Abstract
Cancer is a complex pathology of great heterogeneity and difficulty that makes the constant search for new therapies necessary. A major advance on the subject has been made by focusing on the development of new drugs aimed to alter the metabolism of cancer cells, by generating a disruption of mitochondrial function. For this purpose, several new compounds with specific mitochondrial action have been tested, leading successfully to cell death. Recently, attention has centered on a group of natural compounds present in plants named polyphenols, among which is caffeic acid, a polyphenol that has proven to be a powerful antitumoral agent and a prominent compound for studies focused on the development of new therapies against cancer.
In this review, we revised the antitumoral capacity and mechanisms of action of caffeic acid and its derivatives, with special emphasis in a new class of caffeic acid derivatives that target mitochondria by chemical binding to the lipophilic cation triphenylphosphonium.
Graphical Abstract

Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Background
Noncommunicable diseases represent nowadays the main cause of death in almost every country in the world. Between them, cancer emerges as one of the most significant by reporting an incidence of 24.5 million new cases and 9.6 million deaths in 2017 (Fitzmaurice et al. 2019). In Chile, studies indicate from a total of 106,388 deaths registered in 2017, 27,504 were due to cancer, therefore representing 25.9% of all deaths (Comité Nacional de Estadísticas Vitales 2017) and placing this disease among the pathologies responsible for the largest number of deaths country and worldwide.
Cancer has a great heterogeneity in its origin, course, and response to treatment, which is one of the greatest challenges to achieve, due to different patient response, time-tumor progression, type of tissue, genetic and epigenetic mutations, and metabolic alterations (Prasetyanti and Medema 2017). Due to the high degree of variability, various types of treatments have been developed, chemotherapy being one of the most used of those with clinical efficacy. For instance, main drugs used in chemotherapy are classified according to the type of molecular target and mechanism of action in alkylating agents that cause direct damage to DNA – antimetabolites which correspond to analogs of endogenous molecules that are necessary for DNA and RNA synthesis; mitosis inhibitors which act by altering the formation of the mitotic spindle; and topoisomerase inhibitors that are enzymes that regulate the DNA supercoiling. However, there have been reports of several side effects and drug resistance; therefore, the greatest issue is to improve effectiveness and selectivity against cancerous cells by providing other chemical alternatives. As a result, over the last few years, the search for new pharmacological targets has become relevant in order to develop novel drugs with specific action. Within them, mitochondria have turned out to exhibit special characteristics, allowing the design of selectively targeted drugs (Frattaruolo et al. 2020). As is widely known, this organelle plays a fundamental role in the cell, by participating in vital functions such as ATP production, cycle control, production of metabolic proteins, and cell signaling and death, in addition to being closely related with cellular metabolic stress since it is responsible for reactive oxygen species (ROS) production (Grasso et al. 2020).
2 Mitochondria in Cancer Cells
Cancer cells present various genetic, epigenetic, and metabolic alterations, which lead to the loss of normal cell functions and the acquisition of abnormal survival and proliferation capacities. In this reprogramming phenomenon, the mitochondrion plays a fundamental role by transforming its normal bioenergetic metabolism into an elevated glycolytic metabolism that triggers mitochondrial dysfunction, which is known as the Warburg effect (Grasso et al. 2020; Anderson et al. 2018a).
In cancer, the mitochondria manifest a series of alterations as mutations in mitochondrial DNA (mtDNA), affecting the synthesis of enzymes that participate in the tricarboxylic acid (TCA) cycle and the synthesis of complexes that participates in the electron transport chain (ETC). As a consequence, the production of reductor electron equivalents NADH and FADH2 is altered, resulting in the accumulation of TCA cycle intermediates depending on the affected enzyme. These intermediates can act as oncometabolites, as is the case of fumarate and succinate (Grasso et al. 2020). In addition, tumoral cells present a high rate of ROS production, which is a key feature in carcinogenesis as it correlates with the progression of malignancy, as ROS toxicity may induce the alteration of intracellular pathway signaling (Idelchik et al. 2017). The overproduction of ROS induces the accelerated metabolism and the redox imbalance that cancer cells possess, which prevents the neutralization of ROS. When cells are immersed in hypoxia conditions or a high metabolic rate, electrons can leak from complexes I and III of the ETC and conjugate with O2 forming a superoxide anion (O2−), which is the main cellular ROS. In this sense, if O2− cannot be neutralized, it will lead to the oxidation of cellular macromolecules such as DNA, lipids, and proteins (Grasso et al. 2020). As it is well known, ROS neutralization is given by cellular antioxidant mechanisms such as superoxide dismutases (SODs) – which catalyze the reaction of production of H2O2 from O2− – and the catalase (CAT) and glutathione peroxidase (GSH-Px) enzymes that catalyze reactions where H2O is produced from H2O2– (Idelchik et al. 2017).
