Abstract
β-Galactosidase (β-gal) is an enzyme commonly served as a reporter for the examination of transcription and transfection efficiencies. Due to its overexpression in primary and metastatic ovarian cancers, β-gal was also usually regarded as a molecular target for visualizing peritoneal metastases from ovarian cancers. Moreover, β-gal has been studied as a potential therapeutic target for lactose intolerance via gene replacement therapy in recent years. Interestingly, there were some reports that β-gal has been abnormally accumulated in senescent cells, which allowing this senescence-associated β-gal to be a significant biomarker for senescence. The great significance of β-gal has attracted many researchers’ attentions in developing highly selective and sensitive approaches to monitor the activity of this enzyme in vitro and in vivo. In this review, we reported the recent development of the various materials for β-gal detection and their application in disease progression monitoring, with a focus on fluorescent probe, nanomaterials, and biomolecules. Finally, the trends for the further development of the probe for fluorescence-guided diagnosis in clinical cases and its preclinical potential value were proposed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
β-Galactosidase (β-gal), an enzyme that catalyzes the hydrolysis of a glycosidic bond of β-galactopyranoside within a carbohydrate such as lactose, ganglioside GM1, and lactoceramides, is regarded as a very significant biomarker for cell senescence and primary ovarian cancers [1, 2]. And its deficiency is reported to be associated with β-galactosialidosis and Morquio B syndrome. On the other hand, β-gal serves as an important reporter for verifying the efficiency of transcription and transfection as well. Commonly, it acts as a biomarker to monitor the gene expression of lacZ1. Moreover, β-gal has been studied as a potential therapeutic target for lactose intolerance via gene replacement therapy in recent years [3]. According to the great significance of β-gal, it has attracted many researchers’ attention in developing highly selective and sensitive approaches to monitor the activity of the enzyme in vitro and in vivo. There are various detection techniques, including MR [4], single-photon emission computed tomography (SPECT) [5], positron emission tomography (PET) [6], colorimetric [7], fluorogenic [8], chemiluminescence [9], and bioluminescence [10] approaches. By contrast, fluorescent probes are of great interest owing to their conveniences like high sensitivity, simple handling procedures, inexpensive instruments, and bioimaging ability. Herein, we reported the progress of fluorescent nanomaterials in various fields, especially in chemical sensing and biomedical imaging such as the detection of β-gal, and recent advances related to the nanomaterial for β-gal detection have also been mentioned.
2 Fluorescent Probe for β-Galactosidase Activity Detection
2.1 Fluorescent Probe Based on Coumarins, Fluoresceins, and Rhodamines
Recent research has illustrated that β-gal can be used as a molecular target for visualizing peritoneal metastases originating from primary ovarian cancers. As a result, great efforts have been devoted to developing a real-time tracking method for β-gal. A number of fluorescent probes based on coumarins, fluoresceins, and rhodamines have been synthesized for monitoring β-gal activity.
In 2014, Lee et al. [11] reported a two-photon β-gal fluorescent probe 1 and applied it to the detection of β-gal activity quantitatively in living cells and in aged tissues during cellular senescence. In their strategy, 6-(benzo[d]thiazol-2-yl)-2-(methylamino)-naphthalene was used as the probe fluorophore and β-d-galactopyranoside-derived benzyl carbamate as the β-gal hydrolytic site. After exposing to β-gal, the carbamate compound was cleaved to afford the amino group as the electron donor. An internal charge transfer (ICT) effect was observed to shift the emission to the stronger fluorescent emission. After treating with β-gal in PBS buffer (10 mM, pH 7.4, 37°C), the fluorescent intensity of a 1 μM probe solution increased gradually at 540 nm while decreased at 460 nm. The ratio of the emission intensities at 410–460 nm and 520–570 nm increased by 120-fold upon reaction. Meanwhile, d-galactose, a well-known competitive inhibitor of β-gal, could not trigger this fluorescent emission, indicating that the fluorescent enhancement is specific to β-gal activity. A linear relationship was observed against the β-gal concentration ranging from 0 to 2.0 nM, indicating that probe 1 could quantify β-gal in the low concentration range. Next, the application practicability of 1 has been examined in a representative cell senescence model via monitoring the endogenous SA-β-gal activity. Doubling times and population were recorded in this process, during the middle stages of senescence, suggesting that the probe could sensitively detect the slight increases in SA-β-gal. Further, the probe was successfully applied for tissue imaging. Using 7- and 26-month-old Sprague−Dawley rat skin tissues, the TPM images of probe 1 clearly showed the distribution of β-gal activity at a depth of about 140 μm (Fig. 1).
