsy (Franaszczuk and Bergey 1998). Another more recent measure called transfer entropy (Schreiber 2000) was designed to detect directed exchange of information between two systems by considering the effects of the state of one element on the state transition probabilities of the other element. Seibenhuhner et al. found lower entropy, suggesting decreased MEG signal content, but increased functional connectivity in patients with schizophrenia compared to control subjects (Siebenhühner et al. 2013).
6 Conclusion
The basis of brain functioning is the neuronal oscillations. In this section, we attempted to review several of the most common methods used to measure the brain’s synchronous oscillations which make up the network of brain connectivity. Many of these techniques are currently being used to expand clinical knowledge in many fields with psychiatry being one of the most prominent fields. We have highlighted some of the types of information that can be derived from the varied techniques as well as provided some of the limitations of each technique. In the future, a combined anatomical, functional, and effective connectivity mapping will become the mainstay of the neurosurgeon, neurologist, and psychiatrist for assessing and diagnosing normal and abnormal brain networks. These techniques will not only provide biomarkers of diseases but also help to provide individualized treatment therapies based on pre- and post-treatment connectivity imaging. With the evolution of computers and mathematics, we expect to see more sophisticated and powerful analytical neuroimaging methods developed and applied to the functional neuroimaging data. The primary functional neuroimaging results will continue to be provided from the excellent high temporal resolution of MEG and EEG, and the high spatial resolution of fMRI and PET as well as the anatomical connectivity maps derived from MRI-DTI imaging.
References
Bassett DS, Bullmore E (2006) Small-world brain networks. Neuroscientist 12(6):512–523
Bonita JD, Ambolode LCC II, Rosenberg BM, Cellucci CJ, Watanabe TAA, Rapp PE, Albano AM (2014) Time domain measures of inter-channel EEG correlations: a comparison of linear, nonparametric and nonlinear measures. Cogn Neurodyn 8:1–15
Brovelli A, Ding M, Ledberg A, Chen Y, Nakamura R, Bressler SL (2004) Beta oscillations in a large-scale sensorimotor cortical network: Directional influences revealed by Granger causality. PNAS 101(26):9849–9854
Cabral J, Kringelbach ML, Deco G (2014) Exploring the network dynamics underlying brain activity during rest. Prog Neurobiol 114:102–131
Castellanos NP, Leyva I, Buldu JM, Bajo R, Paul N, Cuesta P, Ordonez VE, Pascua CL, Bocaletti S, Maestu E, del-Pozo F (2011) Principles of recovery from traumatic brain injury: reorganization of functional networks. Neuroimage 55(3):1189–1199
Chana G, Bousman CA, Money TT, Gibbons A, Gillett P, Dean B, Everall IP (2013) Biomarker investigations related to pathophysiological pathways in schizophrenia and psychosis. Front Cell Neurosci 7(95):1–18
Demirci O, Stevense MC, Andreasend NC, Michaela A, Liua J, Whiteh T, Pearlsone JD, Clark VP, Calhouna VD (2009) Investigation of relationships between fMRI brain networks in the spectral domain using ICA and Granger causality reveals distinct differences between schizophrenia patients and healthy controls. Neuroimage 46(2):419–431
Elisevich K, Shukla N, Moran JE, Smith B, Schultz L, Mason K, Barkley GL, Tepley N, Gumenyuk V, Bowyer SM (2011) An assessment of MEG coherence imaging in the study of temporal lobe epilepsy. Epilepsia 52(6):1110–1119
Franaszczuk PJ, Bergey GK (1998) Application of the directed transfer function method to mesial and lateral onset temporal lobe seizures. Brain Topogr 11(1):13–21