Additionally, mitochondria in tumoral cells are characterized by presenting an abnormal inner mitochondrial membrane (IMM) potential that is higher when compared to normal cells, therefore presenting a more electronegative charge (Kalyanaraman et al. 2018; Modica-Napolitano and Weissig 2015).
3 Antineoplastic Agents Targeting Mitochondrial Function
Given the fact that mitochondria participate in multiple metabolic functions, researchers may employ different strategies to develop more effective drugs, such as targeting the TCA cycle, ETC, mitochondrial biogenesis, or the mitochondrial apoptotic pathway (Frattaruolo et al. 2020; Grasso et al. 2020; Anderson et al. 2018a). Among drugs under study or between those already approved by the US Food and Drug Administration (FDA) are those that operate on the TCA cycle such as CB-839, CPI-613, ivosidenib (AG-120), enasidenib (AG-221), and vorasidenib (AG-881) (Frattaruolo et al. 2020; Grasso et al. 2020; Anderson et al. 2018a, b; Konteatis et al. 2020) (Fig. 1).
In this sense, it has been described that CB-839 is a specific inhibitor of glutaminase. This enzyme converts glutamine into glutamate, in what is supposed to be a physiological process in cells, upregulated in cancer. Excess of glutamate has been associated with an increase in cell proliferation and malignancy (Grasso et al. 2020; Chen and Cui 2015). Additionally, CPI-613 corresponds to a lipoate analog that inhibits the pyruvate dehydrogenase and α-ketoglutarate dehydrogenase enzymes, therefore preventing the incorporation to the TCA cycle of carbons derived from glucose or glutamine (Anderson et al. 2018a; Stuart et al. 2014). Further, ivosidenib, enasidenib, and vorasidenib work by inhibiting mutant isocitrate dehydrogenase (mIDH) enzymes. Under normal conditions, isocitrate dehydrogenase should catalyze the conversion of isocitrate to α-ketoglutarate (α-KG); however, when mutated it can catalyze the conversion of α-KG to the oncometabolite 2-hydroxyglutarate (2-HG) (Frattaruolo et al. 2020; Anderson et al. 2018a, b; Abou Dalle and DiNardo 2018). Particularly, ivosidenib inhibits the mutant mIDH1 enzyme, enasidenib inhibits mIDH2, and vorasidenib inhibits both mIDH1 and mIDH2, as described above (Konteatis et al. 2020; Abou Dalle and DiNardo 2018; Popovici-Muller et al. 2018). The mutual mechanism of action consists of blocking the active site that catalyzes the conversion reaction of α-KG to 2-HG (Anderson et al. 2018a, b).
Another strategy that has been developed is to target the different complexes of the ETC (Frattaruolo et al. 2020; Urra et al. 2017; Ashton et al. 2018). As a result, there are several series of drugs described as inhibitors of complex I that can be classified into rotenoids, vanilloids, alkaloids, biguanides, annonaceous acetogenins, and polyphenols (Urra et al. 2017). The latter have been recently attracted attention for their multiple beneficial effects, including antitumoral action (Zhou et al. 2016). Complex I-targeted drugs can inhibit complex I in a competitive or noncompetitive way, being common for competitive compounds to have a hydroquinone/quinone structure, while noncompetitive compounds, such as metformin and other biguanides, can bind non-competitively to different domains of complex I (Frattaruolo et al. 2020). Drugs targeting complex II include α-tocopherol succinate, gracillin, and atpenins. These agents have been described to increase ROS production, leading to apoptosis in cancer cells (Frattaruolo et al. 2020). Among the complex III inhibitor drugs is atovaquone, a ubiquinone analog that can competitively inhibit complex III of ETC as a result of its structural similarity to CoQ10 (Fiorillo et al. 2016). Finally, between drugs whose action is focused on complex IV, the more relevant is arsenic trioxide, a drug used to treat acute promyelocytic leukemia (Ashton et al. 2018).
In addition, researchers have also developed pharmacological compounds interfering with mitochondrial biogenesis, either by preventing the processes of transcription and translation of mtDNA or by altering the fission and fusion dynamics of this organelle (Frattaruolo et al. 2020; Anderson et al. 2018a). Between those that interfere with the DNA-translation process are doxycycline and tigecycline, compounds commonly used as antibiotics that have shown antitumoral effects in many cancer cell lines. As bacteriostatics, they can exert their effect by binding to the 30S ribosomal subunit of bacteria due to the structural similarities between this subunit and mitochondrial 28S ribosomal subunit. Consequently they can block the entry of aminoacyl-tRNA to the A-site of the ribosome, inhibiting the process of elongation and translation of mitochondrial proteins (Dong et al. 2019; Protasoni et al. 2018). Likewise, mitochondrial division inhibitor 1 (MDIVI-1) and indomethacin inhibit mitochondrial fission – an increased process in cancer cells – by blocking dynamin-related protein 1 (DRP-1), which affects mitochondrial dynamics (Frattaruolo et al. 2020; Anderson et al. 2018a; Mazumder et al. 2019).
