Abstract
Although more than 600,000 patients in the USA have now been cured of HCV, the clinical benefits of cure have been seen primarily in patients with more advanced liver disease. This has resulted in a reduced incidence of liver cancers and reduced liver-related mortality. Benefits of cure in patients without cirrhosis have not been seen yet in systemic review, but are likely to be seen in the future years. There remain unresolved issues regarding patients with advanced fibrosis or cirrhosis who are cured of their HCV. It is not clear if fibrosis reverses after cure in everyone, if so by how much, and for how long patients with cirrhosis need to be monitored after cure. We have shown that 62% had improved liver stiffness measured by transient elastography (TE) that was consistent with regression of at least one stage of fibrosis over 1 year. Fifteen patients with matched liver biopsies prior to SVR underwent a biopsy after SVR. However, the post-SVR liver biopsies of only 4 patients showed F1–F2, while 11 patients still showed F3–F4, indicating that TE improvements are overstated when compared to histologic staging and that patients with cirrhosis before DAA therapy need to be monitored for hepatocellular carcinoma after cure.
Commercial Affiliation or Consultancy of Authors: PJP consulting/advisory boards: Gilead, AbbVie, Merck, Intercept, Molecular Stethoscope, Chromis Therapeutics, Genfit, and Prometheus. Speaking/teaching: Gilead, Intercept, Merck, and Abbvie. Research funding to Scripps Health: Gilead, BMS, AbbVie, Conatus, Molecular Stethoscope, Genfit, Prometheus, Intercept, Bayer, and Cepheid.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
More than one million patients with HCV worldwide and over 600,000 patients in the USA have now been cured of HCV infection using DAA therapies. In our own hepatology unit at Scripps Clinic in La Jolla, California, we have successfully cured over 150 patients in clinical trials using DAAs and over 1,000 patients since the approval of the first DAA regimen in 2011. Between 2011 and 2013, we needed to treat 156 patients in order to achieve an SVR in 107 of them, with an SVR rate of less than 70%. In retrospect, these were low rates of cure but represented an incremental improvement over Pegylated interferon (PegIFN) and ribavirin (RBV) regimens which had been in use since 2000. Besides these relatively low SVR rates, the toxicities of the first-line protease inhibitors telaprevir and boceprevir were an extreme challenge to patients and their physicians.
Once combination regimens using sofosbuvir became available in 2014, we witnessed cure rates at or above 95% with minimal side effects and we were successful in treating and curing over 250 patients per year in our practice. The number of patients we treated began to decline in 2016 and has now been reduced to <100 cases annually, as most of our population with HCV have now been cured, transplanted, or died in earlier years. The benefit of cure for these patients has been a remarkable achievement to witness for any provider who has been entrusted with their care. The gains in quality of life, reduced healthcare expenses, reduced hospitalizations, and reduced mortality that cure has given to these patients are incalculable. I would never try to estimate such benefits that have now been shared by more than one million patients.
An evidence-based analysis of literature done by the Cochrane Systematic Review group in 2017 concluded there were no clinical benefits of DAA therapies for chronic HCV [1]. This review was subsequently refuted by a number of societies and physicians as being incorrect [2]. I believe the reason for this discrepancy is relatively simple to explain. The clinical benefits of cure seen thus far have been primarily in patients with more advanced liver disease. A significant number of decompensated HCV patients have now been delisted for transplantation, and there is a reduced incidence of HCV-associated liver cancers as well as reduced liver-related mortality. However, the true benefit of DAAs and cure will likely be seen in the future years and has not been fully realized this soon after the availability of DAAs. The most likely reason why the Cochrane analysis in 2017 concluded there was no evidence of clinical benefit of DAAs is that they were premature in their review. Further, they could only utilize evidence-based literature for their analysis. It may take another 5 years before there are significant clinical benefits seen in the evidence-based literature.
Nonetheless, we are now seeing publications that demonstrate curative therapy has markedly reduced the number of patients listed for transplantation in the USA for HCV [3, 4] (Fig. 1). Recent modeling studies have shown a reduction in the expected death rates in decompensated HCV cirrhosis attributed to DAA therapies [5] (Fig. 2). At our own liver center in Southern California, we have rarely transplanted a patient for decompensated HCV cirrhosis in the last 2 years. This is a remarkable change from the last two decades when decompensated HCV cirrhosis was the major indication for transplant in our center. Nonalcoholic steatohepatitis (NASH) and alcoholic liver disease are now quickly becoming the most common indications for liver transplantation in the USA [3]. SVR is conferring a reduced mortality not just from liver-related deaths but also from cardiovascular-related deaths [6] (Fig. 3a, b). Prior studies have demonstrated a reduced all-cause mortality in HCV patients who were cured with IFN-based regimens, so this is not surprising data [7, 8]. We can expect to eventually see data that shows a reduction in mortality due to non-liver-related cancers as well [9].