French CC, Beaumont JG (1984) A critical review of EEG coherence studies of hemisphere function. Int J Psychophysiol 1(3):241–254
Friston KJ, Frith CD, Liddle PF, Frackowiak RS (1993) Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab 13:5–14
Gaillard R, Dehaene S, Adam C, Clemenceau S, Hasboun D, Baulac M, Cohen L, Naccache L (2009) Converging intracranial markers of conscious access. PLoS Biol 7:472–492
Goense JB, Logothetis NK (2008) Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr Biol 18(9):631–640
Greenblatt RE, Pflieger ME, Ossadtchi AE (2012) Connectivity measures applied to human brain electrophysiological data. J Neurosci Methods 2007(1):1–16
Gross J, Schmitz F, Schnitzler I, Kessler K, Shapiro K, Hommel B, Schnitzler A (2004) Modulation of long-range neural synchrony reflects temporal limitations of visual attention in humans. Proc Natl Acad Sci USA 101(35):13050–13055
Gross J, Timmermann L, Kujala J, Dirks M, Schmitz F, Salmelin R, Schnitzler A (2002) The neural basis of intermittent motor control in humans. PNAS 99(4):2299–2302
Haenschel C, Linden D (2011) Exploring intermediate phenotypes with eeg: working memory dysfunction in schizophrenia. Behav Brain Res 216:481–495
Hamalainen M, Hari R, Ilmoniemi J, Knuutila J, Lounamaa OV (1993) Magnetoencephalography-theory, instrumentation and applications to noninvasive studies of the working human brain. Rev Mod Phys 65(2):413–497
Hinkley LB, Vinogradov S, Guggisberg AG, Fisher M, Findlay AM, Nagarajan SS (2011) Clinical symptoms and alpha band resting-state functional connectivity imaging in patients with schizophrenia: implications for novel approaches to treatment. Biol Psychiatry 70(12):1134–1142
Honey CJ, Kötter R, Breakspear M, Sporns O (2007) Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proc Natl Acad Sci USA 104(24):10240–10245
Horwitz B (2003) The elusive concept of brain connectivity. Neuroimage 19:466–470
Kaminski MJ, Blinowska KJ (1991) A new method of the description of the information flow in the brain structures. Biol Cybern 65(3):203–210
Kelly EF, Lenz JE, Franaszczk PJ, Truong YK (1997) A general statistical framework for frequency-domain analysis of EEG topographic structure. Comput Biomed Res 30:129–164
Kubicki M, Westin CF, McCarley RW, Shenton ME (2005) The application of DTI to investigate white matter abnormalities in schizophrenia. Ann NY Acad Sci 1064:134–148
Lachaux JP, Rodriguez E, Martinerie J, Varela FJ (1999) Measuring phase synchrony in brain signals. Hum Brain Mapp 8:194–208
Le Bihan D, Mangin JF et al (2001) Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 13(4):534–546
Llinas RR (1988) The Intrinsic electrophysiological properties of mammalian neurons: a new insight into CNS function. Science 242(4886):1654–1664
Llinas RR, Grace AA, Yarom Y (1991) In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range. Proc Natl Acad Sci USA 88(3):897–901
Logothetis NK, Pfeuffer J (2004) On the nature of the BOLD fMRI contrast mechanism. Magn Reson Imaging 22(10):1517–1531
Ogawa S, Lee TM, Kay AR, Tank DW (1990) Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA 87:9868–9872
Ponten SC, Bartolemi F, Stam CJ (2007) Small-world networks and epilepsy: graph theoretical analysis of intracerebrally recorded mesial temporal lobe seizures. Clin Neurophysiol 118:918–927
Rotarska-Jagiela A, Schönmeyer R, Oertel V, Haenschel C, Vogeley K, Linden DE (2008) The corpus callosum in schizophrenia-volume and connectivity changes affect specific regions. Neuroimage 39(4):1522–1532
Rowe JB (2010) Connectivity analysis is essential to understand neurological disorders. Front Syst Neurosci 4(144):1–13