Pseudocolored ratiometric TPM images (Fyellow/Fblue) of (a) 7-month-old and (b) 26-month-old Sprague−Dawley rat skin tissues stained with 10 μM SG1 (Reproduced from ref. [11] with permission from ACS)
Later in 2015, Hirabayashi et al. [12] made great efforts in fluorescent detection of β-gal. They reported a silicon-substituted fluorescein, which had a carboxylic group at the 2-position of the benzene moiety, to construct a red-fluorescent probe 2 for β-gal. Firstly, TokyoMagenta (TM), a moiety of fluorescein which has been employed, was modified with enzyme substrates at the 3′ and 6′ positions to form the colorless and nonfluorescent status. Then they applied 2 to test β-gal for the enzymatic reaction. The solution of probe 2 was almost colorless and nonfluorescent initially, but a sharp shift of red color was observed and strongly fluorescence enhancement (>1,000-fold) appeared after the incubation with β-gal. In the same year, Asanuma et al. [13] reported a strategy to synthesize a hydroxymethyl rhodol (HMR) derivative bearing β-gal, HMRef-β-gal (3), which enabled highly sensitive detection of β-gal activity in living cells. The probe exhibited a weak fluorescence at pH 7.4 without β-gal, while an obvious high fluorescence increase (1,400-fold) was observed upon the reaction with β-gal. They further applied probe 3 for imaging several cultured ovarian cancer cells. The results showed that the enhanced fluorescence could be decreased by a competitive β-gal inhibitor, indicating the high specificity of 3 to detect intracellular β-gal activity. Further, SHIN3 cells were used to test the practicability of cancer imaging in a mouse model. The metastases as small as 1 mm were visualized clearly and specifically after injection of 3 in 5 min. This results clearly indicated potential value of the probe for fluorescence-guided diagnosis of peritoneal metastases from ovarian cancers.
In recent years, Zhang et al. [14] reported a small molecule fluorescent probe named 4-hydroxyl-N-butyl-1,8-naphthalimide–β-gal (NI–β-gal, 4) for the detection of β-gal. Upon incubation with β-gal, probe 4 would turn into 4-hydroxyl-N-butyl-1,8-naphthalimide with a large Stokes shift and sharp fluorescent enhancement according to the restoring of ICT character. The synthetic strategy was well prepared so as all the reactions conditions were mild. After a three-step procedure, 4-bromo-1,8-naphthalic anhydride was adopted to afford 4-hydroxyl-N-butyl-1,8-naphthalimide. Upon addition of β-gal into probe 4 in PBS buffer (pH 7.4), a large fluorescence increase was observed at 545 nm and a decrease at 440 nm. Correspondingly, the F545/F440 ratio acted as an enhancement behavior of 680-fold. Subsequently, probe 4 turned out to be qualified to image stable β-gal expression within tumors in living mice, and bright fluorescence could be observed in the transfected tumor at 75 min after administration of 4, whereas the control group exhibits a negative signal. In comparison with non-transfected tumors, transfected tumors showed an approximately tenfold higher fluorescence intensity at 2 h after administration of 4. Therefore, probe 4 maybe a useful tool in biomedical research such as gene therapy for cancer in the future.
2.2 NIR Fluorescent Probe
Conventional fluorophores with wavelengths less than 600 nm exhibited several shortcomings in vivo, such as poor tissue penetration and strong background fluorescence from bio-specimens. In order to overcome preceding drawbacks as well as elevate the signal-to-noise ratio, near-infrared fluorescent probes targeting β-gal have been prepared by incorporating β-galactose residues into near-infrared fluorophores like cyanine and squarylium dye scaffolds.
Redykeisar et al. [15] reported QCy7-based probe 5 targeting β-gal, which possessed long-wavelength fluorescence and a turn-on option. The fluorophore named quinone-cyanine-7 (QCy7), which was prepared by a simple two-step procedure, had strong emission in the NIR region but no emission after linking with β-galactose residues. To assay the turn-on imaging option in vivo, probe 5 were then injected subcutaneously into mice and the signal was monitored over time. The results showed that probe 5 possessed excellent in vivo compatibility and exhibited an enhanced NIR fluorescent emission with the high signal-to-background ratio in mice. In the same year, Han et al. [16] reported a fluorescent turn-on probe termed AcGQCy7 (6) to detect β-gal activity in living cells. Because of its disrupted p-conjugated system, the prepared probe 6 was nonfluorescent in the NIR region, but after the enzymatic cleavage of β-galactose by β-gal under physiological conditions, the probe emitted strong fluorescence. The probe (300 μM) was dissolved in HBSS was tested for the presence or absence of β-gal (15 U mL−1) at 37°C for 30 min. The color of the solution rapidly turned into dark blue when it was in contact with β-gal, and a broad absorbance band covering of 400–580 nm was detected. The fluorescence intensity of the β-gal-treated solution was 110-fold higher than that of the solution without it. The cellular imaging capability of the probe was studied in two cell lines, rat glial tumor C6 (control, no β-galactosidase expression) and its derivative, C6/LacZ, which constitutively expresses β-gal. C6/LacZ or C6 cells were incubated in HBSS buffer containing probe 6 (1 mM) at 37°C for 40 min. As expected, a bright fluorescence signal was detected only in the β-gal-positive C6/LacZ cells but not in the β-gal-negative C6 cells. Co-localization studies indicated that the cleaved product that specifically targeted mitochondria as the red fluorescence from 6 was overlapped with the MTG signal. This finding agrees with the author’s another research of QCy7 as an excellent mitochondria mark.
