Abstract
This chapter reviews the recent literature and highlights the technical and methodological improvements in the analysis of polybrominated diphenyl ethers (PBDEs). Sample preparation, extraction of the analytes and clean-up are discussed with emphasis on recent developments. Gas chromatography coupled with mass spectrometry (GC-MS) is discussed for the instrumental analysis of PBDEs. The most important parameters that may affect accurate measurements of PBDEs are also included. Information related to quality assurance/quality control (QA/QC) procedures used in the analysis of PBDEs, including method validation parameters and possible sources for biased results, is given in detail. An overview of recent inter-laboratory studies on PBDEs and a discussion of the scores and outcomes conclude the chapter.
Graphical Abstract

Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Analytical methods
- Gas chromatography
- Mass spectrometry
- Mass spectrometryPBDEs
- Polybrominated diphenyl ethers
- Quality assurance
- Recent developments
- Review
1 Introduction
Due to their widespread environmental occurrence and their possible adverse effects in organisms, brominated flame retardants (BFRs), such as polybrominated diphenyl ethers (PBDEs), hexabromocyclododecane (HBCD) and tetrabromobisphenol-A (TBBP-A), are being determined in a growing number of laboratories. Analytical methods for BFRs have shown a rapid development and they were in many cases based on protocols previously established for other persistent organic pollutants (POPs), such as organochlorine pesticides (OCPs), polychlorinated biphenyls (PCBs) or polychlorinated dioxins and furans (PCDD/Fs). Although different properties of BFRs (e.g. polarity or vapour pressure) suggest that different procedures should be applied for their analysis, some common approaches can be found depending on the analyte, and the type of sample or detection method [1–4]. Some compounds, such as individual HBCD isomers and TBBP-A, may require specific analytical approaches due to their particular properties [126]. The methods described in the literature for the analysis of PBDEs have been reviewed in the past years [2–5]. This chapter focuses on recent literature until 2010 and highlights the technical and methodological improvements in the analysis of PBDEs. It also gives detailed information on quality assurance issues including results of recent inter-laboratory studies
2 Sample Preparation
Although sampling is a crucial step in the complete analytical process, it is not considered in this chapter since the approach needed for BFRs is not different than for other POPs. The sample preparation will therefore be covered from sample collection onwards. Since the use and production of PBDEs have been restricted and concentrations of some congeners are declining in many regions, scientists were encouraged to search for more sensitive and selective analytical techniques to monitor current environmental levels. This has resulted in a growing number of analytical papers exploring alternate sample preparation methodologies (Table 1) for the analysis of PBDEs in abiotic and biotic matrices. In order of execution, sample preparation usually consists of sample pre-treatment, extraction of the analytes and clean-up of the crude extract.
2.1 Sample Pre-Treatment
If non-polar solvents are used during sample preparation, the matrix has to be water-free to enable extraction of the analytes. Biological samples, such as food and tissue samples, are often subjected to water depletion by mixing the sample with sodium sulphate or by freeze drying. Subsequent thorough mixing ensures a homogeneous and water-free matrix. Soil, sediment, sludge and dust samples should be dried before extraction. In addition, they may be sieved to ensure particle size homogeneity and to facilitate further manipulations of the samples, but this is not imperative for a successful extraction or clean-up procedure.
Analysis of hair requires a different sample pre-treatment [32]. This matrix received increasing attention in medical settings where hair analyses are indicative of pharmaceutical and illicit drug use. The hair has to be washed to eliminate external contamination, dried and cut. Subsequent destruction of the hair matrix is necessary to enable the extraction of the analytes. Destruction can consist of an acid digestion with HCl or a basic digestion with NaOH followed by an overnight incubation at 40–50°C. Tadeo et al. [32] have investigated different sample pre-treatment techniques to analyse the PBDE content of hair. Acidic digestion provided cleaner hair extracts with less interfering compounds than those obtained with alkaline digestion. Moreover, alkaline digestion may degrade some halogenated compounds (such as some OCPs), although this effect was not observed for PBDEs.
2.2 Extraction
There are some particularities in the sample extraction for PBDE analysis that requires additional attention. First, the wide range in molecular weights of the PBDE congeners (between 250 and 960 u) yields different physical behaviours, indicating that extraction techniques may not work equally well for all PBDE congeners alike [37]. Second, the increasing environmental awareness and the need for lower detection limits require adaptations of the existing extraction and clean-up procedures. PBDEs can be successfully extracted by Soxhlet or liquid–liquid extraction (LLE), but this requires lengthy extraction times and high solvent volumes. Advanced extraction techniques, such as pressurised liquid extraction (PLE), ultrasonic-assisted extraction (UAE), microwave-assisted extraction (MAE) and supercritical-fluid extraction (SFE), have recently been introduced to reduce extraction time and solvent consumption, as well as to improve extraction efficiency.
PLE is widely used for the extraction of PBDEs from solid matrices, such as fish and sediment. It provides high extraction yields due to the combination of high temperatures and pressures. The main advantages of this technique are the reduced extraction time and low solvent consumption, compared to Soxhlet or UAE, and the possibility to conduct a large number of unsupervised extractions, providing increased efficiency and better reproducibility. However, solvent type and system- specific parameters, such as pressure, temperature, heating time, static time, flush volume, purge time and static cycles, need to be thoroughly optimised for optimal analyte recoveries. For the analysis of milk samples, an additional advantage of PLE is its capability to disrupt fat globules and release contaminants from milk resulting in improved extraction yields [33]. PLE has been successfully used to extract PBDEs from human and environmental samples, such as milk [33, 38], sediment and fish [38]. Although PLE has many advantages over other extraction techniques, an extensive clean-up of PLE extracts is often required due to the large amounts of co-extracted compounds (Table 1).
MAE is based on the application of microwave energy to heat the extraction solvent, while controlling temperature, pressure and microwave energy. The main advantage of MAE is a significantly reduced solvent consumption, as low as 20 mL of solvent per sample [21]. Temperature and pressure control allow the heating of the solvent system under pressure above its boiling point. Due to the combination of high pressure and high temperature, the solvent remains liquid, promoting analyte diffusion. The extraction time is shortened compared to Soxhlet extraction. However, optimisation of influential factors, such as extraction temperature, particle size, hold-up time and the employed solvent system, is necessary. The addition of polar compounds, such as methanol, is required for microwave absorption and therefore for heating of the extraction solvent. Mixtures of polar with non-polar solvents are commonly used in MAE (e.g. acetone:n-hexane, isopropanol:cyclohexane, xylene:dichloromethane or methanol:toluene). The extraction of BDE-209 from e-waste resulted in low extraction yields due to its high molecular weight and its non-polar nature [22]. Therefore, the extraction of BDE-209 might require additional adaptations, such as higher temperature and longer extraction time.
Matrix solid-phase dispersion (MSPD) is increasingly used for extracting contaminants from a variety of solid and semi-solid matrices. The basic procedure comprises three major steps: (1) sample grinding in the presence of an excess amount of sorbent (e.g. alumina), (2) loading of the ground mixture onto an empty SPE cartridge, and (3) elution with a suitable solvent. Prior to elution, extraction can be facilitated by placing the cartridges in an ultrasonic water bath [17]. The efficiency of the MSPD depends on multiple factors, particularly the sorbent type and the eluting solvent. A careful selection according to the sample matrix and the substance/substance group to be analysed and the sample matrix is therefore critical. The advantages of MSPD are its simplicity, efficiency, low cost, and its speed due to simultaneous extraction and clean-up. MSPD extraction has also been proven useful in the analysis of placental tissue [34], which requires an extraction technique that is vigorous enough to surface the analytes buried in the tissue. The optimised MSPD method performed similarly to the Soxhlet extraction [34].
Solid-phase microextraction (SPME) is increasingly being used for the determination of PBDEs in various samples, such as water, sediment, biota tissue and in manufactured products. In SPME, the analytes are absorbed on a silica fibre coated with a thin layer of polymers. After insertion of the loaded SPME fibre in the gas chromatographic (GC) system, the analytes are readily desorbed from the polymeric fibre, thus eliminating the need for additional sample clean-up. Headspace SPME has been used to determine PBDEs levels in water, soil, sediment and sewage sludge [6]. However, due to the broad range in molecular weight and volatility of PBDEs, relatively high extraction temperatures were required. Room temperature extraction of a broader spectrum of PBDE congeners from natural waters by SPME can be obtained by using the organically modified silicate (Ormosil), an SPME phase that possesses specific selectivity for PBDEs [19]. From sediments, a significantly lower extraction yield of PBDEs compared to that from water has been observed. However, mixing of the sediment with potassium permanganate, sulphuric acid and water had an important positive effect on the extraction yield of SPME for tetra to hexa-BDEs [19].
The applicability of SPME to PBDEs analysis in wastewater influents has recently been questioned, since most PBDE congeners are absorbed to the biosolids during the treatment process, causing a drastic reduction (>95%) of the PBDE levels in the effluent [39]. Hence, if particle adsorption was predominant in aqueous media, it might be the best to collect particles and analyse them separately.
Other extraction techniques. A novel technique referred to as cloud point extraction-ultrasound-assisted back-extraction was recently applied to extract and pre-concentrate PBDEs from water and soil [7]. This technique is based on the induction of a micellar-organised medium by using a non-ionic surfactant to extract PBDEs. To couple this efficient extraction technique with GC analysis, ultrasound-assisted back-extraction (UABE) into an organic solvent was required. The cloud point of a non-ionic surfactants aqueous solution is defined as the temperature at which the solution becomes turbid. The cloud point phenomenon occurs in a narrow temperature range and depends on the nature of the amphiphile and its concentration. The analytes are extracted in the surfactant-rich (coacervate) phase, which is small in volume, and decanted into the bottom of a centrifuge tube. To enable subsequent GC analysis, a simple back-extraction of the coacervate phase with isooctane is sufficient. Main advantages include low organic solvent consumption, low cost, simple set-up, environmental friendliness and no need for sample clean-up. One should mention that the efficiency of the extraction procedure varies with sample pH, concentration of surfactant, equilibration time and temperature, matrix modifiers and ionic strength. Therefore, to achieve a high extraction efficiency of PBDEs from the aqueous bulk, these variables were optimised to establish the optimal working conditions.
2.3 Clean-Up and Fractionation
Clean-up techniques for PBDEs are often based on multiple silica layer columns containing varying amounts of neutral, acid and basic silica, with different degrees of acid or base impregnation. Anhydrous sodium sulphate is often added to ensure a water-free extract after elution with the most suitable organic solvents. Alumina and Florisil® columns are also used and can be eluted with similar solvents. Importantly, the use of pre-packed columns minimises the risk of contamination of the adsorbent by particles from laboratory air, which might contain PBDEs. Both the minimised exposure of the sample to dust and the use of relatively small volumes of solvents in the automated fractionation process should be considered particularly valuable in PBDE analysis since procedural blanks are often a problem (see below). Efficient removal of large amounts of lipid may be necessary when analysing biota or food. This is possible using LLE of the raw extracts with concentrated sulphuric acid [26] or gel permeation chromatography (GPC). A short summary of the existing clean-up procedures and their recent applications is illustrated below and in Table 1.