Annual standardized incidence rates (ASIR) of LT wait-listing per 100,000 US population by etiology of liver disease and indication for wait-listing. X-axis is the year of LT wait-listing registration. PI protease inhibitor, DAA direct-acting antiviral [3]. (a) HCV. (b) HBV. (c) NASH
Observed versus expected deaths in DAA-treated patients [5]. 54 deaths expected from survival model. 1Standardized mortality ratio
(a, b) Disease outcomes after DAA-induced SVR: data from the resist-HCV cohort [6]
There is a remaining issue that confronts a significant number of these patients, especially those who had advanced fibrosis or cirrhosis (METAVIR F3/F4), that is, how long do they require monitoring for complications of portal hypertension (esophageal varices) and HCC? Fibrosis stage and liver stiffness are predictors of adverse outcomes in chronic HCV, but it is not clear if fibrosis reverses after cure in everyone, and if so by how much, and whether their risk for liver cancer is reduced to a level that is safe to stop screening. A comprehensive study utilizing the Veterans Affairs Hospital database in 22,500 patients has now demonstrated a reduced risk for HCC following SVR achieved by DAAs in patients with F3/F4 at the beginning of treatment [10] (Fig. 4). However, this risk has been reduced to 0.5–1.0% per year range but not to zero. Further, patients who start therapy with decompensated cirrhosis do not seem to reduce their HCC risk below 2.5% annually. Neither of these populations can stop their screening according to the American Association for the Study of Liver Disease (AASLD) guidelines [11].
Cumulative incidence of HCC among 22,500 patients treated with DAA agents [10]. SVR sustained virological response
Healthcare providers and patients thus have no guidance as to when to discontinue monitoring for hepatocellular carcinoma and complications from portal hypertension. The most recent estimate of the number of patients that fall into this category is 25–37% of all HCV-infected individuals in the USA [12]. Therefore, this is not a trivial concern as it has significant clinical impact on 200,000 or more successfully cured patients.
Vibration-controlled transient elastography (TE) has been shown to accurately detect advanced fibrosis and cirrhosis in patients with chronic hepatitis C virus (HCV) infections [13]. However, the use of TE is limited by active inflammation and/or edema of the liver, which can cause overestimation of the degree of fibrosis [14]. Other factors known to confound TE include obesity, waist circumference, ascites, hepatic congestion, extrahepatic cholestasis, and eating within 4 h of the exam [15]. Despite these limitations, TE offers a simple and rapid bedside assessment of fibrosis for many patients.
Multiple reports have demonstrated that novel direct-acting antiviral (DAA) therapies for HCV result in dramatic improvement in liver stiffness measured by TE in patients with sustained virologic response (SVR) [16,17,18,19]. Improvements in liver stiffness can be observed as early as the end of treatment and continue even 12 months after therapy completion, with liver stiffness improvements ranging from −2 to −10 kilopascals (kPa) [16] (Fig. 5). This decrease in liver stiffness is associated with lowering of liver enzymes, improvements in FIB-4 and APRI fibrosis scores, and an increase in platelets [18, 19]. A recent report from Georgia [20] demonstrated reversal of TE scores in 304 patients with advanced fibrosis or cirrhosis following SVR at a similar rate to that reported by our group in 2015 [21].
Difference in liver stiffness at different time points in patients who achieve SVR vs. those who do not achieve SVR, demonstrating net decline in liver stiffness [16]
However, a correlation between fibrosis regression by TE and histology is lacking in the DAA era literature. These critical data are needed for physicians who are monitoring patients with advanced fibrosis or cirrhosis after SVR following DAA treatment. Previous evidence shows a lower risk of hepatocellular carcinoma (HCC) and other liver-related complications with fibrosis regression following interferon-based regimens [22], but the regression of fibrosis seems to be a very slow process [23]. Further, some patients did not regress after SVR, and some even worsened, with an increased risk for HCC [24]. This potential complication has created an abundance of caution from clinicians and updates to guidance documents recommending indefinite screening for HCC in patients who achieved SVR who had advanced fibrosis or cirrhosis [25]. The guidelines issued by the European Association for the Study of the Liver (EASL) and the Asociación Latinoamericana para el Estudio del Hígado (ALEH) have indicated that the routine use of noninvasive tests during treatment or after SVR in non-cirrhotic patients does not add to clinical disease management and that the routine use of noninvasive tests after SVR in patients with HCV cirrhosis has a high false-negative rate and cannot be used to determine which patients no longer need HCC screening or for the diagnosis of cirrhosis reversal. They further indicate that the routine use of noninvasive tests after SVR has not yet established thresholds that predict low risk of liver-related events [26]. Despite these recommendations, TE is being done routinely in the community post-SVR, so demonstrating the correlation between biopsy and TE has important implications for clinical practice.