Another pharmacological strategy is to restore mitochondrial-induced apoptosis, as occurs with resveratrol and venetoclax by inducing mitochondrial release of cytochrome c (Cyt c), therefore initiating the apoptotic cascade by activating the caspase pathway (Frattaruolo et al. 2020; Anderson et al. 2018a).
In recent years, drugs that can inhibit mitochondrial ROS (mtROS) production have been proposed as new strategy, especially those based on antioxidant compounds capable of selectively targeting the mitochondria (Grasso et al. 2020). The accumulation of mtROS produces several alterations in various signaling pathways, activating survival and proliferation factors such as hypoxia-induced factor 1 (HIF-1) and hypoxia-induced factor 2 (HIF-2). As a consequence, it has been described that cells present an elevated angiogenesis and glycolytic enzyme activity that allow the maintenance of ATP production for the tumor cell, despite of increased mtROS (Dickerson et al. 2017). Although excessive mtROS production is a key process in the development of cancer since it leads to the oxidation of cellular macromolecules (Grasso et al. 2020) and malignant cell transformation, it has been largely observed that very high increases in its production cause the death of cancer cells (Idelchik et al. 2017; Dickerson et al. 2017). However, mechanisms of action of many drugs that causes mtROS accumulation have not yet been thoroughly elucidated.
4 Polyphenols as Antitumoral Agents
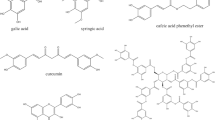
Nowadays, the approach of antitumoral therapy focuses on the beneficial effects of polyphenols, a group of compounds that contain two or more phenolic groups. Given their chemical structure, polyphenols have been extensively described as antioxidant, anti-inflammatory, antimicrobial, and antitumoral agents (Zhou et al. 2016). They can be classified into different categories based on their structure and number of phenolic rings, such as stilbenes, lignans, phenolic alcohols, flavonoids, and phenolic acids. This last group can be subdivided into derivatives of hydroxybenzoic acid or hydroxycinnamic acid (Quiñones et al. 2012). Within hydroxycinnamic acid derivatives is caffeic acid (CA), which exhibits powerful anti-inflammatory, antitumoral, and antioxidant effects, thus controlling oxidative stress by free radicals that constitutes a key process in cancer progression (Caffeic acid 2020). The biological effects exhibited by CA are closely related to its chemical structure. Therefore, its powerful antioxidant effects are associated with the presence of a catechol group in its structure, which is known for its great reducing capacity resulting from two hydroxyl groups (Damasceno et al. 2017).
Antioxidants can be classified as primary or secondary according to their mechanism of action. If they react directly with the radical, they are classified as primary antioxidants, while if they have an indirect effect on radicals, they are classified as secondary antioxidants (Damasceno et al. 2017). CA exhibits both mechanisms. As a primary antioxidant, CA has demonstrated direct neutralization of free radicals by donating protons from its hydroxyl groups to form stable compounds that are not able to produce oxidative damage to cell structures. In the process, CA acquires a semiquinone structure when it has one oxidized group and then an o-quinone structure when both groups are (Damasceno et al. 2017) (Fig. 2). As a secondary antioxidant, CA acts through the chelation of transition metals, which catalyze the decomposition of H2O2 in hydroxyl radical (OH-). This radical has a great redox potential, which is why it produces oxidative damage in the cell by reacting with lipids, proteins, and nucleic acids (Damasceno et al. 2017). In this process, CA also undergoes structural transformation to semiquinone and o-quinone.
Possible mechanism involved in the antioxidant activity of caffeic acid. (Damasceno et al. 2017)
As mentioned above, high concentration CA can behave as prooxidants, and it is mainly to this effect that their effective antitumoral and proapoptotic capacities are associated (Damasceno et al. 2017). Therefore, the administration of high concentrations of CA to cancer cells results in the production of very high quantities of free radicals in presence of O2 or Cu+2, thus causing extensive oxidative damage and consequently triggering death in tumor cells, without significant side effects on non-cancerous cells, since they have a normal antioxidant balance (Damasceno et al. 2017).