Food samples often have high lipid contents and extracts therefore require an extensive clean-up, such as sulphuric acid treatment at 60°C for 1 h [9]. Afterwards, the organic phase can be separated, dried, re-dissolved and fractionated into two parts using a small glass column filled with activated alumina. The first fraction was eluted with n-hexane and contained the non-polar constituents, while the second fraction was eluted with a mixture of DCM and n-hexane (50:50, v/v) and contained the PBDEs. This clean-up procedure combines the advantages of a clean-up with sulphuric acid and SPE fractionation. Such procedure is ideal to obtain clean chromatograms, but requires thorough validation to ensure the stability of the analytes. Extracts from air and dust, with lower lipid content compared to food and serum, were subjected to a clean-up on a mixed acid (44% H2SO4, w/w) and basic silica (33% 1 M NaOH, w/w) column [9], followed by subsequent fractionation on alumina. The hexane fraction was discarded and PBDEs were eluted with 50 mL of n-hexane/DCM (1:1, v/v).
Removal of lipids from marine mussel extracts was tested using a GPC system filled with Bio-Beads S-X3 and elution with DCM [14]. Subsequent fractionation was done on silica and alumina, extracts were then treated with concentrated sulphuric acid and further fractionated using a two-dimensional LC system with two columns coupled in series. GPC has also been used to clean-up soil samples to remove humic substances and sulphur [18]. Subsequently, the collected fractions were eluted with n-hexane through a multilayer silica column. A similar technique was applied to clean-up soil samples, with acetonitrile as the eluting solvent [17]. Afterwards, the extract was filtered before injection.
Lacorte et al. [38] developed a comprehensive, highly sensitive and robust ultra-trace analytical method for the quantification of OH-PBDEs, MeO-PBDEs and their parent PBDE congeners in relevant environmental matrices (sediment, fish and milk), by means of PLE, followed by GPC and Florisil® clean-up. In contrast to previous studies [40, 41] where PBDEs, OH- and MeO-PBDEs were collected in separate fractions, all target compounds were eluted in a single fraction, which was further analysed by GC-HRMS [38].
Another approach modified a clean-up and fractionation technique for PCDD/F purification from environmental matrices (Table 1) [12]. Adaptations include changes in the solvent composition and volume and the elimination of the carbon fractionation step for brominated compounds, which significantly increased the recovery of PBDEs. In another study, a carbon-dispersed column was used in the analytical procedure for brominated compounds in air and dust samples [11]. After clean-up on a mixed silica gel column and elution of the PBDE fraction with hexane:DCM, the eluate was additionally purified on a carbon-dispersed silica column and analytes were further eluted with hexane:DCM and toluene. Using this technique, recoveries for PBDEs and PCBs were 57 and 54%, respectively, while recoveries of HBCDs and PBDD/DFs ranged between 74 and 126%.
Simultaneous analysis of PBDEs and their HO- and MeO-metabolites in the same sample requires extensive clean-up and fractionation procedures, owing to the different nature of the metabolites. Lipids from polar bear tissue extracts were removed with concentrated H2SO4 and protonated MeSO2-PCBs (remaining in the acid phase) were separated from all other contaminants, which migrated to the organic phase [23]. Neutral contaminants (PCBs, PBDEs and MeO-PBDEs) were separated from halogenated phenolic contaminants (HPCs) by aqueous KOH partitioning. The organic phase containing the neutral contaminants was further cleaned on a deactivated Florisil® column (8 g, 1.2% H2O, w/w). The KOH fraction, which contains the HPCs, was then acidified with concentrated H2SO4 and the reprotonated HPCs were extracted with MTBE:n-hexane and derivatised to their MeO-analogues using diazomethane. The MeO-HPCs were purified on a silica column (3g, 22% H2SO4, w/w) and eluted with 15% DCM in n-hexane (v/v). The final extract contained OH-PCBs, OH-PBDEs and other HPCs.
3 State-of-the-Art of GC-MS Analysis
GC has become a routine technique for the analysis of PBDEs. Yet, due to their physico-chemical properties (e.g. vapour pressure or polarity) and to their thermal instability, specific PBDE congeners need special precautions during GC analysis. It was shown that thermal degradation may occur during GC analysis of higher brominated PBDE congeners (especially BDE-209) [42, 43]. Therefore, before selecting an analysis methodology for PBDEs, the characteristics of the GC system should carefully be optimised in agreement with the properties of the analytes. These characteristics include column brand, stationary phase, as well as column length, internal diameter and injection technique may have a very strong influence on the accuracy of PBDE analysis [3, 44]. Finally, the detection technique completes the set of parameters that have to be optimised for an accurate determination of PBDEs.
3.1 GC Separation of PBDEs
Sample injection. Although various injection methods can be used for the analysis of PBDEs, the most common injectors are splitless, on-column and programmable temperature vaporisation (PTV) injection systems. Their advantages and disadvantages derive primarily from their availability, price, detection limits, extract constitution and discrimination of PBDE congeners on the basis of molecular weight.
Due to the relatively low levels of PBDEs in environmental samples, splitless injection is the preferred technique, but special precautions should be taken to minimise thermal degradation and discrimination of higher molecular weight PBDEs. Therefore, the optimal injector temperature and splitless time should be kept as high as possible (e.g. 325°C and 4 min, respectively) to obtain an increased response factor especially for fully brominated PBDE (e.g. BDE-209) [44]. An attractive way to reduce thermal degradation of PBDEs in injection system is to apply on-column injection. Its main advantage is that it shortcuts the injector-interface between extract and column by delivering the extract directly to the capillary column. As a result, a higher precision for the analysis of BDE-209 was observed when such injection was applied [44]. One of the main disadvantages is the need for very clean sample extracts so that potential matrix residues do not reach the capillary column, giving rise to increased noise, peak tailing, retention time shifts, more frequent column trimming and reduced column lifetime. Both splitless and on-column injection methods are limited to volumes from 1 to 3 μL. In comparison, PTV injection may accommodate up to 125 μL after thorough optimisation [45–47]. Although splitless is the most common injection technique, injections in a properly used PTV system may have some advantages, such as minimal degradation of labile PBDEs [3, 42, 46]. Recently, it was shown that especially for the analysis of BDE-209, the use of PTV injector in solvent vent mode may not only positively influence the response factor of BDE-209, but may also reduce the formation of its main thermal degradation product, BDE-207 [42].
Capillary column selection. In general, capillary GC columns offer adequate resolution to determine individual PBDE congeners. To achieve adequate separation of all PBDE congeners present in the sample and possible interferences, there is a need for sufficiently long columns (30–50 m) with small internal diameters (≤0.25 mm). In case of clean extracts, the use of short narrow-bore capillary columns (internal diameter ≤ 0.1 mm) may also provide satisfactory resolution [45]. To facilitate the selection of the most suitable GC columns for developing a congener-specific analysis, the elution order of 126 PBDEs was tested on seven different GC stationary phases [48]. First, less co-elutions for PBDE congeners with different bromine numbers were observed compared to PCBs. Second, a stationary phase-dependent degradation was seen, indicating that column equivalency is not always a suitable criterion for column selection [46, 48]. The most efficient column for PBDE congener-specific separation was found to be a DB-XLB (J&W Scientific) column with a DB-1 (J&W Scientific) column as first runner-up. However, the latter is preferred for routine analysis due to reduced degradation of hepta and higher brominated PBDE congeners.
Even on the most efficient stationary phases, single dimension GC cannot separate all PBDE congeners. Of the 126 PBDE congeners that were tested by Korytár et al., 55 congeners were involved in 22 co-elutions for DB-XLB, which is the most efficient stationary phase [48]. As a consequence, six column combinations were evaluated for GC × GC separation of PBDEs coupled to μ-ECD or time of flight-mass spectrometry (TOF-MS) detectors [49]. It was concluded that a DB-1 × 007-65HT (Quadrex) combination was the most suitable due to (1) the highest number of PBDE congeners separated, (2) less decomposition of higher brominated congeners, and (3) most suitable maximum operating temperature. With this set-up, there were only 17 co-eluting pairs involving 35 congeners.
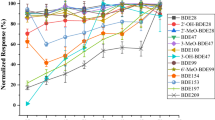
Special precautions are needed for BDE-209 analysis due to its thermal sensitivity and thus its higher susceptibility for degradation in the GC system. Therefore, the GC column should be relatively short (10–15 m) to reduce the residence time in the system [1]. Based on the ability of the low pressure-GC (LP-GC) technique to elute compounds at lower temperatures compared to conventional GC techniques [50], BDE-209 was analysed at temperatures below the degradability limit. It was shown that the low elution temperature of BDE-209 (<295°C), combined with its short residence time in GC lead to minimal thermal degradation [42]. Besides minimal thermal degradation, the analysis of BDE-209 using LP-GC system resulted in an analysis time of merely 6.5 min. Baseline separation of 22 PBDE congeners (major components of PBDE technical mixtures) was possible in less than 12 min using this LP-GC system (Fig. 1).
Mass chromatogram (m/z = 79, 487 and 495) of a standard mixture of 22 PBDE congeners (major components of penta, octa and deca-BDE technical mixtures) injected in the LP-GC-ECNI-MS system (analytical column: 10 m × 0.53 mm ID, df = 0.15 μm AT-5). Peak numbers correspond to PBDE congener nomenclature (modified from [42])
Interferences might not only be due to co-eluting congeners, but can also be caused by other brominated compounds, such as MeO-PBDEs or polybrominated biphenyls (PBBs). Two MeO-PBDEs, namely 5-MeO-BDE-47 and 5-Cl-6-MeO-BDE-47, were found to co-elute with BDE-100 and BDE-99, respectively, using a DB-5 column [40], while BDE-154 and BB-153 were also co-eluting on the same type of column [2]. Therefore, alternative stationary phases have been suggested [2, 40].
3.2 Mass Spectrometric Detection for BFRs
The most widely used detectors for PBDE determination are mass spectrometers, classified into low-resolution (LR) or high-resolution (HR) mass spectrometric (MS) instruments. The LR-MS instruments are operated either in EI or in ECNI mode.
Using EI-MS for PBDE analysis, the major ions formed are [M.]+ and [M-2Br].+, which can be used for their identification and quantification [51]. The main potential interferences that may influence the accuracy originate from chlorinated compounds, such as PCBs. For example, the nominal masses corresponding to ions monitored for di-BDEs and penta-CBs (m/z = 326), and also for tetra-BDEs and hepta-CBs (m/z = 396) are the same and therefore a resolution power of 24,000 is needed to separate them, if they co-elute on the GC column [3]. However, this is not recommended due to the significant loss of sensitivity at this elevated resolution [52].
