Abstract
Background: Due to the advances in catheter-based interventional techniques, a wide range of heart diseases can now be treated with a purely interventional approach. Little is yet known regarding biological effects at the intracardiac implantation site or the effects on endothelialization and vascular inflammation in an in vivo environment. Detailed knowledge of ongoing vascular response, the process of endothelialization, and possible systemic inflammatory reactions after implantation is crucial for the clinical routine, since implants usually remain in the body for a lifetime.
Methods: For this narrative review, we conducted an extensive profound PubMed analysis of the current literature on the endothelialization processes of intracardially implanted devices, such as persistent foramen ovale (PFO) occluders, atrial septal defect (ASD) occluders, left atrial appendage (LAA) occluders, transcatheter aortic valve implantations (TAVIs), and leadless pacemakers. Additionally, the known biological activities of common metallic and synthetic components of intracardiac devices in an “in vivo” setting have been evaluated.
Results: Nitinol, an alloy of nickel and titanium, is by far the most commonly used material found in intracardiac devices. Although allergies to both components are known, implantation can be performed safely in the vast majority of patients. Depending on the device used, endothelialization can be expected within a time frame of 3–6 months. For those patients with a known allergy, gold coating may be considered as a viable alternative.
Conclusion: Based on our analysis, we conclude that the vast majority of devices are made of a material that is both safe to implant and nontoxic in long-term treatment according to the current knowledge. The literature on the respective duration of endothelialization of individual devices however is highly divergent.
The authors declare that there is no conflict of interests regarding the publication of this paper. All companies have granted image rights to depict their devices in this article.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Interventional cardiology is a rapidly evolving field in modern clinical medicine. Although the enormous therapeutic potential was not immediately recognized after Forßmann carried out the first catheterization of the right heart in 1929 in a heroic self-experiment, the idea was taken up and further developed by Cournand et al., who are today regarded as founders of interventional cardiology (Forssmann-Falck 1997; Nicholls 2020).
Grüntzig’s first successful percutaneous coronary intervention (PCI) in 1977 marked the dawn of a new era in clinical cardiology as catheter-based therapy changed from a purely diagnostic tool to an interventional treatment option for acute coronary syndrome (Ar et al. 1979).
During recent years, interventional cardiology has undergone an enormous transformation as catheter technology has developed rapidly and is used in many different cardiological diseases. Today, in addition to coronary heart disease, a wide range of valvular diseases or congenital heart defects, such as atrial septal defect (ASD) (Sievert et al. 1998) and persistent foramen ovale (PFO) can be successfully treated using a catheter-based interventional approach. Even the catheter-based implantation of cardiac pacemakers is widely used in countless clinics today.
Permanent intracardiac placement of devices represents an enormous challenge for product engineers, as all components must offer exceptional stability and durability combined with low weight and small size. In addition, any device exposed to blood flow in an “in vivo” environment must be safe in terms of hemostasiological interactions; more precisely, the device must not initiate thrombogenic or even hemolytic cascades. Furthermore, the materials components must have an extremely low allergic potential, both at the implantation site and, in case of systemic reactions, in the entire organism.
All the devices must undergo extensive testing on safety and durability for market approval in order to achieve CE certification. In addition, an optimally designed intracardiac device should also have acceptable biocompatibility, especially rapid endothelialization, to reduce the duration of anticoagulant use with the goal of reduced bleeding risk and to safely discontinue use of endocarditis prophylaxis.
The aim of this narrative review is to provide an overview of the current state of knowledge on endothelialization in common intracardiac devices as well as an overview of the known in vivo interactions, and the important components of different devices.
2 Intracardiac Devices
2.1 PFO Occluders
The patent foramen ovale (PFO) is an essential component of intrauterine circulation, allowing blood to bypass fetal lungs. Although spontaneous occlusion should occur shortly after birth, this physiological process is absent or incomplete in up to 27.3% of the population (Hagen et al. 1984).
In the vast majority of cases, a PFO does not have a clinical relevance. Nevertheless, it may play a crucial role in the genesis of migraine headaches or even cryptogenic stroke (Saver et al. 2017). In symptomatic patients, interventional treatment with an occluder can be considered to permanently close the opening. Recent studies (CLOSE, REDUCE, RESPECT LT, and DEFENSE PFO) showed that interventional PFO occlusion (Fig. 1a) was associated with a significant reduction in recurrent stroke compared to drug therapy (Mas et al. 2017; Saver et al. 2017; Søndergaard et al. 2017). Based on the long-term results of RESPECT and the results of the REDUCE study, two occluder devices, the AMPLATZER PFO closure and GSO (GORE Medical, Flagstaff, AZ, USA) received US Food and Drug Administration (FDA) approval for secondary stroke prevention in 2016 and 2018, respectively.
The later developed Flex II PFO occluder (Occlutech, Jena, Germany) received CE certification for clinical use in Europe in 2009. According to the manufacturer, more than 33,000 such devices have been delivered worldwide.
2.1.1 Amplatzer PFO Occluder
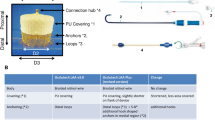
The Amplatzer occluder (Abbott Cardiovascular, North Chicago, Illinois, USA), first implanted in 1997, has been very well described elsewhere (Meier 2005; Madhkour et al. 2019). In brief, it is a double disc made of Nitinol mesh, the inside of which is made of polyester fabric.
A thin neck, which consists of the tightly woven wires of the discs, serves as a connector. The neck is rotated around its longitudinal axis so that it can be extended in principle (Fig. 1b). The two discs are sewn together with polyester fabric for better stabilization (Scalise et al. 2016).
The special feature is the so-called “shape memory,” which means that the device returns to its original shape after being stretched through the guiding catheter.
The Amplatzer PFO Occluder is available in three different sizes (18 mm, 25 mm, 35 mm), whereby the size specification is based on the size of the right-sided disc. The most frequently implanted occluder is the medium size. The small version has its special value in the case of a small PFO with a largely stable septum primum, whereas the 35 mm version is used in the case of an extremely redundant septum primum and possibly in the case of an atrial septal aneurysm.
According to the current literature, the chances of success are very high, so that complete closure of the shunt can be assumed in well over 90% of cases (Bruch et al. 2002; Greutmann et al. 2009).
Residual shunts require surgical intervention only in rare cases, although the actual average duration until complete endothelialization “in vivo” is not completely clear. In a recent position paper, the German Cardiology Society recommends dual antiplatelet therapy with aspirin and clopidogrel for a period of 6 months. Should there be a concomitant indication for oral anticoagulation, this will be given as monotherapy, with NOACs being preferred.
2.1.2 Gore Septal Occluder
The Gore Septal Occluder (GSO) has been approved for the treatment of PFOs in Europe for 10 years now by means of CE certification.
In contrast to the more commonly implanted Amplatzer Occluder, it is intended to offer advantages in difficult anatomical conditions. The device consists of five nitinol wires formed into a left atrial and a right atrial disc. The outer frame is coated with polytetrafluoroethylene film. For implantation, the device comes already loaded on a 10 French introducer catheter. A special safety feature is the integrated retrieval cord, which can be used to retrieve the device if necessary. When fully deployed, two circular discs are formed, facing each other, which can be fixed in place by a locking mechanism in the center.
2.1.3 Flex II PFO Occluder
The Flex II PFO occluder (Occlutech, Jena, Germany) consists of two self-expanding woven nitinol discs (Fig. 1c). The special feature of this device is a central pin on the left atrial disc and also a ball socket joint connection. The complete closure is achieved by two biocompatible polyethylene terephthalate patches.
The superiority of this device is the flexibility and ability to angulate in order to achieve the maximal adaptation to the interatrial septum. This offers an advantage for the complex anatomical variations (Neuser et al. 2016).
2.2 ASD Occluders
Atrial septal defect (ASD) is a relatively common congenital heart defect with a birth prevalence of 1.43:1000 live births and an expected survival rate into adulthood of 97% (Anderson et al. 2002; Anderson 2016; Lee et al. 2018). ASD of the ostium secundum is the most common type, occurring in 70% of all patients with ASD, followed by ASD of the ostium primum (10%) and ASD of the sinus venosus (5–10%) (Moons et al. 2009). The “true atrial septum,” that is, the tissue directly separating the atrial cavities, is restricted to the base of the oval fossa and the surrounding inferoanterior margins. Defects of the true atrial septum are called “secundum defects.” Atrial septal defects are usually well tolerated in children, but can cause significant complications in adults (Campbell 1970). Early closure is therefore recommended and can be achieved using catheter deployment in the majority of cases. A symptomatic benefit can be seen at any age (Komar et al. 2014) Since left ventricular compliance decreases with age or in the presence of conditions that can increase left atrial pressure (e.g., high blood pressure, ischemic heart disease, cardiomyopathy, aortic and mitral valve disease), the left–right shunt can increase due to ASD (Le Gloan et al. 2018; Kumar et al. 2019). However, conditions that reduce right ventricular compliance (e.g., pulmonary arterial hypertension, pulmonary stenosis, right heart disease, tricuspid valve disease) may eventually reverse the shunt and cause cyanosis (Le Gloan et al. 2018).