5 Caffeic Acid and Its Derivatives with Antitumoral Action
Numerous studies have shown that CA and its derivatives have proven to be effective on different types of cancer, exhibiting antiproliferative, proapoptotic, antiangiogenic, and antimetastatic effects (Chiang et al. 2014; Monteiro Espíndola et al. 2019; Kabała-Dzik et al. 2018). As in colorectal cancer, CA derivatives such as caffeic acid phenethyl ester (CAPE) and caffeic acid phenylpropyl ester (CAPPE) (Fig. 3) have been described with antiproliferative effects by inducing cell cycle arrest as resulting from the suppression of the mechanistic target of rapamycin (mTOR) and the phosphoinositide 3-kinase/protein kinase B (PI3-K/Akt) signaling pathways (Chiang et al. 2014). Both targets induce cell proliferation and are overexpressed in cancer. The activation of the PI3-K/Akt pathway improves cell proliferation by increasing levels of cyclin D1, which is involved in the progression of the cell cycle from G1 to S phase, hence the antiproliferative effect resulting from the inhibition of this pathway induced by these derivatives.
Chemical structure of CAPE (a) and CAPPE (b). (Chiang et al. 2014)
In addition, CAPE and CAPPE produce an increased activity of the AMP-activated protein kinase (AMPK), which is defined as an energy sensor involved in the maintenance of cellular energy homeostasis. Increased AMPK activity is inversely associated with the risk of cancer by suppressing mTOR activity and increasing apoptosis in cancer cells (Chiang et al. 2014).
As indicated by Monteiro et al., studies in hepatocarcinoma have demonstrated the antiangiogenic and antimetastatic capacity of CA and CAPE. The latter has shown the activation of intrinsic and extrinsic apoptotic pathways (Monteiro Espíndola et al. 2019). In addition, the antiangiogenic capacity of CAPE is given by the inhibition of HIF-1α, which has an increased expression in tumor cells due to the hypoxic environment in which they develop, thus increasing the expression of vascular endothelial growth factor (VEGF) (Monteiro Espíndola et al. 2019). Furthermore, CAPE has an antimetastatic capacity exerted through inhibiting the expression of matrix metalloproteinase 2 (MMP2) and matrix metalloproteinase 9 (MMP9), by suppressing the expression of nuclear factor kappa B (NFκB) (Monteiro Espíndola et al. 2019). These molecules are known for their role in the degradation of the extracellular matrix, which is why they can promote metastasis as a result of their overexpression in cancer.
Additionally, CAPE successfully altered the mitochondrial membrane potential (MMP) in vitro, causing the release of Cyt c from the MMI. This event increases the activation of caspase 9, promoting apoptosis through the intrinsic pathway. Moreover, CAPE also activated the extrinsic pathway of apoptosis that is mediated by the apoptosis-inducing ligand related to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), through upregulation of the death receptor 5 (DR5) resulting from the activation of mitogen-activated protein kinase (p 38) (Monteiro Espíndola et al. 2019).
In studies with breast adenocarcinoma conducted by Kabała-Dzik et al., CA and CAPE have demonstrated a dose- and time-dependent cytotoxic effect, showing greater effects at 48 h rather than 24 h, with a concentration of 100 μM for CA compared to 10 μM for CAPE (Kabała-Dzik et al. 2018). CAPE showed a more powerful cytotoxic capacity than CA, presenting a lower IC50 (inhibitory concentration 50) value than the one exhibited by CA (Kabała-Dzik et al. 2018). CA and CAPE also induced the inhibition of migratory capacity in a dose-dependent manner, showing again that CAPE was more potent than CA (Kabała-Dzik et al. 2018).
6 Mitochondriotropic Derivatives of Caffeic Acid
Given the extensive evidence promoting caffeic acid and its derivatives as powerful antitumoral agents, the idea of creating mitochondria-targeted compounds based on CA emerged to maximize the arrival at their site of action (Teixeira et al. 2018). To achieve this goal, several methods have been developed including those based on compounds linked to triphenylphosphonium (TPP+) (Zielonka et al. 2017).
TPP+ is a lipophilic cation widely used to direct various molecules to the mitochondria since its delocalized positive charge allows selective accumulation within this organelle. This process occurs in two phases. First, the compound enters the cell guided by the electrical attraction generated by a negative plasma membrane potential (ΔΨp) of 30–60 mV. Second, it enters the mitochondria as a result of the even more negative membrane potential of the MMI (ΔΨm), which is between 150 and 180 mV. Therefore, TPP+ acts as a driving force for its accumulation in the mitochondria against the concentration gradient, reaching intracellular concentrations 100–1000 times higher. This feature leads to micromolar range concentrations of these compounds, thus achieving millimolar concentrations within the mitochondria (Zielonka et al. 2017). Furthermore, the incorporation of an alkyl chain serves as a link between TPP+ and the compound of interest, giving different degrees of lipophilicity depending on its length, being more lipophilic with a longer chain length and vice versa (Zielonka et al. 2017) (Fig. 4).
