Abstract
In this chapter, an overview of the degradation processes of brominated flame retardants is presented, including both chemical and biological processes. BFRs could be affected by different types of reactions, being the photochemical reactions the most widely studied and reported. Also, among the different BFR families, PBDEs, and in particular the deca-BDE, are those that have more studies and therefore more data on degradation pathways and degradation products. Degradation processes may change the structure of the contaminants, as well as its characteristics and properties. In this sense, it is very important to know the potential degradation products, as well as their environmental behavior and toxicology. Research regarding the transformation processes of BFRs and its products is needed for both environmental remediation and health assessments. Conclusions and future perspectives are outlined.
Graphical Abstract

Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Biodegradation
- Brominated flame retardants
- Hexabromocyclododecane
- Photodegradation
- Polybrominated diphenylethers
- Tetrabromobisphenol A
- Thermal degradation
1 Introduction
Persistent organic pollutants (POPs), such as brominated flame retardants (BFRs), are organic compounds that are resistant to environmental degradation through chemical, biological, and photolytic processes. Because of this, they have been observed to persist in the environment, to be capable of long-range transport, to bioaccumulate in human and animal tissue, to biomagnify in food chains, and to have potential significant impacts on human health and the environment. However, there are a number of reactions that can take place once the BFRs are released to the different environmental compartments (air, water, soil, sediment).
While physicochemical properties, long-range transport, bioaccumulation, and toxicity of potential environmental contaminants are described, at least to some extent, the persistence of chemicals is in general less well understood. In order to conduct proper risk assessments for BFRs, more knowledge is needed on their persistence, especially the half-lives of such compounds. Degradation half-life time (t 1/2) is an important parameter to characterize the extent of degradation. Wania and Dugani [1] reported the t 1/2 values for several polybrominated diphenyl ethers (PBDE) congeners at ~150 days except for BDE-28 (~60 days). The United Nations Environment Program on POPs has established criteria for identifying new POPs, and according to these criteria, a substance is classified as persistent if it has a half-life in soil or sediment of >180 days [2]. Under the European Union REACH chemical legislation, the half-life of a substance must be >120 days in freshwater sediment or soil to fulfill the persistent criteria, and to fulfill the very persistent criteria, the half-life must be >180 days in soil or sediment [3].
Depending on the chemical structure, it is possible to identify major potential reaction pathways. Many factors influence the degradation of contaminants, including the presence of light, level of oxygen, temperature, pH, humidity, and microorganism species composition. BFRs could be affected by abiotic oxidation, reductive debromination, hydrolysis, elimination, and substitution reactions. These processes may change the structure of the contaminant, as well as its characteristics and properties. In this sense, it is very important to take care on degradation products. For instance, European Union risk assessment [4] on BDE-209 concluded recently that there is a need for further information on the degradation of BDE-209 into more toxic and bioaccumulative compounds (e.g., reductive debromination to lower brominated congeners).
This chapter discusses some of the environmental degradation resulting from widespread urban chemical release to soil, surface water, sediments, groundwater, and air. Moreover, some degradation studies carried out for developing effective remediation processes for BFRs were also included.
2 Chemical Degradation
The perbromination of BFRs such as PBDEs makes it vulnerable to a range of chemical reactions, such as substitution, reduction, and photolysis. The latter two reaction pathways are leading to, among other products being formed, lower PBDE congeners. From a chemical point of view, deca-BDE-209 is a labile molecule. BDE-209 reacts readily with nucleophiles [5], it is reduced by hydride reagents such as sodium borohydride [6], and it is rapidly photolyzed under UV-B and UV-C irradiation [7]. Data available suggest that photochemical degradation is the main transformation process for PBDEs in the environment. Thus, it is important to understand the photochemical behavior of PBDEs, including both photodegradation kinetics and photoproducts.
2.1 Photochemical Degradation
A large number of halogen-containing organic compounds have been reported to undergo photochemical transformations, both under experimental and natural conditions as reviewed by Méallier [8], Pagni and Sigman [9], and Richard and Grabner [10]. In the environment, photochemical transformations proceed by direct photolysis under the action of solar light and occur in the atmosphere and in surface waters. However, the low volatility and low solubility of many organohalogen compounds make it difficult to study their photolysis in these media in the laboratory. To overcome these constraints, a variety of procedures for measuring the photolysis reactions of organohalogen pollutants have been reported, although differences in the use of solvents, apparatus, and wavelengths can lead to different results being obtained for the same compound.
