Abstract
Every decision a drug discovery scientist makes along the way will impact the ultimate product to emerge from the long and arduous discovery and development process. To meet this challenge, an innovator must have a basic understanding of those steps in this process that demand far more than knowledge of basic bench science. Perhaps the most difficult of these steps involves an understanding of regulatory and clinical development issues that only become relevant years after the potential product has overcome its initial scientific hurdles. This chapter provides a review of currently available clinical development paradigms for antibacterial drugs including non-inferiority trials and various approaches to superiority trials. The thorny problem of how pathogen-specific antibiotics can be developed is explored. The goal of this chapter is simply to familiarize the bench scientist with the challenges ahead for any project and to provide a framework for assessing risk in that context.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Antibiotics
- Antimicrobial resistance
- Bacterial infection
- Clinical development
- Combination therapy
- Drug target
- Enhancers
- Infectious diseases
- Nonclinical development
- PK/PD
- β-lactamase
1 Introduction
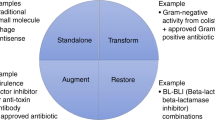
The scientist working on drug discovery at the laboratory bench is frequently in a world unto one's self. Corporate strategy, or even academic laboratory strategy, may seem distant or even irrelevant. This distance is a paradox that can lead to eventual frustration, conflict, and wasted energy and resources. It is critical, therefore, for the discovery scientist to develop a level of understanding of the world they seek to change by their innovative work. To achieve this success, it is key for the researcher to have clearly in mind at the outset the key characteristics of the ultimate product that is the goal of the research. These characteristics usually take the form of a Target Product Profile. An example of a target product profile for a new β-lactamase inhibitor – a current objective of several pharmaceutical companies for the purpose of restoring the antibacterial spectrum of a proven β-lactam antibacterial against multidrug-resistant Gram-negative bacteria – is shown in Scheme 1.
The objective of this chapter is to provide to the bench-level innovators key insights into how their products will – or will not – progress to achieve the benefit they seek to provide ultimately to patients.
2 Novel Targets?
The first consideration is target selection. This consideration is foremost since the choice of target directly influences the likelihood that the ultimate product will be successful. This topic is the subject of the chapter by Sutterlin and colleagues [1]. The advantages in pursuing inhibitors of targets that have never led to a marketed antibacterial product are many. It is likely that the inhibitor of a novel target will belong to a new chemical class, and thus will not demonstrate cross-resistance with antibacterials belonging to known classes. Any new class of antibacterial may offer the potential for novel antibacterial combinations that may have additional advantages over currently used combinations. Finally, there is intrinsic commercial value to a novel class, as demonstrated by the fact that every new antibacterial wants to be known as defining a new class, whether this designation is accurate or not. This raises the question: how do we define a novel target? Could it be new binding sites on the ribosome? What if those binding sites are adjacent to those used by marketed antibiotics? Or does “novel” have to imply a target that has never led to a marketed product? In considering these questions, we must understand that pursuing a non-validated (by achieving market authorization) antibacterial target is a high-risk effort.
The results of decades of efforts to address novel targets have not resulted in a single marketed novel antibacterial for systemic use. On the other hand, at least one novel class of antibacterial has been discovered via brute force screening that turned out to have a novel target – daptomycin, a lipopeptide [2]. Given that the antibiotic wars between microorganisms have been occurring within various ecological niches for billions of years, it may be that the targets that we already know are those targets most likely to yield efficient and safe inhibitors.
The chemical libraries in pharmaceutical companies are probably biased against antibacterial compounds [3, 4], and these libraries leave significant portions of chemical space uncovered. A second issue is safety. It is important to remember the enormity of the task upon which we embark. We are looking for a poison for a living bacterial cell that will not be a poison for our own cells, even though we are related albeit distantly. Most antibiotics fail because of safety either in nonclinical studies or during early clinical development [5]. While we know the safety risks of known classes of antibiotics, the safety risks of new classes are unknown. New classes directed at new targets may present a greater safety risk than new classes directed at known targets.
Novel targets have not borne fruit during the genomics era of the 1990s. A total of 67 high-throughput screens were undertaken at GlaxoSmithKline during the period of 1995 and 2001 against essential gene targets in bacteria [6]. The compound libraries used varied between 260,000 and 530,000 structures. Some screens were run a second time using a different analytical format. Only 16 of these screens identified hits. Of these 16 screens, only five resulted in lead compounds. Empiric screening of a 500,000 compound library against wild-type Staphylococcus aureus and wild-type Escherichia coli was also carried out. The E. coli screen yielded no hits at all. The S. aureus screen yielded a large number of hits, almost all of which turned out to be nuisance compounds and nonspecific membrane-active agents. This experience remains typical of that encountered by antibacterial researchers across both industry and academia even today.