There are only a few studies of CA derivatives linked to a mitochondrial target delivery system such as TPP+. Consequently, Teixeira et al. developed a series of mitochondriotropic antioxidants derived from CA named “AntiOxCINs.” These antioxidants are constituted from a primary compound of CA linked to TPP+ which they have called “compound N° 1.” Then, they synthetized novel compounds that present the catechol characteristic of CA, named as “compounds N° 22,” “N° 23,” and “N° 24.” In addition, they synthetized another group of compounds derived from pyrogallol group named as “compounds N° 25,” “N° 26,” and “N° 27.” Both groups of new compounds are identical, except for the length of the alkyl chain which can be of six, eight, or ten carbons, as shown in Fig. 5 (Teixeira et al. 2017). The results obtained show that AntiOxCINs present a high chelating capacity on transition metals (even similar to the activity exhibited by EDTA, a known chelating agent), and an increased mitochondrial uptake and ability to prevent mitochondrial membrane lipid peroxidation, compared to compound N° 1. In addition, the compounds were shown to induce the opening of the mitochondrial permeability transition pore (mPTP) (Teixeira et al. 2017), which has a known role in cell death. Its opening causes the mitochondrial release of Cyt c, thus initiating apoptotic signaling pathways (Grasso et al. 2020). Additional tests were performed on compounds N° 24 and N° 25, as they proved to be the most promising ones. The results illustrated their ability to prevent ROS and Fe+2-related cytotoxicity in HepG2 cells at concentrations of 2.5 μM (compound N° 24) and 100 μM (compound N° 25), within incubation periods of 48 h. In addition, these derivatives did not induce proapoptotic changes related to nuclear morphology or mitochondrial depolarization in normal cells, suggesting their safe use in every type of cells (Teixeira et al. 2017).
Chemical structure of the caffeic acid mitochondriotropic derivatives “AntiOxCINs” developed by Teixeira et al. (2017). Compounds Nº 1, 22, 23, 24, 25, 26 and 27
Furthermore, in a study developed by Li et al. (2017), mitochondriotropic compounds based on hydroxycinnamic acid derivatives were synthetized, named “MitoHCAs” (Li et al. 2017). The mitochondriotropic character of these compounds was also achieved by binding to TPP+ (Li et al. 2017). In relation to these novel compounds, four new mitochondriotropic compounds based on p-coumaric (Mitop-CoA), caffeic (MitoCaA), ferulic (MitoFA), and cinnamic (MitoCA) acids were synthesized (Fig. 6) and were subjected to several tests evaluating their antioxidant and antiproliferative capacities (Li et al. 2017). The results obtained showed that MitoCaA was the most powerful antioxidant since it inhibits lipid peroxidation and exhibits a concentration-dependent behavior (Li et al. 2017). Subsequently, similar results were obtained when comparing the antioxidant capacity of MitoHCAs against endogenous ROS (H2O2) (Li et al. 2017). In addition – in order to comprehend the antioxidant mechanism by which MitoHCAs reduce H2O2 – results showed that MitoHCAs do not have direct radical elimination capacity and neither exert changes in the production of mitochondrial O2−. However, they do affect the expression and activity of antioxidant enzymes: MitoCaA, MitoFA, and Mitop-CoA increased significantly the activity of the GSH-Px and CAT enzymes, suggesting this is the mechanism by which MitoHCAs decrease H2O2 (Li et al. 2017). Furthermore, both MitoCaA and Mitop-CoA were found to have an inhibitory effect on mitochondrial SOD, while MitoFA did not (Li et al. 2017). Additionally, antiproliferative capacity of MitoHCAs against human hepatoma HepG2 cells and normal cell lines (human liver L02 and WI38 diploid human fibroblasts) was also evaluated, resulting in a selective inhibition of cell viability over HepG2 cancer cells compared to normal cells (Li et al. 2017). MitoCA, MitoFA, and MitoCaA compounds demonstrated the most potent antiproliferative capacity against HepG2 cells at 48 h of cell treatment (Li et al. 2017). In addition, selectivity indexes (SI) for MitoHCAs were calculated, which correspond to the quotient between the antiproliferative activity of the compounds in normal cells and the same activity but in HepG2 cancer cells. The compounds with the higher SI were MitoCA, MitoFA, and MitoCaA. The latter was determined as the most selective (Li et al. 2017). Since MitoCaA was the compound with the highest antioxidant and antiproliferative capacity, additional studies were performed. The results showed that MitoCaA caused mitochondrial fragmentation in HepG2 cells in a dose-dependent manner, causing donut-shaped morphology and a discontinuous mitochondrial network at a concentration of 20 μM. Under the same experimental conditions, the compound failed to produce mitochondrial damage in normal L02 cells (Li et al. 2017), although apoptotic assays did demonstrate that MitoCaA possesses a dose-dependent apoptotic effect (Li et al. 2017). MitoCaA was able to induce the apoptosis mechanism through the opening of mPTP and its consequent release of mitochondrial Cyt c (Li et al. 2017). These results differ from the tests performed by Teixeira et al., where it was reported that CA-based mitochondriotropic derivatives would not have an effect on mPTP opening. The difference could be attributed to structural variability in molecular targets and mechanisms of action, despite they originated from the same compound, which requires further studies.