2.1.1 Polybrominated Diphenyl Ethers
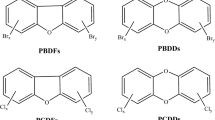
PBDEs belong to the group of organobromine compounds that absorb light in the UV-A spectra. The energy supplied by UV light often results in loss of bromine and thereby also a possibility for rearrangements. Photolytic degradation of organobromines is a well-known type of reaction in basic chemistry. The rate of degradation of PBDEs by UV light in the sunlight region is dependent on the degree of bromination. Hence, lower PBDEs degrade slower than highly brominated congeners. Degradation rates of 15 PBDE congeners, dissolved in 80% methanol exposed to UV light, were determined by Eriksson et al. [11] showing decreasing rates with decreased bromination degree of the diphenyl ethers. The observed rate difference is up to 700 times between the slowest reacting PBDE studied (tetra-BDE-77) and the fastest (deca-BDE-209). Much of these differences can be explained by their absorbance behavior since the higher PBDEs absorb at longer wavelengths. Hexa-BDEs to octa-BDEs and di-BDFs to penta-BDFs were shown. One product was also identified as a methoxylated tetra-BDF.
Many studies have shown that BDE-209 is labile to light, and photolysis is an important degradation pathway for the compound in the environment. The main photoproducts of BDE-209 are lower brominated PBDEs and polybrominated dibenzofurans (PBDFs), which are more persistent, bioavailable, and toxic. The first study, performed in the late 1980s by Watanabe and Tatsukawa [12], indicated the formation of debrominated diphenyl ethers. Octa-BDEs down to tri-BDEs were reported as major products. Furthermore, mono-BDFs to hexa-BDFs were reported as products in their study as well as tetra- and penta-bromobenzenes. Ohta et al. [13] dissolved deca-BDE in toluene to detect a large number of lower PBDEs, mainly mono-BDEs to nona-BDEs. Deca-BDE dissolved in toluene or adsorbed to silica gel, sand, sediment, or soil and subjected to UV light reported similar transformations without matrix-related effects on the product profile (PBDEs and PBDFs) but with effects on BDE-209 degradation rate [7]. Great differences of BDE-209 photolytic rates were found among these different media. The half-life of BDE-209 adsorbed on soil was 600 times longer than that on silica gel. Similarly, it has been shown that sand particles coated with humic acid may decrease degradation rates of deca-BDE when irradiated with UV light [14]. In contrast, Ahn et al. [15] showed increased photolytic transformation rates when deca-BDE was adsorbed to clay minerals (montmorillonite and kaolinte), probably due to the electron-donating ability of the clay minerals.
According to the previous studies, the possible mechanism of BDE-209 photolysis can be proposed as shown in Fig. 1 [16]. The excited BDE-209 molecule ([Ar–Br]*) can undergo debromination processes via C–Br homolysis (step 1) or by the attack of electron donor (step 2). Then the generated aryl radical can get a hydrogen atom from the hydrogen donor (DH) (step 3), or undergo other pathways such as polymerization to form products. Thus, besides the hydrogen-donating and electron-donating efficiency of the reaction media, properties of BDE-209 in different media may affect the photolytic rate, such as the energy for BDE-209 to be exited and the difficulty for C–Br bonds to cleave.
Photolytic mechanism of BDE-209. Reproduced from [16]
Photolysis of deca-BDE yields a wide span of products, from nona-BDEs to hydroxylated bromobenzenes. The photolytic half-life for BDE-209 is longer on more complex matrices, i.e., sediment and soil. The slow rate of photodebromination on soil may cause a continuous production of lower brominated PBDEs, such as BDE-154 and BDE-183, which are more stable to photolytic degradation and more bioaccumulative due to smaller molecular size and lower K ow. Recommendations and perspectives BDE-209, the main constituent of deca-BDE mixture, is primarily forming debrominated diphenyl ethers with higher persistence, which are more bioaccumulative than the starting material when subjected to UV light. Hence, deca-BDE should be considered as a source of these PBDE congeners in the environment.
2.1.2 Hexabromocyclododecane
The information on photodegradation processes of hexabromocyclododecane (HBCD) is very scarce. Recently, the photochemical properties of α-, β-, and γ-HBCD have been investigated [17]. As a result of this study, the UV absorption spectra of the three HBCD diastereosiomers were provided, as well as a detailed assignment of the UV spectral features. The photodegradation and photostereoisomerization trends of HBCDs under the UV illumination with wavelengths shorter than 240 nm were predicted. The study also demonstrated the photostereoisomerization trends. However, more attention should be paid to the photochemical properties for HBCDs.