In May 2017, the American Gastroenterological Association (AGA) published guidelines recommending a TE cutoff of <9.5 kPa to rule out advanced liver fibrosis in non-cirrhotic chronic HCV patients who achieved SVR after antiviral therapy [27]. The cutoff of <9.5 kPa would be expected to misclassify only 1% of patients as not having advanced fibrosis in a low-risk population and 7% in a high-risk population, which would include patients with liver stiffness >12.5 kPa before therapy or other risk factors for chronic liver disease. This conditional recommendation was rated to have low-quality evidence per the GRADE framework, as actual comparative post-SVR data have not been available.
2 Methods
Patients with advanced fibrosis or cirrhosis prior to treatment in the Hepatology division at Scripps Clinic were identified and screened for the study between 2010 and 2015. Patients were included in the study if they were at least 18 years old with chronic HCV with fibrosis of at least F3 staging who achieved SVR at 12 and 24 weeks’ posttreatment. Exclusion criteria included liver transplant prior to therapy, SVR without DAA therapy, failure to achieve SVR, age less than 18 years old, liver fibrosis less than F3, and additional causes of liver fibrosis other than HCV. Patient’s baseline fibrosis prior to DAA therapy was assessed by liver biopsy, TE, and/or clinical signs. Patients with clinical evidence of portal hypertension (varices, portal hypertensive gastropathy, ascites, and/or hepatic encephalopathy) or baseline TE greater than 12 kPa were categorized as having cirrhosis; otherwise, patients were placed in the advanced fibrosis (F3) group.
In patients for whom SVR was achieved at 12 and 24 weeks, fasting TE measurements consisting of ten measurements with an interquartile range of <25% were collected at 6–12-month intervals for up to 5.5 years. Fibrosis staging cutoffs for TE were as follows: F0–F1 < 7 kPa, F2 7–9.4 kPa, F3 9.5–11.9 kPa, and F4 > 12 kPa, derived from values used in meta-analyses and systematic reviews [28, 29]. Hepatocellular carcinoma was screened regularly with ultrasound, CT, and/or MRI as needed.
Fourteen patients who had improvement in their fibrosis by at least one stage by TE had additional informed consent discussions regarding obtaining liver biopsies to confirm improvement. One patient enrolled in the study who showed unchanging liver stiffness also wished to pursue liver biopsy. Liver biopsies were processed and evaluated using standard techniques; sinusoidal fibrosis was additionally evaluated. The biopsies were all read independently by two expert pathologists who were blinded to any clinical information and to the timing with respect to antiviral treatment. Discrepancies were solved by consensus reading.
Liver biopsy fibrosis stage was assessed according to the Batts-Ludwig scoring system as follows [30]: F0, no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis with septal fibrosis; F3, bridging fibrosis with architectural distortion; and F4, cirrhosis. Similarly, hepatic inflammation was evaluated according to both the METAVIR and Batts-Ludwig grading systems [30, 31]. The 11 biopsies used for morphometric measurements were read by a third pathologist using the Batts-Ludwig and Ishak scoring systems.
For quantification of fibrosis, sections were stained with picrosirius red, which binds stoichiometrically to collagen. A digitized image of each entire stained section was acquired using an Aperio/Leica Scanscope XT scanner at 20X magnification. The image analysis process also included a manual editing step to determine the total stained area of the section and to eliminate image artifacts. An area quantification algorithm (Indica Labs, Inc.) was used to quantify number of red-stained pixels of the collagen fibers. Accuracy of classification was confirmed by visual inspection and results expressed as a fraction of the total pixels positive for picrosirius red (communication from Zachery D Goodman).