A left–right shunt leads to right ventricular volume overload, which in turn results in right ventricular dilatation. It is well tolerated throughout childhood, despite a pulmonary–systemic flow ratio that can exceed 3:1. The pulmonary vascular system is also able to absorb the increased blood flow at low pulmonary artery pressure for many years. A persistent large left–right shunt leads to increased right atrial and right ventricular dilatation from late childhood onwards, which in some patients leads to arrhythmia and a progressive increase in pulmonary vascular resistance (Le Gloan et al. 2018). Severe pulmonary vascular disease is rare (<5%), unless there are other associated factors (Nashat et al. 2018).
2.2.1 Amplatzer Septal Occluder
The Amplatzer® Septal Occluder (ASO) (Abbott Cardiovascular, North Chicago, Illinois, USA; former: St. Jude Medical, Inc., St. Paul, Minnesota) consists of nitinol–titanium memory wire mesh infused with polyester patches that facilitate occlusion and endothelialization (Fig. 2a). It consists of a smaller right and a larger left disc connected by a waist; the difference in size of both discs is 4 mm (Nassif et al. 2016).
ASO has been shown to be a practical, safe, and effective treatment option for ASD (Masura et al. 1997; Podnar et al. 2001; Masura et al. 2005; Cardoso et al. 2007; Knepp et al. 2010). Nevertheless, complications such as implant embolization, mispositioning, and fracture may occur in rare cases. Moreover, cases of erosion/perforation, cardiac arrhythmia, cardiac tamponades, and even infectious endocarditis have been reported (Sievert et al. 1998; Chessa et al. 2002; Fischer et al. 2003; Balasundaram et al. 2005; Sadiq et al. 2012).
2.2.2 Occlutech ASD Occluder
The Figulla Occlutech ASD closures (Occlutech, Jena, Germany) consist of individually braided, very thin (40–150 μm or 0.00157–0.00590 inches) nitinol strands. All strands end proximally and therefore do not require clamping to the left disc (Fig. 2b). This results in a smaller amount of uncovered metallic material. The ultrathin fabrics made out of polyethylene terephthalate (PET) of the device promote the endothelial growth after implantation as well as the defect closure (Pedra et al. 2016). The design of the Flex II ASD occluder is intended to allow ideal alignment of the septum, which in turn should increase feasibility and patient safety during implantation. The device is made of Titanium oxide–covered nitinol, which should result in the lowest possible release of nickel.
2.3 Left Atrial Appendage Occluders
The term “LAA occluder” refers to devices that can be used to close the left atrial appendage (LAA). In patients with atrial fibrillation, the LAA is anatomically particularly important, as this is where the vast majority of cardio-embolic strokes originate (Alli and Holmes, 2015). In patients at high risk for bleeding complications, implantation of an occluder allows discontinuation of anticoagulants after the initial endothelialization phase. The most common product is the Watchman Device, which has been CE certified since 2005. Another product, CE certified in 2013, is the Amulet device, which is designed to provide benefits due to its wide range of available sizes, according to the manufacturer.
2.3.1 Watchman Device
The Watchman device (Boston Scientific, Marlborough, MA, USA) consists of a nitinol frame coated with a permeable 160 micron polyethylene terephthalate knit fabric on the left atrial surface (Fig. 3a) (Fountain et al. 2006). It is a parachute-shaped, self-expanding device (Kramer and Kesselheim, 2015). The PET knit fabric facilitates endothelialization over the device and serves as a filter for emboli that originate from the LAA pouch (Della Rocca et al. 2019). After femoral vein access and transseptal puncture, the Watchman device is delivered using a 12-Fr delivery catheter and is then deployed until its titanium dowel pin separates from the catheter (Fig. 3b). The Watchman device is affixed to the LAA wall by 10 fixation barbs, which are arranged around the mid-perimeter. To match different LAA orifice sizes, the device is manufactured in five sizes (21 mm, 24 mm, 27 mm, 30 mm, and 33 mm) to allow adequate placement. An adequate seal is defined as a leakage < 5 mm.
2.3.2 Amulet Occluder
The AMULET is a second-generation Amplatzer Cardiac Plug (Abbott Cardiovascular, North Chicago, Illinois, USA; former: St. Jude Medical, Inc., St. Paul, Minnesota). The self-expanding device is made of flexible, braided nitinol filled with polyester tissue. It consists of a proximal disc and a distal lobe shaped like a hockey puck connected by a flexible waist (Meerkin et al. 2013). The proximal disc covers the LAA orifice and the distal lobe with stabilization hooks that secure the engagement of the occluder to the LAA wall. The LAA Occluder is delivered by a 12-F or 14-F sheath into the left atrium after a transseptal puncture. It is available in eight sizes. Pre-interventional standard imaging, including transesophageal echocardiography and computerized tomography (CT) scan, is performed in order to determine the proper occluder size. The proximal disc is always slightly larger than the lobe and has a central screw.
2.4 Transcatheter Aortic Valve Implantation
Transfemoral aortic valve replacement, first performed in 2001 by Cribier et al., is beyond doubt one of the greatest achievements in interventional cardiology (Cribier et al. 2002). As alternative to conventional surgical aortic valve replacement, a minimally invasive transcatheter implantation of a valve prothesis is possible. The intervention was originally reserved for medium- to high-risk elderly patients, also due to material durability profiles. In recent years, its indication has been extended to younger patients age <75 years. Recently, transcatheter valve replacement has been approved by the FDA as the method of choice for all patients (Edlinger et al. 2020). The most important challenge is the material compatibility and the special required characteristics of the valve. Those consist of “durability, low thrombogenicity, hydrodynamics, hemocompatibility, low calcification susceptibility and crimping and deployment stability” (Rotman et al. 2018).
Various models have been developed over the years, whereby the self-expanding Medtronic valves (CoreValve, Evolut R) (Medtronic, Minneapolis, MN, USA) and the balloon-expanding Edwards valves (Sapien, Sapien XT, Sapien 3) (Edwards Lifesciences, Irvine, CA, USA) are the most widely implanted valve types (Chakos et al. 2017). The SAPIEN 3 and SAPIEN 3 Ultra are the latest generation of balloon-expanding Edwards valves. The Evolut R and Evolut PRO are the latest self-expanding valves from Medtronic (Renker and Kim, 2020). In several hospitals they consist more than 70% of the total transcatheter aortic valve implantation (TAVI) procedures.
Another important aspect concerns the valve-in-valve procedures. Here is the geometric orifice area that plays the most significant role. Therefore a valve-in-valve procedure should only be used after careful planning, because it can diminish the hydrodynamic performance of the valve and significantly reduce the opening area (Rotman et al. 2018).
2.4.1 Edwards SAPIEN 3/SAPIEN 3 Ultra
The Edwards SAPIEN 3 (Fig. 4a) and SAPIEN 3 Ultra (Fig. 4b) consist of a cobalt–chromium stent frame, three leaflets of bovine pericardium, an inner and an outer skirt. The SAPIEN 3 has an internal skirt and an outer sealing skirt made of polyethylene terephthalate (PET) (Jose et al. 2015). In the SAPIEN 3 Ultra, the outer portion is textured PET material with a greater height in comparison to the SAPIEN 3 (Renker and Kim, 2020). The valves are manufactured in four sizes (20 mm, 23 mm, 26 mm, and 29 mm).
2.4.2 Medtronic Evolut R/Evolut PRO
Both valves consist of a self-expandable nitinol stent frame and three leaflets of porcine pericardium positioned in a supra-annular location. In contrast to the Evolut R (Fig. 5a), an external porcine pericardial sleeve has been added to the Evolut PRO (Fig. 5b) as a sealing sleeve. The Evolut R model is manufactured in four sizes: 23 mm, 26 mm, 29 mm, and 34 mm, while the Evolut PRO is only available in 23 mm, 26 mm, and 29 mm.
The valve size selection is of paramount significance for the physician and for the patient and is highly associated with the success of a TAVI procedure. In order to choose the right valve size, an MSCT Scan must be performed. Then the appropriate software should be used to quantify the aortic root.
The cut-off values of the manufacturer’s sizing plan should be considered. There is often a difference between clinical valve selection and selection based on the computer software. The “device–host interaction” is very important in order to prevent complications such as obstruction of the coronary arteries, relevant aortic regurgitation after implantation as well as conduction disorders such as atrioventricular (AV) block (El Faquir et al. 2020).