The caveats noted above should be viewed as just that: caveats. There is nothing wrong with having novel targets as part of an overall strategy, or even as the main strategy, as long as one is cognizant of the risks involved and is prepared to address these risks early in the discovery process. A careful review of previous experience is required in order to avoid the pitfalls that are now well known to the “old hands” of antibiotic hunters. All efforts to discover antibiotics inhibiting novel targets should be balanced with lower risk approaches.
3 Not “Novel” Targets
Two alternatives to novel antibacterial targets include identifying novel inhibitors of known targets or modifying known inhibitors of known targets to improve one or more aspects of their profile [7]. A current example of the former approach is avibactam, a novel β-lactamase inhibitor targeting the same active sites of the same β-lactamases targeted by marketed compounds [8,9,10]. The great advantage of avibactam (and its congener structures) is its ability to increase the spectrum of activity against key β-lactamases like the KPC and OXA-48 carbapenemases, and the class C “cephalosporinases.” Examples of medicinal chemistry efforts towards this latter objective abound and include the advances in cephalosporin structures from the first-generation compounds like cephalothin and cefazolin through the fourth-generation structure, cefepime. Additional examples from other antibacterial classes include tigecycline, eravacycline, telithromycin, and solithromycin.
4 Nonclinical Development
The translation of scientific findings during the early preclinical phase of discovery science into a potential product that is ready to begin its first trials in man is an important process and one that cannot be adequately dealt with in this chapter. Most antibiotics, if they are to fail, will do so during these translational activities or during Phase I trials to establish pharmacokinetics and safety. Three key issues must be clearly resolved during translation studies.
-
Using a variety of approaches, it must be clear that the new product is unlikely to directly select for mutational resistance among targeted pathogens during therapy. There are several approaches to this problem. The discussion presented by Singh et al. [11] provides a reasonable roadmap. Several strains should be tested in this regard since resistance rates are occasionally strain specific, probably related to other genetic characteristics. A no-go decision for a compound should be considered when, in a single step, at a frequency of 10−7–10−8 assessed at 2–4 times the minimum inhibitory concentration (MIC), the resulting MIC jumps to a level above the pharmacokinetic exposure likely to be achieved in humans.
An example of what happens when a compound is studied in the clinic without attention to in vitro data occurred with GSK2251052, a leucyl t-RNA synthase inhibitor. During Phase II studies in complicated urinary-tract infections, highly resistant mutants emerged within 1–2 days of therapy causing recurrent bacteremia [12]. In vitro data had already predicted this possibility. This outcome is not the one you want for your clinical trials.
-
Pharmacokinetic and pharmacodynamic studies of the new compound in animal models are essential to the further development of antibacterial products [13]. These studies set potential efficacious dose levels for the animal models that can be extrapolated to humans [14]. In the nonclinical setting, this is accomplished first by understanding the MIC of the product required to inhibit 90% of key pathogens (MIC90). Next, one must identify the pharmacokinetic parameter that most correlates with in vivo activity. This can be Cmax, area under the concentration curve or time above the MIC. Once this is established, using the appropriate animal model and Monte Carlo simulations, the pharmacokinetic exposure required to inhibit infections caused by 90% of pathogens can be established. This dose can then be extrapolated to the human (in most but not all cases) and be used to estimate an efficacious dose.
-
Nonclinical safety studies carried out under Good Laboratory Practice conditions must establish safe doses of the new compound in animal models [15]. These safe doses can be directly compared to the efficacious dose as determined by the PK/PD studies noted above. Once a safe dose is established in animals, one can calculate the starting dose for the first-in-man trials. The determination of the starting dose for Phase I trials will be a critical result of the safety studies. Clearly, if a safe dose has not been established, or if the ratio of the safe dose to the efficacious dose is 2 or less, it may not be practical to continue on to human studies.