Descriptive statistics were calculated for the primary outcome of changes in liver stiffness and improvements in fibrosis by biopsy. Changes in liver stiffness and liver chemistries following SVR were compared to pre-SVR levels by paired t-tests. Time to improvement, defined as the time from SVR to one stage of fibrosis regression on TE, was assessed in both the cirrhosis and advanced fibrosis groups using Kaplan-Meier curves and compared using the log-rank test. Statistical significance was defined as a p-value <0.05. The primary outcomes were liver stiffness in kPa, and subsequently fibrosis staging predicted by TE after SVR was achieved compared to liver biopsy fibrosis staging and morphometry in a subset of patients. Secondary outcomes included the analysis of pre- and post-therapy FIB-4 and APRI and the prevalence of HCC in this post-SVR population.
3 Results
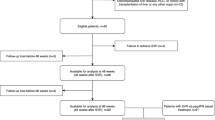
Two hundred twenty-four patients from a single center were originally eligible for the study. Three patients declined to participate. Twenty-eight patients were excluded; 1 had a liver transplant, 10 had multiple etiologies for their liver disease, and 17 were treated without DAA therapy. One hundred and nine patients who were treated in 2015 and later are still in follow-up, pending final analysis. Eighty-four patients were included in this analysis.
The mean age was 60 years old, and mean BMI was 28 kg/m2 (standard deviation 4.6 kg/m2). The study population was non-Hispanic white (87%), Hispanic (10%), or Asian (3%). The majority of patients were genotype 1A or 1B. Fifty-seven percent (57%) of the patients were treatment-experienced, and most had previously received interferon and ribavirin. Fifty-five percent (55%) of patients were treated with sofosbuvir-based regimens. Thirty-five percent (35%) of patients received telaprevir-based therapy, and 10% were in DAA clinical trials, which included NS3/4A protease inhibitors and/or NS5A/B inhibitors, sometimes in combination with ribavirin and/or interferon.
Fifty-six patients were in the cirrhotic group; 28 patients were in the F3 group. The cirrhosis group had a statistically significantly higher mean FIB-4 scores ( p < 0.01) and lower platelets ( p < 0.001) than the F3 group. The cirrhotic group also had a lower mean albumin, but this was not statistically significant. None of the patients had decompensated cirrhosis at the time of enrollment. Three patients developed ascites and hepatic encephalopathy during their antiviral therapy with telaprevir, Pegylated interferon, and ribavirin; however, all three had compensated cirrhosis at the time this study began, and resolution of ascites was documented by ultrasound or CT scan prior to enrollment.
Among the 56 patients who had cirrhosis prior to SVR, 23 patients (41%) continued to have cirrhosis (≥12 kPa) predicted by TE. Thirty-three patients (59%) had improved by at least one stage (<12 kPa), and 27 patients (48%) had improved by at least two predicted stages of fibrosis (<9.5 kPa). The median time to improvement was 1 year (95% confidence interval, 1.2–1.9 years; Figs. 6 and 7). Among the 28 patients who had F3 fibrosis prior to SVR, 9 patients (32%) had either worsened or unchanged stiffness (≥9.5 kPa) by TE. Seven patients progressed to cirrhosis (≥12 kPa), and two patients remained in F3 (9.5–11.9 kPa). Nineteen patients (68%) had improved liver stiffness by at least one stage of fibrosis (<9.5 kPa), and 11 patients (39%) had improved liver stiffness by at least two stages of fibrosis (<7 kPa). Median time to improvement was 1.5 years (95% confidence interval, 1.1–1.8 years; Figs. 6 and 7). Overall, among the 84 patients with advanced fibrosis or cirrhosis, 52 patients (62%) had decreased liver stiffness. There were statistically significant changes in liver chemistries and FIB-4 scores between pre-SVR and the most recent post-SVR values. The mean FIB-4 score among patients with cirrhosis decreased from 4.7 to 2.6 ( p < 0.001). In the F3 group, the FIB-4 score in the advanced fibrosis group decreased from 2.8 to 1.4 ( p < 0.01). Of the 84 patients, 4 (4.8%) were found to have hepatocellular carcinoma (HCC). Prior to DAA, two patients had cirrhosis, and two had F3 fibrosis.