Chemical structure of the hydroxycinnamic acid derivatives “MitoHCAs” developed by Li et al. (2017)
7 Conclusion
Polyphenols and especially CA have been described before as antitumoral, antimicrobial, and antioxidant agents. The novel mitochondriotropic derivatives of caffeic acid have shown to maximize their effects on tumor cells by allowing its selective accumulation within the cancer mitochondria, an organelle on which they exert their effects by altering the redox state and ultimately leading to mitochondrial-mediated apoptosis process. The effects of increased lipophilicity and the mitochondrial targeting product of the link to TPP+ moiety are reflected in more potent compounds than those without TPP+. The selectivity of these compounds for cancer cells is also highlighted, which could be given by the difference in MMP between normal and cancerous cells, the latter exhibiting a higher MMP that leads to the selective accumulation of mitochondrial derivatives. This would indicate a safe use of these compounds, also reducing side effects, a common problem in chemotherapy due to the low selectivity of these drugs. Therefore, in initial studies on tumor cell lines, mitochondriotropic derivatives of CA appear as promising and powerful antitumoral agents for future development of new molecules with targeted approach for cancer therapy, given their increased potency and selectivity.
Abbreviations
- α-KG:
-
Alpha-ketoglutarate
- ΔΨp:
-
Plasma membrane potential
- ΔΨm:
-
Membrane potential of the MII
- 2-HG:
-
2-Hydroxyglutarate
- AMPK:
-
AMP-activated protein kinase
- CA:
-
Caffeic acid
- CAPE:
-
Caffeic acid phenethyl ester
- CAPPE:
-
Caffeic acid phenylpropyl ester
- Cyt c:
-
Cytochrome c
- DR5:
-
Death receptor 5
- DRP-1:
-
Dynamin-related protein 1
- ETC:
-
Electron transport chain
- FDA:
-
Food and Drug Administration
- GSH-Px:
-
Glutathione peroxidase
- HIF:
-
Hypoxia-induced factor
- IMM:
-
Inner mitochondrial membrane
- IC50:
-
Inhibitory concentration 50
- MDIVI-1:
-
Mitochondrial division inhibitor 1
- MitoCaA:
-
Mitochondriotropic caffeic acid
- MitoCA:
-
Mitochondriotropic cinnamic acid
- MitoFA:
-
Mitochondriotropic ferulic acid
- Mitop-CoA:
-
Mitochondriotropic p-coumaric acid
- mIDH:
-
Mutant isocitrate dehydrogenase
- MMP:
-
Mitochondrial membrane potential
- MMP2:
-
Matrix metalloproteinase 2
- MMP9:
-
Matrix metalloproteinase 9
- mtDNA:
-
Mitochondrial DNA
- mtROS:
-
Mitochondrial ROS
- mTOR:
-
Mechanistic target of rapamycin
- NFκB:
-
Nuclear factor kappa B
- O2-:
-
Superoxide anion
- OH-:
-
Hydroxyl radical
- PI3-K/Akt:
-
Phosphoinositide 3-kinase/protein kinase B
- p38:
-
Mitogen-activated protein kinase
- ROS:
-
Reactive oxygen species
- SI:
-
Selectivity indexes
- SODs:
-
Superoxide dismutases
- TCA:
-
Tricarboxylic acid
- TRAIL:
-
Tumor necrosis factor-related apoptosis-inducing ligand
- TPP+:
-
Triphenylphosphonium
- VEGF:
-
Vascular endothelial growth factor
References
Abou Dalle I, DiNardo CD (2018, Jul) The role of enasidenib in the treatment of mutant IDH2 acute myeloid leukemia. Ther Adv Hematol 9(7):163–173. https://doi.org/10.1177/2040620718777467
Anderson RG, Ghiraldeli LP, Pardee TS (2018a) Mitochondria in cancer metabolism, an organelle whose time has come? Biochim Biophys Acta Rev Cancer 1870(1):96–102. https://doi.org/10.1016/j.bbcan.2018.05.005. Elsevier B.V., Aug. 01, 2018
Anderson NM, Mucka P, Kern JG, Feng H (2018b) The emerging role and targetability of the TCA cycle in cancer metabolism. Protein Cell 9(2):216–237. https://doi.org/10.1007/s13238-017-0451-1. Higher Education Press, Feb. 01, 2018