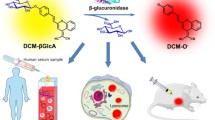
In 2016, Gu et al. [17] reported a ratiometric near-infrared probe (DCM-β-gal) which was able to be activated by β-gal for the real-time fluorescent quantification in vivo and in situ. They designed 7 by grafting a β-gal activatable unit onto an NIR fluorophore DCM-OH. Upon excitation of the new absorption peak at 535 nm, a strong NIR fluorescence enhancement was observed with a peak at 685 nm. The color changed from faint yellow to rose red, which allows the colorimetric detection of β-gal using the naked eye. Furthermore, owing to the alteration in concentration-dependent ratio, the detection limit of DCM-β-gal was 1.7 × 10−4 U mL−1. In order to get living cells generating endogenous β-gal, lacZ gene was introduced into 293T cells through a gene transfection method to overexpress β-gal. After incubation with 7 (10 μM) at 37°C for 30 min, the lacZ-(+) 293T cells successfully overexpressed β-gal and were observed to exhibit a decreased fluorescence in the green channel as well as a concomitant increase in the red channel. These results suggested that 7 could be specifically activated in β-gal-expressing cells, hence offering a ratiometric and light-up NIR readout for the in situ quantitative tracking and visualization of endogenous β-gal in living cells (Figs. 2 and 3).
Confocal and ratiometric images of 293T and OVCAR-3 cells incubated with DCM-β-gal (10 μM) for 30 min: (a–g) lacZ-(−) 293T cells without overexpressed β-gal and (h–n) lacZ-(+) 293T cells with overexpressed β-gal pretreated with inhibitor for 30 min (Reproduced from ref. [17] with permission from ACS)
2.3 ESIPT and AIE Fluorescent Probes
Besides ICT mentioned above, the β-gal could also be detected through varieties of other mechanisms, such as excited-state intramolecular proton transfer (ESIPT), the aggregation-induced emission (AIE), and so on. ESIPT is a fast photochemical process in which a proton transfers from a hydroxyl (or amino) unit to a carbonyl oxygen (or imine nitrogen) atom in the excited state of a fluorophore [18, 19]. ESIPT compounds generally have high fluorescence quantum yield, large Stokes shift, and dual-wavelength emission (emission of enol and ketone form). Hence ESIPT-based fluorophores are ideal candidates for acting as a signal component to detect biomolecules [20]. In recent years, several ESIPT fluorophores, including 2-(benzothiazol-2′-yl)-phenols, and salicylaldehyde azine (SA) derivatives have been developed to constitute fluorescent probes for the β-gal detection [21,22,23]. For example, Otsubo et al. [21] selected 2-(benzothiazol-2-yl)-phenol derivatives as the substrates to synthesize a series of fluorescence dyes for β-gal detection. At first, they found the fluorescent wavelength could be controlled by adjusting the electronic character of phenol or benzothiazole ring. Compared with the compound with no substituent, an electron-donating effect at the meta-position of phenolic hydroxyl moiety could cause an obvious blue shift of the fluorescent wavelength. On the contrary, the electron-donating effect at para-position made the fluorescent wavelength shift toward the longer side. Moreover, the further shift to the longer-wavelength end could be caused by the reduction of electron density of the benzothiazole ring. Then, these substrates were coupled with 2,3,4,6-tetra-O-acetyl-d-galactopyranosyl bromide and converted to 2-(benzothiazol-2-yl)-phenyl-2,3,4,6-tetra-O-acetyl-β-d-galactopyranoside derivatives; afterward, all the acetyl groups were removed and the fluorescent probes for β-gal detection have been successfully synthesized. These probes exhibited no or weak fluorescence in solution because their ESIPT effect was blocked; however, after reacting with β-gal from Aspergillus oryzae, the fluorescent probes were hydrolyzed and the ESIPT effect could be restored, resulting in greatly enhanced fluorescence intensities. Their ratios of fluorescence intensity before and after enzyme reaction were high enough to detect β-gal accurately. In addition, it was worth mentioning that these probes could trace β-gal at low pH, and because of their immobility or poor solubility to water, the probes might stain live cells on the outer surface or interior of a cell membrane without fixing or permeabilizing in the future.
In 2014, Cellier et al. [22] designed and synthesized a series of 2-arylbenzothiazole derivatives-enzyme substrates as fluorogenic probes in order to detect β-gal and other enzymatic activities. The core 2-arylbenzothiazole derivatives were highly fluorescent, and this had been rationalized by ESIPT process. When combined with the enzymic substrates, the ESIPT effect of 2-arylbenzothiazole derivatives was blocked because there were no more hydroxyl protons at the ortho-position. However, enzyme-induced hydrolysis of weakly fluorescent enzyme substrate derivatives respectively liberated either fluorescent compounds. In addition, the coliform bacteria had also been detected because they could grow in the presence of a β-gal substrate which was transformed by β-gal into a fluorescent product that could readily be detected. Moreover, Wei et al. [23] successfully reported a series of ESIPT-based 2-(benzothiazol-2′-yl)-phenol fluorogenic substrates for β-gal detection in 2017. They developed a novel efficient method for the synthesis of important indoxyl glycoside substrates by using 1-acetylindol-3-ones as intermediates and synthesized new precipitating fluorogenic substrates for β-gal detection based on 2-(benzothiazol-2′-yl)-phenols and the ESIPT effect. And they have also assessed the application of the fluorogenic substrates in the detection of foodborne pathogenic bacteria.