A rare but sometimes life-threatening complication is the transcatheter heart valve migration into the outflow tract of the left ventricle. In this case is a balloon repositioning of the valve necessary as well as possibly a valve-in-valve procedure in order to prevent severe aortic regurgitation (Ito et al. 2017).
One of the deciding mortality as well as success parameters of a TAVI procedure – also long term – is the paravalvular regurgitation. This reinforces the importance of correct valve selection and the experience of the physician. In several publications, it is observed that in the second part of the study or cohort there are a greater number of good final results as the technique of the interventional cardiologist improves exponentially (Wang et al. 2021).
2.5 Leadless Pacemakers
Within the last decade, single-chamber leadless pacemaker devices have been developed, which are implanted to the inner side of the right ventricle via a steerable catheter insertion system (Reynolds et al. 2016). Currently, the most common device in clinical use is the MicraTM (Medtronic Inc., Minneapolis, MN, USA). Despite its small size of 0.8 m3, a light capsule weight of 2.0 g, a length of 25.9 mm, and an outer diameter of 6.7 mm, it has all the features of a conventional single-chamber pacemaker system (Reddy et al. 2015). The Micra pacemaker itself consists of nitinol, gold, steel, titanium, and tungsten (Figs. 6a and 6b). The tines, with which the device is affixed to the endocardium of the right ventricle, consist entirely of nitinol.
There are now numerous clinical studies that prove both, the effectiveness and safety of the product. Medtronic postulates that the device would float in an in vivo environment at the inside of the right ventricle. According to the manufacturer, the only connection to the endothelium is through the pacemaker’s anchoring system. As lead-free pacemaker technology is a relatively new topic, the effects on the intracardiac endothelium at the anchor point are not yet well understood. However, individual cases have also been published in which unexpected encapsulation was observed. . First data from autopsies show partial or even complete encapsulation (Tjong et al. 2015; Kypta et al. 2016). In the two published cases, complete endothelialization or even encapsulation is reported after 12 months and 19 months, respectively. Within one of our prior in vitro studies, we could identify a potential impact of the tungsten component in these processes of endothelialization (Edlinger et al. 2019).
3 Common Device Components
3.1 Nitinol
Nitinol, a material used in the majority of intracardiac devices, is a 55:45 nickel–titanium alloy. It is of enormous value for medical applications due to its thermal shape–memory effect and super elasticity (Ryhänen et al. 1998). Nitinol is not considered cytotoxic or thrombogenic, although its individual components, nickel and titanium, can be detected in peripheral blood samples (Shayan and Chun 2015).
It is assumed that the immunological effects are comparable to those of stainless steel without any toxic effects (Ryhänen et al. 1998). Nitinol is widely used, for example, in many PFO and ASD occluders, as well as the tines of the Micra pacemaker are made of this material.
3.2 Titanium
Due to its good in vivo compatibility, titanium is a widely used component for implants and alloys (Ungersboeck et al. 1995). Concentrations of 50–150 μg/l can be measured in peripheral blood samples, a level considered nontoxic (Ipach et al. 2012). Data from orthopedic studies could show an increase in classical inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin-1beta (IL-1β) and IL-6 in patients with titanium implants. However, there is no evidence of toxicity to date (Sun et al. 2000; Östberg et al. 2015). In cardiology, for example, titanium is used in pacemaker generators with no long-term adverse effects. It is particularly important as a component of the nitinol alloy.
3.3 Tungsten
Tungsten is another component that is commonly used in medical devices. Certain publications point to toxic effects during long-term treatments (Witten et al. 2012). However, according to the current knowledge, no toxic effects are expected at normal corrosivity rates and slightly elevated serum levels are considered as normal (Peuster et al. 2003). It is considered to be a very strong and durable material as well as very resistant to corrosion. In cardiology, tungsten is widely used as a component of pacemaker probes. The Micra pacemaker also partly consists of this material.
3.4 Gold
Implants made of gold and its nanoparticles are known to be noncytotoxic and nonimmunogenic (Shukla et al. 2005). Cytokine elevations of interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-α), interleukin 1-beta (IL-1β), and mononuclear chemotactic protein 1 (MCP-1) are frequently found. However, these cytokine reactions are insignificant and have no influence on the cell viability (Zhang et al. 2011). For this reason, gold is of paramount value as a reserve material if the patient to be implanted suffers from a previously known allergy, for example to titanium. This is a rare allergy, which affects only the 0.6% of the total population. There are reports of patients who have been successfully implanted with a gold-plated leadless pacemaker for this indication. This was described in a 65-year-old man with a type IV allergy to titanium in the case report by Kypta et al. (Kypta et al. 2015; Goli et al. 2012).
3.5 Steel
Steel is considered to be a material of manufacturing precision, good hygiene, as well as high resistance against corrosion. It has been reported that the toxic effects of steel implants are higher than those of other components (Haynes et al. 1998). Lacey et al. observed a decrease in monocyte and macrophage survival in response to steel (Lacey et al. 2009), as well as a reduced leukocyte migration to the implant site or prosthetic implants. In addition, steel has been shown to induce abundant elevation of cytokines such as IL-1β (Haynes et al. 1998).
4 Cardiac Injury, Wound Healing, and Regeneration – A Brief Overview
Cardiac injury causes a severity-related damage of the myocardium, followed by cardiac repair or wound healing where damaged tissue is usually replaced by a fibrotic scar, as described in the literature (Deb and Ubil 2014; Talman and Ruskoaho 2016). Moreover, it has recently been reported that adult cardiomyocytes (CMs) have a slight ability to proliferate, raising the promise of promoting cardiac regeneration in humans (Beltrami et al. 2001; Bergmann et al. 2009; Mollova et al. 2013). However, the feasibility of cardiac regeneration is largely dependent on the type and extent of immune responses, thus leading to an inflammatory response (Sattler and Rosenthal 2016; Cheng et al. 2017).
Although the implantation of an intracardiac device is necessary for the maintenance of proper cardiac pacing in various cardiac diseases, it is also accompanied by the injury of the cardiac tissue at the site of implantation. This inevitable injury initiates a complex series of tissue repair processes that comprise of the interaction and timely coordination of several cell types, cytokines, chemokines, and signaling cascades. Furthermore, there is a host reaction following the implantation of foreign biomaterial into the cardiac implantation site. This also includes blood–material interactions, inflammation, granulation, provisional matrix formation, and the fibrotic remodeling of the injured area (Gretzer et al. 2006; Luttikhuizen et al. 2006).
Generally, the immune response to cardiac injury is accomplished by the innate and the adaptive immune systems in synergy and can be divided into three phases: the pro-inflammatory phase, the proliferative phase, and the reparative phase (Lai et al. 2019).
4.1 Pro-inflammatory Phase
In the very early process after the implantation of a foreign biomaterial, a material–blood interaction occurs, whereby proteins from the blood adhere to the implant’s surface. A provisional matrix based on the blood’s components forms, that is, the initial thrombus or blood clot where further protein adsorption proceeds. This provisional matrix and the injured tissue are responsible for the recruitment of structural, biochemical, and cellular compartments that are essential for wound healing (Gristina 1994; Gretzer et al. 2006; Luttikhuizen et al. 2006). In this period, inflammatory cells are recruited to the site of injury to clear the damaged wound of dead cells and tissue, as well as to degrade the matrix debris. Furthermore, it initiates the processes necessary to form the reparative scar. However, it has been described that prolonged or excessive inflammation is accompanied by a poor tissue remodeling and worse outcomes in patients or animal models with myocardial infarction (MI) (Timmers et al. 2008; Arslan et al. 2010; Frangogiannis 2012).
The initial immune response is driven by molecules released from necrotic cells, the so-called damage associated patterns (DAMPs) (Arslan et al. 2010). In addition, during tissue death, dying proteases, hydrolases, and mitochondrial reactive oxygen species (ROS) are also released into the extracellular space, generating further DAMPs that trigger the inflammatory response (Kono and Rock 2008). Subsequently, these DAMP molecules bind to pattern recognition receptors (PRRs), including toll-like receptors (TLRs) and the receptor for advanced glycation end products (RAGE), that are expressed by both tissue resident cells and recruited leukocytes (Muzio et al. 2000; Chavakis et al. 2004). Among other DAMPs present in cardiac inflammation, high-mobility group B1 (HMGB1) is one of the best characterized (Andrassy et al. 2008). HMGB1 is responsible for the initiation of inflammation in myocardial infarction (MI) and cardiac ischemia by promoting the migration of immune cells through its interaction with PRRs, such as TLR2/4 (most abundant TLRs in the heart) and RAGE (Nishimura and Naito 2005; Klune et al. 2008; Sims et al. 2009). Moreover, it induces tissue healing by changing the macrophages’ phenotype, favoring neoangiogenesis and promoting stem cell activation and proliferation (Bianchi et al. 2017).