LR-EI-MS provides a higher selectivity compared to LR-ECNI-MS, since for the latter only the bromine trace can be monitored (m/z 79 and 81). However, LR-EI-MS (with quadrupole as mass analyser) is not routinely used for PBDE analysis due to its relatively low sensitivity, especially when measuring PBDE congeners with more than six bromine atoms. Recently, GC-EI-MS operated in SIM mode was used to analyse PBDEs in human hair samples with limit of quantification (LOQ) as low as 0.3–0.6 ng/g of sample for tri to hepta-BDEs and 3 ng/g of sample for BDE-209 [32]. Determination of mono to deca-PBDE congeners together with PBBs in electrical and electronic equipments was also achieved by GC-EI-MS [53]. Method limits of detection (LOD) ranged between 0.2 and 8 ng/g for mono to hepta-BDE, and 70 ng/g for BDE-209, respectively.
In contrast to EI, the low-energy electrons (thermal electrons) generated in ECNI by interactions between a high-energy electron beam and a moderating gas (e.g. methane), react with the analytes to form negative ions. The electron energy should be low to facilitate electron capture and the specific energy required for electron capture depends on the molecular structure of the analyte. PBDEs do normally not form molecular ions or other diagnostic ions under ECNI conditions (except BDE-209), thus only bromide ions (m/z 79 and 81) may be monitored.
Compared to EI-MS, ECNI-MS is less selective because non-specific bromide ions \( {[{\text{Br}}]^{-} } \) at m/z 79 and 81 are used for the quantification of all homologue groups, except BDE-209, which gives rise to specific fragments ([C6Br5O]−; m/z 486.7). However, ECNI-MS is a very sensitive method with LODs that are one order of magnitude lower than those for LR-EI-MS. Therefore, this technique proves to be suitable for the analysis of low contaminated samples, such as human serum and milk. Reliable results have also been obtained for the quantification of selected PBDEs in river water and sediment samples. Detection limits ranged from 3 to 160 pg/L and 5 to 145 pg/g for river water and sediment, respectively [8]. The use of GC-ECNI-MS in combination with stir-bar sorptive extraction and automated thermal desorption/cryotrapping was applied for the simultaneous determination of PBDEs and PBBs in sheep and human serum samples [36].
Selectivity of LR-ECNI-MS can be improved under optimised conditions. By optimising the electron energy, emission current, source temperature and system pressure, the relative abundances of the larger molecular fragments \( {[{\text{M}} - x{\text{H}} - y{\text{Br}}]^{-} } \) increases and therefore the technique can be used for the monitoring of each homologue group instead of the non-specific bromide ions [54]. Monitoring high mass fragments under optimised ion source conditions was successfully applied for the determination PBDEs in environmental and biological samples at concentration levels <0.01 pg in one single instrumental run was recently described [55]. Good repeatabilities (1.7–9.1%) and reproducibilities (4.1–20%), and low LODs (e pg/mL) were obtained, allowing PBDEs quantification in snow and human serum samples.
Besides MS operated in either EI or ECNI modes, other MS type detectors were used to analyse PBDEs. PCBs and PBDEs were simultaneously determined in soil by GC-TOF-MS. Method detection limits ranged between 0.1 and 0.4 mg/kg for PCBs and 0.1 and 0.6 mg/kg for PBDEs with RSD < 7.3% for PCBs and <6.3% for PBDEs [56]. Another multi-residue method for the quantification of 30 organohalogenated compounds in human breast tissue using GC-triple quadrupole MS has recently been developed. Analyses were performed in both EI and ECNI modes and the acquisition of two transitions (in EI) or two ions (in ECNI) per analyte were acquired. This approach allowed confirmation through matching of ion ratios between the quantification and the confirmation transitions (EI) or ions (ECNI) [57]. To analyse tri to hexa-BDEs in sediments, GC combined with an ion trap mass analyser operated in EI mode was applied [19]. All PBDEs showed a common MS/MS transition consisting in the elimination of two Br atoms. Instrumental LOQs ranged from 0.05 pg/μL (BDE-47) to 0.5 pg/μL (BDE-154) for an injection volume of 2 μL.
4 Analysis of PBDEs in Polymers
The European Commission Directive 2002/95/EC on the "restriction of the use of certain hazardous substances in electrical and electronic equipment" (RoHS) prohibits the usage of several BFRs in electric and electronic devices. As of 1 July 2006, The Commission Decision 2005/681/EC specifies a limit of 1 g/kg for the sum of PBDEs and PBBs in plastics. Proper enforcement of this regulation requires reliable testing of products for their content of BFRs.
PBDEs were analysed in matrix polymers by Raman spectroscopy without any sample pre-treatment [58]. The LOD was approximately 100 μg/g and the analysis took only 1 min. Deca-BDE could be identified based on distinctive bands. Energy dispersive X-ray fluorescence (ED-XRF) analysis, GC-MS and infrared spectroscopy techniques have also been evaluated for the analysis of polymers after various extraction procedures [59]. A portable XRF analyser was successfully used for non-destructive semi-quantitative determination and screening purposes for the presence of BFRs in consumer products [60]. During validation, XRF-measured bromine was highly correlated with the GC-MS-measured bromine for furniture foam and plastics of electronic consumer products. In the field study phase, the XRF-measured bromine in room furniture was highly correlated with the penta-BDE concentrations in room dust. This technique has shown great potential as a fast field-applicable screening tool.
Although having low recoveries for styrenic polymers, UAE can be an easy, fast and robust analytical tool for the determination of BFRs in polypropylene (PP) and polyethylene (PE) [61]. The characterisation of the two certified reference materials (CRMs; ERM-590 and ERM-591) for BFRs in polymer materials indicates that several laboratories successfully applied UAE during the characterisation measurements [62, 63]. Apart from UAE, other extraction techniques were used, such as Soxhlet extraction, PLE, static extraction and even complete dissolution of the polymer. Details of all analytical protocols used in this certification study are given in Table 2.
In another study, a flow-injection (FI) system was coupled to inductively coupled plasma-mass spectrometry (ICP-MS) and used for the detection of bromine traces in polymers, plastic paints and enamels containing BFRs [64]. Using this approach, individual PBDEs cannot be unambiguously identified, but materials can be rapidly screened for bromine-containing compounds. Sample preparation is based on MAE and after appropriate optimisation of the digestion procedure and the ICP-MS detection, a detection limit of 4.2 mg/kg was obtained for synthesised polyurethane standards containing known amounts of bromine. The precision of the proposed method, evaluated as the RSD of signals obtained from three replicate analyses of polymeric standard BFRs at the normative EU level, was as low as 3.6%. The proposed system provides rapid binary (yes/no) overall responses, being appropriate for the screening of bromine above a pre-set threshold. The unreliability region (UR), given by the probability of false positives and false negatives (set at 5% in both cases), was in the range of 442 and 678 mg/kg of bromine (at a cut-off level of 0.1% in BFRs by weight of homogeneous material fixed by the EU normative). Finally, the applicability of the proposed screening system was tested for the reliable control of bromine in different commercial samples including flame-retarded paints and enamels.
5 Quality Assurance Parameters: How to Tweak the Quality of Your Analytical Results
Every step in the analysis of BFRs has critical parameters that need optimisation to minimise the uncertainty of the final result and thus improve the quality of the data. The ISO/IEC 17025 standard, which describes general requirements for the competence of calibration and testing laboratories, requires that accredited laboratories use validated methods, demonstrate traceability of calibrations and apply an appropriate quality control programme. Over the years, intensive effort was put in mapping possible and specific pitfalls in PBDE analysis, which improved the quality of the released data over time.
Reference materials and method validation. The access to analytical standards of sufficient purity (i.e. reference materials), both “external” calibrants and (mass-labelled) internal standards, is a key aspect for reliable quantification of PBDEs. In the last 10 years, almost all individual or mixtures of native and numerous 13C12-labelled PBDE congeners became available as standard solutions or as neat crystals. These commonly used standards are often not very well characterised and a large uncertainty is associated with the PBDE concentration of solutions (up to 5%) and with the purity of the solid substance (in some cases exceeding 5%). A recently organised CCQM study pointed at the necessity to verify and correct if necessary the purity of the calibrants indicated by the suppliers or to incorporate this uncertainty into the uncertainty budget of the measurements [65].
Extensive sample preparation is often needed in PBDE analysis and demands for internal standards (IS) and syringe standards (SS) to compensate for losses of target analytes during sample preparation and for inter-injection fluctuations. Several PBDE congeners (such as BDE 77, 116 and 126, but also others) are most likely to combine all the above-mentioned characteristics and are therefore suited as IS [66]. Preferably, the selected IS should be a 13C12-labelled analogue, but this limits the choice of detection to EI-MS (except for BDE-209, see below). The use of BDE-138 should be discouraged, since it has been reported in the technical mixture Bromkal 70-5 DE [67]. Alternatively, PBBs not present in the Firemaster® mixtures can be used. BB-103 and BB-155 were also used as IS for different levels of bromination of PBDE homologues [68]. BB-209 has been proposed as IS for the determination of BDE-209 in sediments. Although BB-209 was produced commercially until 2000 in small amounts in France [69], it could not be identified in a large number of sediment and biota samples from the Netherlands [70]. Alternatively, the use of 13C12-BDE-209 as IS for BDE-209 analysis by ECNI-MS is possible when m/z 484.7/486.7 and 494.7/496.7 are monitored for BDE-209 and 13C12-BDE-209, respectively.
Recently, fluorinated derivatives (F-PBDEs) became available and it was proven that they are suitable IS or SS for the analysis of PBDEs [71]. Although the use of mass-labelled IS should be encouraged, a recent publication has shown that results obtained by using isotopically labelled internal standards do not render superior results per se compared to those obtained with other carefully selected suitable internal standards (such as, e.g. PCB-209, other PBDE congeners, polychlorinated diphenyl ethers (PCDEs) or F-PBDEs) [65]. Despite the limited amount of data sets for either approach, it could be seen that (1) the within-laboratory intermediate precision data were well comparable for all analytes and were about the same using either isotope dilution MS (IDMS) or other methodology, (2) mean results compared well for all analytes except BB-209, and (3) the standard deviation of the mean of means for both data sets and all analytes was comparable. Although these conclusions were based upon data provided by National Metrology Institutes, which spend more time on QA/QC compared to regular analytical laboratories, it shows that the analytical process can be controlled even without mass-labelled IS if proper measures are taken. Additional recommendations regarding internal standards were given in the literature [72].
Monitoring the ion intensity ratios in EI-MS is widely used to verify identification of target analytes. If the isotopic ratio of the quantitation and confirmation ion differ more than 15% [73, 74] or 20% [45], results should be flagged as questionable and should not be used to check compliance with limit or threshold values. For ECNI-MS, relatively good specificity is obtained by measuring the bromine trace (m/z 79 and 81) and possible co-elutions are limited to compounds yielding bromine fragments upon ionisation.