5 Clinical Development Considerations
Notwithstanding the importance of the safety and efficacy activities comprising “preclinical development,” recent experiences in antibacterial development emphasize the critical importance of correctly defining the objectives of the clinical development strategy. Here I provide a brief statement of the range of clinical development designs corresponding to a range of exploratory antibacterial mechanisms. These designs include:
-
A standard antibiotic undergoing non-inferiority type trials versus a marketed comparator
-
A fixed-dose combination study. For example, the pairing of a marketed antibacterial (such as a β-lactam) with an enhancer (such as a β-lactamase inhibitor), using modified non-inferiority trials
-
A new “enhancer” that could be combined with a number of marketed antibacterials to increase efficacy
-
A new antibiotic optimized for activity only against a single bacterial species, such as Pseudomonas aeruginosa or Acinetobacter baumannii
5.1 Non-inferiority Trials
Non-inferiority trials have remained the accepted design for testing new antibiotics since the 1950s [16]. These trials are the most risk-free route to secure the regulatory approval necessary to enter the marketplace. The reason that non-inferiority trials are the standard design is the recognition – with good reason! – that it is unethical to withhold efficacious therapy from patients with serious bacterial infections. A design comparing a new antibiotic to a placebo would fall under this “unethical” umbrella. The non-inferiority trial targets a clinical indication such as urinary-tract infection, skin and skin-structure infection, intra-abdominal infection, or pneumonia. In this trial design, the bacterial pathogen itself is a secondary consideration. The selection of the comparator antibacterial is paramount. The comparator should be a generally accepted (clinically) standard, or first-line, antibacterial for the clinical condition being studied. The comparator should have clinical approval in most (or all of the) countries where the trials will be conducted.
Non-inferiority trials are designed to provide a statistical margin that demonstrates that the new antibacterial is not inferior in efficacy to the marketed comparator antibacterial [16, 17]. This margin, or M2 in statistical parlance, derives from two design criteria. The first criterion is the estimated activity of the comparator compared to placebo (M1). The second criterion is the number of patients that it is feasible to enroll in a trial within a reasonable time period. The comparator must have a treatment effect that is greater than placebo. For most infections, the treatment effect is estimated by comparing data from the pre-antibiotic era to data from clinical trials of antibacterials in the modern era. The treatment effect has varied from about 20% to 70% [18, 19]. The M2 margin generally should not be more than one-half of the treatment effect. In reality, it is rarely more than 10% for the US FDA and 12.5% for the European regulators (see reference [16] for the reasoning behind these values). The M2 margin value is a critical consideration because it is the primary determinant of the number of patients who must be enrolled in the clinical trial. Given this margin value, and with consideration to the various other factors (such as the evaluation of the patients enrolled), the typical non-inferiority trial requires approximately 800 patients. The cost of this trial will be roughly $25–$50 million. Trials in nosocomial pneumonia tend to require greater expense. Two such trials (plus the subjects studied in the Phase I and Phase II trials) define a safety database of close to 2,000 individuals. A safety database of 1,500–2,000 individuals traditionally has been considered by the regulatory agencies as adequate for antibacterial development [18, 19].
5.2 Fixed-Dose Combination Antibacterials
There is a long history of the use of this approach. These clinical trials have all been studied in the context of proof of non-inferiority. Examples include sulfamethoxazole-trimethoprim, quinuprisitin–dalfopristin, and all marketed β-lactam–β-lactamase inhibitor combinations. The non-inferiority approach to the fixed-dose combination is unique for antibacterials. In contrast, antiviral and oncology combination drugs are generally studied in the context of superiority trials, where the combination is thought to be more efficacious than either of its components, or than other combinations already marketed. The unique considerations of antibiotic therapy, in which placebos are not allowed and most existing therapies are already highly efficacious, preclude the use of a superiority criterion. The one area where there is opportunity to look for superiority is among patients infected with resistant pathogens. However, enrolling such patients into the clinical trial is very difficult unless the majority of such infections are already due to the resistant pathogen. The best example of this situation is the global pandemic of methicillin-resistant S. aureus infection where in many countries (including the USA), where up to 70% of strains were resistant. In this situation, enrolling patients with resistant infections is relatively straightforward. But who wants to wait for a global pandemic of resistance to enable as possible this approach to antibacterial clinical design?
Fixed-dose combination study in the future might correspond to an already marketed antibiotic plus a compound that enhances its activity. Such a combination can be studied in traditional non-inferiority trials. If both compounds are safe and non-inferior to a reasonable comparator, ready approval may follow. A fixed-dose combination that includes two experimental agents is more challenging and requires much more preclinical (as well as additional clinical) effort [20]. But the approach is not impossible, and such a combination could still be studied ultimately in standard non-inferiority trials.
This assessment brings us to consideration of the clinical approach to β-lactam–β-lactamase inhibitor combinations. These fixed-dose combination drugs target a very specific resistance mechanism – the mechanism arising due to the presence of a bacterial β-lactamase. Here, non-inferiority trials must produce some minimum number of patients infected with infections caused by pathogens resistant to the β-lactam drug in the combination, but susceptible to the drug combined with the inhibitor in the combination. In a certain way of thinking, this circumstance allows confirmation of the superiority of the combination against resistant strains, without the necessity of carrying out a superiority trial solely consisting of resistant infection. This approach leans heavily on a partner antibacterial that has been previously marketed and has well-understood characteristics, corresponding to a clear regulatory label. This approach has worked well for the development of all currently approved β-lactam–β-lactamase inhibitor combinations. The approach becomes more challenging when the target organisms are encountered less frequently in the clinic. Good examples are the carbapenem plus β-lactamase inhibitors currently still in development (imipenem–cilastatin–relebactam and meropenem–vaborbactam) that target pathogens resistant to the carbapenem alone, as a result of the presence of carbapenemase enzymes. The recruitment of even small numbers of patients infected with these pathogens into traditional non-inferiority trials has proven difficult. Nevertheless, regulators seem ready to accept in vitro, animal model and pharmacokinetic/pharmacodynamics data in support of these combinations instead of the clinical data they required previously [21]. This demonstratres the importance of preclinical data.