Physiologically, the extracellular matrix (ECM) is responsible for the support and the maintenance of the heart’s structural integrity. However, during inflammation the ECM is degraded by matrix metalloproteinases (MMPs), activated by necrotic cells, neutrophils, and macrophages. This degraded ECM can in turn act as a DAMP, driving the inflammatory pathway forward (Dobaczewski et al. 2010a). In the context of cardiac injury, a switch to a transient fibrin-based ECM is achieved (González-Rosa et al. 2011; Frangogiannis 2017), further modulating and guiding inflammatory cells through TLRs (Corbett and Schwarzbauer 1998; Smiley et al. 2001; Flick et al. 2004) and promoting the proliferation of endothelial cells and fibroblasts (Frangogiannis 2017).
As mentioned above, dying cardiomyocytes release intracellular components, such as deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), as well as intracellular components like adenosine triphosphate (ATP) and heat shock proteins (HSPs), that might accelerate the ongoing immune response (Arslan et al. 2011; Kono et al. 2014). Furthermore, reactive oxygen species (ROS), which stem from mitochondria of necrotic cells or are secreted by neutrophils, constitute a key player in the promotion of immune cells to infiltrate the injured tissue. ROS contributes to the onset of the nuclear factor kappa light chain enhancer of activated B-cells (NF-κB), a main chemotactic and pro-inflammatory protein complex (Thannickal and Fanburg 2000; Gloire et al. 2006), and directly activates the so-called inflammasome, as well as cardiac resident cells, such as fibroblasts and mast cells (Gilles et al. 2003; Kawaguchi et al. 2011). The inflammasome, a multiprotein complex of receptors and cytokines, in turn promotes the immune response and triggers the expression and activation of other cytokines (Latz et al. 2013).
After the immune response has been initiated by damage-associated molecular patterns (DAMPs) and related pattern recognition receptors (PRRs), resident immune cells and nonimmune cells, such as resident macrophages, endothelial cells (ECs), and fibroblasts, drive the expression of pro-inflammatory cytokines and chemokines. In cardiac device implantation, the extent of immune responses is primarily mediated by the extent of injury that happened during the implantation procedure (Zdolsek et al. 2007; Tang et al. 1998). In the presence of DAMPs, cytokines, chemokines, activated platelets, and histamine, neutrophils are the first innate immune cells that are rapidly recruited to the injured tissue (Mcdonald et al. 2010; Soehnlein and Lindbom 2010). Contemporaneously, the cardiac endothelium is activated by pro-inflammatory cytokines, such as TNF-α, IL-1β, and histamine (Duperray et al. 1995; Dewald et al. 2004; Debrunner et al. 2008). This ensemble of pro-inflammatory cytokines constitutes the inflammasome and facilitates the neutrophil transmigration between and through the endothelial wall to the site of tissue injury (Frangogiannis et al. 1998; Singh and Saini 2003). Furthermore, IL-6 seems to be a main mediator of tissue injury, since it is expressed by CMs and recruited neutrophils and macrophages (Youker et al. 1992). IL-6 in turn upregulates intercellular adhesion molecule 1 (ICAM-1) on CMs that mediates neutrophil binding and is associated with cytotoxic events (Entman et al. 1992; Youker et al. 1992).
4.2 Cell Proliferation Phase
The cellular proliferative phase is the second phase and is characterized by the expansion of neutrophils and macrophages that degrade dead cells and the matrix debris, further promoting the expression of cytokines and growth factors. Due to the pro-inflammatory and cytotoxic activity of neutrophils, excessive amounts or a prolonged presence of neutrophils have been associated with remodeling and a poor prognosis after MI (Mocatta et al. 2007; Akpek et al. 2012). On the other hand, they constitute a key factor in the resolution of inflammation and lead to a shift of the macrophages’ phenotype to a reparative one (Čulić et al. 2002; Pase et al. 2012). Furthermore, they contribute to the initiation of angiogenesis during inflammation by expressing vascular endothelial growth factor (VEGF) (Gong and Koh 2010).
Generally, monocytes are a type of leukocytes that have the ability to differentiate into macrophages and dendritic cells. There are two different subpopulations of monocytes present in cardiac inflammation: the Ly6Chigh and Ly6Clow (Hettinger et al. 2013; Yona et al. 2013). Ly6Chigh monocytes belong to the primary subset that is recruited to the injured heart, driven by MCP-1. Therefore, Ly6Chigh monocytes are commonly active in the early pro-inflammatory phase and are responsible for proteolytic and inflammatory processes. In contrast, Ly6Clow are sometimes known as resident monocytes due to their appearance of not being actively recruited into the injured myocardium (Geissmann et al. 2003; Nahrendorf et al. 2007). They have been shown to emerge later, in the resolution phase, demonstrating decreased inflammatory properties, as well as the expression of VEGF (Yao et al. 2012). It is not definitively clear if Ly6Clow arise from differentiation of Ly6Chigh (Hanna et al. 2011; Yona et al. 2013), although it has been speculated that they arise from the same progenitor cells (Hettinger et al. 2013; Yona et al. 2013). In addition, two macrophage subsets (M1 and M2 macrophages) correspond with these different monocyte concentrations. M1 macrophages are present early after heart injury and are known to secrete pro-inflammatory cytokines, like IL-1β, TNF-α, IL-6, and IL-10 (Dewald et al. 2005), whereas M2 monocytes become active at the later stage of reparative heart tissue healing (Nahrendorf et al. 2010). However, the simple division of macrophages into two subsets should be considered due to the great variety of macrophage phenotypes (Martinez and Gordon 2014). The initial acute inflammatory response usually resolves within 1 week after device implantation, though it is also dependent on the extent of injury at the implant site (Gretzer et al.).
4.3 Endothelialization and Resolution of Inflammation
Finally, the conversion from inflammation to the repair phase is crucial for wound healing as a prolonged inflammatory response would lead to CM death, excessive fibrosis, cardiac remodeling, and damage. In cardiac device implantation, acute inflammation is often followed by a chronic inflammation period that is characterized by the presence of mononuclear cells, such as monocytes and lymphocytes. This chronic inflammation lasts for a short time of approximately 2 weeks and is strictly located to the site of implantation. The prolongation of the inflammation phase for greater than 3 weeks, usually a device infection is indicated (Luttikhuizen et al. 2006). Once the inflamed/injured area is cleared of apoptotic cells, the repair process is initiated and a new ECM is produced (Frangogiannis 2014). The resolution phase is mainly characterized by the recruitment of lymphocytes, the activation of fibroblasts, and the proliferation of ECs, as well as the activation of smooth muscle cells (SMCs).
Originally, it was thought that circulating endothelial progenitor cells (EPCs), as progenitors of ECs, were a source of new endothelial cells, as first described by Asahara et al. in 1997 (Asahara et al. 1997). They originate from different hematopoietic progenitor cells located in the bone marrow, such as hematopoietic stem cells, myeloid precursors, and mesenchymal stem cells (Balistreri et al. 2015). However, EPCs also stem from different nonhematopoietic tissues, such as the umbilical cord etc. (Ingram et al. 2004; Mund et al. 2012; Chan et al. 2013). In response to tissue damage, they are released into the circulation and invade the site of injury attracted to inflammatory cytokines and chemoattractant proteins. As progenitor cells, EPCs constitute a source for ECs by differentiation and further promoting the proliferation of resident ECs (Buijs et al. 2004; Li et al. 2012). Furthermore, they release several growth factors, such as VEGF and angiopoietins, and other pro-endothelial factors that promote the healing process (MCP-1), stromal cell-derived factor 1, insulin-like growth factor 1, platelet-derived growth factor (PDGF), and macrophage inflammatory protein 1a (Rehman et al. 2003; Caiado et al. 2008). In turn, these factors stimulate ECM proteins and the proliferation of SMCs. An overview of the factors released by ECs, SMCs, and inflammatory cells was already provided by Welt and Rogers (Welt and Rogers 2002).