2.1.3 Tetrabromobisphenol A
A large number of halogen-containing organic compounds have been reported to undergo photochemical transformations, under both experimental and natural conditions. For phenolic compounds, such as tetrabromobisphenol A (TBBPA), the rate of photodegradation is highly dependent on the pH [10] as the absorption of the associated and dissociated forms can be very different. Photolytic decomposition of TBBPA in the environment occurs in the atmosphere and in surface waters. Eriksson et al. [11] have reported that, at pH values below its pKa of ~7.4, the quantum yield of TBBPA photodecomposition decreases with decreasing pH, while at pH values above its pKa, photodecomposition is independent of pH. There are several reported studies on TBBPA decomposition pathways [11, 18, 19], which suggest that the most important routes involve debromination and scission reactions that yield phenols. Radical reactions are thought to be responsible for the formation of the intermediates in the TBBPA decomposition pathways.
Eriksson et al. [11] developed a method for studies of the phototransformation at UV irradiation of aqueous solutions of TBBPA and related compounds at various pHs. They found that the rate of decomposition of TBBPA was six times higher at pH 8 than at pH 6. Identification of the degradation products of TBBPA and Tri-BBPA, by gas chromatography (GC)–mass spectrometry (MS) analysis and by comparison to synthesized reference compounds, indicated that TBBPA and Tri-BBPA decompose via different mechanisms. Three isopropylphenol derivatives, 4-isopropyl-2,6-dibromophenol, 4-isopropylene-2,6-dibromophenol, and 4-(2-hydroxyisopropyl)-2,6-dibromophenol, were identified as major degradation products of TBBPA, while the major degradation product of Tri-BBPA was tentatively identified as 2-(2,4-cyclopentadienyl)-2-(3,5-dibromo-4-hydroxyphenyl)propane (Fig. 2).
Proposed degradation path for TBBPA. Reproduced from [11]
In the environment, photochemical transformations proceed by direct photolysis under the action of solar light. In sensitized redox processes under aerobic conditions, the active reaction intermediates participating in the transformation of the pollutant may be the electronically excited sensitizer molecule or the solvated electron as well as reactive oxygen species such as singlet oxygen (1O2) or the superoxide anion radical (O2•−) [20, 21]. Han et al. [22] explored the possibility of photosensitized degradation of TBBPA mediated by singlet oxygen or free radicals. While direct photodegradation may be environmentally important, particularly in alkaline waters, the authors suggest that reaction with 1O2 may be an alternative pathway. Because TBBPA is a stable compound that at neutral pH does not absorb much of the atmosphere-filtered solar radiation, its photosensitized oxidation by 1O2 may be the key reaction initiating or mediating TBBPA degradation in the natural environment. Han et al. [22] concluded that because the quantum yield of generation of self-sensitized singlet oxygen by TBBPA is low, it is unlikely to play a role in the degradation of the retardant. However, previous studies have shown that there are environmental sources of singlet oxygen such as the humic acids [23] which could contribute to the destruction of TBBPA.
2.2 Thermal Degradation
Some studies have focused on the thermolysis of certain BFRs. Results showed the formation of polybrominated dibenzo-p-dioxins (PBDDs) and PBDFs. In particular, experiments using brominated aromatics that could be considered as PBDD/PBDF precursors, such as PBDEs or polybrominated biphenyls (PBBs), resulted in high yields of PBDDs/PBDFs. Weber and Kuch [24] discussed four categories of thermal processes according to their potential for PBDD/PBDF generation: thermal stress, pyrolysis/gasification, insufficient combustion conditions, and controlled combustion conditions.
Under thermal stress situations, as they may occur in production or recycling processes, PBDDs/PBDFs precursors like PBDEs can have a relevant potential for PBDD/PBDF formation via a simple elimination. From a mechanistic point of view, the formation of PBDFs from PBDEs requires only an intramolecular elimination of Br2 or HBr. It is generally observed that the yield of PBDD/PBDFs in pyrolytic residues decreases from penta-BDEs, octa-BDEs, to deca-BDEs. Luijk et al. [25] suggested that the higher yield of PBDFs from low brominated PBDEs is due to the energetically favorable elimination of HBr in lower brominated DPE (requirement of an hydrogen position in ortho-position of the PBDEs), compared to the energetically less favorable elimination of two bromine substituents in highly brominated DE. An alternative explanation for the low PBDD/PBDF formation potential of higher brominated PBDEs may be the steric hindrance in the formation of CC bond, when bromine substitution results in 1,9-substituted PBDF: the bromine substituents in 1,9 position in PBDFs cause a “steric crowding.” Therefore, the formation of PBDFs with a hydrogen substituent in 9-position is favored (requirement of a hydrogen position in meta-position of PBDEs) (Fig. 3).