5.3 Development of an “Enhancer” Compound as a Stand-Alone Agent
This possibility is likely neither feasible nor commercially desirable. The enhancer in this case is a compound simply added to whatever cocktail of antibiotics is thought to be the best available therapy to treat an infection, such as urinary-tract infection. The cocktail plus enhancer is compared to the cocktail alone in a randomized active-control superiority trial. Because the control cocktail (or single antibiotic, such as a carbapenem) in general is so effective in these infections, achieving superiority will require such a large number of patients as to render the study infeasible. Of course, if resistance to the antibiotics in the cocktail was common and if the enhancer allowed these drugs to overcome that resistance, such a trial might be feasible. Nonetheless, the enrollment of sufficient patients with resistance into a clinical trial will be exceedingly difficult. There are two primary reasons for this difficulty. Many patients will present a severe underlying illness that will exclude them from the trial. Many (if not most) patients will have been treated previously with other antibiotics and would also be excluded from participation (see the section on pathogen-specific antibiotics and superiority trials below).
Even if such a study could be conducted, how would such a drug be used in the clinic? Useful instruction is provided by the attempt by Pfizer to market the β-lactamase inhibitor sulbactam as a single agent for physicians to add to whichever β-lactam partner they desired in the treatment of various infections [22]. This marketing effort (with sulbactam marketed as “Combactam”) was undertaken in Germany and Austria. The problem was that this approach required physicians to understand which β-lactamases might be present in the infection they were treating, and what the appropriate dosage of Combactam was required for combination with their selection of a β-lactam. These requirements were too much for the majority of practicing physicians. Combactam sales suffered. The attempt was an abortive one. Would an enhancer drug fare better in the marketplace?
5.4 Pathogen-Specific Antibiotics
I exclude from this discussion a consideration of compounds targeting Clostridium difficile and Mycobacterium tuberculosis, given the exceptional circumstances of these infections. While antibiotics active against specific genera or species of bacteria are seen by many as being highly desirable from the point of view of antimicrobial stewardship and sparing the microbiome, such products are difficult to discover and even more challenging to develop. Most antibiotics that are discovered, regardless of the screening program used, are active against a fairly broad spectrum of bacterial pathogens. Most hits are broadly active against Gram-positive bacteria. Some compounds – but these compounds are rare – are restricted to activity against Gram-negative pathogens only. A more likely scenario is the discovery of a compound with broad activity that has additionally a particular advantage against a specific genera or species. Examples might include some tetracyclines like minocycline, tigecycline, and eravacycline that have enhanced activity against Acinetobacter spp. compared to their activity against other Gram-negative species. Another example might be the carbapenem–β-lactamase inhibitor combinations noted earlier, having broad-spectrum activity attributed to the carbapenem but with activity targeted towards certain carbapenem-resistant strains attributable to the β-lactamase inhibitor. As noted above, such compounds or combinations are much more straightforward to develop and bring to market more than something that targets only a specific genera or species.
The problem for truly pathogen-specific antibiotics remains the clinical trial design. To carry out the clinical trial, a sufficient number of enrollable patients with serious infections caused by the pathogen in question must be identified. A very instructive hypothetical case example was examined at a recent FDA workshop [21]. This case example involved a fictitious antibiotic (called X-1) exquisitely but specifically active against only P. aeruginosa. In attempting to design a clinical trial for X-1, previous trials that enrolled at least some patients with Pseudomonas infections were examined. In nosocomial pneumonia, about 15% of patients enrolled were infected with Pseudomonas. For urinary-tract infection and intra-abdominal infection, the numbers were around 3% and 7%, respectively. One can see the challenge already. To get sufficient numbers of patients for a non-inferiority trial, enrollment of thousands of infected patients would be required to secure a sufficient number of evaluable patients with actual Pseudomonas infections. If one assumes that 200–300 patients with such infections are required, given the statistical requirements discussed above, a total enrollment of 3,000–5,000 patients would be necessary (depending on the exclusion criteria used). The largest antibacterial trial of this sort that was undertaken using this approach compared linezolid against vancomycin for nosocomial pneumonia [23]. That trial included around 1,184 patients and took 5 years to complete. It demonstrated statistical superiority of linezolid in the context of a non-inferiority trial. The problem is that outside non-inferiority trials for antibacterial drugs, there is almost no other such clinical experience. Accordingly, a clear and feasible pathway to regulatory approval using a non-inferiority design approach is lacking.