Both lymphocytes, B- and T-cells, comprise the main cellular components of the adaptive immune system. T-cells are further divided into CD8+ and CD4+ subsets, whereas CD4+ T cells are the main actors in the healing process. According to their secreted cytokines they are further classified into Th1 (IL-2, TNF-α, and interferon gamma (IFN-γ); Th2 (IL-4, IL-4, IL-13); Th17 (IL-17, IL-21, IL-22); and regulatory T-cells (Treg) (transforming growth factor beta (TGF-β), IL-35) (Hofmann and Frantz 2015). More precisely, especially Tregs play a key role in the healing phase through suppressing the immune response in the damaged tissue, promoting revascularization, and initiating the shift to a reparative phenotype of the macrophages (M2 microphages; as mentioned above) (Zouggari et al. 2009; Dobaczewski et al. 2010b; Weirather et al. 2014). Particularly, TGF-β was shown to be mainly responsible for the deactivation of inflammatory macrophages in MI (Dobaczewski et al. 2011). These M2 macrophages then have the ability to express high amounts of several different MMPs and secrete anti-inflammatory cytokines, such as IL-4, IL-13, and mainly IL-10 (Frangogiannis et al. 2000). Consequently, the extent of IL-4 and IL-13 expression also determines the extent and duration of the inflammatory response. Furthermore, it was found that myeloid–epithelial–reproductive tyrosine kinase (MERTK) (Wan et al. 2013) and platelet-derived growth factor (PDGF) (Zymek et al. 2006) are crucial for the transition to a reparative status too. Furthermore, TGF-β signaling and the decline in pro-inflammatory cytokine signaling result in the activation of interstitial and perivascular fibroblasts, EC proliferation, followed by reparative myocardial fibrosis and angiogenesis (Chen and Frangogiannis 2013). Any failure of accurate regulation of Treg or TGF-β signaling may lead to excessive scar formation, an ongoing chronic inflammation (Kypta et al. 2016), such as described in the case report by Kypta et al. (Dobaczewski et al. 2011).
5 Discussion
In the last decades, an immense increase in the number of implanted intracardiac devices could be observed (Mond and Proclemer, 2011). At the same time, patients implanted with a device are getting older and therefore may live for decades with this foreign material embedded in the endocardium and exposed to blood flow (Leon et al. 2010; Proclemer et al. 2010). This is of relevance insofar as little is known about long-term toxic effects of implantable devices (Eliaz 2019; Nasakina et al. 2019). Furthermore, at present it is not known with certainty whether there is a “critical concentration” of the metallic components which, if exceeded, can be expected to cause consequential damage to health. Nitinol, by far the most commonly used alloy for intracardiac devices, appears to have a number of good properties especially during implantation and durability in long-term treatment. On the one hand, it is highly malleable, which is of enormous importance in the context of implantation (Stoeckel et al. 2004; Henderson et al. 2011; Maleckis et al. 2018); on the other hand, it is considered to be extremely durable with overall good tolerance (Eliaz 2019).
We know from numerous preliminary reports that as a result of intracardiac positioning, endothelialization on nitinol surfaces is expected to occur after only a few weeks (Zahn et al. 2001; Sigler et al. 2005a; Schwartz et al. 2010) and depends on numerous factors, such as the size of the device or, in the case of occluders, the primary interventional outcome (Granier et al. 2018). Incomplete endothelialization could lead to complications at site of the implantation, such as thrombus formation (Sigler et al. 2005b; Sellers et al. 2019). Moreover, patient-specific factors must be taken into consideration. For instance, there are known cases of patients with multiple allergies where excessive endothelialization was found in the autopsy (Kypta et al. 2016). Vice versa, it seems conceivable that endothelialization processes or the healing phase can be negatively influenced by the intake of immunosuppressive substances such as glucocorticoids (Radovsky et al. 1988) or TNF-α inhibitors (Sandberg et al. 2012). The same is conceivable for patients in whom cytotoxic substances or radiation therapies are used (Hopewell 1990). As a wide overall variation in anatomic conditions is to be expected in PFO, ASD, or LAA occlusions, positional control by transesophageal echocardiography appears essential (Krizanic et al. 2010; Saw et al. 2016).
Intracardiac pacemakers are a special case in this context, as no endothelial surface usually forms over the implanted cardiac device due to direct contact with the blood flow (Jana 2019). According to the manufacturers, the devices should only be anchored to the endocardium at their base, while the majority of the device should remain floating in the blood flow. However, there are now several published cases reporting the contrary. Namely, a complete or at least partial endothelialization/encapsulation of the device in the right ventricular wall (Candinas et al. 1999; Esposito et al. 2002; Tjong et al. 2015; Keiler et al. 2017). Interestingly, there are also reports from autopsies where histological processing has shown a clear evidence of inflammatory processes around the encapsulated pacemaker (Dvorak et al. 2012). Exact knowledge of any expected endothelialization is of enormous clinical relevance, since an influence on the stimulation threshold is at least conceivable through the encapsulation (Stokes et al. 1991). There are also issues of what to do in the event of battery exhaustion. An extraction, as originally intended by the developers, seems unlikely in the case of complete encapsulation. It remains to be seen whether the limited space at the surface of the inner heart is sufficient to safely implant an additional device. For the Micra pacemaker, it could be shown in an animal model that up to three devices can be implanted without any problems (Omdahl et al. 2016).
Another major uncertainty is the importance of allergies in long-term use. Nitinol is an alloy which consists of 45–50% nickel (Eliaz 2019), a relevant allergen. Nickel allergies are type IV allergies, that is, a contact allergy caused by long-term exposure, usually after 24 h to a few days (Tramontana et al. 2020), and are relatively common with a prevalence of approximately 8% to 19% in adults (Diepgen et al. 2016). Whether the allergenic potential within the blood flow is particularly high, or whether a weakening occurs once endothelialization has been achieved, remains completely unclear to date. Allergies are also known to occur with exposure to titanium, which is the other component of the nitinol alloy (Fage et al. 2016). However, the incidence is significantly lower for titanium; consequently therefore the clinical relevance is probably of secondary importance (Grosse Meininghaus et al. 2020). In case of a confirmed allergy, it is possible to coat the device with a less/nonallergenic substance such as gold, which has already been done in individual cases (Kypta et al. 2015). The measurement of any metal released from cardiac devices has already been performed (Ries et al. 2003; Saylor et al. 2018). An open question for the future will be whether there are measurable parameters that can be used to estimate the degree of endothelialization. For example, it is conceivable that the metallic components could be measured as nanoparticles in peripheral blood, but their concentration would decrease during the healing phase. It may also be assumed, that with complete endothelialization achieved, the metal content might fall below the detection limit, which in turn provides important additional information for the estimated duration of the healing process.
6 Conclusion
In summary, we conclude that the vast majority of intracardiac devices meet very high safety standards from a hemostasiological point of view, and that there is currently no evidence of any therapy-limiting toxic effects in long-term treatment. Nitinol, as a component of many devices, is of particular importance in this context. However, there are currently gaps in knowledge for patients who are under immunosuppressive medication. Moreover, the impact of an optimal implantation technique on the initial healing phase and endothelialization phase has not been fully understood in many cases.
For these reasons, it seems indispensable to us that patients continue to be treated at the respective healthcare center after primary implantation and are followed up by means of imaging, so that individualized coagulation management can be determined if necessary.
7 Limitations
We could only include devices which are already in broad clinical use. Nevertheless, a number of less-established devices in the field of interventional cardiology might already have received market approval or might be in the preclinical testing phase. However, in our opinion, the current knowledge of the established devices provide a fundamental basis for the above review and the obtained conclusions.
Contributions
C.E planned and coordinated the study, compiled and analyzed data, wrote the manuscript, and contributed in the final submission. V.P., E.B., S.H.K., and M.B. analyzed data, prepared figures, and contributed to manuscript preparation. F.K. analyzed data and contributed to final submission. V.D. contributed in data acquisition. K.K. carried out English-language editing of the paper. E.L., C.B., and U.C.H. revised the article critically for the content. M.L. planned the study and provided the final approval of the article.