Ashton TM, Gillies McKenna W, Kunz-Schughart LA, Higgins GS (2018) Oxidative phosphorylation as an emerging target in cancer therapy. Clin Cancer Res 24(11):2482–2490. https://doi.org/10.1158/1078-0432.CCR-17-3070. American Association for Cancer Research Inc., Jun. 01, 2018
***, “Caffeic acid | C9H8O4 – PubChem.” https://pubchem.ncbi.nlm.nih.gov/compound/689043#section=Pharmacology-and-Biochemistry. Accessed 29 July 2020
Chen L, Cui H (2015) Targeting glutamine induces apoptosis: a cancer therapy approach. Int J Mol Sci 16(9):22830–22855. https://doi.org/10.3390/ijms160922830. MDPI AG, Sep. 22, 2015
Chiang E-PI et al (2014, Jun) Caffeic acid derivatives inhibit the growth of colon cancer: involvement of the PI3-K/Akt and AMPK signaling pathways. PLoS One 9(6):e99631. https://doi.org/10.1371/journal.pone.0099631
Comité Nacional de Estadísticas Vitales (2017) ANUARIO DE ESTADÍSTICAS VITALES, 2017 Período de información : 2017. Anu. Estad. Vitales
Damasceno SS, Dantas BB, Ribeiro-Filho J, Antônio D, Araújo M, da Costa JGM (2017) Chemical properties of caffeic and ferulic acids in biological system: implications in cancer therapy. A review. Curr Pharm Des 23(20):3015–3023. https://doi.org/10.2174/1381612822666161208145508
Dickerson T, Jauregui CE, Teng Y (2017) Friend or foe? Mitochondria as a pharmacological target in cancer treatment. Fut Med Chem 9(18):2197–2210. https://doi.org/10.4155/fmc-2017-0110. Future Medicine Ltd., Dec. 01, 2017
Dong Z et al (2019) Biological functions and molecular mechanisms of antibiotic tigecycline in the treatment of cancers. Int J Mol Sci 20(14). https://doi.org/10.3390/ijms20143577. MDPI AG, Jul. 02, 2019
Fiorillo M et al (2016, Jun) Repurposing atovaquone: targeting mitochondrial complex III and OXPHOS to eradicate cancer stem cells. Oncotarget 7(23):34084–34099. https://doi.org/10.18632/oncotarget.9122
Fitzmaurice C et al (2019, Dec) Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol 5(12):1749–1768. https://doi.org/10.1001/jamaoncol.2019.2996
Frattaruolo L, Brindisi M, Curcio R, Marra F, Dolce V, Cappello AR (2020) Targeting the mitochondrial metabolic network: a promising strategy in cancer treatment. Int J Mol Sci 21(17):1–21. https://doi.org/10.3390/ijms21176014. MDPI AG, Sep. 01, 2020
Grasso D, Zampieri LX, Capelôa T, Van De Velde JA, Sonveaux P (2020) Mitochondria in cancer. Cell Stress 4(6):114–146. https://doi.org/10.15698/cst2020.06.221. Isfahan University of Medical Sciences (IUMS), Jun. 01, 2020
Idelchik M d PS, Begley U, Begley TJ, Melendez JA (2017, Dec) Mitochondrial ROS control of cancer. Semin Cancer Biol 47:57–66. https://doi.org/10.1016/j.semcancer.2017.04.005
Kabała-Dzik A, Rzepecka-Stojko A, Kubina R, Wojtyczka RD, Buszman E, Stojko J (2018, Dec) Caffeic acid versus caffeic acid phenethyl ester in the treatment of breast cancer MCF-7 cells: migration rate inhibition. Integr Cancer Ther 17(4):1247–1259. https://doi.org/10.1177/1534735418801521
Kalyanaraman B et al (2018) A review of the basics of mitochondrial bioenergetics, metabolism, and related signaling pathways in cancer cells: therapeutic targeting of tumor mitochondria with lipophilic cationic compounds. Redox Biol 14:316–327. https://doi.org/10.1016/j.redox.2017.09.020. Elsevier B.V., Apr. 01, 2018
Konteatis Z et al (2020, Feb) Vorasidenib (AG-881): a first-in-class, brain-penetrant dual inhibitor of mutant IDH1 and 2 for treatment of glioma. ACS Med Chem Lett 11(2):101–107. https://doi.org/10.1021/acsmedchemlett.9b00509
Li J et al (2017, Jan) Synthesis of hydroxycinnamic acid derivatives as mitochondria-targeted antioxidants and cytotoxic agents. Acta Pharm Sin B 7(1):106–115. https://doi.org/10.1016/j.apsb.2016.05.002
Mazumder S et al (2019, May) Indomethacin impairs mitochondrial dynamics by activating the PKCζ-p38-DRP1 pathway and inducing apoptosis in gastric cancer and normal mucosal cells. J Biol Chem 294(20):8238–8258. https://doi.org/10.1074/jbc.RA118.004415