Time to improvement from SVR by one stage of fibrosis estimated by transient elastography. Median time to improvement was 1.5 years for F3–F4 (F3–F4, 1.1–1.8 years, 95% CI) and 1 year for the cirrhosis group (1.2–1.9 years, 95% CI). Log-rank statistic p-value = 0.08 [32]
Pre- and post-SVR transient elastography values (kPa) for cirrhotic group and F3 group. Box plots of pre-SVR and post-SVR TE values from 0.5 to 2 years after SVR. Box consists of first to third quartile values, with the median line marked. Whiskers extend to the farthest data point within 1.5 of the interquartile range. Outliers outside of 1.5 of the interquartile range are marked with a circle, and the mean of each group is marked by an “X.” The three asterisks denote statistical significance with p-value <0.001 for a paired t-test comparison between the pre-SVR and 0.5 years after post-SVR samples. Unfortunately, there was only one baseline TE reading in the F3 group, so statistical testing was unable to be done [32]
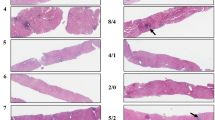
Fifteen patients underwent a repeat liver biopsy after achieving SVR. Prior to DAA therapy, eight patients had cirrhosis, and seven patients had F3 fibrosis. In the cirrhosis group, the highest level of fibrosis observed on repeat biopsy after SVR was F3, and the lowest was F0–F1. These biopsied cirrhotic patients were predicted per AGA guidelines by TE to have at least one stage improvement (<12.5 kPa). Six cirrhotic patients were predicted by TE to have F0–F1 fibrosis (<7 kPa) and two cirrhotic patients to have F2–F3 fibrosis (7–11.9 kPa) after SVR. Their liver biopsies revealed that two patients had F1–F2 fibrosis and six patients had features of F3–F4 fibrosis. In this group with dramatic improvements on TE, the pathologist also reported that three patients had reduced sinusoidal fibrosis compared to their prior biopsy, and two patients did not have any sinusoidal fibrosis in their current biopsy (but their prior biopsy was not available to determine sinusoidal fibrosis regression). Among the seven F3 patients, six patients were predicted by TE to have F0–F1 fibrosis (<7 kPa), but only two post-SVR liver biopsies were noted to be F1–F2. The other five post-SVR biopsies contained features of F3 fibrosis. A reduction in sinusoidal fibrosis was appreciated in three patients in the F3 group. Of the 15 patients with cirrhosis and advanced fibrosis, 13 had significant improvements in their liver stiffness by TE (<9.5 kPa) after SVR was achieved. The liver biopsies of four patients had a staging of F1–F2; the other nine patients had liver biopsies with features of F3 or F4 fibrosis (Fig. 8).
Post-SVR fibrosis stage predicted by TE compared to liver biopsy [32]
Morphometric analysis was done on the first 11 of the 15 patients who underwent post-SVR biopsy. Of these 11 patients, 10 had a decline in collagen by an average of 46% over varying time intervals (Figs. 9 and 10). The one subject with the increase in collagen had the lowest amount of baseline collagen and had demonstrated improvements in liver biopsy (F3 to F1–F2) and TE (post-SVR TE <9.5 kPa). The mean percent collagen decreased from 7.1 to 3.8% ( p < 0.01).
Collagen quantitation morphometric analysis before and after SVR [32]
Pre- and post-SVR liver biopsies show regression of fibrosis [32]
4 Discussion
TE with a cutoff of 9.5 kPa and 12.5 kPa has been recommended to stratify patients into advanced fibrosis and cirrhosis, respectively, including patients who have been treated with DAAs [27]. In our study, 13 of the 15 patients with advanced fibrosis or cirrhosis who underwent a repeat liver biopsy had liver stiffness <9.5 kPa at the time of their repeat biopsy. However, the biopsies of only four patients (31%) had fibrosis without F3–F4 features. In the two patients with liver stiffness >9.5 kPa, both continued to have F3 fibrosis, suggesting that TE remains an effective tool for confirming advanced fibrosis but may not be sensitive enough to confirm resolution of advanced fibrosis.
A previous histologic study utilizing morphometric analysis in paired liver biopsies from 37 patients with cirrhosis due to HCV demonstrated reversal of fibrosis and morphometry scores following SVR after interferon-based therapy [33]. The regression of area of fibrosis measurement in this previous study was similar to that reported in our study. These findings suggest that, similar to patients treated with interferon-based regimens, the degree of liver fibrosis regression from DAA therapy is overestimated by TE compared to liver biopsy, which remains the only reliable and practical approach to stage liver fibrosis after SVR is achieved.
Hepatocellular carcinoma (HCC) screening should still be considered in non-cirrhotic patients with HCV status post-SVR after DAA therapy, despite their liver stiffness being <9.5 kPa, because they may continue to have F3 fibrosis and thus be at increased risk of HCC development. Four patients were found to have HCC. The two patients who had cirrhosis at baseline did not demonstrate liver stiffness improvement, but the two F3 patients with HCC diagnosed at 6 months and 2 years after therapy demonstrated liver stiffness improvement consistent with F0–F1 staging.
