Abstract
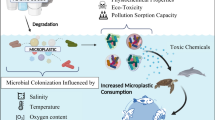
The occurrence of microplastics (MPs) in the terrestrial and marine environment has been gaining global attention. These microparticles carry biofilm communities that are distinct from the surrounding environment. MP-colonizing microorganisms are important links for the fate of MPs in different ecosystems. However, the influence of plastic-colonizing microorganisms on the fate of microplastics is largely unknown. Here we review the formation of biofilms and dynamic variation on the surfaces of microplastics together with the main research methodologies for biofilm analysis. The potential impacts of biofilm formation on the environmental fate of microplastics caused by MP-colonizing microorganisms such as weathering processes, vertical transport, sorption and release of contaminants, trophic transfer of MP particles, and potential environmental toxicity of MPs in the marine ecosystem are also reviewed. Future studies are needed on the processes and mechanisms of microplastic and biofilm interactions in the terrestrial environment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Biodegradation
- Biofilms
- Extracellular polymeric substances
- Microplastics
- Toxicity
- Vertical transport
- Weathering
1 Introduction
According to the International Union of Pure and Applied Chemistry (IUPAC), biofilms are defined as aggregates of microorganisms in which cells that are frequently embedded within a self-produced matrix of extracellular polymeric substances (EPSs) adhere to each other and/or to a surface. Biofilms may form on living or nonliving surfaces and can be prevalent in the marine and terrestrial environments. Due to the large specific surface area of microplastics (MPs), many microorganisms including bacteria, fungi, algae, and protists can easily colonize the surfaces of microplastics in the form of biofilms. The formation and development of biofilms on the surfaces of microplastics may change the morphology and physicochemical properties of MPs in the environment, thus leading to diverse physical, chemical, and biological influences on the environmental fate of MPs such as weathering, vertical transportation, co-migration with chemical pollutants and pathogens, as well as biodegradation. In this chapter the methodologies and processes of biofilm formation and development on the surface of MPs are reviewed, and the different influences of biofilm formation on the properties of MPs are also investigated with the aim of better understanding the fate of MPs in the terrestrial environment.
2 Formation and Development of Biofilms on the Surfaces of Microplastics
2.1 Major Stages of Biofilm Formation
Biofilms are formed by EPS secreted by microorganisms including proteins, glycoproteins, and glycolipids which form a matrix around the microbes and enable them to attach to a variety of different biological and abiotic surfaces. Continuous changes in bacterial colonization of artificial surfaces (such as glass, stainless steel, and polycarbonate sheets) have been confirmed in seawater [1]. Different scholars divide the formation of biofilms into different stages from the core flora and time series.
Biofilm formation is divided into early stage (1–14 weeks), mid-stage (14–35 weeks), and late stage (35–45 weeks) based on changes in the core flora of the biofilm on the surface of plastic flakes exposed at the bottom of the harbor [2]. The formation process of biofilm on the surface of plastic flakes in the real marine environment is constructed. Wimpenny [3] gives a classic biofilm formation process in chronological order:
-
1.
Rapid formation of organic molecular layers on clean solid surfaces.
-
2.
Colonization by bacteria loosely attached to solid surfaces.
-
3.
Colonization by bacteria more firmly attached, forming microbial communities and producing EPS.
-
4.
Communities stretching outward to form regular and irregular structures.
-
5.
Biofilms mature, new species enter the biofilm and grow, and organic or inorganic fragments are combined to form a solution gradient resulting in spatial heterogeneity of the biofilm.
-
6.
Protozoa that phagocytose bacteria may prey on biofilms.
-
7.
Mature biofilms may peel off and this cycle alternates or forms a top-level community.
Lennox [4] divides biofilm formation into five processes: (1) mucosal formation, (2) bacterial proximity and touching, (3) reversible and irreversible attachment, (4) exogenous species supplementation and growth, and (5) diffusion. Some researchers have also divided biofilm formation into four processes: (1) adsorption of dissolved organic molecules, (2) colonization by prokaryotes, (3) colonization by single-cell eukaryotes, and (4) colonization by invertebrate larvae and algal spores. These four processes may occur simultaneously or independently on the surface of the microplastic [5].
2.2 Factors Affecting Biofilm Formation on Microplastics
A conditioning layer comprising organic and inorganic materials is formed by adsorption within a few minutes of the first contact of the plastic surface with the surrounding water. Microorganisms are in contact with the surface through repulsive or attractive interactions between cell walls and media surfaces. The initial condition layer may have the ability to control colonization by altering material-specific surface properties [6]. Biofilm formation is a multistage process mediated by a variety of factors including surface properties, nutrient solution, pH, and temperature [7]. The environment surrounding the matrix and the conditions of cell growth (such as temperature, carbon source, fluid flow, composition of nutrient media, and growth factors) are complex factors that affect the attachment of bacteria to the surfaces of MPs [8]. There are a variety of attachment mechanisms between microbes and matrices that increase the adhesion of the substrate surface through pili, bristles, flagella, and adjustment of EPS yield [9, 10]. The initial condition layer and the colonizer alter the surface properties of the material and promote the colonization of other organisms. Microbial cells can attach to the surface through specific and non-specific interactions, both depending on surface hydrophobicity/hydrophilicity, roughness, electric charge, and functional groups. The chemical properties of the condition layers are related to the roughness or hydrophobicity of the initial matrix surface and are important for biological sedimentation, indicating the importance of the first adsorption process [11]. Hook et al. [12] believe that surface hydrophobicity and polymer morphology do not affect the adhesion of bacteria to polymers. In contrast, Sanni et al. [13] propose a strong correlation between bacterial sedimentation and hydrophobicity, molecular flexibility parameters in the specific condition of poly(meth)acrylate.
3 Methodology of Microplastic-Associated Biofilm Research
3.1 Scanning Electronic Microscopy
Scanning electron microscopy (SEM) is a tool for observing the surface morphology of samples using secondary electron signal imaging [14] and is widely used in biological, medical, materials, geological, environmental, and other research fields. Energy-dispersive spectroscopy (EDS) combined with scanning electron microscopy (SEM/EDS) is a commonly used elemental microanalytical method that identifies and quantifies the target surface elements of a sample surface [15]. At present, SEM has become a common method for the study of morphology with MPs and their surface biofilms (Fig. 1). EDS is used to analyze the elemental composition of specific areas of MPs to characterize the aging and adsorption characteristics of MPs in the environment.