The FDA workshop, in the context of the fictional X-1, hypothesized a novel diagnostic test that could help by enriching the trial for those patients actually infected with P. aeruginosa – the target of X-1. Not only does this test not exist but such a test is not even on the near-term horizon. Such a test would almost certainly have to be a bedside or point-of-care test. That means it would have to be waived from the Clinical Laboratory Improvements Act (CLIA) that requires most diagnostic tests to be conducted in certified medical laboratories [24]. In order to achieve this status, the test would need to be simple such that untrained personnel would be able to carry out the test reliably, and that the specificity and sensitivity of the test would remain the same across operators with widely varying training and skill sets. The reason that such a test would be required has to do with the time required to enroll patients in trials. A test that is sent to the laboratory will require hours to complete and report back to the physicians, just given hospital logistics (transport, lab protocols, and so forth). But for serious infections, delays in antibiotic therapy can be deleterious.
New guidelines that may be forthcoming from FDA may help to ameliorate this situation, though. The Clinical Trials Transformation Initiative has proposed streamlining these trials by pre-enrolling patients at risk of serious infections such as those caused by P. aeruginosa [25]. Such pre-enrollment and prior evidence of colonization by Pseudomonas would eliminate the need for an enriching rapid diagnostic test. Such an approach, if adopted by the FDA (which I believe is likely), might be a major step forward for the study of pathogen-specific antibacterials in nosocomial pneumonia. At this point, I will note that trials for drugs against even less frequent pathogens like A. baumannii will be even more challenging.
At the X-1 workshop, I suggested a potential trial design based on superiority (for a review of the superiority trial approach to bacterial infections, see references [26, 27]). The basis of my suggestion involved including external or historical controls. The reason for this inclusion is that since all (or the vast majority of) patients are treated with the experimental therapy, you only have to enroll about half the number of patients compared to the number that would be required if half were treated with a comparator or standard of care cocktail.
The video presentation by Ellenberg [28] given at an NIH conference on trial designs for emerging infectious diseases is highly recommended with respect to the consideration of external controls in clinical design. This presentation is very informative. In designing trials to address rare infections, rare pathogens, and pathogen-specific indications, patient numbers may not support a randomized design. We might not even be able to achieve statistical inference with an externally controlled design. Nonetheless, in my opinion this design is where we will have to go. According to Byar [29] and later Elllenberg [28], an externally controlled trial design can be justified if the conditions listed below can be met.
-
A randomized trial is infeasible because of the rarity of the condition under study.
-
Sufficient experience exists to ensure that patients not receiving therapy will have a uniformly poor prognosis.
-
The therapy must not be expected to have substantial side effects.
-
There must be a justifiable expectation that the potential benefit to the patient will be sufficiently large to make interpretation of the results of a non-randomized trial unambiguous.
-
The scientific rationale for the treatment must be sufficiently strong that a positive result would be widely expected.
I would argue that a new antibiotic expected to be active against resistant pathogens would meet these criteria, assuming that it was shown to be safe in a sufficient number of volunteers/patients. The data supporting a lack of efficacy of antibiotics where the exposure obtained is insufficient to meet the pharmacodynamic target required for the pathogen are clear and overwhelming. While the statistical problems to this approach are numerous, they hinge on a single assumption: that the distribution of patients with good versus poor prognoses will be the same in the experimental and control groups. This assumption is a key basis for preferring a randomized trial but may be subject to quantitative interrogation.
Most of the failures of externally controlled trials to provide reliable results have resulted from inadequate controls:
-
Controls had been derived from a different time such that control therapy had changed by the time the actual trial was conducted.
-
Or supportive care had changed altering prognosis for controls.
-
Or the effect size in controls had simply been underestimated for other reasons.
How can we overcome these obstacles for antibacterial drugs? The key features that will contribute to future successful antibacterial clinical include:
-
Providing the resources needed for comprehensive PK/PD studies.
-
Having clearly and adequately designed PK/PD targets.
-
Making certain that adequate PK is achieved in the patient population to be studied (possibly including the study of the PK of the new antibacterial as an add-on to the standard-of-care control in the patient population to be studied for efficacy later).
-
Consider a small, open label Phase II study to help convince physicians and regulators that a new antibiotic will indeed benefit patients as expected based on PK/PD considerations. This study will also bolster related PK/PD arguments and will provide at least some data on efficacy.