The sensitivity and specificity of TE to determine the correct fibrosis stage typically ranges from 78 to 89%, depending on the actual stage seen on liver biopsy in viremic HCV patients [28]. Although there is a range of cutoffs that may be used for fibrosis stages, the discordance between TE and liver biopsy after SVR from DAA therapy in this study exceeds expectations (Fig. 8). These findings suggest that a distinction must be made between total amount of hepatic fibrosis reflected in liver stiffness measurement and the histopathologic features seen on liver biopsies used in staging.
In our study, the repeat biopsies of seven patients revealed major reductions in sinusoidal fibrosis. Sinusoidal fibrosis regression was recently correlated with patients who had significant TE decreases in a study where liver transplant patients underwent DAA therapy for recurrent HCV [34]. Current histologic staging systems do not utilize sinusoidal fibrosis, which has been traditionally associated with non-alcoholic steatohepatitis and perivenular fibrosis. However, hepatitis C is associated with sinusoidal fibrosis, which has been suggested to be an early histopathologic sign of hepatitis C recurrence in liver transplant recipients [35]. The reduction in sinusoidal fibrosis may reflect an overall reduction in total collagen and hepatic fibrosis; sinusoidal fibrosis has previously been shown to be an independent variable that correlates with hepatic fibrosis [36]. This hypothesis is further supported by the morphometric analysis that was completed on the first 11 biopsied patients from the present study, where all but 1 had significant reductions in total collagen. Although there may be sampling variability, morphometric analysis has been shown to be a more sensitive tool for tracking changes in fibrosis than numerical scoring systems [37]. Although morphometry is a more specific and quantifiable measurement, the lack of its widespread availability makes it an impractical test for clinical use.
The limitations of our study included limited sample size of patients undergoing a repeat biopsy and consequently possible sampling error. It is feasible that a larger study would show a closer correlation between TE scores <9.5 kPa after SVR and resolution of advanced fibrosis or cirrhosis. However, it may prove impractical or even impossible to convince a significant number of patients who have accomplished a virologic cure and have no evidence of portal hypertension to undergo a liver biopsy years after SVR. Necro-inflammation and transaminase flares before treatment could lead to an increase of stiffness not related to liver fibrosis (confounders). However, acute inflammation is unlikely to have a role in the TE and biopsy changes because the findings were consistent over a prolonged period during normal AST/ALT levels (Fig. 2) and because the average time to follow-up biopsy was 3 years. Unfortunately, no TE studies were performed immediately after DAA therapy, but previous studies have shown that there is an immediate improvement of 2 or more kPa due to resolution of inflammation during antiviral treatment [34, 38]. TE studies were not conducted prior to antiviral therapy in patients who were treated before 2013 when FibroScan® was approved for use in the USA. This limited the ability to assess TE measurement and histology pre-therapy, but the aim of this study was to assess fibrosis reversal after therapy. Multiple studies have previously shown that this correlation in chronic HCV pre-therapy is valid [13,14,15, 25,26,27,28,29].
Ours is the first study that has also paired liver biopsies showing a regression of liver fibrosis with histology as well. By obtaining follow-up liver biopsies on a select group of patients with dramatic TE improvements, this study also demonstrated that these TE-predicted improvements, especially with cutoffs of <9.5 kPa, were not seen in histologic staging but in morphometric analysis. Of the 13 patients who had a repeat biopsy and displayed significant improvement in liver stiffness (<9.5 kPa), only 31% showed improvement to less than F3 staging on pathology. There is a discordance between the level of liver stiffness improvement measured by TE and fibrosis regression seen on liver biopsies, using these simple histologic scoring systems. Morphometry demonstrates 46% reduction in fibrosis with SVR over a relatively short time period and is a more accurate measure of improvement in fibrosis regression. However, morphometry is not a practical test for the clinic as it is done by only a few specialty centers and pathologists in the world.
It is therefore currently impossible for one to determine the ultimate benefit of cure in patients who had advanced fibrosis or cirrhosis before their cure. I anticipate that this issue will not be resolved until enough patients have been followed for enough time that we can conclusive say they are free of liver-related mortality. Hopefully this will be clearer within the next decade.
Until that time patients with TE scores <20 kPa and platelet counts ≥150,000 can be monitored without repeat endoscopy, however all patients will continue to require HCC screening at 6–12-month intervals.