When observing the microplastic surface biofilm, the SEM sample preparation is usually subjected to cell fixation, dehydration, drying, and then sample analysis according to the SEM method [16, 17]. Cell fixation is an important step in sample preparation. During cell dehydration or drying, cells lose water and undergo structural changes, resulting in distortion of results [18]. Generally, glutaraldehyde or its combination with citric acid is used to fix microbial cells [19]. Sample drying methods generally include freeze-drying, room temperature or oven drying, and CO2 critical point drying [17, 19, 20].
SEM can be used to visually identify microbial morphology and posture, characterizing the biodiversity on microplastic surfaces [21], or to analyze the surface morphology of MPs to understand the process of change for weathering and fragmentation of MPs in the environment [20] and helps to distinguish MPs from organic particles [22]. SEM coupled with EDS analysis can be used to identify microplastic samples, especially to distinguish carbon-dominated plastics from inorganic particles [23]. In addition, EDS is also a means of detecting harmful substances such as potentially toxic metals from the environment adsorbed on the surfaces of the MPs.
3.2 Crystal Violet Staining
Crystal violet is a staining solution commonly used in tissue or cell staining to stain the nucleus a deep purple color. Crystal violet is a basic dye that binds to DNA in the nucleus and binds to negatively charged surface molecules and polysaccharides in the extracellular matrix [24] while simultaneously allowing proteins to be stained. It is therefore often used as a biofilm semiquantitative method to characterize the biofilm formation process. Crystal violet staining is simple to operate, but it cannot distinguish the living status of cells. According to the mature state of the cells, potassium hydroxide is added to adjust the pH of the dye solution to 6.0–8.0. The lower pH dye solution is used for fresh cell staining, while the higher pH dye solution is suitable for matured cells. The pH can also be adjusted with aniline or pyridine to enhance the dyeing ability of the dye solution for old cells [25]. In addition, the combination of crystal violet and ammonium oxalate to view biofilms can improve the quality of protein-selective staining and enhance coloration and optical effects [26]. Cells stained with crystal violet can be decolorized with a solution such as sodium dodecyl sulfate solution, acetic acid, or ethanol [27,28,29], and the absorbance of the decolorizing solution is measured and can indirectly represent the total amount of biofilm. Moreover, since the light could strongly interfere with the crystal violet staining effects, special care is needed to avoid light contamination during the preparation, storage and usage of crystal violet dye.
3.3 Laser Confocal Scanning Microscopy
Laser confocal scanning microscopy (LCSM) is a recent technique developed for the study of histomorphology. It can perform layered scanning on light-transmitting samples and is often used for the morphological study of the three-dimensional structure of bacterial biofilms [30]. LCSM is developed based on fluorescence microscopy technology and is mainly composed of a laser light source, a scanning device, a detector, a computer system, an image output device, an optical device, and a confocal system. The imaging principle is to use a laser scanning beam to form a point light source through a grating pinhole and scan the optical signal of the collecting point by point on the focal plane of the fluorescent marking specimen to reach the photomultiplier tube (PWT) through the detecting pinhole and then display the signal on the computer through signal processing. An image is formed on the screen. The term “confocal” refers to the LCSM having a pinhole light source in front of the illumination source and in front of the detector. After a series of lenses, it is finally focused on the pinhole of the light source and the pinhole [31].
LCSM can provide three-dimensional information about different cell and polymeric biofilm components such as phototrophic organisms, bacteria, and EPS [32]. In addition, the continuous development of fluorescent markers makes it possible for fluorescent dyes to target specific components of biofilms such as nucleic acids and protein residues and even to identify specific cellular physiological states, providing further description of the natural structure, composition, and cellular tissues of biofilms. According to the purpose of the research, the specific fluorescent dye to stain the sample can be selected [29], and the biofilm image along the Z-axis direction in 3D mode can be collected to obtain a complete series of stack format images. The three-dimensional structure of the biofilm can be calculated quantitatively using Imaris and ImageJ software [33]. It should be noted that the fluorescent dye should be stored at a suitable temperature according to the product description and should be protected from light during storage and use.
3.4 Flow Cytometry Combined with viSNE
Flow cytometry uses a device for automated analysis and sorting of cells. It can quickly measure, store, and display a series of important biophysical and biochemical parameters of dispersed cells suspended in a liquid. Flow cytometry and mass spectrometry flow cytometry are powerful analytical tools for simultaneously studying ten extrinsic markers in a single cell to identify rare subtypes and complex cellular states in heterogeneous populations. These single-cell multiparametric extrinsic measurements have been used in many applications in biology and medicine [34]. Flow cytometry combined with microscopic observations reveal that micro(nano)plastics form agglomerates with mucus matter and associated microbial communities in seawater [35]. Dussud et al. [36] used 1 mmol L−1 pyrophosphate for cell detachment pretreatment and ultrasonication with an ultrasonic probe. The cell-separated sample was fixed with 1% (v/v) (final concentration) glutaraldehyde. The cells were then stained with a nucleic acid dye in the dark after which the cells were counted using a flow cytometer.
Visual stochastic network embedding (viSNE) is a tool for nonlinear dimensionality reduction and high-dimensional data visualization. It was originally used to visualize mass spectrometry flow cytometry data from healthy and leukemia blood samples, qualitatively distinguishing blood cell types and detecting abnormal phenotypic changes in blood cell populations. The optimized viSNE program can be used to distinguish species and different phenotypes present in biofilms. Flow cytometry is used in combination with viSNE, which quantifies the survival of large cells after cell decay and temperature stress, while in the field it detects changes in community structure driven by known environmental factors (flow conditions, dissolved organic carbon, calcium) and plastic contamination [37].