-
Define inclusion/exclusion criteria early. I would advise being expansive rather than constrictive with respect to these criteria. One does not want a lot of amendments in the middle of a pivotal trial, as this trial is not non-inferiority.
-
Carry out a retrospective (within the previous year or two) observational study of the key patient population treated with standard-of-care or with comparator drug to define control level of response. This retrospective study should utilize the same inclusion and exclusion criteria to be used for the trial and should be done in centers likely to participate in the trial, so as to remove as much as possible center-to-center bias.
-
Early in the trial, carry out a prospective study of standard-of-care or comparator to validate the assumptions you have made about controls during your retrospective standard-of-care. Obviously this study must be done in the centers actually participating (and contributing patients to) the ongoing trial.
-
Alternatively, randomize patients in a 4:1 ratio of experimental therapy versus standard of care, simply to validate the external controls you are using in the trial.
The design I proposed at the workshop involves using external controls. Patients would be those with either nosocomial pneumonia or complicated urinary-tract infection caused by P. aeruginosa. Controls would come from a retrospective study of such patients treated with a carbapenem antibiotic, with or without the addition of an aminoglycoside antibiotic. The retrospective study would focus on outcome (clinical cure in my view) in those patients found to have infections with carbapenem-resistant pathogens. The literature supports the expectation of roughly a 50% clinical cure rate under these circumstances [30]. Carbapenem-resistant Pseudomonas, in general, already comprises about 15%–20% of strains in most hospitals. If our experimental therapy gave us an 80% cure rate, with a 30% absolute difference we might be able to see an important trend towards superiority in as few as 30 treated patients compared to about 100 external control patients. To obtain 30 patients infected with carbapenem-resistant Pseudomonas, we might expect to enroll 300 patients total. Most of these patients would not have Pseudomonas infections, or their Pseudomonas would not be resistant to carbapenem antibiotics. This number would be more than adequate to establish a safety database under the FDA guidance on antibacterials for unmet needs [31], especially given patients who would have been exposed to the drug in Phase I and possibly in Phase II trials as well. If the assumptions regarding efficacy of our new therapy and control (carbapenem) are correct, in such a trial we should be able to prove superiority 90% of the time at the P = 0.05 level (caveat: I am not a statistician).
As noted in the workshop, two companies, Achaogen and The Medicines Company, are conducting superiority trials for their new products (plazomicin and meropenem-vaborbactam, respectively). Both companies have also carried out non-inferiority trials in complicated urinary-tract infection as well. Their second superiority trial will lean heavily on their non-inferiority trial results to support both efficacy and safety. Both superiority trials have been problematic. Achaogen recently announced top-line results for their trial comparing plazomicin to colistin in the treatment of nosocomial pneumonia caused by carbapenem-resistant pathogens [32]. Notwithstanding the small patient numbers in their trial, the trial data themselves are informative: Day 28 all-cause mortality or significant disease related complications (primary endpoint); 4/17 (23.5%) for plazomicin versus 10/20 (50.0%) for colistin, corresponding to a difference of 26.5% (90% CI: 0.7, 51.2%); Day 28 all-cause mortality 2/17 (11.8%) for plazomicin versus 8/20 (40.0%) for colistin, corresponding to a difference 28.2% (90% CI: 0.7, 52.5%). As is evident, these results do not reach statistical significance at the P = 0.05 level. Nevertheless, they are indicative of the results the FDA may expect from pathogen-specific trials targeting resistant infections.
As it stands as of this writing, no such trial has ever been carried out as a stand-alone pivotal trial for approval of an antibacterial drug. As noted at the beginning, there is no established regulatory pathway for approval of a pathogen-specific antibacterial drug. In addition, the regulatory landscape for antibacterials is changing rapidly. There are clearly efforts within the FDA to look at how to obtain and use real-world data [33]. These efforts could have an impact on the selection and use of external controls in future trials. Such efforts might help lead to the regulatory pathway we need. In addition, the recent passage of the 21st Century Cures Act will further spur the agency to develop these needed pathways [34].