Recently, although some fluorescent probes for β-gal have been developed, most of the reported sensors were fabricated with traditional fluorophores, which suffered from ACQ effect [17]. It causes the fluorescence in the aggregate state to be weaker than with that in solution. Therefore, it is still highly demanded to explore β-gal fluorescent probes, which could accumulate in living cells or tumor tissue imaging without ACQ effect. In 2001, Tang’s group firstly found and reported the phenomenon of “aggregation-induced emission (AIE)” [24]. The compounds with AIE effect are almost nonluminescent when they are dissolved in a good solvent but emit intensely in a poor solvent. Meanwhile, the compounds with AIE effect have significant advantages, such as bright luminescent in the aggregate state and large Stokes shift [25,26,27]; thus, they have been greatly developed for detection of β-gal [28, 29]. In 2015, Peng et al. [28] reported a salicylaldehyde azine derivative SA-β-gal (11) for light-up detection of β-gal activity in living cells based on both AIE and ESIPT effects. When the hydroxyl groups at the ortho-position on the benzene ring of salicylaldehyde azine were substituted by β-galactopyranoside, ESIPT process was blocked. Upon the addition of β-gal, the β-galactopyranoside group on SA-β-gal was cleaved, and the restored hydroxyl group occurred in ESIPT to regain the AIE characteristics, resulting in bright fluorescence. Contrary to traditional β-gal fluorescent sensors, SA-β-gal could emit strongly in the aggregation state, which can well avoid the ACQ phenomenon. The probe showed a large Stokes shift, a high light-up ratio, and a high sensitivity (0.014 UmL−1) toward β-gal. Moreover, it was worth mentioning that the probe could also be well retrained in living cells emitting strong fluorescence (Fig. 4).
Imaging b-galactosidase activity in cells. Images of probe SA-β-gal (50 mM) in (a) C6/LacZ cells and (b) HeLa cells for 2 h at 37°C; the excitation was set at 405 nm (Reproduced from ref. [28] with permission from RSC)
In 2017, Jiang et al. [29] designed and synthesized a tetraphenylethylene-based turn-on probe TPE-Gal (12) for β-gal detection with AIE effect in aqueous samples and in living cells. TPE-Gal was designed to bear a positively charged pyridinium pendant. And a substrate of β-galactosidase-d-galactose residue was conjugated to the terminal of the pyridinium pendant. In the presence of β-gal, the d-galactose residue was cleaved and resulted in a phenolate intermediate. Then the intermediate could undergo 1,6-elimination of p-quinone methide to generate a tetraphenylethylene-pyridinium compound with poor solubility, resulting in the appearance of aggregation effect and a light-up fluorescence of the TPE moiety based on the AIE effect. The TPE-Gal displayed distinguishable advantages such as high specificity toward β-gal, no self-quenching at high concentration, large Stokes shift and so on. Moreover, the researchers also tested the applicability of TPE-Gal for sensing β-gal not only in aqueous solution but also in living cells. The results showed that TPE-Gal could exhibit excellent fluorescence properties in these environments (Fig. 5).
3 Nanomaterial for β-Galactosidase Activity Detection
The nanomaterial has attracted considerable attentions from the early 1980s due to its special physical and chemical properties and promising application. Entering the twenty-first century, the researches of nanomaterials have been extended in various fields, especially in chemical sensing and biomedical imaging such as the detection of β-gal, and some studies related to the nanomaterials for β-gal detection have also been reported.
3.1 Thiolated Copper Nanoclusters (CuNCs) and Silica Nanoclusters
Huang et al. [30] used glutathione (GSH) as the protecting ligand and reducing agent to successfully synthesize thiolate-protected copper nanoclusters (CuNCs) with aggregation-induced luminescence, which can self-assemble into dots by aluminum cations via a coordination reaction and then emit bright red luminescence. CuNCs are one of the most emerging luminescent materials because of their AIE properties [31,32,33] and long decay times in microseconds. And unlike organic AIE dots, thiolated CuNCs are better at avoiding the interference of autofluorescence in biosystems for their long emission times and red/NIR emission. However, the extremely low emission efficiency in neutral solution and poor stability to temperature, pH, and solvent greatly limit its application. Therefore, Chen et al. [34] utilized glutathione as a protector for CuNCs allowing their good stability in not only neutral but also weak alkaline solutions, which enabled their practicability under physiological conditions. And aluminum cations were found to be able to induce the dispersed CuNCs’ (only emit very faint light) assembly into red bright luminescent dots quite effectively in water, which was probably due to the strong coordination of aluminum ions to GSH on the surface of the CuNCs. And the formation of a huge amount of coordinate bonds between GSH and aluminum ions limited the vibration and rotation of the GSH ligands and then reduced the non-radiative decay rate, allowing the enhancement of emission. According to transmission electron microscopy (TEM) images, the mean diameter of CuNCs that existed dispersedly in water was about 2–5 nm, and the dots were mono-dispersive both in luminescence and in size, which benefit for analytical applications a lot. Furthermore, GSH-protected CuNCs were reported that their luminescence intensity was responsive to changes in pH. Huang believed this phenomenon was mostly because of the alternation of pH value, leading to the change of the aggregation state of the CuNCs. At lower pH, the CuNCs aggregated with intensive luminesce, while at higher pH the CuNCs were dispersed, showing weak emission. Interestingly, the reversibility of this pH-driven luminescence switch was observed clearly, as it didn’t fatigue after five continuous cycles. Hence, Huang claimed that CuNCs would make an excellent luminescent pH indicator and a potential β-galactosidase probe. Huang and his team found that the luminescence of the CuNC dots assembled by Al3+ was gradually quenched by increasing amount of p-nitrophenol, indicating that there was significant interaction between these two taking place. The coordination between Al3+ and p-nitrophenol was owing to the highly efficient electron transfer between the CuNC dots and p-nitrophenol. And based on that, Huang reckoned that it could be utilized to assay β-gal using 4-nitrophenyl-b-d-galactopyranoside (NPGal) as the substrate. NPGal would rapidly hydrolyzed into galactose and p-nitrophenol in the presence of β-gal, and the latter was going to attach on the surface of CuNC dots via a coordinate bond, causing luminescence quenching. And the quenching behavior corresponds to the amount of β-gal, illustrating that CuNC dots could serve as a potential monitor of β-gal in a continuous and real-time way.