3.5 DNA Extraction and High-Throughput Sequencing
High-throughput sequencing (HTS), also known as next-generation sequencing (NGS), can sequence up to tens of millions of DNA strands in parallel at one time. It has become a common research tool in the life sciences and has been widely used in genomics, sequencing, epigenomics, and functional genomics. High-throughput sequencing can complete a variety of sequencing tasks including genome-wide, transcriptome, and macrogenome and bring new methods for functional genomics analysis.
DNA extraction is a preliminary step for high-throughput sequencing. In contrast to natural media such as water and soil, MPs are highly polymeric, and the microbial content on the surface is low. It was found that the particle size, quantity, type, and physicochemical properties of MPs affect DNA extraction [29]. Commercial kits can be selected to extract whole-genomic DNA from microplastic surfaces to increase productivity. The extracted product is subjected to purity evaluation by agarose gel electrophoresis, and its quality is evaluated by NanoDrop [38]. According to the research needs, the appropriate primer template is selected for PCR amplification, and the amplified product can be sequenced on the machine after passing the test.
Zettler et al. [46] used high-throughput sequencing technology for the first time to analyze the microbial community diversity of six microplastic surfaces, and they found that the average number of microbial species per surface exceeded 1,000. Since then, more studies have focused on the microbial community structure and diversity of biofilms on MP surfaces and spatiotemporal variability of microbial community structures on biofilms on the surfaces of MPs [38,39,40].
Some typical biofilms formed on the surface of plastic and non-plastic materials are listed in Table 1.
4 Biofilms on Plastic Surfaces and Their Physicochemical Implications
4.1 Weathering
Plastic weathering is the process by which the physical integrity of a material is lost through the influence of abiotic and biological factors. Photooxidation is the most common non-biodegradable pathway and can be divided into three main steps: initiation (polymer chain breakage and radical formation induced by UV light), propagation (auto-oxidation), and termination (forming inert products). Weathered surfaces may exhibit changes in shape, increased surface roughness, and chemical changes (e.g., become more polar due to the formation of carbonyl groups) [6]. Over time the surface area of plastics which is available for microbial colonization increases [50], thus increasing the effects of microplastic biodegradation. On the other hand, the formation of biofilms alleviates the ultraviolet degradation by sunlight of plastics which hinders the physicochemical weathering process [51].
Biodegradation of polymers occurs in addition to physical weathering [52]. Flemming [53] reported a variety of patterns in which biofilms disrupt the structure and function of synthetic polymeric materials, namely, (1) fouling surfaces, altering surface properties, and contaminating adjacent media such as water by released microbes; (2) increased leaching of additives and monomers from the polymer matrix by microbial degradation; (3) attacking polymers and additives by enzymes or biological groups, resulting in loss of embrittlement and mechanical stability; (4) hydration and fungal hyphal penetration of the polymer matrix, causing expansion and increasing conductivity; and (5) degradation of the polymer color by excretion of lipophilic microbial pigments. Gewert et al. [54] investigated the biodegradation pathways and products of six plastic polymers. The six plastics were divided into two categories according to the main chain components. One has a carbon chain as the main skeleton (PE, PP, PS, and PVC), and the other contains heterocyclic atoms (PET and PU). Ultraviolet radiation and oxygen are the main factors leading to the fracture of the C-C skeleton in the initial stages of microplastic degradation. The small molecular polymers after fracture may be further degraded by microbial intracellular or extracellular enzymes.
4.2 Vertical Transport
The vertical transport of MPs in the ocean is influenced by multiple physical, chemical, and biological processes [55]. Density is an important parameter to control the vertical migration of MPs. Plastic density is commonly 0.85–1.41 g cm−3. Low-density plastics (density less than seawater) float on the surface of seawater for migration, medium-density plastics (density close to seawater) are suspended in seawater, and high-density plastics (density greater than seawater) migrate on the seabed by suspension or mass transfer [5]. Reisser et al. [56] analyzed the distribution of low-density plastic particles below 0–5 m depth in the sea. It was found that the concentration of plastic particles decreased exponentially with increasing water depth and the smaller the particles, the easier it was for them to migrate vertically. MPs are affected by physical and biological processes during migration and by density changes. A survey of the North Atlantic found that the density of oceanic MPs increased significantly compared to nearshore MPs, mainly due to biofouling [57]. On one hand, biofilms may increase the density of MPs causing them to sink. On the other hand, biofilms may increase the buoyancy of plastic particles with higher density than water, and they more readily float [6]. With the impacts from biofilms, physical and chemical processes such as flocculation occur between the microplastic particles and the agglomerates formed settle to the seabed. Some plankton ingest MPs coated with biofilms which in turn release plastic particles with altered physical and chemical properties, increasing their sinking rate [58]. The plastic particles that converge on the bottom layer are reduced in density due to the feeding of benthic organisms on their surface biofilms, thus regaining buoyancy [59].
Numerical simulation is the main research method for studying the vertical migration of MPs in the ocean. Kukulka et al. [60] used a turbulent mixing model to simulate the migration of plastic particles in the vertical direction under buoyancy and turbulence. Isobe et al. [61] established a vertical two-dimensional particle tracking model to simulate the migration of plastic particles in coastal waters. The sediment deposition model can be used for the simulation of high-density MPs. Ballent et al. [62] used the Mohid model (a general three-dimensional numerical calculation model) and the experimentally obtained sedimentation-resuspension parameters to simulate the migration of high-density MPs in the Nazaré canyon and found the MPs moving up and down in the canyon under tidal currents. After the model is established in the actual research, the parameters of the MP migration process need to be obtained and verified to identify the rationality of the simulation results.
4.3 Transport of Plastic-Associated Pollutants Through Biofilms
MPs have a large specific surface area and readily adsorb different pollutants including persistent organic pollutants, potentially toxic metals, and pathogens. Additives are certain chemicals added to the molecular structure of plastics to improve their properties. They have hydrophilic groups and metabolic properties and are difficult to leach with weak solvents. Plastic additives may leach and migrate as the environment changes, for example, bisphenol A and nonylphenol, which are highly hydrophilic [5]. Jang et al. [63] found the brominated flame retardant HBCD and bisphenol A on PS foam collected on the Korean coast. Plastics can adsorb persistent organic pollutants (POPs) and can act as important carriers for the transportation and diffusion of organic pollutants. Bakir et al. [64] studied the potential of microplastic transport and removal of hydrophobic organic pollutants (HOCs) in estuarine environments and found that the potential for PE transport and removal of phenanthrene and 4,4′-DDT is much greater than that for PP and PVC. Potentially toxic metals are also common contaminants adsorbed on microplastic surfaces. For example, the detection rate for Cd and Pb in the biofilms of microplastic samples was 6.9% and 7.5%, respectively, from two beaches in southwest England [65]. In addition, chemical contaminants such as drugs and antibiotics were also detected on microplastic fragments.