The imprecision level of PBDE determination depends on the analyte (between 10 and 20% for tri to hepta-BDEs and around 25% for BDE 209 and HBCD). The first worldwide inter-laboratory studies on BFRs showed that laboratories should improve their method precision [66]. Since then a lot of effort has been invested in pinpointing the pitfalls in BFR analysis [4], which resulted in improved performance of analytical laboratories if proper procedures and protocols are used [65].
Although trueness assessment during validation is preferably done by means of a CRM, this is not always possible for BFRs due to limited availability of adequate reference materials. Standard addition procedures are accepted in this case as a valid approach to evaluate the trueness of the analytical procedure [72]. Recovery measurements of spiked samples (especially solid samples, such as sediments and sewage sludge) may yield higher recoveries of analytes due to a possibly easier extraction from these spiked samples, since they are not naturally incurred in the matrix. When possible, proper incubation and ageing of the spiked samples should be carried out so that the spiked compounds mimic as much as possible the behaviour of the naturally incurred analytes [75]. The best procedure to evaluate the trueness of analytical methods used for PBDE determination is the analysis of CRMs. Stapleton recently reported on the mass fractions of PBDEs on several existing NIST SRMs [76]. Few environmental reference materials certified for PBDE content exist, such as NIST SRM 2585 (indoor dust), NIST SRMs 1957 and 1958 (human serum), NIST SRMs 1953 and 1954 (human milk), NIST SRMs 2257 and 2258 (organic solution), NIST SRM 2977 (mussel tissue) and NIST SRM 1945 (whale blubber). While the number of these materials is currently limited, it is anticipated that with time their number will increase.
The method stability and reliability (maintenance of its performance over time) should be assessed through regular analysis of standard solutions, procedural blanks, duplicate samples, in-house prepared reference materials or CRMs (in order of increasing importance). The use of such materials with each analytical batch is encouraged seeing the numerous possible problems associated with PBDE analysis. Recoveries can be influenced by a variety of parameters, such as adsorption to glass (higher brominated BDEs), UV-degradation (BDE-209) or losses caused by evaporation (BDE-28). In addition to the internal QA/QC measures, laboratories are encouraged to participate in inter-laboratory studies and proficiency tests.
Integrity of the analytes . To eliminate potential sources of error during sample preparation, some treatments should be avoided to preserve the integrity of particular PBDEs. High temperatures and/or extensive saponification times can result in decomposition of higher brominated BDEs [1]. BDE-209 and possibly other higher brominated BDE congeners are photosensitive and thus direct exposure to UV light should be avoided. Wrapping the containers, extraction funnels and solvent receptacles with aluminium foil or using amber glassware are probably the simplest preventive measures. The use of UV filters on laboratory windows and fluorescent lighting is also highly recommended. Both photolytic and thermal degradation may lead to degradation products formed through debromination. A combination of exposure to daylight and poor solubility may even result in the complete disappearance of BDE-209 from solutions placed on laboratory benches and directly exposed to sunlight [4].
The extended exposure of PBDEs to elevated temperatures can be avoided through various means, such as (1) using short and narrow-bore GC columns with thin films, which reduces the residence time of the compounds in the GC system, (2) using short injector residence times, e.g. by means of cold or pressure-pulse injection modes or by using on-column injection, and (3) guaranteeing a clean GC-injector liner free of matrix residues that could retain the analytes and enhance thermal degradation in the injector. Yet, the stability of the GC system deteriorates over time by accumulation of matrix particles in the inlet and column, which requires regular replacement of injector liners and column trimming.
Because BDE-209 is most sensitive to thermal degradation, it can be used as an indicator of the system performance status. Several degradation products, such as BDE-206 and BDE-207 can be used to monitor the stability of the system [42, 65]. Preventing degradation is important because it cannot fully be compensated for by using labelled IS (that undergo a similar degradation). Thermal degradation that occurs during analysis was shown to be also concentration dependent [77].
In contrast to the above-mentioned analyte degradation, signal enhancement has also been reported [17], probably caused by an increased protection of the analytes from adsorption in the injector when matrix residues are present in the extract. Sellström has reported a similar effect for fish tissue for which the recovery of BDE-209 was higher when a matrix was present [51]. If relative recoveries from spiked samples are acceptable and not statistically different from recoveries calculated from standard solutions, calibration curves can be made from standard solvent-based solutions [78]. If matrix effects are pronouncedly present, calibration curves should be established by means of matrix-matched spiked standards.
Complete evaporation of the extracts to dryness should be avoided as PBDEs tend to adsorb to glass even more strongly than PCBs, which may result in incomplete dissolution upon reconstitution. Reconstitution time should therefore be sufficiently long and validated during method optimisation. Extended evaporation of the extracts might also lead the selective loss of certain compounds that are relatively more volatile than others, i.e. BDE-28. For analysis of BDE-209, the use of solvents such as toluene, DCM or acetone:n-hexane mixtures is preferred, because of its limited solubility in other organic solvents [2, 79].
Background contamination. An important QC measure to be considered on a routine basis includes the regular analysis of reagent and procedural blanks. Reagent blanks should be run regularly not only to check for possible contamination of solvents but also to check the status of the instrument, which is closely related to the integrity of the analytes as discussed previously. Procedural blanks should be analysed to monitor and compensate for a possible background contamination (whether it is noticed or not depends mainly on the LOQ of the instrumental method, see below).
Guidelines are available on how to minimise background contamination of PBDEs in the analytical laboratory [2–4, 66]. The use of plastics should be reduced to a minimum. Moreover, significant concentrations of BDE-47 and BDE-99 have been identified in laboratory air [80]. Dust and air-borne particles are a known carrier of high loads of PBDEs, especially of BDE-209 [81]. If possible, a dedicated PBDE sample preparation environment where special measures are implemented should be installed (clean room). Any materials that are not needed in the laboratory should be avoided (e.g. packaging materials, upholstered chairs, etc.) [4]. (Cross-) contamination can be avoided by implementing strict and validated washing and cleaning procedures. It is suggested to wash all glassware in 2.5% RBS 25 foaming cleaner, followed by rinsing with distilled water and subsequently heating at 450°C for 4 h [80, 82]. Glassware that cannot be washed after use, e.g. Soxhlet coolers, should be thoroughly rinsed with one or more organic solvents to prevent cross-contamination.
Due to the specific problems associated with, e.g. BDE-209, procedural blanks should be implemented at a much more regular interval as for any other organic analysis, i.e. for each analytical batch or at least every ten samples [4, 81]. A high background contamination with BDE-209 can compromise more than just the result of BDE-209 itself, since (non-systematic) degradation of BDE-209 can result in formation of other lower brominated BDEs that can compromise the trueness of those results as well. Furthermore, where solution blanks have a shorter residence time at the bench than the sample extracts, BDE-209 may migrate to and be retained on the glass wall of recipients, whereas it will migrate into the extracts where the storage period is longer. This gives rise to unrealistically clean blanks and more contaminated samples [4]. A 13C12-labelled IS for BDE-209 is therefore highly recommended.
Up-to-date, no uniform approach has been adopted or implemented to deal with laboratory background contamination of PBDEs. While some laboratories consistently measure (low) blank values and correct for them to enhance the trueness of their results [83–85], others report that blanks are below LOD [86–88]. Vonderheide summarised this discrepancy, stating that low sample intake necessitates a very sensitive method to allow accurate determination of blank contamination, while a high sample intake procedure is relatively less sensitive to blank problems [37]. Although less sensitive to blank problems, a high sample intake procedure, however, might suffer from other drawbacks, such as clean-up and chromatographic separation difficulties.
The LOD and the LOQ as defined by IUPAC [89] may be estimated in different ways. Current state-of-the-art equipment is able to accurately measure amounts that are several times lower than what is commonly present in the blanks, which renders instrumental LOQ obsolete. When reporting low mass fractions, it is prerequisite to actually measure the background contamination to assess its potential contribution to the total analyte signal. When this approach is not followed, values just above the LOQ (mainly background contamination) can be reported, which clearly should be avoided if data of an acceptable quality are desired. One might argue that whenever blanks cannot be measured due to lack of sensitivity of the method used, the uncertainty of the measurements should be adapted accordingly. For the analysis of serum samples, various authors used different blank samples, such as calf serum [90], sterilised water containing 0.9% NaCl [91] or water [92]. It appears necessary to include more information regarding blank composition and preparation in scientific manuscripts because such information may collectively allow researchers to better pinpoint the source of contamination.
Taking into account the above considerations, blank measurements can be subtracted from the measured sample values before final reporting under the conditions the blank is under control, i.e. a stable measurement is obtained over time (e.g. RSD of 10 determination <30%). Finally, the variation of blank values should be incorporated into the LOQ. According to literature, PBDE mass fractions should only be reported when exceeding the levels found in procedural blanks by a minimum factor of two [72]. Alternatively, it was proposed that reported levels of PBDEs should be above a value equal to the procedural blank value plus ten times [93], five times [79] or three times [84, 94] the SD of the procedural blanks. Some authors applied a correction for procedural blanks if the blank value was between 10 and 20% of the measured value in the sample [73]. If the procedural blank value was less than 10%, no corrective action was taken. Data were excluded from further consideration if the blank value exceeded 30% of sample measurements. Some analytical alternatives to reduce blank values have been recommended in the literature [72]. Some authors have set the LOQ at five times the blank level, if blank interference was present [95, 96]. This correction method resulted in many unusable data, since blank interference raised the LOQ to higher levels than those present in most samples. Given the low PBDE levels that often need to be measured in environmental or food samples, the use of a method blank cut-off value equal to three times SD of the blank measurement (after subtraction of the blank value) is a good compromise between detection power and data quality. Table 3 briefly summarises specific PBDE QA/QC measures, their impact and the possibilities to implement them.
6 Recent Inter-Laboratory Studies on BFRs
During the last decade, laboratories have increasingly become involved in the analysis of BFRs in environmental, human and food samples. To assure as well as to improve the quality of BFR analyses, a series of international inter-laboratory exercises has been organised.
The first worldwide inter-laboratory study on PBDE analysis was conducted in 1999/2000 and involved five biological samples, two sediments and two standard solutions sent to 26 participants in nine countries [66]. The results reported for BDE-47 were considered acceptable with a range of RSDs of 17–40%, while results for BDE-99 (25–77%), BDE-100 (19–48%), BDE-153 (30–48%) and BDE-154 (25–43%) showed that further improvement was needed. Analysis of BDE-209 was not under control of the participating laboratories at that time.