Abbreviations
- ASD:
-
Atrial Septal Defect
- ASO:
-
Amplatzer Septal Occluder
- ATP:
-
Adenosine Triphosphate
- CMs:
-
Cardiomyocytes
- DAMPs:
-
Damage-Associated Patterns
- ECs:
-
Endothelial Cells
- ECM:
-
Extracellular Matrix
- EPCs:
-
Endothelial Progenitor Cells
- GSO:
-
Gore Septal Occluder
- HMGB1:
-
High-mobility Group B1
- HSPs:
-
Heat Shock Proteins
- ICAM-1:
-
Intercellular Adhesion Molecule 1
- IFN-γ:
-
Interferon Gamma
- LAA:
-
Left Atrial Appendage
- MERTK:
-
Myeloid–Epithelial–Reproductive Tyrosine Kinase
- MMPs:
-
Matrix Metalloproteinases
- MI:
-
Myocardial Infarction
- NOAC:
-
New/“Non-Vitamin K” – Oral Anticoagulants
- PCI:
-
Percutaneous Coronary Intervention
- PDGF:
-
Platelet-Derived Growth Factor
- PET:
-
Polyethylene Terephthalate
- PFO:
-
Persistent Foramen Ovale
- PRRs:
-
Pattern Recognition Receptors
- RAGE:
-
Receptor for Advanced Glycation End Products
- ROS:
-
Reactive Oxygen Species
- SMCs:
-
Smooth Muscle Cells
- TAVI:
-
Transcatheter Aortic Valve Implantation
- TGF-β:
-
Transforming Growth Factor Beta
- TLRs:
-
Toll-Like Receptors
- Treg:
-
Regulatory T-cells
- VEGF:
-
Vascular Endothelial Growth Factor
- VSD:
-
Ventricular Septal Defect
References
Akpek M, Kaya MG, Lam YY, Sahin O, Elcik D, Celik T, Ergin A, Gibson CM (2012) Relation of neutrophil/lymphocyte ratio to coronary flow to in-hospital major adverse cardiac events in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Am J Cardiol 110:621–627
Alli O, Holmes DR (2015) Left atrial appendage occlusion for stroke prevention. Curr Probl Cardiol 40:429–476
Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, Kaya Z, Buss S, Autschbach F, Pleger ST, Lukic IK (2008) High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation 117:3216
Anderson RH (2016) Development of the atrial septum. Heart 102:481
Anderson RH, Brown NA, Webb S (2002) Development and structure of the atrial septum. Heart 88:104–110
Ar IG, Senning A, Siegenthaler WE (1979) Non-operative dilatation of coronary artery stenosis. N Engl J Med 301:61–68
Arslan F, De Kleijn DP, Pasterkamp G (2011) Innate immune signaling in cardiac ischemia. Nat Rev Cardiol 8:292
Arslan F, Smeets MB, O’Neill LA, Keogh B, Mcguirk P, Timmers L, Tersteeg C, Hoefer IE, Doevendans PA, Pasterkamp G, De Kleijn DP (2010) Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation 121:80–90
Asahara T, Murohara T, Sullivan A, Silver M, Van Der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275:964–966
Balasundaram RP, Anandaraja S, Juneja R et al (2005) Infective endocarditis following implantation of amplatzer atrial septal occluder. Indian Heart J 57:167–169
Balistreri CR, Buffa S, Pisano C, Lio D, Ruvolo G, Mazzesi G (2015) Are endothelial progenitor cells the real solution for cardiovascular diseases? Focus on controversies and perspectives. Biomed Res Int:2015
Beltrami AP, Urbanek K, Kajstura J, Yan S-M, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA (2001) Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med 344:1750–1757
Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H (2009) Evidence for cardiomyocyte renewal in humans. Science 324:98–102
Bianchi ME, Crippa MP, Manfredi AA, Mezzapelle R, Rovere Querini P, Venereau E (2017) High-mobility group box 1 protein orchestrates responses to tissue damage via inflammation, innate and adaptive immunity, and tissue repair. Immunol Rev 280:74–82
Bruch L, Parsi A, Grad MO, Rux S, Burmeister T, Krebs H, Kleber FX (2002) Transcatheter closure of interatrial communications for secondary prevention of paradoxical embolism: single-center experience. Circulation 105:2845–2848
Buijs JOD, Musters M, Verrips T, Post JA, Braam B, Van Riel N (2004) Mathematical modeling of vascular endothelial layer maintenance: the role of endothelial cell division, progenitor cell homing, and telomere shortening. Am J Phys Heart Circ Phys 287:H2651–H2658
Caiado F, Real C, Carvalho T, Dias S (2008) Notch pathway modulation on bone marrow-derived vascular precursor cells regulates their angiogenic and wound healing potential. PLoS One 3:e3752
Campbell M (1970) Natural history of atrial septal defect. Heart 32:820–826
Candinas R, Duru F, Schneider J et al (1999) Postmortem analysis of encapsulation around long-term ventricular endocardial pacing leads. Mayo Clin Proc 74:120–125
Cardoso CO, Rossi Filho RI, Machado PR et al (2007) Effectiveness of the Amplatzer device for transcatheter closure of an ostium secundum atrial septal defect. Arq Bras Cardiol 88:384–389
Chakos A, Wilson-Smith A, Arora S et al (2017) Long term outcomes of transcatheter aortic valve implantation (TAVI): a systematic review of 5-year survival and beyond. Ann Cardiothorac Surg 6:432–443
Chan JKY, Patel J, Seppanen E, Chong MS, Yeo JS, Teo EY, Fisk NM, Khosrotehrani K (2013) Prospective surface marker-based isolation and expansion of fetal endothelial colony-forming cells from human term placenta. Stem Cells Transl Med 2(11):839–847
Chavakis T, Bierhaus A, Nawroth PP (2004) RAGE (receptor for advanced glycation end products): a central player in the inflammatory response. Microbes Infect 6:1219–1225
Chen W, Frangogiannis NG (2013) Fibroblasts in post-infarction inflammation and cardiac repair. Biochimica et Biophysica Acta (BBA)-Mol Cell Res 1833:945–953
Cheng B, Chen H, Chou I, Tang TW, Hsieh PC (2017) Harnessing the early post-injury inflammatory responses for cardiac regeneration. J Biomed Sci 24:1–9
Chessa M, Carminati M, Cao QL et al (2002) Transcatheter closure of congenital and acquired muscular ventricular septal defects using the Amplatzer device. J Invasive Cardiol 14:322–327
Corbett SA, Schwarzbauer JE (1998) Fibronectin–fibrin cross-linking: a regulator of cell behavior. Trends Cardiovasc Med 8:357–362
Cribier A, Eltchaninoff H, Bash A et al (2002) Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 106:3006–3008
Čulić O, Eraković V, Čepelak I, Barišić K, Brajša K, Ferenčić Ž, Galović R, Glojnarić I, Manojlović Z, Munić V (2002) Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects. Eur J Pharmacol 450:277–289
Deb A, Ubil E (2014) Cardiac fibroblast in development and wound healing. J Mol Cell Cardiol 70:47–55
Debrunner M, Schuiki E, Minder E, Straumann E, Naegeli B, Mury R, Bertel O, Frielingsdorf J (2008) Proinflammatory cytokines in acute myocardial infarction with and without cardiogenic shock. Clin Res Cardiol 97:298–305
Della Rocca DG, Prete AD, Di Biase L, Horton RP, Al-Ahmad A, Bassiouny M, Mohanty S, Trivedi C, Romero J, Gianni C, Burkhardt JD, Gallinghouse GJ, Sanchez JE, Versaci F, Natale A (2019) Current endocardial approaches for left atrial appendage closure. Eur J Arrhythm Electrophysiol 5(1):40–46
Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, Tincey S, Michael LH, Entman ML, Frangogiannis NG (2004) Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol 164:665–677
Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG (2005) CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res 96:881–889
Diepgen TL, Ofenloch RF, Bruze M et al (2016) Prevalence of contact allergy in the general population in different European regions. Br J Dermatol 174:319–329
Dobaczewski M, Chen W, Frangogiannis NG (2011) Transforming growth factor (TGF)-β signaling in cardiac remodeling. J Mol Cell Cardiol 51:600–606
Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG (2010a) The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol 48:504–511
Dobaczewski M, Xia Y, Bujak M, Gonzalez-Quesada C, Frangogiannis NG (2010b) CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am J Pathol 176:2177–2187
Duperray A, Mantovani A, Introna M, Dejana E (1995) Endothelial cell regulation of leukocyte infiltration in inflammatory tissues. Mediat Inflamm 4(5):322–330
Dvorak P, Novak M, Kamaryt P et al (2012) Histological findings around electrodes in pacemaker and implantable cardioverter-defibrillator patients: comparison of steroid-eluting and non-steroid-eluting electrodes. Europace 14:117–123
Edlinger C, Paar V, Tuscher T et al (2019) Potential mechanisms of endothelialisation in individuals implanted with a leadless pacemaker systems: an experimental in vitro study. J Electrocardiol 55:72–77
Edlinger C, Krizanic F, Butter C, Bannehr M, Neuss M, Fejzic D, Hoppe UC, Lichtenauer M (2020) Economic assessment of traditional surgical valve replacement versus use of transfemoral intervention in degenerative aortic stenosis. Minerva Med 112(3):372–383