The distribution and diffusion of the various abovementioned pollutants in MPs and the surrounding water environment may be affected by biofilms. On one hand, biofilms may enhance the adsorption capacity of pollutants on the surface of MPs. On the other hand, specific microbes in the biofilm can metabolize and degrade organic pollutants adsorbed on the MPs [6]. Biofilms are an organic phase composed of water, lipids, and proteins, and they can adsorb water, inorganic and organic solutes, and particles [66], representing a potential barrier to the adsorption, diffusion, and release of chemicals. The viscosity of EPS contributes to the ability of biofilm-coated MPs and heteropolymers to adsorb contaminants [67]. Biofilms can increase the mass transfer resistance of pollutants to the contact with and exit from the plastic polymers [68]. Kinetic laboratory study of HOCs adsorbed onto MPs shows that when microplastic surfaces are in the presence of biofilms, the diffusion coefficient is reduced by approximately four orders of magnitude [69]. A range of bacteria, fungi, and algae in the biofilms can degrade HOCs [70], with the additives released from MPs being used as a nutrient source to promote microbial growth [71].
5 Biofilms on Plastic Surfaces and Their Biological Effects
5.1 Microbial Community Structure
MPs have become a popular topic in microbial colonization research because of their small particle size, wide distribution, and large specific surface area. Once released into the environment, MPs are rapidly colonized by microorganisms such as fungi and bacteria and by diatoms or that form biofilms on the plastic surface [2, 72]. Because of the unique surface properties of MPs, the microbial communities colonizing the surface are different from those in the surrounding environment. MPs provide a unique microhabitat that supports the growth of some microbial consortia [73]. Thus, Zettler et al. [46] introduced the term “plastisphere” to describe the environmental niche formed by these plastics.
Microplastic surfaces in aquatic ecosystems are novel ecological habitats for marine organisms, and the composition and diversity of biofilm communities have been investigated in numerous studies [21, 46, 74]. Different methods have been used to study the bacterial composition of the plastisphere. With the development of molecular biology technology, high-throughput sequencing technology has been widely used to reveal the composition and diversity of microbial communities on the surfaces of MPs. Some studies find that microbial abundance and diversity on the surface of MPs are lower than those in the surrounding water or sediments [74, 75]. The microbial community structure of the plastisphere is largely influenced by geographical factors, spatial location, and exposure time [2, 76,77,78]. In addition, different types of polymers and environmental factors also have a significant impact [79, 80]. Miao et al. [81] evaluated the effects of substrate type on microbial communities and found altered metabolic pathways in microbiomes colonizing MPs. Similar results have also been found in the study of the composition and function of PE MPs communities in soil ecosystems by Huang et al. [39]. Compared to natural matrices, microbial communities colonizing the surfaces of MPs exhibit different functions and may trigger different ecological effects on the environmental fates of MPs. Further investigations are therefore needed to illustrate the potential effects of the structure and function of microorganisms colonizing the surfaces of MPs, especially the ecological effects in aquatic systems and the soil environment.
5.2 Trophic Transfer
Due to their small size and widespread presence in the marine environment, MPs can be ingested by a series of marine organisms such as zooplankton, invertebrates, crustaceans, and fish [82, 83] and can be transmitted along the food chain through predation [83,84,85]. Intake of MPs may interfere with the food chain as low-nutrient organisms are predated by high-nutrient organisms and then transmitted along the food chain [86, 87]. In contrast to marine microplastic contamination, the distribution and potential impact of MPs in soil ecosystems are poorly understood. Studies show that earthworms and collembolans can transport MPs in soils and increase their mobility [88,89,90]. Zhu et al. [91] found that predator-prey relationships among different trophic levels can increase the migration of MPs in soils. Moreover, the movement of MPs by soil fauna may affect the bioavailability of MPs to other soil organisms [92]. In addition, most studies have focused on virgin MPs ingested by organisms along the food chain, neglecting the fact that most of the surfaces of MPs in the environment are weathered and covered by biofilms [6]. There have been few studies on the bioaccumulation of MPs and MP particles attached to biofilms at the nutritional level. Microorganisms such as bacteria and algae attached to the surface of MPs may be taken up as food by predators such as fish, thus increasing the risk of ingesting MPs [93]. In addition, the buoyancy of MPs adhering to biofilms may change, allowing them to migrate from surface waters to the bottom of the water column, thereby increasing the chance of being ingested by benthic organisms [58, 79, 94]. In summary, the formation of biofilms on the surfaces of MPs may affect the feeding preference for MPs ingested by organisms through alteration of physical and chemical properties or increasing the bioavailability of MPs [6]. Considering the actual environment, future studies should focus on the role of microorganisms and surface biofilms in the effects of MPs on nutrient transfer.
5.3 Toxicity and Adverse Effects
MPs are usually made from highly hydrophobic materials and chemical additives and are thus susceptible to contamination by a number of chemical pollutants such as POPs, potentially toxic elements, antibiotic resistance genes (ARGs), and pathogens [46, 73, 95,96,97]. MPs are colonized by diverse and metabolically complex microbial consortia and can be regarded as a novel microbial niche and may serve as a vector for chemical pollutants which may increase the environmental risk from the adsorbed chemical pollutants [98,99,100]. Environmental MPs are available to every level of the food web from primary producers to higher trophic-level organisms [101]. After a long process from source to sink, MPs are colonized by microorganisms and wrapped by biofilms [102]. The migration of hydrophobic organic pollutants (HOCs) between plastic debris and water may be affected by biofilms which have the ability to metabolize HOCs [6]. MPs have been reported to exhibit concentrations of POPs up to six orders of magnitude greater than the background concentration in the surrounding seawater [103]. Gong et al. found potentially pathogenic bacteria on LDPE MPs exposed in lake water and considered that MPs could serve as transfer vectors for harmful microorganisms in water [104]. Similarly, Wu et al. [73] compared biofilms on MPs with two natural substrates (rocks and leaves), finding that specific ARG subtypes and several pathogenic bacterial hosts were selectively enriched by MP biofilms. Diffusion of specific microorganisms (especially pathogenic microorganisms) in MP biofilms may increase the risk of disease to other organisms including humans. However, the link between the toxicity and adverse effects on MPs and biofilms is still not fully understood. In conclusion, MPs and their associated biofilms represent ecological risks and potentially adverse effects on the environmental safety and health. Future studies are required to clarify the mechanisms of interactions among MPs, biofilm-colonizing microorganisms, and chemical pollutants.