Following this first study, three inter-laboratory exercises were organised under the Quality Assurance of Information for Marine Environmental Monitoring in Europe (QUASIMEME) project [4]. These exercises conducted between 2001 and 2004 included eel, mussel, lake trout, salmon, herring, mackerel, cormorant liver, porpoise liver, porpoise blubber, capelin oil, sewage sludge, sediments, sediment extracts, human milk and test solutions of undisclosed concentrations. The targeted congeners were BDEs-28, 47, 99, 100, 153, 154, 183 and 209, but HBCD and TBBP-A were also included. The authors stressed that it was very difficult to establish any kind of quality-related trend in the data set due to the numerous parameters that varied from round to round. But nevertheless, they identified a number of specific problems and drew some clear conclusions [4]. The Horwitz function [97], which states that the analytes’ mass fractions are inversely correlated with the associated RSD of the measurements, was also applied to this data set. Tables 4 and 5 summarise the results of the following eight rounds held by QASIMEME between 2005 and 2009 including harbour, coastal and open sea sediments as well as dab, plaice, salmon, shrimp, and mussel tissue [98–105]. Statistical evaluation and laboratory assessment in these inter-laboratory studies were done using the Cofino Model [106, 107] and robust statistics according to the German Standard DIN 38402-45 [108].
Ten inter-comparison studies on PBDEs in sediment were evaluated (Table 4). The number of participants ranged from 9 to 16. There were no significant temporal trends as regards laboratory performance but according to the Horwitz equation there was a negative correlation of RSD and PBDE concentration. The median RSDs over ten rounds conducted from 2005 to 2009 for BDE28, 47, 99, 100, 153, 154, 183 and 209 were 46, 29, 30, 39, 40, 31, 53 and 51%, respectively. The high median RSD seen for BDE28 might be attributed to its very low concentration in most of the inter-comparison samples. However, the median RSD of 51% observed for BDE209 still reflects unsatisfactory performance of the participating laboratories. Results for BDE183 are difficult to assess but low levels present in the inter-comparison samples seem at least partly to explain the high RSD.
Just like for the analysis of PBDEs in sediments ten inter-comparison studies on PBDE in marine biota samples have been evaluated (Table 5). The number of participating laboratories in these exercises ranged from 13 to 18. In the five rounds organised in 2005 and 2006, fish tissue samples including common dab, common plaice, and salmon were distributed to the participants. In the following five inter-comparisons, mussel tissue and one shrimp homogenate had to be analysed. As PBDE levels in fish are distinctly higher than those in mussels the results for fish tissue and mussel/shrimp homogenates were evaluated separately. Median RSDs for six tri to hexa-BDEs in fish ranged from 22% for BDE-47 to 36% for BDE-28 indicating acceptable performance of the participating laboratories. No assigned value could be calculated for BDE-183 and BDE-209 due to the very few numerical results reported. Unlike laboratory performance for PBDE in fish samples, laboratory performance for the analysis of PBDE in mussels was generally disputable except for the major congener BDE-47 for which a median RSD of 33% was calculated. Actually, the majority of laboratories were not in a position to produce reliable results for samples containing very low PBDE concentrations.
Takahashi et al. [109] reported the results of an inter-laboratory comparison between six Japanese laboratories on the analysis of PBDEs, polybrominated and monobromo-polychlorinated dibenzodioxins and furans in a test solution of undisclosed concentrations and one air-dried sediment sample. All laboratories used isotopic dilution HRGC-HRMS operated in the EI mode. RSDs for the individual PBDEs in the standard mixture were between 9 and 24%, while those for the sediment samples ranged from 17 to 39%, except for BDE-100, for which an RSD of 74% was reported. Average concentrations for tri to hexa-BDE congeners ranged from 0.029 to 0.38 ng/g dw, whereas those for BDE-183 and BDE-209 were 2 and 170 ng/g dw, respectively. The strong variation in BDE-100 results was due to the poor separation of BDE-100 from interferences when using a DB-17HT capillary column. There were indications that adding copper may lead to formation of lower brominated PBDE congeners and PBDFs due to degradation of BDE-209 during Soxhlet extraction [109].
Since 2000, the Norwegian Institute for Public Health (NIPH) has been organising inter-laboratory studies on the analysis of dioxins and dioxin-like PCBs in frequently consumed foods (chicken meat, trout fillet and palm oil) [110–112]. In the fifth round organised in 2004, the participants were for the first time asked to voluntarily report concentrations of PBDEs and HBCD in food samples. Laboratories were requested to determine BDEs-28, 47, 99, 100, 153, 154, 183 and 209. Analysis was performed using the laboratory’s own methods for sample preparation and instrumental analysis, their own reference standards and quantification procedures, and their own method for lipid determination. Since then, PBDEs have been included in this yearly programme for a number of environmentally relevant matrices, in particular fish tissue (trout, halibut, salmon, eel, and herring) and have attracted a large number of laboratories [112–117]. Usually around 40 participants reported results for tri to hepta-BDEs. In the first years, the calculated consensus values for BDE-209 were only indicative as too few laboratories had reported this congener but later the number of reported results increased. To our knowledge, the reports of these inter-comparison studies provide the most comprehensive database on laboratory performance in PBDE analysis in biological matrices available at the moment. It is important to note that the majority of participating laboratories have used isotopic dilution HRGC-HRMS.
Here, we report only on the results of PBDEs in fish tissue from 2004 to 2009 (Table 6) as this is an important matrix for monitoring spatial and temporal trends in the aquatic environment as well as for checking compliance with environmental quality standards, threshold values or background reference concentrations. Other matrices included in these inter-comparisons were chicken, reindeer, deer and beef meat, palm oil, cod liver oil, butter oil, egg yolk, breast milk, butter, and cream. There was a clear tendency towards lower RSDs for the sum of tri to hepta-BDEs over time. While the average RSD in 2004 was 40%, it dropped down to 25% in 2009. This improvement was independent of the PBDE concentration in the sample. These results provide evidence that the performance of governmental, private and research laboratories in conducting PBDE analysis (tri to hepta-BDEs) in fish samples has significantly improved.
Results for BDE-209 are difficult to interpret due to the extremely low concentrations of this congener in the inter-comparison samples and the fairly high number of outliers as well as left-censored values (Table 6). Yet, RSDs of 50–75% still indicate unsatisfactory performance. Hence, when interpreting PBDE levels in fish tissue the high uncertainty associated with results of BDE-209 needs definitely to be considered. There was no trend in the quality of results of the standard solutions (Table 7). RSDs were satisfactory even for BDE-209 and varied between 8 and 18% and were much lower than those reported by de Boer and Wells [4].
To assist environmental monitoring programmes as well as for the purpose of certification of reference materials, the National Institute of Standards and Technology (NIST) regularly conducts inter-laboratory exercises that also comprise PBDEs [118–121]. The materials distributed for the 2007 exercise included SRM 1945 Organics in Whale Blubber, SRM 1958 Human Serum, a homogenised blubber control material “Marine Mammal Quality Assurance Exercise Homogenate VIII” (Homogenate VIII) from a female pilot whale and “Marine Mammal Control Material 1-Serum” (MMCM1-serum) also derived from a female pilot whale [121]. Twelve laboratories reported data for BDEs-47, 99, 100, 153 and 154. Median mass fractions reported for SRM 1945 were within the uncertainties of the certified value for BDE-99, BDE-100 and BDE-154, and slightly below and above for BDE-47 and BDE-153, respectively. These discrepancies were mainly due to the low uncertainties of the certified values of these two congeners. RSDs ranged from 20 to 47% with highest values for BDE-153. RSDs for the blubber homogenate were between 12 and 21%, except for BDE-99 (39%), indicating excellent performance of the participating laboratories [121].
An inter-laboratory study comprising two rounds on the analysis of BDE-209 in environmental samples has been conducted within the European project NORMAN (Network of Reference Laboratories and Related Organisations for Monitoring and Biomonitoring of Emerging Environmental Pollutants) [122, 123]. In the first round, six laboratories experienced in PBDE analysis measured BDE-209 in a test solution of undisclosed concentration and a dust sample (NIST SRM 2585). Each laboratory was allowed to use its own analytical methodology. Rigorous implementation of previously agreed recommendations including the obligatory use of 13C12-labelled BDE-209 as internal standard resulted in relative reproducibility and repeatability standard deviations below 10% for both samples. All reported results for the dust sample were in agreement with the certified value of the NIST SRM 2585 [122].
In a follow-up study, expert and other laboratories were involved (ten participants in total). Prior to the second round, a meeting with the participants was held to discuss analytical difficulties and problems. Experiences from the first round as well as recommendations from the literature were exchanged and solutions discussed. The laboratories were given advice on the use of the preferred conditions for extraction, clean-up and GC. In this exercise, a test solution of undisclosed concentration, a dust sample (NIST SRM 2585) and a low contaminated marine sediment had to be analysed [123]. The reproducibility variation coefficients for the sediment, dust and the test solution were 31, 20 and 13%, respectively. The repeatability variation coefficients were less than 10% for all samples. The performance of the participating laboratories was surprisingly good given the differences in their experience to analyse BDE 209.
The measurement of PBDE in polymers is similar to that in environmental samples, but due to the high concentrations of PBDEs that are present in plastics, sample intake can be significantly reduced compared to the environmental analyses described above and thus little effort has to be made with regard to the clean-up. This can result in simple analytical procedures, and hence, better laboratory performances than for environmental samples might be expected. However, in a recent inter-comparison exercise on analysis of PBDEs in PET spiked with technical mixtures of penta-BDE, octa-BDE, and deca-BDE as well as deca-BB at 0.4–0.8 g/kg, high RSDs for the major constituents BDE-47, 99, 183, 209, and BB-209 of 33, 27, 40, 47 and 44%, respectively, have been observed [124].
The German Federal Environment Agency commissioned the Federal Institute for Materials Research and Testing to develop and validate a method for the analysis of technical mixtures of penta and octa-BDE in polymers. The method was subjected to an inter-laboratory study with 18 participants from seven countries which had to analyse four polymers spiked with technical Penta- or Octa-BDE at 1 g/kg [125]. The relative reproducibility standard deviations for the sum of congeners representing penta-BDEs in the epoxy resin and polyurethane sample were 15% for either type of polymer while for the sum of octa-BDEs in poly(acrylonitrile, butadiene, styrene) (ABS) copolymer and in polystyrene, relative reproducibility standard deviations of 27% and 26%, respectively, were obtained.
A recent certification inter-laboratory study involving 16 selected laboratories demonstrated that BDEs-28, 47, 99, 100, 153, 154, 183, 197 + 204, 209, and BB-209 can be measured in spiked PP and PE samples with high accuracy. After elimination of technically doubtful results, the RSDs between laboratories ranged from 3% for BDE-209 to 12% for BDE-47 and BB-209 [63]. To the best of our knowledge, these are the best results ever obtained in an inter-laboratory study on the analysis of PBDEs.