Formation pathways of PBDDs and PBDFs from deca-BDE during thermal degradation. Reproduced from [24]
For most BFRs, formation of PBDDs/PBDFs by a simple elimination or condensation step is not possible. TBBPA is one example in this respect. Although formation of PBDDs and PBDFs has been observed during thermolysis of TBBPA [26], the yields were orders of magnitudes lower compared to PBDEs or bromophenols. Dettmer [27] investigated the thermal degradation of TBBPA during thermal degradation and observed the formation of large amounts of brominated phenols (up to 17%) and to a lesser amount brominated benzenes (up to 0.5%). The amount of brominated phenols and benzenes detected in the condensates showed a good quantitative correlation to the amount of PBDDs and PBDFs formed. The formation of PBDDs/PBDFs from TBBPA proceeds therefore, most probably, in two steps: (a) the generation of precursors (polybrominated phenols and polybrominated benzenes) during thermal degradation/incineration of the polymer/TBBPA and (b) dimerization/condensation of the precursors.
Under insufficient combustion conditions as they are present in, e.g., accidental fires and uncontrolled burning as well as gasification/pyrolysis processes, considerable amounts of PBDDs/PBDFs can be formed from BFRs, preferably via the precursor pathway. In contrast, under controlled combustion conditions, BFRs and PBDDs/PBDFs can be destroyed with high efficiency.
3 Biological Degradation
BFRs have been shown to be susceptible to several metabolic processes including oxidative debromination, reductive debromination, oxidative CYP enzyme-mediated biotransformation, and Phase II conjugation (glucuronidation and sulfation) [28]. Thus, biodegradation can be one of the most important processes that reduce concentrations of organic chemicals in the environment. Aerobic biodegradation processes can predominate in surface waters, surface soils, and the aeration basins of wastewater treatment plants (WWTPs). Anaerobic processes, in contrast, can occur in aquatic sediments, groundwater, and anaerobic digestion units of WWTPs. Anaerobic degradation in sediments, soils, and sewage sludge has been frequently reported for organohalogen compounds other than BFRs. Reductive dehalogenation (e.g., substitution of Br or Cl by a hydrogen atom) has been shown to be an important mechanism [29, 30]. Thereby, halogenated compounds serve as electron acceptors in respiratory or cometabolic processes. Examples include reduction of polychlorinated biphenyls (PCBs) and PBBs in anaerobic sediment enrichment cultures [31, 32].
Biotransformation, an alternative to degradation, alters the compound without making substantial changes to the carbon skeleton of the substrate. Microbial O-methylation is one such biotransformation reaction commonly observed for many halogenated phenolic compounds [33]. The microbial biotransformation products may differ greatly in their chemical characteristics (e.g., water solubility, partitioning onto solids) and more importantly their bioaccumulation potential and toxicity. Thus, it is important to know the different biotransformation mechanisms to elucidate potential risks to environmental and human health due to transformation products.
3.1 Polybrominated Diphenyl Ethers
Debromination of deca-BDE and octa-BDE mixture was observed with anaerobic bacteria including Sulfurospirillum multivorans and Dehalococcoides species [34] (Fig. 4). Hepta- and octa-BDEs were produced by the S. multivorans culture when it was exposed to deca-BDE, although no debromination was observed with the octa-BDE mixture. In contrast, a variety of hepta- through di-BDEs were produced by Dehalococcoides-containing cultures exposed to octa-BDE mixture, despite the fact that none of these cultures could debrominate deca-BDE. The more toxic hexa-BDE-154, penta-BDE-99, tetra-BDE-49, and tetra-BDE-47 were identified among the debromination products.
Identified PBDE substrates (black) and debromination products (red) detected for ANAS195 amended with the octa-BDE mixture. Arrows connect substrates with potential debromination products assuming no isomerization. Reproduced from [34]
Kim et al. [35] reported an aerobic degradation pathway for diphenyl ether used for the biotransformation of selected PBDEs by an isolated Sphingomonas strain. Sphingomonas sp. PH-07 was isolated from activated sludge samples of a WWTP using diphenyl ether as sole carbon and energy source. In liquid cultures, this strain mineralized 1 g of diphenyl ether per liter completely within 6 days. The metabolites detected and identified corresponded with a feasible degradative pathway. However, the strain PH-07 even catabolized several brominated congeners such as mono-, di-, and tri-PBDEs thereby producing the corresponding metabolites.