5.4 Biodegradation
Plastics exposed to the environment may undergo either weathering or biodegradation processes under the complex influences of physical, chemical, and biological factors. The biodegradation of plastics is driven mainly by multiple degradation pathways [55]. Biodegradation of long-chain polymers is usually limited due to their large molecular weight and lack of efficient microorganisms for degradation. The biodegradation process of petroleum-sourced plastics usually includes [105, 106] (1) biofilm formation on the plastic surface, (2) depolymerization, (3) catabolism of the depolymerization by-products, and (4) biomineralization of organic matter.
The biodegradation of plastics has been reported in several studies over the last 30 years. However, there is general agreement that the process is extremely slow under normal conditions [107,108,109,110]. Biodegradation requires a crucial initial step that is the formation and development of a microbial biofilm either at the surfaces or directly into the cracks in the MPs [111]. MPs act as a novel, functionally important microhabitat in aquatic and terrestrial ecosystems and exhibit a distinctive microbial community structure which is markedly different from the surrounding environment [75, 76, 78]. Compared with planktonic bacteria, plastic-related bacterial biofilms have stronger ability to degrade plastics [112]. Delacuvellerie et al. [72] found several genera of hydrocarbon-degrading bacteria enriched on several plastics, and these bacteria are potential players in plastic degradation. Yoshida et al. [113] screened a novel bacterium, Ideonella sakaiensis 201-F6, that is able to biodegrade poly(ethylene terephthalate). More plastic-degrading microorganisms have subsequently been found in the environment [111, 114,115,116,117,118]. Although several microorganisms are involved in the degradation of plastics, it remains a challenge to obtain a strain suitable for commercial exploitation. Moreover, efficient screening techniques are a prerequisite for the isolation of highly efficient MP-degrading bacterial strains or consortia. To date, few studies have focused on the degradation of MPs by microbial consortia.
Given the importance of biofilms in changing the physicochemical properties and environmental fate of MPs, further studies are needed to investigate the biofilm-mediated sorption of hazardous chemical contaminants, pathogens, and ARGs. Studies on mechanisms of interaction, combined biological toxicities, and ecological risks between MPs and their associated biofilms are also needed. In addition, biofilm maturity (dynamic formation processes) may have a great influence on these aspects. Moreover, the screening, isolation, and characterization of high-efficiency plastic-degrading microorganisms from biofilms, together with their enzymatic and molecular mechanisms for plastic biodegradation, are needed toward a better understanding of microplastic pollution and bioremediation in the terrestrial environment.
References
Keswani A, Oliver DM, Gutierrez T et al (2016) Microbial hitchhikers on marine plastic debris: human exposure risks at bathing waters and beach environments. Mar Environ Res 118:10–19
De Tender CA, Devriese LI, Haegeman A et al (2017) The temporal dynamics of bacterial and fungal colonization on plastic debris in the North Sea. Environ Sci Technol 51(13):7350–7360
Wimpenny JWT (1996) Laboratory growth systems in biofilm research. Cells Mater 6(1):221–232
Lennox JE, Ross JR (2011) Biofilms: the hypertext book. https://www.hypertextbookshop.com/biofilmbook/v004/r003. Accessed 22 Feb 2020
Luo YM (2019) Pollution and management of microplastics in marine and coastal environments. Science Press, Beijing
Rummel CD, Jahnke A, Gorokhova E et al (2017) Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ Sci Technol Lett 4(7):258–267
Sauer K (2003) The genomics and proteomics of biofilm formation. Genome Biol 4(6):219
Renner LD, Weibel DB (2011) Physicochemical regulation of biofilm formation. MRS Bull 36(05):347–355
Rosenberg M, Bayer EA, Delarea J et al (1982) Role of thin fimbriae in adherence and growth of Acinetobacter calcoaceticus RAG-1 on hexadecane. Appl Environ Microbiol 44(4):929–937
Haiko J, Westerlund-Wikström B (2013) The role of the bacterial flagellum in adhesion and virulence. Biology 2(4):1242–1267
Lorite GS, Rodrigues CM, de Souza AA et al (2011) The role of conditioning film formation and surface chemical changes on Xylella fastidiosa adhesion and biofilm evolution. J Colloid Interf Sci 359(1):289–295
Hook AL, Chang CY, Yang J et al (2012) Combinatorial discovery of polymers resistant to bacterial attachment. Nat Biotechnol 30(9):868–875
Sanni O, Chang CY, Anderson DG et al (2015) Bacterial attachment to polymeric materials correlates with molecular flexibility and hydrophilicity. Adv Healthc Mater 4(5):695–701
Smith KCA, Oatley CW (1955) The scanning electron microscope and its fields of application. Br J Appl Phys 6(11):391–399
Newbury DE, Ritchie NWM (2013) Is scanning electron microscopy/energy dispersive X-ray spectrometry (SEM/EDS) quantitative? Scanning 35(3):141–168
Ozturk S, Aslim B, Suludere Z (2009) Evaluation of chromium (VI) removal behaviour by two isolates of Synechocystis sp. in terms of exopolysaccharide (EPS) production and monomer composition. Bioresour Technol 100(23):5588–5593
Tu C, Liu Y, Wei J et al (2018) Characterization and mechanism of copper biosorption by a highly copper-resistant fungal strain isolated from copper-polluted acidic orchard soil. Environ Sci Pollut Res 25:24965–24974