6 Conclusions
Everything the discovery scientist does from the outset will ultimately influence the risks that will be encountered on the way from the laboratory bench to regulatory approval and the marketplace. A clearly delineated Target Product Profile giving the desired and the acceptable characteristics of the compound to be discovered, even in a preliminary way, will help the team keep the goal in focus and keep the research on track. Accepting targets that are novel or that guarantee a pathogen-specific focus will increase the risk, but should not necessarily be discounted based on the increased risk. The risks must simply be balanced within the context of an overall program. The regulatory landscape is changing quickly. It will behoove the scientist to be aware of this evolution as it proceeds. In all cases, the burden of preclinical testing will surely increase as pharmacokinetic and pharmacodynamic data assume greater importance for future regulatory filings. A strategy to identify enhancer compounds that could be combined with any of a number of potential partners and to develop the enhancers as stand-alone products is especially risky given the requirement for superiority trials, and given the difficulty in marketing such compounds. By contrast, enhancers or resistance inhibitors (such as β-lactamase inhibitors) that can be partnered with a single compound and then developed as a fixed-dose combination minimize risk. Pathogen-specific antibacterials do not fit well with traditional development pathways such as non-inferiority trials, and as such will probably have to be studied using superiority designs. These superiority designs for antibacterials have not been clearly delineated by regulatory authorities and remain untested. Strong PK/PD data will be required to justify such trials. But I am optimistic that such pathways will be available soon given the recent FDA workshop on unmet needs for antibacterial drugs, FDA’s efforts at defining how to examine real-world data and the passage of the 21st Century Cures Act.
References
Sutterlin HA, Malinverni JC, Lee SH, Balibar CJ, Roemer T (2017) Antibacterial new target discovery: sentinel examples, strategies and surveying success. Top Med Chem. (this volume). doi:10.1007/7355_2016_31
Silver LL (2016) Appropriate targets for antibacterial drugs. Cold Spring Harb Perspect Med 6:a030239. doi:10.1101/cshperspect.a030239
O’Shea R, Moser HE (2008) Physicochemical properties of antibacterial compounds: implications for drug discovery. J Med Chem 51(10):2871–2878. doi:10.1021/jm700967e
Silver LL (2016) A gestalt approach to gram-negative entry. Bioorg Med Chem 24:6379–6389. doi:10.1016/j.bmc.2016.06.044
Sertkaya A, Eyraud JT, Birkenbach A, Franz C, Ackerley N, Overton V, Outterson K (2014) Analytical framework for examining the value of antibacterial products. https://aspe.hhs.gov/report/analytical-framework-examining-value-antibacterial-products. Accessed 24 Jan 2017
Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL (2007) Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov 6:29–40. doi:10.1038/nrd2201
Walsh CT, Wencewicz TA (2014) Prospects for new antibiotics: a molecule-centered perspective. J Antibiot 67:7–22. doi:10.1038/ja.2013.49
Livermore DM, Mushtaq S, Warner M, Zhang J, Maharjan S, Doumith M, Woodford N (2011) Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 55:390–394. doi:10.1128/AAC.00756-10
Stachyra T, Péchereau MC, Bruneau JM, Claudon M, Frère JM, Miossec C, Coleman K, Black MT (2010) Mechanistic studies of the inactivation of TEM-1 and P99 by NXL104, a novel non-β-lactam β-lactamase inhibitor. Antimicrob Agents Chemother 54:5132–5138. doi:10.1128/AAC.00568-10
Mushtaq S, Warner M, Livermore DM (2010) In vitro activity of ceftazidime+NXL104 against Pseudomonas aeruginosa and other non-fermenters. J Antimicrob Chemother 65:2376–2381. doi:10.1093/jac/dkq306
Singh MP, Petersen PJ, Weiss WJ, Janso JE, Luckman SW, Lenoy EB, Bradford PA, Testa RT, Greenstein M (2003) Mannopeptimycins, new cyclic glycopeptide antibiotics produced by Streptomyces hygroscopicus LL-AC98: antibacterial and mechanistic activities. Antimicrob Agents Chemother 47:62–69. doi:10.1128/AAC.47.1.62–69.2003
O’Dwyer K, Spivak AT, Ingraham K, Min S, Holmes DJ, Jakielaszek C, Rittenhouse S, Kwan AL, Livi GP, Sathe G, Thomas E, Van Horn S, Miller LA, Twynholm M, Tomayko J, Dalessandro M, Caltabiano M, Scangarella-Oman NE, Brown JR (2015) Bacterial resistance to leucyl-tRNA synthetase inhibitor GSK2251052 develops during treatment of complicated urinary tract infections. Antimicrob Agents Chemother 59:289–298. doi:10.1128/AAC.03774-14
Van Bambeke F, Tulkens PM (2016) Editorial commentary: colistin and a new paradigm in drug development. Clin Infect Dis 62:559–560. doi:10.1093/cid/civ968