Abbreviations
- DAA:
-
Direct-acting antiviral therapy
- F3:
-
Advanced fibrosis
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- kPa:
-
Kilopascals
- SVR:
-
Sustained virologic response
- TE:
-
Vibration-controlled transient elastography
References
Jakobsen JC, Nielsen EE, Feinberg J et al (2017) Direct-acting antivirals for chronic hepatitis C. Cochrane Database Syst Rev 9:CD012143
Kwo PY, Shiffman ML, Bernstein DE (2018) The cochrane review conclusion for hepatitis C DAA therapies is wrong. Am J Gastroenterol 113(1):2–4
Flemming JA, Kim WR, Brosgart CL, Terrault NA (2017) Reduction in liver transplant wait-listing in the era of direct acting anti-viral therapy. Hepatology 65(3):804–812
Belli LS, Berenguer M, Cortesi PA et al (2016) Delisting of liver transplant candidates with chronic hepatitis C after viral eradication: a European study. J Hepatol 65:524–531
Kim WR, Osinusi A, Mannalithara A et al (2018) Survival benefits of direct-acting antiviral therapy in patients with decompensated hepatitis C cirrhosis. J Hepatol 68(4):S84
Calvaruso V, Pe'a S, Cacciola I, on behalf of resist-HCV et al (2018) Disease outcomes after DAA-induced SVR: data from the resist-HCV cohort. J Hepatol 68(4):S83
van der Meer AJ, Wedemeyer H, Feld JJ et al (2014) Life expectancy in patients with chronic HCV infection and cirrhosis compared with a general population. JAMA 312(18):1927–1928
Lee M-H, Yang P, Chen C et al (2012) Chronic hepatitis C virus infection increases mortality from hepatic and extrahepatic diseases: a community-based long-term prospective study. J Infect Dis 206:469–477
Allison RD, Tong X, Moorman AC et al (2015) Increased incidence of cancer and cancer-related mortality among persons with chronic hepatitis C infection, 2006-2010. J Hepatol 63(4):822–828
Kanwal F, Kramer J, Asch S et al (2017) Risk of HCC in HCV patients treated with direct acting antiviral agents. Gastroenterology 153(4):996–1005
Bruix J, Sherman M (2011). Hepatology 53:1020–1022
Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW (2010) Aging of hepatitis C (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology 138:513–521
Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F et al (2005) Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 41:48–54
Tapper EB, Cohen EB, Patel K, Bacon B, Gordon S, Lawitz E et al (2012) Levels of alanine aminotransferase confound use of transient elastography to diagnose fibrosis in patients with chronic hepatitis C infection. Clin Gastroenterol Hepatol 10:932–937
Castera L (2012) Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology 142:1293–1302
Singh S, Facciorusso A, Loomba R, Falck-Ytter YT (2018) Magnitude and kinetics of decrease in liver stiffness after anti-viral therapy in patients with chronic hepatitis C: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 16:27–38
Bachofner JA, Valli PV, Kröger A, Bergamin I, Künzler P, Baserga A et al (2017) DAA treatment of chronic hepatitis C results in rapid regression of transient elastography and fibrosis markers FIB-4 and APRI. Liver Int 37:369–376
Tada T, Kumada T, Toyoda H, Mizuno K, Sone Y, Kataoka S et al (2017) Improvement of liver stiffness in patients with hepatitis C virus infection who received direct-acting antiviral therapy and achieved sustained virological response. J Gastroenterol Hepatol 32:1982–1988
Knop V, Hoppe D, Welzel T, Vermehren J, Herrmann E, Vermehren A et al (2016) Regression of fibrosis and portal hypertension in HCV-associated cirrhosis and sustained virologic response after interferon-free antiviral therapy. J Viral Hepat 23:994–1002
Dolmazashvili E, Abutidze A, Chkhartishvili N, Karchava M, Sharvadze L, Tsertsvadze T (2017) Regression of liver fibrosis over a 24-week period after completing direct-acting antiviral therapy in patients with chronic hepatitis C receiving care within the national hepatitis C elimination program in Georgia: results of hepatology clinic HEPA experience. Eur J Gastroenterol Hepatol 29:1223–1230
Crissien AM, Minteer WB, Pan JJ, Waalen J, Frenette CT, Paul PJ (2015) Regression of advanced fibrosis or cirrhosis measured by elastography in patients with chronic hepatitis C who achieved sustained virologic response after treatment for HCV. Hepatology 62:264A–265A