Sun ZP, Li J, Liu HH et al (2013) The impact of different processing techniques on the original form of environmental scanning electron microscope bacteria. J Yangzhou Univ (Agric Life Sci Edn) 34(1):41–43
Ji M, Jin LY, Zhao JP (2019) A sample preparation technique for scanning electron microscope of free cell. J Chin Electron Microsc Soc 38(1):72–74
Zhou Q, Zhang HB, Fu CC et al (2018) The distribution and morphology of microplastics in coastal soils adjacent to the Bohai Sea and the Yellow Sea. Geoderma 322:201–208
Reisser J, Shaw J, Hallegraeff G et al (2014) Millimeter-sized marine plastics: a new pelagic habitat for microorganisms and invertebrates. PLoS One 9(6):e100289
Cooper DA, Corcoran PL (2010) Effects of mechanical and chemical processes on the degradation of plastic beach debris on the island of Kauai, Hawaii. Mar Pollut Bull 60(5):650–654
Wang ZM, Wagner J, Ghosal S et al (2017) SEM/EDS and optical microscopy analyses of microplastics in ocean trawl and fish guts. Sci Total Environ 603-604:616–626
Negri M, Gonçalves V, Silva S et al (2010) Crystal violet staining to quantify Candida adhesion to epithelial cells. Br J Biomed Sci 67(3):120–125
Chance HL (1952) Crystal violet as a nuclear stain for Gaffkya Tetragena and other bacteria. Biotech Histochem 27(5):253–258
Hinds IL (1983) Oxalic acid-crystal violet staining method for demonstration of amyloid. Lab Med 14(5):295–297
Bonnekoh B, Wevers A, Jugert F et al (1989) Colorimetric growth assay for epidermal cell cultures by their crystal violet binding capacity. Arch Dermatol Res 281(7):487–490
Stepanović S, Vuković D, Dakić I et al (2000) A modified microtiter-plate test for quantification of staphylococcal biofilm formation. Microbiol Methods 40(2):175–179
Chen T (2018) Formation of biofilm on microplastics and its influences on physicochemical properties of microplastics in the coastal sea. University of Chinese Academy of Sciences, Beijing
Hu JS, Chen HT, Zhang J et al (2010) Advances in the common identification methods of bacterial biofilm. Chin Vet Sci 40(11):1194–1199
Wang WB, Qi PS (2008) Application of electron microscopy in the biofilm’s characteristic analysis. Ind Saf Environ Prot 34(7):15–16
Kamjunke N, Spohn U, Füting M et al (2012) Use of confocal laser scanning microscopy for biofilm investigation on paints under field conditions. Int Biodeterior Biodegradation 69:17–22
Bridier A, Briandet R (2014) Contribution of confocal laser scanning microscopy in deciphering biofilm tridimensional structure and reactivity. Methods Mol Biol 1147:255–266
Apichitsopa N, Jaffe A, Voldman J (2018) Multiparameter cell-tracking intrinsic cytometry for single-cell characterization. Lab Chip 18(10):1430–1439
Summers S, Henry T, Gutierrez T (2018) Agglomeration of nano- and microplastic particles in seawater by autochthonous and de novo-produced sources of exopolymeric substances. Mar Pollut Bull 130:258–267
Dussud C, Hudec C, George M et al (2018) Colonization of non-biodegradable and biodegradable plastics by marine microorganisms. Front in Microbiol 9:1571
Sgier L, Freimann R, Zupanic A et al (2016) Flow cytometry combined with viSNE for the analysis of microbial biofilms and detection of microplastics. Nat Commun 7:11587
Zhang M, Zhao Y, Qin X et al (2019) Microplastics from mulching film is a distinct habitat for bacteria in farmland soil. Sci Total Environ 688:470–478
Huang Y, Zhao Y, Wang J et al (2019) LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ Pollut 254(Pt A):112983
Jin Y, Lu L, Tu W et al (2019) Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci Total Environ 649:308–317
Lobelle D, Cunliffe M (2011) Early microbial biofilm formation on marine plastic debris. Mar Pollut Bull 62(1):197–200
Lehtola MJ, Miettinen IT, Keinänen MM et al (2004) Microbiology, chemistry, and biofilm development in a pilot drinking water distribution system with copper and plastic pipes. Water Res 38(17):3769–3779
Carpenter EJ, Anderson SJ, Harvey GR et al (1972) Polystyrene spherules in coastal waters. Science 178(4062):749–750
Webb HK, Crawford RJ, Sawabe T et al (2009) Poly(ethylene terephthalate) polymer surfaces as a substrate for bacterial attachment and biofilm formation. Microbes Environ 24:39–42
Jones PR, Cottrell MT, Kirchman DL (2006) Bacterial community structure of biofilms on artificial surfaces in an estuary. Microb Ecol 53(1):153–162
Zettler ER, Mincer TJ, Amaral-Zettler LA (2013) Life in the “plastisphere”: microbial communities on plastic marine debris. Environ Sci Technol 47(13):7137–7146
Carpenter EJ, Smith Jr KL (1972) Plastics on the Sargasso Sea surface. Science 175(4027):1240–1241
Jackson CR, Churchill PF, Roden EE (2001) Successional changes in bacterial assemblage structure during epilithic biofilm development. Ecology 82(2):555–566
Lee JW, Nam JH, Kim YH et al (2008) Bacterial communities in the initial stage of marine biofilm formation on artificial surfaces. J Microbiol 46(2):174–182
Andrady AL (2015) Degradation of plastics in the environment. In: Andrady AL (ed) Plastics and environmental sustainability. Wiley, Hoboken, pp 145–184
Jiang PL (2018) Microplastic-associated bacterial assemblages in some coastal areas of Southeast China. East China Normal University, Shanghai
Harshvardhan K, Jha B (2013) Biodegradation of low-density polyethylene by marine bacteria from pelagic waters, Arabian Sea, India. Mar Pollut Bull 77(1–2):100–106
Flemming HC (1998) Relevance of biofilms for the biodeterioration of surface of polymeric materials. Polym Degrad Stab 59(1–3):309–315
Gewert B, Plassmann MM, MacLeod M (2015) Pathways for degradation of plastic polymers floating in the marine environment. Environ Sci: Processes Impacts 17(9):1513–1521