The debromination pathways of seven PBDEs (BDE-47, -99, -153, -183, -196, -197, and -203) by three different cultures of anaerobic dehalogenating bacteria were investigated by Robroch et al. [36] (Fig. 5). Dehalogenating cultures evaluated were a trichloroethene-enriched consortium containing multiple Dehalococcoides species and two pure cultures, Dehalobacter restrictus PER-K23 and Desulfitobacterium hafniense PCP-1. All studied congeners were debrominated to some extent by the three cultures and all exhibited similar debromination pathways with preferential removal of para and meta bromines. Debromination of the highly brominated congeners was extremely slow, with usually less than 10% of nanomolar concentrations of PBDEs transformed after 3 months. In contrast, debromination of the lesser brominated congeners, such as penta-BDE-99 and tetra-BDE- 47, was faster, with some cultures completely debrominating nanomolar levels of tetra-BDE-47 within weeks.
PBDE debromination pathway by different cultures. Highlighted molecules are those that were applied as initial substrate. The cultures that produced each congener are listed by the reaction arrows. Asterisk (*) indicates congener that is presumptively identified due to lack of available standards. Reproduced from [36]
White rot fungi are known to degrade a wide variety of recalcitrant pollutants. Zhou et al. [37] studied for the first time BDE-209 transformation by fungi. White rot fungi can rapidly oxidize and mineralize a broad spectrum of diverse aromatic compounds, such as PCBs, which have similar structures as PBDEs. However, the biodegradation of PBDEs is limited by their low bioavailability resulting from extremely low water solubility. Many researches have examined the possibility of enhancing the bioavailability of low solubility and highly sorptive compounds by adding a “solubilization” agent such as a surfactant or cyclodextrin to the system. Tween 80 is a kind of nonionic surfactant which was used widely in the degradation of hydrophobic or insoluble organic compounds. Cyclodextrins are cyclic, nonreducing maltooligosaccharides produced from the enzymatic degradation of starch and related compounds by certain bacteria that contain the cyclodextrin glycosyltransferases. Zhou et al. [37] showed that BDE-209 could be degraded by white rot fungi. Tween 80 at appropriate concentration was found capable of significantly enhancing the biodegradation of BDE-209 by white rot fungi. But Tween 80 at a high concentration will restrain the fungal growth and the degradation of BDE-209. Cyclodextrins could also improve the BDE-209 degradation by white rot fungi. It is a promising bioavailability-enhancing agent for the treatment of BDE-209 contaminations, not only for its positive effects on the BDE-209 degradation, but also for its partial biodegradability, nontoxicity, and relatively low cost.
3.2 Hexabromocyclododecane
Anaerobic degradation of technical HBCD mixture has been reported by Davis et al. [38]. Soil and sediment microcosms were used to evaluate the environmental lifetime of HBCD under realistic environmental concentrations. HBCD loss was observed in both viable and abiotic soils and sediments, although the rates were appreciable faster in the viable reaction mixtures. Biologically mediated transformation processes (i.e., biotransformation) accelerated the rate of loss of HBCD when compared to the biologically inhibited (i.e., heat-treated) soils and sediments. Biotransformation half-lives for HBCD were determined to be 63 and 6.9 days in the aerobic and anaerobic soils, respectively, while biotransformation half-lives for HBCD in the two river systems ranged from 11 to 32 days and 1.1 to 1.5 days under aerobic and anaerobic conditions, respectively. Brominated degradation products were not detected in any of the soils or sediment microcosms during the course of this study.
In a later work, Davis et al. [39] identified major intermediate metabolites formed during HBCD biodegradation. Substantial biological transformation of α-, β-, and γ-HBCD diastereomers was observed in wastewater (i.e., digester) sludge and in freshwater aquatic sediment microcosms prepared under aerobic and anaerobic conditions. Concomitant with the loss of HBCD in these matrixes, there was a concurrent production of three products. These metabolites were identified as tetrabromocyclododecene, dibromocyclododecadiene, and cyclododecatriene. These results demonstrate that microorganisms naturally occurring in aquatic sediments and anaerobic digester sludge mediate complete debromination of HBCD.