Agostini et al. [35] also used nanoclusters targeting SA-β-gal presenting specifically in senescent cells to deliver drugs for the purpose of avoiding senescence-related disease. The activity of SA-β-gal is encoded by the GLB1 gene [36, 37], which is a biomarker for increased lysosome number or activity and has been associated with replicative senescence [38, 39] and organismal aging [40, 41] for a long time. And the nano-device involved was mesoporous silica nanoparticles (MSNs) capped with a galacto-oligosaccharide (GOS). The structure of MSNs contained unique mesoporous materials with large specific volume and easer functionalization [42]. It could be absorbed by living cells through endocytosis. Moreover, using molecular/supramolecular ensemble on the external surface of MSNs could make them functional to develop gated MSNs, which released their cargo in response to external stimulant while not releasing the payload if there was only the hybrid material alone, and the latter phenomenon was called “zero delivery.” In their study, Auvray et al. [43] selected MCM-41-based MSNs as the scaffold and loaded with Rhodamine B as a model drug after calcining. Then the oligosaccharide derivative GOS was capped onto MSNs to obtain the final product (S1). The MSN S1 nanoparticles were roughly spherical with a diameter of about 100 nm and an average pore diameter of 2.5 nm, and the maximum loading of Rhodamine B was approximately 0.14 g per gram SiO2. Moreover, S1 was capable of releasing their cargo selectively in SA-β-gal-positive cells like β-gal yeast cells and human fibroblasts DC1787. While interesting, no drug release in cells without SA-β-gal such as wild-type yeast cells was observed. Therefore, the MSN S1 nanoparticles were highly specific but no detectable toxicity for imaging SA-β-gal in senescent cells (Fig. 6).
Internalization and release of cargo in β-gal overexpressing yeast cells and human senescent cells. (a) Controlled release of Rhodamine-loaded S1 nanoparticles in wild-type (WT) and β-gal overexpressing yeast cells. (b) Quantitation of β-gal activity in WT and β-Galoe yeast cells (Reproduced from ref. [35] with permission from Wiley)
3.2 Carbon Quantum Dots
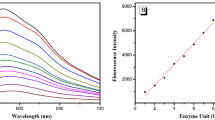
Other than being an important biomarker for senescence, β-gal has been a biomarker for visualizing peritoneal metastases from ovarian cancers where it’s overexpressed [2, 13]. Tang et al. [44] developed functional carbon quantum dots (CQDs) to assay glycosidase enzymes based on a combined host-guest recognition and specific static quenching-induced signal transduction mechanism. Compared with conventional fluorescent probes, CQDs possess much superiority including excellent biocompatibility, stable light emitting, and good photostability [45,46,47], for which it’s believed to be alternatives to molecular fluorophores. β-Gal, which could catalyze the hydrolysis of 4-nitrophenyl-β-d-galactopyranoside (NPGal) into the corresponding glycose and p-nitrophenol, was picked up as a glycosidase example. Then NPGal selectively associated with β-cyclodextrin linking to CQD (β-CD-CQDs) for the hydrophobicity and agreeable size match of the cavity. And the formed nonfluorescent inclusion complex rendered the fluorescence quenching with high efficiency. On the contrary, the presence of β-gal could trigger the preceding processes and finally resulted in a sharp change in the fluorescence signal. Through the intracellular sensing and cytotoxicity tests, β-CD-CQDs nanoprobe exhibited excellent biocompatibility, membrane permeability, and capability of imaging β-gal in the OVCAR3 cells. The detection limit of β-CD-CQDs for β-gal could be lower up to 0.6 UL−1, and the real-time monitoring of the β-gal level in ovarian cancer cells was also achieved. It’s promising that the preceding established detection strategy could be expanded as a universal approach.
4 Targeted Fluorescent Probes for β-Galactosidase Detection
Targeted imaging by NIR fluorescent probes has been developed as an efficient alternative to the conventional methods for improving the diagnosis of a particular disease and therapeutic response of applicable drugs. A variety of targeting moieties, including antibodies, peptides, and low molecular weight non-peptide ligands, have been used in various formulations to selectively target the tumor site for the delivery of diagnostic agents and/or drugs.