El Faquir N, Rocatello G, Rahhab Z, Bosmans J, De Backer O, Van Mieghem NM, Mortier P, De Jaegere PP (2020) Differences in clinical valve size selection and valve size selection for patient-specific computer simulation in transcatheter aortic valve replacement (TAVR): a retrospective multicenter analysis. Int J Card Imaging 36:123–129
Eliaz N (2019) Corrosion of metallic biomaterials: a review. Materials (Basel) 12(3):407
Entman ML, Youker K, Shoji T, Kukielka G, Shappell SB, Taylor AA, Smith CW (1992) Neutrophil induced oxidative injury of cardiac myocytes. A compartmented system requiring CD11b/CD18-ICAM-1 adherence. J Clin Invest 90:1335–1345
Esposito M, Kennergren C, Holmström N et al (2002) Morphologic and immunohistochemical observations of tissues surrounding retrieved transvenous pacemaker leads. J Biomed Mater Res 63:548–558
Fage SW, Muris J, Jakobsen SS et al (2016) Titanium: a review on exposure, release, penetration, allergy, epidemiology, and clinical reactivity. Contact Dermatitis 74:323–345
Fischer G, Stieh J, Uebing A et al (2003) Experience with transcatheter closure of secundum atrial septal defects using the Amplatzer septal occluder: a single Centre study in 236 consecutive patients. Heart 89:199–204
Flick MJ, Du X, Witte DP, Jiroušková M, Soloviev DA, Busuttil SJ, Plow EF, Degen JL (2004) Leukocyte engagement of fibrin (ogen) via the integrin receptor α M β 2/Mac-1 is critical for host inflammatory response in vivo. J Clin Invest 113:1596–1606
Forssmann-Falck R (1997) Werner Forssmann: a pioneer of cardiology. Am J Cardiol 79:651–660
Fountain RB, Holmes DR, Chandrasekaran K et al (2006) The PROTECT AF (WATCHMAN left atrial appendage system for embolic PROTECTion in patients with atrial fibrillation) trial. Am Heart J 151:956–961
Frangogiannis NG (2012) Regulation of the inflammatory response in cardiac repair. Circ Res 110:159–173
Frangogiannis NG (2014) The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol 11:255–265
Frangogiannis NG (2017) The extracellular matrix in myocardial injury, repair, and remodeling. J Clin Invest 127:1600–1612
Frangogiannis NG, Lindsey ML, Michael LH, Youker KA, Bressler RB, Mendoza LH, Spengler RN, Smith CW, Entman ML (1998) Resident cardiac mast cells degranulate and release preformed TNF-α, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation 98:699–710
Frangogiannis NG, Mendoza LH, Lindsey ML, Ballantyne CM, Michael LH, Smith CW, Entman ML (2000) IL-10 is induced in the reperfused myocardium and may modulate the reaction to injury. J Immunol 165:2798–2808
Geissmann F, Jung S, Littman DR (2003) Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19:71–82
Gilles S, Zahler S, Welsch U, Sommerhoff CP, Becker BF (2003) Release of TNF-α during myocardial reperfusion depends on oxidative stress and is prevented by mast cell stabilizers. Cardiovasc Res 60:608–616
Gloire G, Legrand-Poels S, Piette J (2006) NF-κB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol 72:1493–1505
Goli A, Shroff S, Osman MN, Lucke J (2012) A case of gold-coated pacemaker for pacemaker allergy. The Journal 945
Gong Y, Koh D-R (2010) Neutrophils promote inflammatory angiogenesis via release of preformed VEGF in an in vivo corneal model. Cell Tissue Res 339:437–448
González-Rosa JM, Martín V, Peralta M, Torres M, Mercader N (2011) Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development 138:1663–1674
Granier M, Laugaudin G, Massin F et al (2018) Occurrence of incomplete endothelialization causing residual permeability after left atrial appendage closure. J Invasive Cardiol 30:245–250
Gretzer C, Emanuelsson L, Liljensten E, Thomsen P (2006) The inflammatory cell influx and cytokines changes during transition from acute inflammation to fibrous repair around implanted materials. J Biomater Sci Polym Ed 17:669–687
Greutmann M, Greutmann-Yantiri M, Kretschmar O, Senn O, Roffi M, Jenni R, Luescher TF, Eberli FR (2009) Percutaneous PFO closure with Amplatzer PFO occluder: predictors of residual shunts at 6 months follow-up. Congenit Heart Dis 4:252–257
Gristina AG (1994) Implant failure and the immuno-incompetent fibro-inflammatory zone. Clin Orthop Relat Res 298:106–118
Grosse Meininghaus D, Kruells-Muench J, Peltroche-Llacsahuanga H (2020) First-in-man implantation of a gold-coated biventricular defibrillator: difficult differential diagnosis of metal hypersensitivity reaction vs chronic device infection. HeartRhythm Case Rep 6:304–307
Hagen PT, Scholz DG, Edwards WD (1984) Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc 59:17–20
Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, Hedrick CC (2011) The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C− monocytes. Nat Immunol 12:778
Haynes DR, Boyle SJ, Rogers SD et al (1998) Variation in cytokines induced by particles from different prosthetic materials. Clin Orthop Relat Res:223–230
Henderson E, Nash DH, Dempster WM (2011) On the experimental testing of fine Nitinol wires for medical devices. J Mech Behav Biomed Mater 4:261–268
Hettinger J, Richards DM, Hansson J, Barra MM, Joschko A-C, Krijgsveld J, Feuerer M (2013) Origin of monocytes and macrophages in a committed progenitor. Nat Immunol 14:821–830
Hofmann U, Frantz S (2015) Role of lymphocytes in myocardial injury, healing, and remodeling after myocardial infarction. Circ Res 116:354–367
Hopewell J (1990) The skin: its structure and response to ionizing radiation. Int J Radiat Biol 57:751–773
Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC (2004) Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 104:2752–2760
Ipach I, Schäfer R, Mittag F et al (2012) The development of whole blood titanium levels after instrumented spinal fusion - is there a correlation between the number of fused segments and titanium levels? BMC Musculoskelet Disord 13:159
Ito M, Tada N, Hata M (2017) Balloon repositioning of Transcatheter Aortic Valve after migration into the left ventricular outflow tract, followed by Valve-in-Valve procedure. Tex Heart Inst J 44:274–278
Jana S (2019) Endothelialization of cardiovascular devices. Acta Biomater 99:53–71
Jose J, Richardt G, Abdel-Wahab M (2015) Balloon- or self-expandable TAVI: clinical equipoise? Interv Cardiol 10:103–108
Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J (2011) Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation 123:594–604
Keiler J, Schulze M, Sombetzki M et al (2017) Neointimal fibrotic lead encapsulation - clinical challenges and demands for implantable cardiac electronic devices. J Cardiol 70:7–17
Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A (2008) HMGB1: endogenous danger signaling. Mol Med 14:476–484
Knepp MD, Rocchini AP, Lloyd TR et al (2010) Long-term follow up of secundum atrial septal defect closure with the amplatzer septal occluder. Congenit Heart Dis 5:32–37
Komar M, Przewlocki T, Olszowska M, Sobien B, Podolec P (2014) The benefit of atrial septal defect closure in elderly patients. Clin Interv Aging 9:1101
Kono H, Kimura Y, Latz E (2014) Inflammasome activation in response to dead cells and their metabolites. Curr Opin Immunol 30:91–98
Kono H, Rock KL (2008) How dying cells alert the immune system to danger. Nat Rev Immunol 8:279–289
Kramer DB, Kesselheim AS (2015) The Watchman saga--closure at last? N Engl J Med 372:994–995
Krizanic F, Sievert H, Pfeiffer D et al (2010) The Occlutech Figulla PFO and ASD occluder: a new nitinol wire mesh device for closure of atrial septal defects. J Invasive Cardiol 22:182–187
Kumar P, Sarkar A, Kar SK (2019) Assessment of ventricular function in patients of atrial septal defect by strain imaging before and after correction. Ann Card Anaesth 22:41
Kypta A, Blessberger H, Kammler J, Lichtenauer M, Lambert T, Silye R, Steinwender C (2016) First autopsy description of changes 1 year after implantation of a leadless cardiac pacemaker: unexpected ingrowth and severe chronic inflammation. Can J Cardiol 32:1578. e1571–1578. e1572
Kypta A, Blessberger H, Lichtenauer M, Lambert T, Kammler J, Steinwender C (2015) Gold-coated pacemaker implantation for a patient with type IV allergy to titanium. Indian Pacing Electrophysiol J 15:291–292
Lacey DC, De Kok B, Clanchy FI et al (2009) Low dose metal particles can induce monocyte/macrophage survival. J Orthop Res 27:1481–1486
Lai S-L, Marín-Juez R, Stainier DY (2019) Immune responses in cardiac repair and regeneration: a comparative point of view. Cell Mol Life Sci 76:1365–1380
Latz E, Xiao TS, Stutz A (2013) Activation and regulation of the inflammasomes. Nat Rev Immunol 13:397–411