Gerecke et al. [40] studied the degradation of HBCD under anaerobic conditions in digested sewage sludge. The half-life of technical HBCD mixture was 0.66 day. Moreover, experiments with (±)-α-, (±)-β-, and (±)-γ-HBCD incubated in separate experiments showed that (±)-β- and (±)-γ-HBCD degraded more rapidly than (±)-α-HBCD by an estimated factor of 1.6 and 1.8, respectively. The fact that (±)-α-HBCD exhibited an almost doubled half-life compared to (±)-β-HBCD and (±)-γ-HBCD is an important finding with respect to the discussion on the persistence of individual HBCD diastereoisomers and the reports on strong relative enrichment of α-HBCD in biota. Finally, no statistically significant enantioselective degradation of α-, β-, or γ-HBCD was found.
3.3 Tetrabromobisphenol A
Various redox zones exist in estuarine sediments, depending upon the location and pollutant input. Nitrate-reducing, sulfate-reducing, iron(III)-reducing, and methanogenic conditions could be encountered within sediment layers. Dehalogenation, a process through which dehalogenating bacteria utilize halogenated compounds as electron acceptors, can be enhanced or inhibited by other electron acceptors present in the sediment [41]. For example, studies have found that under sulfate-reducing conditions (the primary electron-accepting process in the top layers of marine and estuarine sediments), the process of dehalogenation may be inhibited. Dehalogenation may be suppressed by the following: direct inhibition of dehalogenases by sulfate, sulfite, or hydrogen sulfide; preferential use of sulfate (over the halogenated compound) as an electron acceptor within the same organism; or successful competitive exclusion of dehalogenating bacteria through competition for electron donor by sulfate-reducing bacteria [42]. Biotransformation of TBBPA, and their ultimate biodehalogenation product, bisphenol A (BPA), was examined in anoxic estuarine sediments [43]. Dehalogenation of TBBPA was examined under conditions promoting either methanogenesis or sulfate reduction as the primary terminal electron-accepting process. Complete dehalogenation of TBBPA to BPA with no further degradation of BPA was observed under both methanogenic and sulfate-reducing conditions. Dehalogenation of TBBPA under both methanogenic and sulfate-reducing conditions resulted in the accumulation of a persistent dichlorinated bisphenol A isomer, while no BPA was formed. The dehalogenation of TBBPA and the potential for accumulation of BPA in anoxic sediments are significant, given the widespread use of these chemicals. The persistence of BPA in anoxic sediments under a variety of electron-accepting conditions is thus a concern. BPA has been detected in anoxic marine sediments [44], and it will likely persist for an extended period of time. Formation of BPA through the dehalogenation of TBBPA could potentially increase concentrations of this compound in anoxic environments. Combined, the concerns regarding the potential estrogenic effects of BPA and the largely unknown effects of TBBPA, and the potential for the accumulation of BPA under anoxic conditions justify vigilance and the continued study of the environmental fate of this flame retardant.
Although TBBPA dimethyl ether is not produced in industry, it has been detected in samples of terrestrial and aquatic sediments, as well as biological samples. It was hypothesized that the TBBPA dimethyl ether may be a product of microbial transformation. Allard et al. [45] first demonstrated that bacterial cultures were capable of O-methylating TBBPA although the reaction proceeded at a relatively slow rate. George and Häggblom [46] showed that two mycobacterium isolates known for their ability to O-methylate chlorophenols transform TBBPA to the corresponding mono- and dimethylated ethers. Additionally, this study demonstrated the microbially mediated O-methylation of TBBPA in sediment microcosms, suggesting that O-methylation of TBBPA may be an environmentally significant process. With the addition of two hydrophobic methyl groups, TBBPA dimethyl ether is more lipophilic than its parent compound. This characteristic increases its potential for bioconcentration in fatty tissue. Currently, little is known about the toxicology of TBBPA mono- and dimethylated ether. Given the suspected prevalence of bacterial O-methylation, additional environmental and toxicological data should be collected for these derivatives.
4 Conclusions
Large variations were found in the degradability among the different BFRs, and generally, degradation was faster in aerobic than in anaerobic conditions. The degradation rates of PBDEs were not significant, or low, in both aerobic and anaerobic conditions. TBBPA also degraded slowly in anaerobic soil. However, degradation rates in the environment will also be affected by factors such as temperature, presence of light, humidity, and the microorganism flora.
The degradation process more widely studied is the photochemical degradation. Different studies revealed the degradation rates, degradation pathways, and degradation products. It is important to pay attention to these new chemicals formed under different processes. Degradation products are formed and often have similar properties as the original substances (persistence, bioaccumulation potential, toxicity), and may accumulate in the system. If a degradation product is, at the same time, present in the environment in relevant quantities and has high bioaccumulation potential and toxicity, not taking this degradation product into account might lead to an underestimation of the hazard and risk of the parent compound. For example, PBDDs and PBDFs are degradation products of thermal PBDE degradation, and they have greater toxicity than PBDEs themselves. Another example is the BPA, resulting from the TBBPA degradation, and a well-known endocrine disruptor. Thus, the transformation products of BFRs may prove to be important for health risk evaluation. Further research regarding the transformations of BFRs is needed for both environmental remediation and health assessments.