Mallet V, Gilgenkrantz H, Serpaggi J, Verkarre V, Vallet-Pichard A, Fontaine H, Pol S (2008) Brief communication: the relationship of regression of cirrhosis to outcome in chronic hepatitis C. Ann Intern Med 149:399–403
Shiratori Y, Imazeki F, Moriyama M, Yano M, Arakawa Y, Yokosuka O et al (2000) Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med 132:517–524
Tachi Y, Hirai T, Miyata A, Ohara K, Iida T, Ishizu Y et al (2015) Progressive fibrosis significantly correlates with hepatocellular carcinoma in patients with a sustained virological response. Hepatol Res 45:238–246
European Association for the Study of Liver (2015) EASL recommendations on treatment of hepatitis C. J Hepatol 63:199–236
European Association for the Study of the Liver, Asociación Latinoamericana para el Estudio del Hígado (2015) EASL-ALEH clinical practice guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol 63:237–264
Lim JK, Flamm SL, Singh S, Falck-Ytter YT (2017) American Gastroenterological Association Institute guideline on the role of elastography in the evaluation of liver fibrosis. Gastroenterology 152:1544–1577
Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK (2011) Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol 54:650–659
Poynard T, Vergniol J, Ngo Y, Foucher J, Munteanu M, Merrouche W et al (2014) Staging chronic hepatitis C in seven categories using fibrosis biomarker (FibroTest™) and transient elastography (FibroScan®). J Hepatol 60:706–714
Batts KP, Ludwig J (1995) Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol 19:1409–1417
Bedossa P, Poynard T (1996) An algorithm for the grading of activity in chronic hepatitis C. Hepatology 24:289–293
Pan JJ, Bao F, Du E, Skillin C, Frenette C, Waalen J, Alaparthi L, Goodman ZD, Pockros PJ (2018) Transient Elastography Overestimates Fibrosis Regression from Sustained Virologic Response to Direct-Acting Antivirals for Hepatitis C. Hepatol Commun. https://doi.org/10.1002/hep4.1228
D’Ambrosio T, Aghemo A, Fraquelli M, Rumi MG, Donato MF, Paradis V et al (2013) The diagnostic accuracy of Fibroscan for cirrhosis is influenced by liver morphometry in HCV patients with a sustained virological response. J Hepatol 59:251–256
Donato MF, Cristina R, Invernizz F, Colucci G, Fraquelli M, Maggioni M et al (2016) Paired liver biopsy, Fibrotest and Fibroscan before and after treatment with DAA in liver transplanted recipients with recurrent hepatitis C: diagnostic accuracy and concordance. Hepatology 64:134A–135A
Marino Z, Mensa L, Crespo G, Miquel R, Bruquera M, Perez-Del-Pulqar S et al (2014) Early periportal sinusoidal fibrosis is an accurate marker of accelerated HCV recurrence after liver transplantation. Hepatology 61:270–277
Chevallier M, Guerret S, Chossegreos P, Gerard F, Grimaud JA (1994) A histological semiquantitative scoring system for evaluation of hepatic fibrosis in needle liver biopsy specimens: comparison with morphometric studies. Hepatology 20:349–355
Goodman ZD, Becker RL, Pockros PJ, Afdhal NH (2007) Progression of fibrosis in advanced chronic hepatitis C: evaluation by morphometric image analysis. Hepatology 45:886–894
Chan J, Gogela N, Zheng H, Lammert S, Ajayi T, Fricker Z et al (2018) Direct-acting antiviral therapy for chronic HCV infection results in liver stiffness regression over 12 months post-treatment. Dig Dis Sci 63:486–492
Compliance with Ethical Standards
Conflict of Interest Paul J. Pockros has received research grants from Gilead, AbbVie, BMS, Merck, Conatus and has received a honorarium for speaking and consulting to PJP from Gilead, AbbVie, Merck, Conatus. He is Intercept Board of Directors member at Conatus.
Ethical Approval
All procedures were performed in accordance with the ethical standards of the Scripps institutional research committee and with the 1964 Helsinki declaration and its comparable standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study which was approved by the Scripps Human Subject committee IRB.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Pockros, P.J. (2019). The Benefit of Direct-Acting Antiviral HCV Cure Therapies. In: Sofia, M. (eds) HCV: The Journey from Discovery to a Cure. Topics in Medicinal Chemistry, vol 32. Springer, Cham. https://doi.org/10.1007/7355_2018_52
Download citation
DOI: https://doi.org/10.1007/7355_2018_52
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-28399-5
Online ISBN: 978-3-030-28400-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)