Kim et al. [48] developed a ratiometric fluorescent probe (DCDHF-β-gal, 13) for β-gal visualization in vivo. Probe 13 possessed β-d-galactopyranoside unit which not only behaved as a substrate of β-gal but acted as a ligand of asialoglycoprotein receptor (ASGPR). The β-d-galactopyranoside bond was cleaved by intracellular β-gal, thereby releasing NIR fluorophore and exhibiting ratiometric optical response. Initial fluorescence emission at 615 nm of probe 13 would red-shifted to 665 nm upon the incubation with the enzyme. Cellular studies further indicated that DCDHF-β-gal had great capability to target hepatocytes via ASGPRs without significant cytotoxicity. Furthermore, as a ratiometric fluorescent probe, the fluorescent spectra changes of 13 could be measured at two different emission bands, which renders relatively higher sensitivity and accuracy with concentration independence of probes or environmental conditions. Taken together, the novel ratiometric fluorogenic DCDHF-β-gal with NIR emission wavelength could be selectively delivered into ASGPR-positive cells, where it allowed effective noninvasive visualization of the β-gal activity (Fig. 7).
Cell-specific targeting of DCDHF-β-gal. Cellular fluorescence images of DCDHF-β-gal in HepG2, A549, and KB cells. Cells were treated with 10 mM of DCDHF-β-gal for 24 h (Reproduced from ref. [48] with permission from Elsevier)
Prost and Hasserodt [49] reported a new kind of activatable imaging pre-pro-fluorescent probe applying the concept of “AND-type logic gate,” which meant it emitted fluorescent signal only after being consecutively converted by two different enzymes. A β-gal unit was linked via an eliminating para-hydroxybenzyloxycarbonyl spacer to the leucine unit, which in return was linked to the silenced fluorophore through aminomethylpiperidine cyclizing spacer [50]. The fluorescent signal was observed only at the presence of both enzymes. The probe’s simplicity indicated that there was a possible extension to triple-gating probes but still much remains to be explored.
5 Conclusion and Prospective
In general, it has been demonstrated that β-gal is a significant biomarker for cell senescence and primary ovarian cancers. Many efforts have been devoted to treating β-gal as an enzymatic target and visualizing its activity in preclinical diagnosis with fluorescent probes. Molecular bioimaging of enzyme activity in vivo is rapidly emerging as a powerful strategy for accurate disease diagnostics. The development of “smart” noninvasive imaging reagents for the determination of specific enzyme activity in vitro and in vivo is vitally required for cancer diagnosis. Moreover, at the side of the single-wavelength fluorescence-intensity-based systems, ratiometric fluorescence probes are of crucial practical advantages like enhanced signal-to-background ratio, in which the detectable ratio signal can be obtained via two isolated read-out channels of activated versus unreacted probes and bringing about improved and reliable signal quantification.
Nanomaterials has been regarded as one of most emerging biomedical imaging probes because of their special properties are being more and more studied for the detection of β-gal. Compared to conventional fluorescent probes, nanomaterials possess unique superiority such as smaller size, higher selectivity, and better biocompatibility. For example, pH-driven-luminescence GSH-protected CuNCs with AIE property and long decay time are promising to monitor β-gal continuously, and GOS-capped MSNs loaded with certain drugs are potential probes targeting SA-β-gal as well as device for drug delivery. Another used nanoprobe is β-CD-CQDs, which is based on a combined host-guest recognition and specific static quenching-induced signal transduction mechanism, because of their stable fluorescence emitting, excellent photostability, and low detection limit.
There are a number of fluorogenic probes being developed for detecting β-gal, but their applications are limited by a few drawbacks such as poor cellular permeability, narrow emission wavelength, and low penetration depth. Meanwhile, NIR fluorescent probes have reduced tissue absorbance, high photostability, large Stokes shift, and long emission wavelengths, which make them highly suitable for noninvasive in vivo imaging of specific target. Interestingly, a novel activatable imaging pre-pro-fluorescent probe is being researched using a concept of “AND-type logic gate.” That is the probe emits fluorescent signal only after being consecutively converted by two different enzymes and allegedly it’s of great possibility of increased precision in the assay of β-gal. In this paper, we summarized diverse selective fluorescent probes targeting β-gal, all of which have their own superiority, yet there is inferiority holding back their application or some of them still remain to be further studied. Nevertheless, it’s promising and thriving that fluorescent probes targeting β-gal would be put into application not only in molecular imaging but also in drug delivery systems, location of disease, and so on.