As was reviewed in this chapter, some data related with chemical and biological degradation of selected BFRs, such as PBDEs, HBCD, or TBBPA, are available. However, in addition to these more studied BFRs, other BFRs have entered the market in recent years (see chapter by de Wit, this volume) [47], and they are being found in the environment. Thus, more data on these new BFRs are needed to evaluate their degradation rates as well as their degradation products.
Abbreviations
- BFR:
-
Brominated flame retardant
- BPA:
-
Bisphenol A
- GC:
-
Gas chromatography
- HBCD:
-
Hexabromocyclododecane
- MS:
-
Mass spectrometry
- PBB:
-
Polybrominated biphenyl
- PBDD:
-
Polybrominated dibenzo-p-dioxins
- PBDE:
-
Polybrominated diphenyl ether
- PBDF:
-
Polybrominated dibenzofuran
- PCB:
-
Polychlorinated biphenyl
- POP:
-
Persistent organic pollutant
- TBBPA:
-
Tetrabromobisphenol A
- WWTP:
-
Wastewater treatment plant
References
Wania F, Dugani CB (2003) Environ Toxicol Chem 22:1252
UNEP (2001) Final act of the conference of plenipotentiaries on the Stockholm convention on persistent organic pollutants. United Nations Environment Program, Geneva, Switzerland
European Union (2006) Annex XIII. Criteria for the identification of persistent, bioaccumulative and toxic substances, and very persistent and very bioaccumulative substances. Official Journal of the European Union L396, 383–385. Brussels, Belgium
Hansen BG, Munn SJ, de Bruijn J, Luotamo M, Pakalin S, Berthault F, Vegro S, Pellegrini G, Allanou R, Scheer S (eds) (2002) EUR 20402 ENsEuropean Union Risk Assessment Report bis(pentabromophenyl) Ether, Volume 17; European Commission, Luxembourg
Rahm S, Jakobsson E (2001) Reactivity of brominated diphenyl ethers vs. methane thiolate. Proceedings of the second international workshop on brominated flame retardants, Stockholm. Swedish National Chemicals Inspectorate, Solna, Sweden, pp 227–228
Eriksson J, Eriksson L, Marsh G, Bergman A (2003) Organohalogen Compd 61:191
Söderström G, Sellström U, de Wit CA, Tysklind M (2004) Environ Sci Technol 38:127
Méallier P (1999) Phototransformation of pesticides in aqueous solution. In: Boule P (ed) Environmental photochemistry, vol 2, part l. Springer Verlag, Berlin, Heidelberg, Germany, p 241
Pagni RM, Sigman ME (1999) The photochemistry of PAHs and PCBs in water and on solids. In: Boule P (ed) Environmental photochemistry, vol 2, part l. Springer Verlag, Berlin, Heidelberg, Germany, p 139
Richard C, Grabner G (1999) Mechanism of phototransformation of phenol and derivatives in aqueous solution. In: Boule P (ed) Environmental photochemistry, vol 2, part l. Springer Verlag, Berlin, Heidelberg, Germany, p 218
Eriksson J, Rahm S, Green N, Bergman A, Jakobsson E (2004) Chemosphere 54:117
Watanabe I, Tatsukawa R (1987) Bull Environ Contam Toxicol 39:953
Ohta S, Nishimura H, Nakao T, Aozasa O, Miyata H (2001) Organohalogen Compounds 52:321
Hua I, Kang N, Jafvert CT, Fabrega-Duque JR (2003) Environ Toxicol Chem 22:798
Ahn MY, Filley TR, Jafvert CT, Nies L, Hua I, Bezares-Cruz J (2006) Environ Sci Technol 40:215
Xie Q, Chen J, Shao J, Chen C, Zhao H, Hao C (2009) Chemosphere 76:1486
Zhao Y, Zhang X, Sojinu S (2010) Chemosphere 80:150
Barontini F, Cozzani V, Marsanich K, Raffa V, Petarca L (2004) J Anal Appl Pyrolysis 72:41
Marsanich K, Zanelli S, Barontini F, Cozzani V (2004) Thermochim Acta 421:95
Haag WR, Hoigne J (1986) Water Chlorination 5:1011
Baxter RM, Carey JH (1983) Nature 306:575
Han SK, Bilski P, Karriker B, Sik RH, Chignell CF (2008) Environ Sci Technol 42:166