Andrady AL (2011) Microplastics in the marine environment. Mar Pollut Bull 62(8):1596–1605
Reisser J, Slat B, Noble K et al (2015) The vertical distribution of buoyant plastics at sea: an observational study in the North Atlantic Gyre. Biogeosciences 12(4):1249–1256
Morét-Ferguson S, Law KL, Proskurowski G et al (2010) The size, mass, and composition of plastic debris in the western North Atlantic Ocean. Mar Pollut Bull 60(10):1873–1878
Cole M, Lindeque PK, Fileman E et al (2016) Microplastics alter the properties and sinking rates of zooplankton faecal pellets. Environ Sci Technol 50(6):3239–3246
Ye S, Andrady AL (1991) Fouling of floating plastic debris under Biscayne Bay exposure conditions. Mar Pollut Bull 22(12):608–613
Kukulka T, Proskurowski G, Morét-Ferguson S et al (2012) The effect of wind mixing on the vertical distribution of buoyant plastic debris. Geophys Res Lett 39:L07601
Isobe A, Kubo K, Tamura Y et al (2014) Selective transport of microplastics and mesoplastics by drifting in coastal waters. Mar Pollut Bull 89(1–2):324–330
Ballent A, Pando S, Purser A et al (2013) Modelled transport of benthic marine microplastic pollution in the Nazaré Canyon. Biogeosciences 10(12):7957–7970
Jang M, Shim WJ, Han GM et al (2016) Styrofoam debris as a source of hazardous additives for marine organisms. Environ Sci Technol 50(10):4951–4960
Bakir A, Rowland SJ, Thompson RC (2012) Competitive sorption of persistent organic pollutants onto microplastics in the marine environment. Mar Pollut Bull 64(12):2782–2789
Turner A (2016) Heavy metals, metalloids and other hazardous elements in marine plastic litter. Mar Pollut Bull 111(1–2):136–142
Hans-Cur F (1995) Sorption sites in biofilms. Water Sci Technol 32(8):27–33
Wang J, Tan Z, Peng J et al (2016) The behaviors of microplastics in the marine environment. Mar Environ Res 113:7–17
Lissalde S, Charriau A, Poulier G et al (2016) Overview of the Chemcatcher® for the passive sampling of various pollutants in aquatic environments part B: field handling and environmental applications for the monitoring of pollutants and their biological effects. Talanta 148:572–582
Wicke D, Böckelmann U, Reemtsma T (2008) Environmental influences on the partitioning and diffusion of hydrophobic organic contaminants in microbial biofilms. Environ Sci Technol 42(6):1990–1996
Debajyoti G, Shreya G, Dutta TK et al (2016) Current state of knowledge in microbial degradation of polycyclic aromatic hydrocarbons (PAHs): a review. Front Microbiol 7:1369
Wen G, Kötzsch S, Vital M et al (2015) BioMig – a method to evaluate the potential release of compounds from, and the formation of biofilms on polymeric materials in contact with drinking water. Environ Sci Technol 49(19):11659–11669
Delacuvellerie A, Cyriaque V, Gobert S et al (2019) The plastisphere in marine ecosystem hosts potential specific microbial degraders including Alcanivorax borkumensis as a key player for the low-density polyethylene degradation. J Hazard Mater 380:120899
Wu X, Pan J, Li M et al (2019) Selective enrichment of bacterial pathogens by microplastic biofilm. Water Res 165:114979
McCormick A, Hollein TJ, Mason SA et al (2014) Microplastic is an abundant and distinct microbial habitat in an urban river. Environ Sci Technol 48(20):11863–11871
De Tender CA, Devriese LI, Haegeman A et al (2015) Bacterial community profiling of plastic litter in the Belgian part of the North Sea. Environ Sci Technol 49(16):9629–9638
Amaral-Zettler LA, Zettler ER, Slikas B et al (2015) The biogeography of the plastisphere: implications for policy. Front Ecol Environ 13(10):541–546
Jiang P, Zhao S, Zhu L et al (2018) Microplastic-associated bacterial assemblages in the intertidal zone of the Yangtze Estuary. Sci Total Environ 624:48–54
Oberbeckmann S, Loeder MGJ, Gerdts G et al (2014) Spatial and seasonal variation in diversity and structure of microbial biofilms on marine plastics in Northern European waters. FEMS Microbiol Ecol 90(2):478–492
Lagarde F, Olivier O, Zanella M et al (2016) Microplastic interactions with freshwater microalgae: hetero-aggregation and changes in plastic density appear strongly dependent on polymer type. Environ Pollut 215:331–339
Oberbeckmann S, Kreikemeyer B, Labrenz M (2018) Environmental factors support the formation of specific bacterial assemblages on microplastics. Front Microbiol 8:2709
Miao L, Wang P, Hou J et al (2019) Distinct community structure and microbial functions of biofilms colonizing microplastics. Sci Total Environ 650:2395–2402
Betts K (2008) Why small plastic particles may pose a big problem in the oceans. Environ Sci Technol 42(24):8995
Thompson RC, Moore CJ, vom Saal FS (2009) Plastics, the environment and human health: current consensus and future trends. Philos Trans R Soc B Biol Sci 364(1526):2153–2166
Lusher AL, Mchugh M, Thompson RC (2013) Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar Pollut Bull 67(1–2):94–99
Murray F, Cowie PR (2011) Plastic contamination in the decapod crustacean Nephrops norvegicus (Linnaeus, 1758). Mar Pollut Bull 62(6):1207–1217
Auta HS, Emenike CU, Fauziah SH (2017) Distribution and importance of microplastics in the marine environment: a review of the sources, fate, effects, and potential solutions. Environ Int 102:165–176
Carbery M, O’Connor W, Palanisami T (2018) Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ Int 115:400–409
Huerta Lwanga E, Gertse H, Gooren H et al (2017) Incorporation of microplastics from litter into burrows of Lumbricus terrestris. Environ Pollut 220:523–531
Maaß S, Daphi D, Lehmann A et al (2017) Transport of microplastics by two collembolan species. Environ Pollut 225:456–459