Drusano GL (2016) From lead optimization to NDA approval for a new antimicrobial: use of pre-clinical effect models and pharmacokinetic/pharmacodynamic mathematical modeling. Bioorg Med Chem 24:6401–6408. doi:10.1016/j.bmc.2016.08.034
Guidance for Industry (2010) M3(R2) nonclinical safety studies for the conduct of human clinical trials and marketing authorization for pharmaceuticals. http://www.fda.gov/downloads/drugs/guidances/ucm073246.pdf. Last accessed 23 Jan 2017
Shlaes DM (2010) Antibiotics – the perfect storm. Springer-Verlag, New York, NY
Spellberg B, Lewis RJ, Boucher HW, Brass EP (2011) Design of clinical trials of antibacterial agents for community-acquired bacterial pneumonia. Clin Investig (Lond) 1:19–32. doi:10.4155/CLI.10.1
Guidance for Industry (2014) Hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia: developing drugs for treatment. Draft guidance. http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm234907.pdf
Guidance for Industry (2013) Acute bacterial skin and skin structure infections: developing drugs for treatment. http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm071185.pdf
Guidance for Industry (2013) Codevelopment of two or more new investigational drugs for use in combination. U.S. Department of Health and Human Services Food and Drug Administration. Center for Drug Evaluation and Research (CDER). http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm236669.pdf+%26cd=1%26hl=en%26ct=clnk%26gl=us. Last accessed 30 Dec 2016
US Food and Drug Administration (2016) Facilitating antibiotic drug development for patients with unmet need and developing antibacterial drugs that target a single species. http://www.fda.gov/Drugs/NewsEvents/ucm497650.htm. Last accessed 28 Dec 2016
Dougherty TJ, Pucci MJ (2011) Antibiotic discovery and development. Springer Science & Business Media, New York, NY
Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, McGee WT, Reisman A, Chastre J (2012) Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 54(5):621–629. doi:10.1093/cid/cir895
Clinical Laboratory Improvement Act (1988) https://wwwn.cdc.gov/clia/. Last accessed 23 Jan 2017
Clinical trials transformation initiative (2016) Streamlining hospital acquired and ventilator associated bacterial pneumonia trials. https://www.ctti-clinicaltrials.org/projects/streamlining-habpvabp-trials. Last accessed 23 Jan 2017
Shlaes DM, Spellberg B (2012) Overcoming the challenges to developing new antibiotics. Curr Opin Pharmacol 12:522–526. doi:10.1016/j.coph.2012.06.010
Infectious Diseases Society of America (2012) White paper: recommendations on the conduct of superiority and organism-specific clinical trials of antibacterial agents for the treatment of infections caused by drug-resistant bacterial pathogens. Clin Infect Dis 55:1031–1046. doi:10.1093/cid/cis688
Clinical trials for emerging infectious diseases (2015) https://videocast.nih.gov/summary.asp?Live=17597&bhcp=1. Last accessed 28 Dec 2016
Byar DP, Schoenfeld DA, Green SB, Amato DA, Davis R, De Gruttola V, Finkelstein DM, Gatsonis C, Gelber RD, Lagakos S et al (1990) Design considerations for AIDS trials. N Engl J Med 323(19):1343–1348. doi:10.1056/NEJM199011083231912
Bass SN, Bauer SR, Neuner EA, Lam SW (2015) Impact of combination antimicrobial therapy on mortality risk for critically ill patients with carbapenem-resistant bacteremia. Antimicrob Agents Chemother 59:3748–3753. doi:10.1128/AAC.00091-15
Guidance for Industry (2013) Antibacterial therapies for patients with unmet medical need for the treatment of serious bacterial diseases. Draft guidance. http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm359184.pdf
Achaogen Press Release (2016) Achaogen announces positive results in phase 3 cUTI and CRE clinical trials of plazomicin. http://investors.achaogen.com/releasedetail.cfm?ReleaseID=1003671. Last accessed 30 Dec 2016
Sherman RE, Anderson SA, Dal Pan GJ, Gray GW, Gross T, Hunter NL, LaVange L, Marinac-Dabic D, Marks PW, Robb MA, Shuren J, Temple R, Woodcock J, Yue LQ, Califf RM (2016) Real-world evidence – what is it and what can it tell us. N Engl J Med 375:2293–2297. doi:10.1056/NEJMsb1609216
The 21st Century Cures Act (2016) A section-by-section summary. https://rules.house.gov/sites/republicans.rules.house.gov/files/114/PDF/114-SAHR34-Sxs.pdf. Last accessed 30 Dec 2016
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Shlaes, D.M. (2017). The Clinical Development of Antibacterial Drugs: A Guide for the Discovery Scientist. In: Fisher, J.F., Mobashery, S., Miller, M.J. (eds) Antibacterials. Topics in Medicinal Chemistry, vol 25. Springer, Cham. https://doi.org/10.1007/7355_2017_8
Download citation
DOI: https://doi.org/10.1007/7355_2017_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-68096-5
Online ISBN: 978-3-319-68097-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)