In 2009, the Organic Analysis Working Group of CCQM (Consultative Committee for Amount of Substance – Metrology in Chemistry) carried out a laboratory inter-comparison (pilot study P114) for assessing the current state-of-the-art measurement capabilities of National Metrology Institutes (NMIs) to accurately quantify representative PBDE and PBB congeners in a polymer sample [65]. The study involved eight NMIs, which were asked to analyse BDEs-47, 183, 206 and 209 and BB-209 in a commercially prepared PP granulate material and a test solution of undisclosed concentration. The same PP sample as in the certification inter-laboratory study mentioned above was used. Results for the test solution of undisclosed concentration were in good agreement with the certified values. The analytical procedures used to analyse PP in this CCQM study differed substantially. Nevertheless, results were all in agreement, except some technical exceptions. Since the same PP material was used in two independent inter-laboratory exercises, results could be compared. Good agreement between the two data sets (study means versus certified values) has been achieved for all four PBDE congeners and BB-209 [65]. Within-lab repeatability of the NMIs was ≤10% for all labs and all congeners. Expanded uncertainties as estimated by the NMIs were in the range of about 5–10% in most cases and significantly lower than those reported by the laboratories that participated in the certification round.
7 Future Perspectives
Due to their recent restriction in usage and bans from EU and other markets (e.g. USA, Canada, Asia), it is expected that PBDEs will be (if not already done) added on priority monitoring lists (e.g. food and environmental control, Water Framework Directive). Therefore, accurate analytical methodologies are necessary to control the effectiveness of the implementation of legal regulations and emission reduction measures, as well as to estimate human exposure to these chemicals. As results of PBDE measurements have to comply with environmental quality standards, limit or threshold values the regulatory requirements as regards the tolerable uncertainty of PBDE data are high. Hence, it is of utmost importance that all steps of the analytical method are carefully addressed following the recommendations given in the QA/QC section and that laboratories involved in PBDE analysis operate comprehensive QA/QC programmes to demonstrate the reliability of the data they produce. A recent trend was seen towards the introduction of new analytical methodologies for the analysis of PBDE and other BFRs based on LC/MS [126]. These new methods may lead to similar low detection limits as the GC-based methods, but mostly include simpler protocols for the sample preparation, making them attractive for routine analysis.
References
de Boer J, Allchin C, Law R, Zegers B, Booij JP (2001) Method for the analysis of polybrominated diphenyl ethers in sediments and biota. Trends Anal Chem 20:591–599
Covaci A, Voorspoels S, de Boer J (2003) Determination of brominated flame retardants, with emphasis on polybrominated diphenyl ethers (PBDEs) in environmental and human samples – a review. Environ Int 29:735–756
Covaci A, Voorspoels S, Ramos L, Neels H, Blust R (2007) Recent developments in the analysis of brominated flame retardants and brominated natural products. J Chromatogr A 1153:145–171
de Boer J, Wells DE (2006) Pitfalls in the analysis of brominated flame retardants in environmental, human and food samples – including results of three international inter-laboratory studies. Trends Anal Chem 25:364–372
Stapleton HM (2006) Instrumental methods and challenges in quantifying polybrominated diphenyl ethers in environmental extracts: a review. Anal Bioanal Chem 386:807–817
Li MKY, Lei NY, Gong C, Yu Y, Lam KH, Lam MHW, Yu H, Lam PKS (2009) An organically modified silicate molecularly imprinted solid-phase microextraction device for the determination of polybrominated diphenyl ethers. Anal Chim Acta 633:197–203
Fontana AR, Silva MR, Martínez LD, Wuilloud RG, Altamirano JC (2009) Determination of polybrominated diphenyl ethers in water and soil samples by cloud point extraction-ultrasound-assisted back-extraction-gas chromatography–mass spectrometry. J Chromatogr A 1216:4339–4346
Labadie P, Tlili K, Alliot F, Bourges C, Desportes A, Chevreuil M (2010) Development of analytical procedures for trace-level determination of polybrominated diphenyl ethers and tetrabromobisphenol A in river water and sediment. Anal Bioanal Chem 396:865–875
Fromme H, Korner W, Shahin N, Wanner A, Albrecht M, Boehmer S, Parlar H, Mayer R, Liebl B, Bolte G (2009) Human exposure to polybrominated diphenyl ethers (PBDE), as evidenced by data from a duplicate diet study, indoor air, house dust, and biomonitoring in Germany. Environ Int 35:1125–1135
Chen D, Bi X, Zhao J, Chen L, Tan J, Mai B, Sheng G, Fu J, Wong M (2009) Pollution characterization and diurnal variation of PBDEs in the atmosphere of an E-waste dismantling region. Environ Pollut 157:1051–1057
Takigami H, Suzuki G, Hirai Y, Sakai S (2009) Brominated flame retardants and other polyhalogenated compounds in indoor air and dust from two houses in Japan. Chemosphere 76:270–277
Wyrzykowska B, Tabor D, Gullett BK (2009) Same-sample determination of ultratrace levels of polybrominated diphenyl ethers, polybromodibenzo-p-dioxins/furans, and polychlorodibenzo-p-dioxins/furans from combustion flue gas. Anal Chem 81:4334–4342
Schecter A, Shah N, Colacino JA, Brummitt SI, Ramakrishnan V, Harris RT, Päpke O (2009) PBDEs in US and German clothes dryer lint: a potential source of indoor contamination and exposure. Chemosphere 75:623–628
Hong SH, Munschy C, Kannan N, Tixier C, Tronczynski J, Héas-Moisan K, Shim WJ (2009) PCDD/F, PBDE, and nonylphenol contamination in a semi-enclosed bay (Masan Bay, South Korea) and a Mediterranean lagoon (Thau, France). Chemosphere 77:854–862
Liu G, Zhang G, Jin Z, Li J (2009) Sedimentary record of hydrophobic organic compounds in relation to regional economic development: a study of Taihu Lake, East China. Environ Pollut 157:2994–3000
Chen L, Huang Y, Peng X, Xu Z, Zhang S, Ren M, Ye Z, Wang X (2009) PBDEs in sediments of the Beijiang River, China: levels, distribution, and influence of total organic carbon. Chemosphere 76:226–231
Sanchez-Brunete C, Miguel E, Tadeo JL (2009) Analysis of polybrominated diphenyl ethers in sewage sludge by matrix solid-phase dispersion and isotope dilution GC–MS. J Sep Sci 32:109–117
Wang P, Zhang Q, Wang Y, Wang T, Li X, Li Y, Ding L, Jiang G (2009) Altitude dependence of polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in surface soil from Tibetan Plateau, China. Chemosphere 76:1498–1504
Montes R, Rodríguez I, Cela R (2010) Solid-phase microextraction with simultaneous oxidative sample treatment for the sensitive determination of tetra- to hexabrominated diphenyl ethers in sediments. J Chromatogr A 1217:14–21
Petreas M, Oros D (2009) Polybrominated diphenyl ethers in California wastestreams. Chemosphere 74:996–1001
Ranz A, Maier E, Trampitsch C, Lankmayr E (2008) Microwave-assisted extraction of decabromodiphenylether from polymers. Talanta 76:102–106
Vilaplana F, Ribes-Greus A, Karlsson S (2009) Microwave-assisted extraction for qualitative and quantitative determination of brominated flame retardants in styrenic plastic fractions from waste electrical and electronic equipment (WEEE). Talanta 78:33–39
Gebbink WA, Sonne C, Dietz R, Kirkegaard M, Riget FF, Born EW, Muir DCG, Letcher RJ (2008) Tissue-specific congener composition of organohalogen and metabolite contaminants in East Greenland polar bears (Ursus maritimus). Environ Pollut 152:621–629
Meng X, Blasius ME, Gossett RW, Maruya KA (2009) Polybrominated diphenyl ethers in pinnipeds stranded along the southern California coast. Environ Pollut 157:2731–2736
Jin J, Wang Y, Yang C, Hu J, Liu W, Cui J, Tang X (2009) Polybrominated diphenyl ethers in the serum and breast milk of the resident population from production area, China. Environ Int 35:1048–1052
Sagerup K, Helgason LB, Polder A, Strøm H, Josefsen TD, Skåre JU, Gabrielsen GW (2009) Persistent organic pollutants and mercury in dead and dying glaucous gulls (Larus hyperboreus) at Bjørnøya (Svalbard). Sci Total Environ 407:6009–6016
Losada S, Roach A, Roosens L, Santos FJ, Galceran MT, Vetter W, Neels H, Covaci A (2009) Biomagnification of anthropogenic and naturally-produced organobrominated compounds in a marine food web from Sydney Harbour, Australia. Environ Int 35:1142–1149
Haraguchi K, Koizumi A, Inoue K, Harada KH, Hitomi T, Minata M, Tanabe M, Kato Y, Nishimura E, Yamamoto Y, Watanabe T, Takenaka K, Uehara S, Yang H, Kim M, Moon C, Kim H, Wang P, Liu A, Hung NN (2009) Levels and regional trends of persistent organochlorines and polybrominated diphenyl ethers in Asian breast milk demonstrate POPs signatures unique to individual countries. Environ Int 35:1072–1079
Frederiksen M, Thomsen M, Vorkamp K, Knudsen LE (2009) Patterns and concentration levels of polybrominated diphenyl ethers (PBDEs) in placental tissue of women in Denmark. Chemosphere 76:1464–1469
Zhao YX, Qin XF, Li Y, Liu PY, Tian M, Yan SS, Qin ZF, Xu XB, Yang YJ (2009) Diffusion of polybrominated diphenyl ether (PBDE) from an e-waste recycling area to the surrounding regions in southeast China. Chemosphere 76:1470–1476
Abaurrea MP, Weijs L, Ramos L, Borghesi N, Corsolini S, Neels H, Blust R, Covaci A (2009) Anthropogenic and naturally-produced organobrominated compounds in bluefin tuna from the Mediterranean Sea. Chemosphere 76:1477–1482
Tadeo JL, Sánchez-Brunete C, Miguel E (2009) Determination of polybrominated diphenyl ethers in human hair by gas chromatography-mass spectrometry. Talanta 78:138–143
Lacorte S, Guillamon M (2008) Validation of a pressurized solvent extraction and GC–NCI–MS method for the low level determination of 40 polybrominated diphenyl ethers in mothers’ milk. Chemosphere 73:70–75
Priyanthi RMA, Dassanayake S, Wei H, Chen RC, Li A (2009) Optimization of matrix solid phase dispersion extraction procedure for the analysis of polybrominated diphenyl ethers in human placenta. Anal Chem 81:9795–9801