References
Alam J, Cook JL (1990) Reporter genes: application to the study of mammalian gene transcription. Anal Biochem 188(2):245
Chatterjee SK, Bhattacharya M, Barlow JJ (1979) Glycosyltransferase and glycosidase activities in ovarian cancer patients. Cancer Res 39(6):1943
Salehi S, Eckley L, Sawyer GJ et al (2009) Intestinal lactase as an autologous beta-galactosidase reporter gene for in vivo gene expression studies. Hum Gene Ther 20(1):21
Kodibagkar VD, Yu J, Liu L et al (2006) Imaging β-galactosidase activity using 19F chemical shift imaging of LacZ gene-reporter molecule 2-fluoro-4-nitrophenol-β-d-galactopyranoside. Magn Reson Imaging 24(7):959
Van Dort ME, Lee KC, Hamilton CA et al (2008) Radiosynthesis and evaluation of 5-[125i]iodoindol-3-yl-β-d-galactopyranoside ([125i]IBDG) as a β-galactosidase imaging radioligand. Mol Imaging 7(4):187
Celen S, Deroose C, De Groot T et al (2008) Synthesis and evaluation of F-18- and C-11-labeled phenyl-galactopyranosides as potential probes for in vivo visualization of LacZ gene expression using positron emission tomography. Bioconjug Chem 19(2):441
Yamamoto A, Adachi S, Kawamura S et al (1974) Localized β-galactosidase deficiency. Arch Intern Med 134(4):627
Zhang YZ, Naleway JJ, Larison KD et al (1991) Detecting lacZ gene expression in living cells with new lipophilic, fluorogenic beta-galactosidase substrates. FASEB J 5(15):3108
Liu Y, Feng B, Cao X et al (2019) A novel “AIE + ESIPT” near-infrared nanoprobe for the imaging of γ-glutamyl transpeptidase in living cells and the application in precision medicine. Analyst 144:5136–5142. https://doi.org/10.1039/c9an00773c
Wehrman TS, Von DG, Krutzik PO et al (2006) Luminescent imaging of beta-galactosidase activity in living subjects using sequential reporter-enzyme luminescence. Nat Methods 3(4):295
Lee HW, Heo CH, Sen D et al (2014) Ratiometric two-photon fluorescent probe for quantitative detection of β-galactosidase activity in senescent cells. Anal Chem 86(20):10001
Hirabayashi K, Hanaoka K, Takayanagi T et al (2015) Analysis of chemical equilibrium of silicon-substituted fluorescein and its application to develop a scaffold for red fluorescent probes. Anal Chem 87(17):9061
Asanuma D, Sakabe M, Kamiya M et al (2015) Sensitive β-galactosidase-targeting fluorescence probe for visualizing small peritoneal metastatic tumours in vivo. Nat Commun 6:6463
Zhang XX, Wu H, Li P et al (2016) A versatile two-photon fluorescent probe for ratiometric imaging E. coli β-galactosidase in live cells and in vivo. Chem Commun 52(53):8283
Redykeisar O, Kisinfinfer E, Ferber S et al (2014) Synthesis and use of QCy7-derived modular probes for the detection and imaging of biologically relevant analytes. Nat Protoc 9(1):27
Han J, Han MS, Tung CH (2013) A fluorogenic probe for β-galactosidase activity imaging in living cells. Mol BioSyst 9(12):3001
Gu K, Xu Y, Li H et al (2016) Real-time tracking and in vivo visualization of β-galactosidase activity in colorectal tumor with a ratiometric near-infrared fluorescent probe. J Am Chem Soc 138(16):5334
Berezin MY, Achilefu S (2010) Fluorescence lifetime measurements and biological imaging. Chem Rev 110(5):2641
Hsieh CC, Cheng YM, Hsu CJ et al (2008) Spectroscopy and femtosecond dynamics of excited-state proton transfer induced charge transfer reaction. J Phys Chem A 112(36):8323
Murale DP, Kim H, Choi WS et al (2013) Highly selective excited state intramolecular proton transfer (ESIPT)-based superoxide probing. Org Lett 15(15):3946
Otsubo T, Minami A, Fujii H et al (2013) 2-(Benzothiazol-2-yl)-phenyl-β-d-galactopyranoside derivatives as fluorescent pigment dyeing substrates and their application for the assay of β-d-galactosidase activities. Bioorg Med Chem Lett 23(7):2245
Cellier M, Fazackerley E, James AL, Orenga S, Perry JD, Turnbull G, Stanforth SP (2014) Synthesis of 2-arylbenzothiazole derivatives and their application in bacterial detection. Bioorg Med Chem 22(4):1250
Wei X, Wu Q, Zhang J et al (2016) Synthesis of precipitating chromogenic/fluorogenic β-glucosidase/β-galactosidase substrates by a new method and their application in the visual detection of foodborne pathogenic bacteria. Chem Commun 53(1):103
Mei J, Leung NLC, Kwok RTK et al (2015) Aggregation-induced emission: together we shine, united we soar! Chem Rev 115(21):11718
He XP, Zang Y, James TD et al (2016) Fluorescent glycoprobes: a sweet addition for improved sensing. Chem Commun 53(1):82
Dong Y, Wang W, Zhong C et al (2014) Investigating the effects of side chain length on the AIE properties of water-soluble TPE derivatives. Tetrahedron Lett 55(8):1496
Cao D, Yang L, Wang L (2014) Application of aggregation-induced emission (AIE) systems in sensing and bioimaging. Curr Org Chem 18(8):1028
Peng L, Gao M, Cai X et al (2015) A fluorescent light-up probe based on AIE and ESIPT processes for β-galactosidase activity detection and visualization in living cells. J Mater Chem B 3(47):9168