Modica-Napolitano JS, Weissig V (2015) Treatment strategies that enhance the efficacy and selectivity of mitochondria-targeted anticancer agents. Int J Mol Sci 16(8):17394–17421. https://doi.org/10.3390/ijms160817394. MDPI AG, Jul. 29, 2015
Monteiro Espíndola KM et al (2019) Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front Oncol 9(JUN):541. https://doi.org/10.3389/fonc.2019.00541. Frontiers Media S.A., 2019
Popovici-Muller J et al (2018, Apr) Discovery of AG-120 (Ivosidenib): a first-in-class mutant IDH1 inhibitor for the treatment of IDH1 mutant cancers. ACS Med Chem Lett 9(4):300–305. https://doi.org/10.1021/acsmedchemlett.7b00421
Prasetyanti PR, Medema JP (2017) Intra-tumor heterogeneity from a cancer stem cell perspective. Mol Canc 16(1):41. https://doi.org/10.1186/s12943-017-0600-4. BioMed Central Ltd., Feb. 16, 2017
Protasoni M, Kroon AM, Taanman JW (2018, Sep) Mitochondria as oncotarget: a comparison between the tetracycline analogs doxycycline and COL-3. Oncotarget 9(73):33818–33831. https://doi.org/10.18632/oncotarget.26107
Quiñones M, Miguel M, Aleixandre A (2012) Los polifenoles, compuestos de origen natural con efectos saludables sobre el sistema cardiovascular. Nutr Hosp organo Of la Soc Espa??ola Nutr Parenter y Enter 27(1):76–89. https://doi.org/10.3305/nh.2012.27.1.5418
Stuart SD et al (2014) A strategically designed small molecule attacks alpha-ketoglutarate dehydrogenase in tumor cells through a redox process. Cancer Metab 2(1):4. https://doi.org/10.1186/2049-3002-2-4
Teixeira J et al (2017) Development of a mitochondriotropic antioxidant based on caffeic acid: proof of concept on cellular and mitochondrial oxidative stress models. J Med Chem 60(16):7084–7098. https://doi.org/10.1021/acs.jmedchem.7b00741
Teixeira J, Deus CM, Borges F, Oliveira PJ (2018) Mitochondria: targeting mitochondrial reactive oxygen species with mitochondriotropic polyphenolic-based antioxidants. Int J Biochem Cell Biol 97:98–103. https://doi.org/10.1016/j.biocel.2018.02.007. Elsevier Ltd, Apr. 01, 2018
Urra FA, Muñoz F, Lovy A, Cárdenas C (2017) The mitochondrial complex(I)ty of cancer. Front Oncol 7(JUN):118. https://doi.org/10.3389/fonc.2017.00118. Frontiers Media S.A., Jun. 08, 2017
Zhou Y et al (2016) Natural polyphenols for prevention and treatment of cancer. Nutrients 8(8). https://doi.org/10.3390/nu8080515. MDPI AG, Aug. 22, 2016
Zielonka J et al (2017) Mitochondria-targeted triphenylphosphonium-based compounds: syntheses, mechanisms of action, and therapeutic and diagnostic applications. Chem Rev 117(15):10043–10120. https://doi.org/10.1021/acs.chemrev.7b00042. American Chemical Society, Aug. 09, 2017
Acknowledgments
This review was supported by Fondo Nacional de Ciencia e Investigación (FONDECYT) grant 11160281 (M.C.).
Supplementary Materials
Not applied.
Author Contributions
H.B., G.A.V., and M.C. wrote the manuscript. H.B. and G.A.V. did all the figures and table. G.A.V., G.C., J.A.J., and MC edited the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bastidas, H., Araya-Valdés, G., Cortés, G., Jara, J.A., Catalán, M. (2022). Pharmacological Effects of Caffeic Acid and Its Derivatives in Cancer: New Targeted Compounds for the Mitochondria. In: Turksen, K. (eds) Cell Biology and Translational Medicine, Volume 17. Advances in Experimental Medicine and Biology(), vol 1401. Springer, Cham. https://doi.org/10.1007/5584_2022_718
Download citation
DOI: https://doi.org/10.1007/5584_2022_718
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-20513-2
Online ISBN: 978-3-031-20514-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)