Rubinov M, Knock SA, Stam CJ, Micheloyannis S, Harris AWF, Williams LM, Breakspear M (2009) Small world properties of nonlinear brain activity in schizophrenia. Hum Brain Mapp 30:403–416
Sakkalis V (2011) Review of advanced techniques for the estimation of brain connectivity measured with EEG/MEG. Comput Biol Med 41:1110–1117
Sameshima K, Baccala LA (1999) Using partial directed coherence to describe neuronal ensemble interactions. J Neurosci Meth 94(1):93–103
Sauseng P, Klimesch W (2008) What does phase information of oscillatory brain activity tell us about cognitive processes? Neurosci Biobehav Rev 32(5):1001–1013
Schreiber T (2000) Measuring information transfer. Phys Rev Lett 85:461–464
Siebenhühner F, Weiss SA, Coppola R, Weinberger DR, Bassett DS (2013) Intra- and inter-frequency brain network structure in health and schizophrenia. PLoS ONE 8(8):1–13
Smith SM, Miller KL, Moeller S, Xu J, Auerbach EJ, Woolrich MW, Beckmann CF, Jenkinson M, Andersson J, Glasser MF, Van Essen D, Feinberg D, Yacoub E, Ugurbil K (2012) Temporally-independent functional modes of spontaneous brain activity. PNAS 109(8):3131–3136
Song J, Tucker DM, Gilbert T, Hou J, Mattson C, Luu P, Holmes MD (2013) Methods for examining electrophysiological coherence in epileptic networks. Front Neurol 4:55
Sporns O (2007) Brain connectivity. Scholarpedia, 2(10):4695
Stam CJ, Jones BF, Nolte G, Breakspear M, Scheltens PH (2007) Small-world networks and functional connectivity in Alzheimer’s disease. Cereb Cortex 17:92–99
Stam CJ, Reijneveld JC (2007) Graph theoretical analysis of complex networks in the brain. Nonlin Biomed Phys 1(3):1–18
Stephan KE, Friston KJ, Frith C (2009) Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull 35:509–527
Towle VL, Hunter JD, Edgar JC, Chkhenkeli SA, Castelle MC, Frim DM, Kohrman M, Hecox KE (2007) Frequency domain analysis of human subdural recordings. J Clin Neurophysiol 24(2):205–213
Uhlhaas PJ, Roux F, Rodriguez E, Rotarska-Jagiela A, Singer W (2009) Neural synchrony and the development of cortical networks. Trends in Cogn Sci 14(2):72–80
Uhlhaas PJ, Singer Wolf (2010) Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci 11(2):100–113
Van Essen DC, Smith SM, Barch DM, Behrens TEJ, Yacoub E, Ugurbil K, for the WU-Minn HCP Consortium (2013) The WU-Minn human connectome project: an overview. NeuroImage 80:62−79
Varela F, Lachaux JP, Rodriguez E, Martinerie J (2001) The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci 2(4):229–239
Wang XJ (2010) Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev 90:1195–1268
Wedeen VJ, Wanga RP, Schmahmannb JD, Bennera T, Tsengc WYI, Daia G, Pandyad DN, Hagmanne P, D’Arceuila H, de Crespignya AJ (2008) Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage 41(4):1267–1277
White T, Magnotta VA, Bockholt HJ, Williams S, Wallace S, Ehrlich S, Mueller BA, Ho BC, Jung RE, Clark VP, Lauriello J, Bustillo JR, Schulz SC, Gollub RL, Andreasen NC, Calhoun VD, Lim KO (2011) Global white matter abnormalities in schizophrenia: a multisite diffusion tensor imaging study. Schizophr Bull 37(1):222–232
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Bowyer, S.M. (2014). Connectivity Measurements for Network Imaging. In: Kumari, V., Bob, P., Boutros, N. (eds) Electrophysiology and Psychophysiology in Psychiatry and Psychopharmacology. Current Topics in Behavioral Neurosciences, vol 21. Springer, Cham. https://doi.org/10.1007/7854_2014_348
Download citation
DOI: https://doi.org/10.1007/7854_2014_348
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-12768-2
Online ISBN: 978-3-319-12769-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)