Jiang G, Zeng G, Zhu W et al (2017) A selective and light-up fluorescent probe for β-galactosidase activity detection and imaging in living cells based on an AIE tetraphenylethylene derivative. Chem Commun 53(32):4505
Huang Y, Feng H, Liu W et al (2017) Cation-driven luminescent self-assembled dots of copper nanoclusters with aggregation-induced emission for β-galactosidase activity monitoring. J Mater Chem B 5:5120
Jia X, Li J, Wang E (2013) Cu nanoclusters with aggregation induced emission enhancement. Small 9(22):3873
Jia X, Yang X, Li J et al (2014) Stable Cu nanoclusters: from an aggregation-induced emission mechanism to biosensing and catalytic applications. Chem Commun 50(2):237
Wu Z, Liu J, Gao Y et al (2015) Assembly-induced enhancement of Cu nanoclusters luminescence with mechanochromic property. J Am Chem Soc 137(40):12906
Chen PC, Li YC, Ma JY et al (2016) Size-tunable copper nanocluster aggregates and their application in hydrogen sulfide sensing on paper-based devices. Sci Rep 6:24882
Agostini A, Mondragón L, Bernardos A et al (2012) Targeted cargo delivery in senescent cells using capped mesoporous silica nanoparticles. Angew Chem Int Ed 51(42):10556
Antropova YG, Bryantsev AL, Kalinina NI et al (2002) Proliferative activity and expression of cyclin-dependent kinase inhibitor p21WAF1 and p53 protein in endothelial cells of human aorta during replicative aging in vitro. Bull Exp Biol Med 134(1):81
Lee BY, Han JA, Im JS et al (2006) Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell 5(2):187
Leckaczernik B, Moerman EJ, Shmookler Reis RJ et al (1997) Cellular and molecular biomarkers indicate precocious in vitro senescence in fibroblasts from SAMP6 mice. Evidence supporting a murine model of premature senescence and osteopenia. J Gerontol 52(6):B331
Gu Y, Wu Q, Zhu M et al (2011) Efficient pipeline configuration in distributed heterogeneous computing environments. Aging Cell 10(2):338
Chigira S, Sugita K, Kita K et al (2003) Increased expression of the Huntingtin interacting protein-1 gene in cells from Hutchinson Gilford Syndrome (Progeria) patients and aged donors. J Gerontol 58(10):B873
Cabrera S, Haskouri JE, Guillem C et al (2000) Generalised syntheses of ordered mesoporous oxides: the atrane route. Solid State Sci 2(4):405
Vallet-Regí M, Balas F, Arcos D (2007) Mesoporous materials for drug delivery. Angew Chem Int Ed 46(40):7548
Auvray X, Petipas C, Anthore R et al (1995) X-ray diffraction study of the ordered lyotropic phases formed by sugar-based surfactants. Langmuir 11(2):433
Tang C, Zhou J, Qian Z et al (2017) A universal fluorometric assay strategy for glycosidases based on functional carbon quantum dots: β-galactosidase activity detection in vitro and in living cells. J Mater Chem B 5(10):1971
Chen Y, Zhu C, Yang Z et al (2012) A new “turn-on” chemodosimeter for Hg2+: ICT fluorophore formation via Hg2+-induced carbaldehyde recovery from 1,3-dithiane. Chem Commun 48(42):5094
Zheng XT, Ananthanarayanan A, Luo KQ et al (2015) Glowing graphene quantum dots and carbon dots: properties, syntheses, and biological applications. Small 11(14):1620
Lim SY, Shen W, Gao Z (2015) Carbon quantum dots and their applications. Chem Soc Rev 44(1):362
Kim EJ, Kumar R, Sharma A et al (2017) In vivo imaging of β-galactosidase stimulated activity in hepatocellular carcinoma using ligand-targeted fluorescent probe. Biomaterials 122:83
Prost M, Hasserodt J (2014) “Double gating” – a concept for enzyme-responsive imaging probes aiming at high tissue specificity. Chem Commun 50(94):14896
Thorn-Seshold O, Vargas-Sanchez M, McKeon S et al (2012) A robust, high-sensitivity stealth probe for peptidases. Chem Commun 48(50):6253
Acknowledgments
We are grateful for the financial supports from the National Natural Science Foundation of China (81971678, 81671756), Key Research Project of Science and Technology Foundation of Hunan Province (2017SK2093 and 2019SK2211), Key Research Project of Science and Technology Foundation of Changsha (kq1801063), and Fundamental Research Funds for the Central Universities of Central South University (2018zzts041, 2018dcyj067).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Ethics declarations
Conflict of Interest: There are no conflicts to declare.
Ethical Approval: There is no ethical approval to declare.
Informed Consent: There is no informed consent to declare.
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bi, A. et al. (2019). Fluorescent Probes for Diagnostics of β-Galactosidase: From Micro to Macro. In: Cheng, Z. (eds) Fluorescent Imaging in Medicinal Chemistry . Topics in Medicinal Chemistry, vol 34. Springer, Cham. https://doi.org/10.1007/7355_2019_87
Download citation
DOI: https://doi.org/10.1007/7355_2019_87
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-46706-7
Online ISBN: 978-3-030-46707-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)