Loconto PR, Isenga D, O'Keefe M, Knottnerus M (2008) Isolation and recovery of selected polybrominated diphenyl ethers from human serum and sheep serum: coupling reversed-phase solid-phase disk extraction and liquid-liquid extraction techniques with a capillary gas chromatographic electron capture negative ion mass spectrometric determinative technique. J Chromatogr Sci 46:53–60
Loconto PR (2009) Evaluation of automated stir bar sorptive extraction-thermal desorption-gas chromatography electron capture negative ion mass spectrometry for the analysis of PBDEs and PBBs in sheep and human serum. J Chromatogr Sci 47:656–669
Vonderheide AP (2009) A review of the challenges in the chemical analysis of the polybrominated diphenyl ethers. Microchem J 92:49–57
Lacorte S, Ikonomou MG, Fischer M (2010) A comprehensive gas chromatography coupled to high resolution mass spectrometry based method for the determination of polybrominated diphenyl ethers and their hydroxylated and methoxylated metabolites in environmental samples. J Chromatogr A 1217:337–347
Vonderheide AP, Mueller KE, Meija J, Welsh GL (2008) Polybrominated diphenyl ethers: causes for concern and knowledge gaps regarding environmental distribution, fate and toxicity. Sci Total Environ 400:425–436
Marsh G, Athanasiadou M, Bergman Å, Asplund L (2004) Identification of hydroxylated and methoxylated polybrominated diphenyl ethers in Baltic Sea salmon (Salmo salar) blood. Environ Sci Technol 38:10–18
Sinkkonen S, Rantalainen A-L, Paasivirta J, Lahtiperä M (2004) Polybrominated methoxy diphenyl ethers (MeO-PBDEs) in fish and guillemot of Baltic, Atlantic and Arctic environments. Chemosphere 56:767–775
Dirtu AC, Ravindra K, Roosens L, van Grieken R, Neels H, Blust R, Covaci A (2008) Fast analysis of decabrominated diphenyl ether using low-pressure gas chromatography – electron-capture negative ionization mass spectrometry. J Chromatogr A 1186:295–301
Roosens L, Dirtu AC, Goemans G, Belpaire C, Gheorghe A, Neels H, Blust R, Covaci A (2008) Brominated flame retardants and organochlorine contaminants in fish from the Scheldt River, Belgium. Environ Int 34:976–983
Björklund J, Tollbäck P, Hiarne C, Dyremarck E, Östman C (2004) Influence of the injection technique and the column system on gas chromatographic determination of polybrominated diphenyl ethers. J Chromatogr A 1041:201–210
Covaci A, de Boer J, Ryan JJ, Voorspoels S, Schepens P (2002) Determination of polybrominated diphenyl ethers and polychlorinated biphenyls in human adipose tissue by large-volume injection – narrow-bore capillary gas chromatography/electron impact low-resolution mass spectrometry. Anal Chem 74:790–798
Björklund J, Tollbäck P, Östman C (2003) Large volume injection GC-MS in electron capture negative ion mode utilizing isotopic dilution for the determination of polybrominated diphenyl ethers in air. J Sep Sci 26:1104–1110
Tollbäck P, Björklund J, Östman C (2003) Large-volume programmed-temperature vaporiser injection for fast gas chromatography with electron capture and mass spectrometric detection of polybrominated diphenyl ethers. J Chromatogr A 991:241–253
Korytár P, Covaci A, de Boer J, Gelbin A, Brinkman UATh (2005) Retention-time database of 126 polybrominated diphenyl ether congeners and two Bromkal technical mixtures on seven capillary gas chromatographic columns. J Chromatogr A 1065:239–251
Korytár P, Covaci A, Leonards PEG, de Boer J, Brinkman UATh (2005) Comprehensive two-dimensional gas chromatography of polybrominated diphenyl ethers. J Chromatogr A 1100:20–31
Ravindra K, Dirtu AC, Covaci A (2008) Low-pressure gas chromatography: recent trends and developments. Trends Anal Chem 27:291–303
Sellström U (1999) Determination of some polybrominated flame retardants in biota, sediment and sewage sludge. PhD Thesis, University of Stockholm, Sweden
Eljarrat E, de la Cal A, Barcelo D (2003) Potential chlorinated and brominated interferences on the polybrominated diphenyl ether determinations by gas chromatography-mass spectrometry. J Chromatogr A 1008:181–192
Li Y, Wang T, Hashi Y, Li H, Lin J-M (2009) Determination of brominated flame retardants in electrical and electronic equipments with microwave-assisted extraction and gas chromatography-mass spectrometry. Talanta 78:1429–1435
Ackerman LK, Wilson GR, Simonich SL (2005) Quantitative analysis of 39 polybrominated diphenyl ethers by isotope dilution GC/low-resolution MS. Anal Chem 77:1979–1987
Vizcaino E, Arellano L, Fernandez P, Grimalt JO (2009) Analysis of whole congener mixtures of polybromodiphenyl ethers by gas chromatography-mass spectrometry in both environmental and biological samples at femtogram levels. J Chromatogr A 1216:5045–5051
Park H-M, Hong S-M, Agustin-Camacho MR, Dirwono W, Lee K-B (2009) Pressurized liquid extraction for the simultaneous analysis of polychlorinated biphenyls and polybrominated diphenyl ethers from soil by GC-TOF-MS detection. J Chromatogr Sci 47:681–688
Medina CM, Pitarch E, Portolés T, López FJ, Hernández F (2009) GC-MS/MS multi-residue method for the determination of organochlorine pesticides, polychlorinated biphenyls and polybrominated diphenyl ethers in human breast tissues. J Sep Sci 32:2090–2102
Kikuchi S, Kawauchi K, Ooki S, Kurosawa M, Honjo H, Yagishita T (2004) Non-destructive rapid analysis of brominated flame retardants in electrical and electronic equipment using Raman spectroscopy. Anal Sci 20:1111–1112
Altwaiq AM, Wolf M, van Eldik R (2003) Extraction of brominated flame retardants from polymeric waste material using different solvents and supercritical carbon dioxide. Anal Chim Acta 491:111–123
Allen JG, McClean MD, Stapleton HM, Webster TF (2008) Linking PBDEs in house dust to consumer products using X-ray fluorescence. Environ Sci Technol 42:4222–4228
Vilaplana F, Karlsson P, Ribes-Greus A, Ivarsson P, Karlsson S (2008) Analysis of brominated flame retardants in styrenic polymers – comparison of the extraction efficiency of ultrasonication, microwave-assisted extraction and pressurised liquid extraction. J Chromatogr A 1196:139–146
Linsinger TPJ, Birgersson-Liebich A, Pellizzato F, Venelinov T, Voorspoels S, Lamberty A (2009) EUR 23677 EN, ISBN 978-92-79-11105-1
Linsinger TPJ, Birgersson-Liebich A, Lamberty A, Pellizzato F, Venelinov T, Voorspoels S (2009) Development of the first certified reference materials for several brominated flame retardants in polymers. Anal Chem 81:3792–3800
Vazquez AS, Costa-Fernandez JM, Encinar JR, Pereiro R, Sanz-Medel A (2008) Bromine determination in polymers by inductively coupled plasma-mass spectrometry and its potential for fast first screening of brominated flame retardants in polymers and paintings. Anal Chimica Acta 623:140–145
Zeleny R, Voorspoels S, Ricci M, Becker R, Jung C, Bremser W, Sittidech M, Panyawathanakit N, Wong WF, Choi SM, Lo KC, Yeung WY, Kim DH, Han J, Ryu J, Mingwu S, Chao W, Schantz MM, Lippa KA, Matsuyama S (2008) Evaluation of the state-of-the-art measurement capabilities for selected PBDEs and decaBB in plastic by the international intercomparison CCQM-P114 (2010). Anal Bioanal Chem 396:1501–1511
de Boer J, Cofino WP (2002) First world-wide inter-laboratory study on PBDEs. Chemosphere 46:625–633
Sjödin A, Jakobsson E, Kierkegaard A, Marsh G, Sellström U (1998) Gas chromatographic identification and quantification of polybrominated diphenyl ethers in a commercial product, Bromkal 70-5DE. J Chromatogr A 822:83–89
Voorspoels S, Covaci A, Maervoet J, Schepens P (2004) PBDEs in marine and freshwater sediments from Belgium: levels, profiles and relations with biota. J Environ Monitor 6:914–918
de Boer J, de Boer K, Boon JP (2000) Polybrominated Biphenyls and Diphenylethers. In: Paasivirta J (ed) The Handbook of Environmental Chemistry. New Types of Persistent Halogenated Compounds. Part 3K, Springer-Verlag, Berlin/Heidelberg, pp 61–95. ISBN: 978-3-540-65838-2
de Boer J, Wester PG, van der Horst A, Leonards PEG (2003) Polybrominated diphenyl ethers in influents, suspended particulate matter, sediments, sewage treatment plant and effluents and biota from the Netherlands. Environ Pollut 122:63–74
Luthe G, Leonards PEG, Rijerink GS, Liu HL, Johansen JE, Robertson LW (2006) Monofluorinated analogues of polybrominated diphenyl ethers as analytical standards: synthesis, NMR, and GC-MS characterization and molecular orbital studies. Environ Sci Technol 40:3023–3029
Päpke O, Furst P, Herrmann T (2004) Determination of polybrominated diphenylethers (PBDEs) in biological tissues with special emphasis on QC/QA measures. Talanta 63:1203–1211
Dodder N, Strandberg B, Hites RA (2002) Concentrations and spatial variations of polybrominated diphenyl ethers in fish and air from the North-Eastern United States. Environ Sci Technol 36:146–151
Luross JM, Alaee M, Sergeant DB, Cannon CM, Whittle DM, Solomon KR, Muir DCG (2002) Spatial distribution of polybrominated diphenyl ethers and polybrominated biphenyls in lake trout from the Laurentian Great Lakes. Chemosphere 46:665–672
Martinez A, Ramil M, Montes R, Hernanz D, Rubi E, Rodriguez I, Cela R (2005) Development of a matrix solid-phase dispersion method for the screening of polybrominated diphenyl ethers and polychlorinated biphenyls in biota samples using gas chromatography with electron-capture detection. J Chromatogr A 1072:83–91
Stapleton HM, Keller JM, Schantz MM, Kucklick JR, Leigh SD, Wise SA (2007) Determination of polybrominated diphenyl ethers in environmental standard reference materials. Anal Bioanal Chem 387:2365–2379
Voorspoels S (2006) Environmental distribution of brominated flame retardants in Belgium. PhD Dissertation, University of Antwerp, Belgium
Thomsen C, Leknes H, Lundanes E, Becher G (2002) A new method for determination of halogenated flame retardants in human milk using solid-phase extraction. J Anal Toxicol 26:129–138
Sjödin A (2000) Occupational and dietary exposure to organohalogenated substances with special emphasis on polybrominated flame retardants. PhD Thesis, Stockholm University, Sweden
Thomsen C, Leknes H, Lundanes E, Becher G (2001) Brominated flame retardants in laboratory air. J Chromatogr A 923:299–304
Wilford BH, Thomas GO, Jones KC, Davidson B, Hurst DK (2008) Decabromodiphenyl ether (deca-BDE) commercial mixture components, and other PBDEs, in airborne particles at a UK site. Environ Int 34:412–419
Thomsen C, Lundanes E, Becher G (2001) A simplified method for determination of tetrabromobisphenol A and polybrominated diphenyl ethers in human plasma and serum. J Sep Sci 24:282–290