Sandvik SLH, Bilski P, Pakulski JD, Chignell CF, Coffin RB (2000) Mar Chem 69:139
Weber R, Kuch B (2003) Environ Int 29:699
Luijk R, Wever H, Olie K, Govers HAJ (1991) Chemosphere 23:1173
Wichmann H, Dettmer FT, Bahadir M (2002) Chemosphere 47:349
Dettmer FT (2001) Bromorganische Flammschutzmittel-Analytische Anforderungen und thermische Bildung von polybromierten Dibenzo-p-dioxinen und Dibenzofuranen. Dissertation, University of Braunschweig
Hakk H, Letcher RJ (2003) Environ Int 29:801
Suflita JM, Horowitz A, Shelton DR, Tiedje JM (1982) Science 218:1115
Fetzner S (1998) Appl Microbiol Biotechnol 50:633
Bedard DL, van Dort HM, May RJ, Smullen LA (1997) Environ Sci Technol 31:3308
Abraham WR, Nogales B, Golyshin PN, Pieper DH, Timmis KN (2002) Curr Opin Microbiol 5:246
Häggblom MM (1992) FEMS Microbiol Rev 9:29
He JZ, Robrock KR, Alvarez-Cohen L (2006) Environ Sci Technol 40:4429
Kim YM, Nam IH, Murugesan K, Schmidt S, Crowley DE, Chang YS (2007) Appl Microbiol Biotechnol 77:187
Robrock K, Korytar P, Alvarez-Cohen L (2008) Environ Sci Technol 42:2845
Zhou J, Jiang W, Ding J, Zhang X, Gao S (2007) Chemosphere 70:172
Davis, JW, Gonisor SJ, Marty G, Friederich U, Ariano JM (2004) Investigation of the biodegradation of [14C]hexabromocyclododecane in sludge, sediment, and soil. In: The third international workshop on brominated flame retardants. Book of Abstracts, p 239
Davis JW, Gonsior SJ, Markham DA, Friederich U, Hunziker RW, Ariano JM (2006) Environ Sci Technol 40:5395
Gerecke AC, Giger W, Hartmann PC, Heeb NV, Kohler HPE, Schmid P, Zennegg M, Kohler M (2006) Chemosphere 64:311
Häggblom MM, Milligan PW (2000) Anaerobic degradation of halogenated pesticides: influence of alternate electron acceptors. In: Bollag JM, Stotzky G (eds) Soil biochemistry, vol 10. Marcel Dekker, New York, pp 1–34
Gerritse J, Drzyzga O, Kloetstra G, Keijmel M, Wiersum LP, Hutson R, Collins MD, Gottschal JC (1999) Appl Environ Microbiol 65:5212
Voordeckers JW, Fennell DE, Jones K, Häggblom MM (2002) Environ Sci Technol 36:696
Khim JS, Kannan K, Villeneuve DL, Koh CH, Giesy JP (1999) Environ Sci Technol 33:4199
Allard A, Remberger M, Neilson AH (1987) Appl Environ Microbiol 53:839
George KW, Haggblom M (2008) Environ Sci Technol 42:5555
de Wit CA, Kierkegaard A, Ricklund N, Sellström U (2010) Emerging brominated flame retardants (BFRs) in the environment. In: Eljarrat E, Barceló D (eds) Brominated flame retardants, The handbook of environmental chemistry. Springer-Verlag, Berlin Heidelberg
Acknowledgments
This research work was founded by the Spanish Ministry of Science and Innovation through the projects CEMAGUA (CGL2007-64551/HID) and Consolider-Ingenio 2010 (CSD2009-00065), by the CSIC through the project Intramural (ref. 200880I096) and by the Fundación BBVA under the BROMACUA project (Evaluación del impacto ambiental de los retardantes de llama bromados en ecosistemas acuáticos de América Latina). María Luisa Feo acknowledges CSIC for providing training and specialization of staff investigator through the JAE-Doc program.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Eljarrat, E., Feo, M.L., Barceló, D. (2011). Degradation of Brominated Flame Retardants. In: Eljarrat, E., Barceló, D. (eds) Brominated Flame Retardants. The Handbook of Environmental Chemistry(), vol 16. Springer, Berlin, Heidelberg. https://doi.org/10.1007/698_2010_96
Download citation
DOI: https://doi.org/10.1007/698_2010_96
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-19268-5
Online ISBN: 978-3-642-19269-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)