Rillig MC, Ziersch L, Hempel S (2017) Microplastic transport in soil by earthworms. Sci Rep 7(1):1362
Zhu D, Bi QF, Xiang Q et al (2018) Trophic predator-prey relationships promote transport of microplastics compared with the single Hypoaspis aculeifer and Folsomia candida. Environ Pollut 235:150–154
Rodriguez-Seijo A, Lourenço J, Rocha-Santos TAP et al (2017) Histopathological and molecular effects of microplastics in Eisenia andrei Bouché. Environ Pollut 220:495–503
Carson HS (2013) The incidence of plastic ingestion by fishes: from the prey’s perspective. Mar Pollut Bull 74(1):170–174
Gorokhova E (2015) Screening for microplastic particles in plankton samples: how to integrate marine litter assessment into existing monitoring programs? Mar Pollut Bull 99(1–2):271–275
Ashton K, Holmes L, Turner A (2010) Association of metals with plastic production pellets in the marine environment. Mar Pollut Bull 60(11):2050–2055
Cole M, Lindeque P, Halsband C et al (2011) Microplastics as contaminants in the marine environment: a review. Mar Pollut Bull 62(12):2588–2597
Kirstein IV, Kirmizi S, Wichels A et al (2016) Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio spp. on microplastic particles. Mar Environ Res 120:1–8
Alimi OS, Farner Budarz J, Hernandez LM et al (2018) Microplastics and nanoplastics in aquatic environments: aggregation, deposition, and enhanced contaminant transport. Environ Sci Technol 52(4):1704–1724
Bakir A, Rowland SJ, Thompson RC (2014) Transport of persistent organic pollutants by microplastics in estuarine conditions. Estuar Coast Shelf Sci 140:14–21
Turner A, Holmes LA (2015) Adsorption of trace metals by microplastic pellets in fresh water. Environ Chem 12(5):600–610
Oliveira M, Ribeiro A, Guilhermino L (2012) Effects of exposure to microplastics and PAHs on microalgae Rhodomonas baltica and Tetraselmis chuii. Comp Biochem Physiol A Mol Integr Physiol 163:S19–S20
Schlute J, Nadell CD, Bassler BL et al (2015) Adhesion as a weapon in microbial competition. ISME J 9(1):139–149
Lee H, Shim WJ, Kwon JH (2014) Sorption capacity of plastic debris for hydrophobic organic chemicals. Sci Total Environ 470–471:1545–1552
Gong M, Yang G, Zhuang L et al (2019) Microbial biofilm formation and community structure on low-density polyethylene microparticles in lake water microcosms. Environ Pollut 252:94–102
Artham T, Doble M (2008) Biodegradation of aliphatic and aromatic polycarbonates. Macromol Biosci 8(1):14–24
Sen SK, Raut S (2015) Microbial degradation of low density polyethylene (LDPE): a review. J Environ Chem Eng 3(1):462–473
Gu JD (2003) Microbiological deterioration and degradation of synthetic polymeric materials: recent research advances. Int Biodeterior Biodegradation 52(2):69–91
Hakkarainen M, Albertsson AC (2004) Environmental degradation of polyethylene. In: Albertsson AC (ed) Long term properties of polyolefins. Advances in polymer science, vol 169. Springer, Heidelberg, pp 177–200
Arutchelvi J, Sudhakar M, Arkatkar A et al (2008) Biodegradation of polyethylene and polypropylene. Indian J Biotechnol 7(1):9–22
Bryant JA, Clemente TM, Viviani DA et al (2016) Diversity and activity of communities inhabiting plastic debris in the North Pacific Gyre. mSystems 1(3):e00024–e00016
Mercier A, Gravouil K, Aucher W et al (2017) Fate of eight different polymers under uncontrolled composting conditions: relationships between deterioration, biofilm formation, and the material surface properties. Environ Sci Technol 51(4):1988–1997
Wilkes RA, Aristilde L (2017) Degradation and metabolism of synthetic plastics and associated products by Pseudomonas sp.: capabilities and challenges. J Appl Microbiol 123(3):582–593
Yoshida S, Hiraga K, Takehana T et al (2016) A bacterium that degrades and assimilates poly (ethylene terephthalate). Science 351(6278):1196–1199
Yang J, Yang Y, Wu WM et al (2014) Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environ Sci Technol 48(23):13776–13784
Yang Y, Yang J, Wu WM et al (2015) Biodegradation and mineralization of polystyrene by plastic-eating mealworms. 1. Chemical and physical characterization and isotopic tests. Environ Sci Technol 49(20):12080–12086
Yang Y, Yang J, Wu W et al (2015) Biodegradation and mineralization of polystyrene by plastic-eating mealworms. 2. Role of gut microorganisms. Environ Sci Technol 49(20):12087–12093
Auta HS, Emenike CU, Fauziah SH (2017) Screening of Bacillus strains isolated from mangrove ecosystems in Peninsular Malaysia for microplastic degradation. Environ Pollut 231:1552–1559
Syranidou E, Karkanorachaki K, Amorotti F et al (2019) Biodegradation of mixture of plastic films by tailored marine consortia. J Hazard Mater 375:33–42
Acknowledgments
The authors gratefully acknowledge financial support from the National Key Research and Development Program of China (Grant No. 2016YFC1402202), the Key Research Program of Frontier Sciences, Chinese Academy of Sciences (Grant No. QYZDJ-SSW-DQC015), and the External Cooperation Program of BIC, Chinese Academy of Sciences (Grant No. 133337KYSB20160003). We appreciate the linguistic revision by Dr. Peter Christie, Institute of Soil Science, Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tu, C., Zhou, Q., Zhang, C., Liu, Y., Luo, Y. (2020). Biofilms of Microplastics. In: He, D., Luo, Y. (eds) Microplastics in Terrestrial Environments. The Handbook of Environmental Chemistry, vol 95. Springer, Cham. https://doi.org/10.1007/698_2020_461
Download citation
DOI: https://doi.org/10.1007/698_2020_461
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-56270-0
Online ISBN: 978-3-030-56271-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)