Kuiper RV, Vethaak AD, Cantón RF, Anselmo H, Dubbeldam M, van den Brandhof EJ, Leonards PEG, Wester PW, van den Berg M (2008) Toxicity of analytically cleaned pentabromodiphenylether after prolonged exposure in estuarine European flounder (Platichthys flesus), and partial life-cycle exposure in fresh water zebrafish (Danio rerio). Chemosphere 73:195–202
Voorspoels S, Covaci A, Lepom P, Escutenaire S, Schepens P (2006) Remarkable findings concerning PBDEs in the terrestrial top-predator red fox (Vulpes vulpes). Environ Sci Technol 40:2937–2943
Covaci A, Voorspoels S, Roosens L, Jacobs W, Blust R, Neels H (2008) Polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in human liver and adipose tissue samples from Belgium. Chemosphere 73:170–175
Bodin N, Abarnou A, Fraisse D, Defour S, Loizeau V, Guellec AML, Philippon X (2007) PCB, PCDD/F and PBDE levels and profiles in crustaceans from the coastal waters of Brittany and Normandy (France). Mar Pollut Bull 67:657–668
Verreault J, Shahmiri S, Gabrielsen GW, Letcher RJ (2007) Organohalogen and metabolically-derived contaminants and associations with whole body constituents in Norwegian Arctic glaucous gulls. Environ Int 33:823–830
Raab U, Preiss U, Albrecht M, Shahin N, Parlar H, Fromme H (2008) Concentrations of polybrominated diphenyl ethers, organochlorine compounds and nitro musks in mother's milk from Germany (Bavaria). Chemosphere 72:87–94
IUPAC (1976) Nomenclature, symbols, units and their usage in chemical analysis: data interpretation. Anal Chem 48:2294–2296
Bloom M, Spliethoff H, Vena J, Shaver S, Addink R, Eadon G (2008) Environmental exposure to PBDEs and thyroid function among New York anglers. Environ Toxicol Pharmacol 25:386–392
Thomsen C, Liane VH, Becher G (2007) Automated solid-phase extraction for the determination of polybrominated diphenyl ethers and polychlorinated biphenyls in serum—application on archived Norwegian samples from 1977 to 2003. J Chromatogr B 846:252–263
Jaraczewska K, Lulek J, Covaci A, Voorspoels S, Kaluba-Skotarczak A, Drews K, Schepens P (2006) Distribution of polychlorinated biphenyls, organochlorine pesticides and polybrominated diphenyl ethers in human umbilical cord serum, maternal serum and milk from Wielkopolska region, Poland. Sci Total Environ 372:20–31
Pirard C, de Pauw E, Focant JF (2006) Suitability of tandem-in-time mass spectrometry for polybrominated diphenylether measurement in fish and shellfish samples: comparison with high resolution mass spectrometry. J Chromatogr A 1115:125–132
Stapleton HM, Baker JE (2003) Comparing polybrominated diphenyl ether and polychlorinated biphenyl bioaccumulation in a food web in Grand Traverse Bay, Lake Michigan. Arch Environ Contam Toxicol 45:227–234
Sjödin A, Carlsson H, Thuresson K, Sjölin S, Bergman Å (2001) Flame retardants in indoor air at an electronics recycling plant and at other work environments. Environ Sci Technol 35:448–454
Jakobsson K, Thuresson K, Rylander L, Sjödin A, Hagmar L, Bergman Å (2002) Exposure to polybrominated diphenyl ethers and tetrabromobisphenol A among computer technicians. Chemosphere 46:709–716
Horwitz W, Kamps LR, Boyer KW (1980) Quality assurance in the analysis of foods for trace constituents. J Assoc Off Anal Chem 63:1344–1354
QUASIMEME Laboratory Performance Studies, DE-8 Brominated Flame Retardants, Round 41, Exercise 656, 2005, Report, Issue 1: 161205
QUASIMEME Laboratory Performance Studies, DE-8 Development Exercise Brominated Flame Retardants in Sediment, Round 45, Exercise 719, 2006, Report, Issue 1: 26090
QUASIMEME Laboratory Performance Studies, BS-1 Brominated Flame Retardants, Round 46, Exercise 729, 2006, Report, Issue 1: 050107
QUASIMEME Laboratory Performance Studies, BS-1 Brominated Flame Retardants, Round 48, Exercise 748, 2007, Report, Issue 1: 150607
QUASIMEME Laboratory Performance Studies, BS-1 Brominated Flame Retardants, Round 50, Exercise 773, 2007, Report, Issue 1: 031207
QUASIMEME Laboratory Performance Studies, BS-1 Brominated Flame Retardants, Round 52, Exercise 790, 2008, Report, Issue 1: 170608
QUASIMEME Laboratory Performance Studies, BS-1 Brominated Flame Retardants, Round 54, Exercise 818, 2008, Report, Issue 1: 191208
QUASIMEME Laboratory Performance Studies, BS-1 Brominated Flame Retardants, Round 56, Exercise 835, 2009, Report, Issue 1: 25052009
Cofino WP, van Stokkum IHM, Wells DE, Ariese F, Wegener JWM, Peerboom RAL (2000) A new model for the inference of population characteristics from experimental data using uncertainties. Application to inter-laboratory studies. Chemometr Intell Lab Syst 53:37–55
Cofino WP, van Stokkum IHM, van Steenwijke J, Wells DE (2005) A new model for the inference of population characteristics from experimental data using uncertainties – Part II. Application to censored datasets. Anal Chim Acta 533:31–39
DIN 38402-45: German standard methods for the examination of water, waste water and sludge – general information (group A) – Part 45: Inter-laboratory comparisons for proficiency testing of laboratories (A 45), 2003
Takahashi S, Sakai S-I, Watanabe I (2006) An intercalibration study on organobromine compounds: results on polybrominated diphenylethers and related dioxin-like compounds. Chemosphere 64:234–244
Becher G, Haug LS, Thomsen C (2004) World-wide comparison on the quality of analytical determinations of PCDDs/PCDFs and dioxin-like PCBs in food. Talanta 63:1115–1122
Haug L, Thomsen C, Becher G (2005) The quality of PBDE analysis in food – results from an inter-laboratory comparison study. Organohalogen Compd 67:622–625
Haug LS, Becher G (2004) Inter-laboratory comparison on dioxins in food 2004 – sixth round of an international study, report 2004:6, ISBN 82-8082-100-7. Norwegian Institute of Public Health, Oslo, Norway
Haug LS, Becher G (2005) Inter-laboratory comparison on dioxins in food 2005 – sixth round of an international study, report 2005:7, ISBN 82-8082-145-7. Norwegian Institute of Public Health, Oslo, Norway
Haug LS, Becher G (2006) Inter-laboratory comparison on dioxins in food 2006 – seventh round of an international study, report 2006:7, ISBN 82-8082-178-3. Norwegian Institute of Public Health, Oslo, Norway
Liane VH, Becher G (2007) Inter-laboratory comparison on dioxins in food 2007 – eight round of an international study, report 2007:7, ISBN 978-82-8082-216-1. Norwegian Institute of Public Health, Oslo, Norway
Liane VH, Bjellaas T, Becher G (2008) Inter-laboratory comparison on dioxins in food 2008 – ninth round of an international study, report 2008:13, ISBN 978-82-8082-286-4. Norwegian Institute of Public Health, Oslo, Norway
Liane VH, Becher G (2009) Inter-laboratory comparison on dioxins in food 2009 – tenth round of an international study, report 2009:10, ISBN 978-82-8082-367-0. Norwegian Institute of Public Health, Oslo, Norway
Stapleton HM, Keller JM, Schantz MM, Kucklick JR, Wise SA (2005) NIST Inter-comparison exercise program for polybrominated diphenyl ethers (PBDES) in marine sediment: description and results of the 2004 intercomparison exercise. National Institute of Standards and Technology, Gaithersburg, MD, NISTIR 7278
Kucklick JR, Pugh RS, Becker PR, Schantz MM, Wise SA, Rowles TK (2005) Description and results of the 2003 NIST/NOAA inter-laboratory comparison exercise program for organic contaminants in marine mammal tissues. National Institute of Standards and Technology, Gaithersburg, MD, NISTIR 7269
Kucklick JR, Pugh RS, Becker PR, Schantz MM, Poster DL, Wise SA, Rowles TK (2007) Description and results of the 2005 NIST/NOAA inter-laboratory comparison exercise program for organic contaminants in marine mammal tissues, National Institute of Standards and Technology, Gaithersburg, MD, NISTIR 7410
Schantz MM, Parris RM, Wise SA (2008) Description and results of the 2007 NIST/NOAA inter-laboratory comparison exercise program for organic contaminants in marine mammal tissues. National Institute of Standards and Technology, Gaithersburg, MD, NISTIR 7501
Duffek A, Sawal G, Warmbrunn-Suckrow E, Lepom P (2007) Inter-laboratory study on DecaBDE in dust within the EU-Project NORMAN. Abstracts of the 4th International Workshop on Brominated Flame Retardants BFR 2007, Amsterdam, The Netherlands, April 24–27, http://www.bfr2007.com/abstracts/mainb.html?p=3
Duffek A, Leonards P, Lepom P (2008) Inter-laboratory study on Deca-BDE analysis in environmental samples involving routine laboratories. Organohalogen Compd 70:2025–2028
Linsinger TPJ, Voorspoels S, Liebich A (2008) Results of an inter-laboratory comparison on the determination of polybrominated flame retardants in poly(ethyleneterephthalate). Anal Bioanal Chem 390:399–409
Kemmlein S, Bergmann M, Jann O (2005) Standard measurement method for the determination of polybrominated flame retardants (pentabromodiphenylether, octabromodiphenylether) in products. UBA-Texte 31/05, http://www.umweltdaten.de/publikationen/fpdf-l/2971.pdf
Eljarrat E, Covaci A, Guerra P, Barcel莘 D (2010) Recent methodologies for BFR determinations by means of LC-MS. Hdb Env Chem (in this volume)
Acknowledgements
Dr Alin C. Dirtu acknowledges financial support from the University of Antwerp, Belgium. Dr Adrian Covaci was financially supported by the Research Council of the University of Antwerp and by a postdoctoral fellowship from the Research Scientific Foundation – Flanders (FWO). Prof. Dr Hendrik Emons (IRMM) is acknowledged for the improvement of the metrological aspects.
Disclaimer for the EC. Certain commercial equipment, instruments, and materials are identified in this paper to specify adequately the experimental procedure. In no case does such identification imply recommendation or endorsement by the European Commission, nor does it imply that the material or equipment is necessarily the best available for the purpose.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Covaci, A., Dirtu, A.C., Voorspoels, S., Roosens, L., Lepom, P. (2010). Sample Preparation and Chromatographic Methods Applied to Congener-Specific Analysis of Polybrominated Diphenyl Ethers. In: Eljarrat, E., Barceló, D. (eds) Brominated Flame Retardants. The Handbook of Environmental Chemistry(), vol 16. Springer, Berlin, Heidelberg. https://doi.org/10.1007/698_2010_81
Download citation
DOI: https://doi.org/10.1007/698_2010_81
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-19268-5
Online ISBN: 978-3-642-19269-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)