Le Gloan L, Legendre A, Iserin L, Ladouceur M (2018) Pathophysiology and natural history of atrial septal defect. J Thorac Dis 10:S2854
Lee PH, Song JK, Kim JS et al (2018) Cryptogenic stroke and high-risk patent foramen Ovale: the DEFENSE-PFO trial. J Am Coll Cardiol 71:2335–2342
Leon MB, Smith CR, Mack M et al (2010) Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 363:1597–1607
Li D-W, Liu Z-Q, Wei J, Liu Y, Hu L-S (2012) Contribution of endothelial progenitor cells to neovascularization. Int J Mol Med 30:1000–1006
Luttikhuizen DT, Harmsen MC, Luyn MJV (2006) Cellular and molecular dynamics in the foreign body reaction. Tissue Eng 12:1955–1970
Madhkour R, Wahl A, Praz F, Meier B (2019) Amplatzer patent foramen ovale occluder: safety and efficacy. Expert Rev Med Devices 16:173–182
Maleckis K, Anttila E, Aylward P et al (2018) Nitinol stents in the Femoropopliteal artery: a mechanical perspective on material, design, and performance. Ann Biomed Eng 46:684–704
Martinez FO, Gordon S (2014) The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000prime Rep 6
Mas JL, Derumeaux G, Chatellier G (2017) Trials of patent foramen Ovale closure. N Engl J Med 377:2599–2600
Masura J, Gavora P, Formanek A et al (1997) Transcatheter closure of secundum atrial septal defects using the new self-centering amplatzer septal occluder: initial human experience. Catheter Cardiovasc Diagn 42:388–393
Masura J, Gavora P, Podnar T (2005) Long-term outcome of transcatheter secundum-type atrial septal defect closure using Amplatzer septal occluders. J Am Coll Cardiol 45:505–507
Mcdonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P (2010) Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330:362–366
Meerkin D, Butnaru A, Dratva D et al (2013) Early safety of the Amplatzer cardiac plug™ for left atrial appendage occlusion. Int J Cardiol 168:3920–3925
Meier B (2005) Closure of patent foramen ovale: technique, pitfalls, complications, and follow up. Heart 91:444–448
Mocatta TJ, Pilbrow AP, Cameron VA, Senthilmohan R, Frampton CM, Richards AM, Winterbourn CC (2007) Plasma concentrations of myeloperoxidase predict mortality after myocardial infarction. J Am Coll Cardiol 49:1993–2000
Mollova M, Bersell K, Walsh S, Savla J, Das LT, Park S-Y, Silberstein LE, Dos Remedios CG, Graham D, Colan S (2013) Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci 110:1446–1451
Mond HG, Proclemer A (2011) The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009–a world Society of Arrhythmia’s project. Pacing Clin Electrophysiol 34:1013–1027
Moons P, Sluysmans T, De Wolf D et al (2009) Congenital heart disease in 111 225 births in Belgium: birth prevalence, treatment and survival in the 21st century. Acta Paediatr 98:472–477
Mund JA, Estes ML, Yoder MC, Ingram DA Jr, Case J (2012) Flow cytometric identification and functional characterization of immature and mature circulating endothelial cells. Arterioscler Thromb Vasc Biol 32:1045–1053
Muzio M, Polntarutti N, Bosisio D, Prahladan M, Mantovani A (2000) Toll like receptor family (TLT) and signalling pathway. Eur Cytokine Netw 11:489–490
Nahrendorf M, Pittet MJ, Swirski FK (2010) Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation 121:2437–2445
Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo J-L, Libby P, Weissleder R, Pittet MJ (2007) The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204:3037–3047
Nasakina EO, Sudarchikova MA, Sergienko KV et al (2019) Ion release and surface characterization of nanostructured Nitinol during long-term testing. Nanomaterials (Basel):9
Nashat H, Montanaro C, Li W, Kempny A, Wort SJ, Dimopoulos K, Gatzoulis MA, Babu-Narayan SV (2018) Atrial septal defects and pulmonary arterial hypertension. J Thorac Dis 10:S2953
Nassif M, Abdelghani M, Bouma BJ, Straver B, Blom NA, Koch KT, Tijssen JG, Mulder BJ, De Winter RJ (2016) Historical developments of atrial septal defect closure devices: what we learn from the past. Expert Rev Med Devices 13:555–568
Neuser J, Akin M, Bavendiek U, Kempf T, Bauersachs J, Widder JD (2016) Mid-term results of interventional closure of patent foramen ovale with the Occlutech Figulla® Flex II Occluder. BMC Cardiovasc Disord 16:1–7
Nicholls M (2020) André F. Cournand for cardiac catheterization: mark Nicholls focusses on the role of Professor André F. Cournand in the development of cardiac catheterization and the award of the 1956 Nobel Prize. Oxford University Press
Nishimura M, Naito S (2005) Tissue-specific mRNA expression profiles of human toll-like receptors and related genes. Biol Pharm Bull 28:886–892
Omdahl P, Eggen MD, Bonner MD et al (2016) Right ventricular anatomy can accommodate multiple Micra Transcatheter pacemakers. Pacing Clin Electrophysiol 39:393–397
Östberg AK, Dahlgren U, Sul YT et al (2015) Inflammatory cytokine release is affected by surface morphology and chemistry of titanium implants. J Mater Sci Mater Med 26:155
Pase L, Layton JE, Wittmann C, Ellett F, Nowell CJ, Reyes-Aldasoro CC, Varma S, Rogers KL, Hall CJ, Keightley MC (2012) Neutrophil-delivered myeloperoxidase dampens the hydrogen peroxide burst after tissue wounding in zebrafish. Curr Biol 22:1818–1824
Pedra CA, Pedra SF, Costa RN, Ribeiro MS, Nascimento W, Campanhã LOS, Santana MVT, Jatene IB, Assef JE, Fontes VF (2016) Mid-term outcomes after percutaneous closure of the secundum atrial septal defect with the Figulla-Occlutech Device. J Interv Cardiol 29:208–215
Peuster M, Fink C, Von Schnakenburg C (2003) Biocompatibility of corroding tungsten coils: in vitro assessment of degradation kinetics and cytotoxicity on human cells. Biomaterials 24:4057–4061
Podnar T, Martanovic P, Gavora P et al (2001) Morphological variations of secundum-type atrial septal defects: feasibility for percutaneous closure using Amplatzer septal occluders. Catheter Cardiovasc Interv 53:386–391
Proclemer A, Ghidina M, Gregori D et al (2010) Trend of the main clinical characteristics and pacing modality in patients treated by pacemaker: data from the Italian pacemaker registry for the quinquennium 2003-07. Europace 12:202–209
Radovsky AS, et al (1988) Paired comparisons of steroid-eluting and nonsteroid endocardial pacemaker leads in dogs: electrical performance and morphologic alterations. Pacing Clin Electrophysiol 11(7):1085–1094. https://doi.org/10.1111/j.1540-8159.1988.tb03955.x. PMID: 2457888
Reddy VY, Exner DV, Cantillon DJ et al (2015) Percutaneous implantation of an entirely Intracardiac leadless pacemaker. N Engl J Med 373:1125–1135
Rehman J, Li J, Orschell CM, March KL (2003) Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 107:1164–1169
Renker M, Kim WK (2020) Choice of transcatheter heart valve: should we select the device according to each patient’s characteristics or should it be “one valve fits all”? Ann Transl Med 8:961
Reynolds D, Duray GZ, Omar R, Soejima K, Neuzil P, Zhang S, Narasimhan C, Steinwender C, Brugada J, Lloyd M, Roberts PR, Sagi V, Hummel J, Bongiorni MG, Knops RE, Ellis CR, Gornick CC, Bernabei MA, Laager V, Stromberg K, Williams ER, Hudnall JH, Ritter P (2016) Micra Transcatheter Pacing Study Group A leadless intracardiac transcatheter pacing system. N Engl J Med 374:533–541. https://doi.org/10.1056/NEJMoa1511643
Ries MW, Kampmann C, Rupprecht HJ et al (2003) Nickel release after implantation of the Amplatzer occluder. Am Heart J 145:737–741
Rotman OM, Bianchi M, Ghosh RP, Kovarovic B, Bluestein D (2018) Principles of TAVR valve design, modelling, and testing. Expert Rev Med Devices 15:771–791
Ryhänen J, Kallioinen M, Tuukkanen J et al (1998) In vivo biocompatibility evaluation of nickel-titanium shape memory metal alloy: muscle and perineural tissue responses and encapsule membrane thickness. J Biomed Mater Res 41:481–488
Sadiq M, Kazmi T, Rehman AU et al (2012) Device closure of atrial septal defect: medium-term outcome with special reference to complications. Cardiol Young 22:71–78
Sandberg O, et al (2012) Etanercept does not impair healing in rat models of tendon or metaphyseal bone injury. Acta Orthop 83(3):305–310. https://doi.org/10.3109/17453674.2012.693018. Epub 2012 May 23. PMID: 22616743; PMCID: PMC3369160
Sattler S, Rosenthal N (2016) The neonate versus adult mammalian immune system in cardiac repair and regeneration. Biochimica et Biophysica Acta (BBA)-Mol Cell Res 1863:1813–1821
Saver JL, Committee RTS (2017) Trials of patent foramen Ovale closure. N Engl J Med 377:2600
Saw J, Lopes JP, Reisman M et al (2016) Cardiac computed tomography angiography for left atrial appendage closure. Can J Cardiol 32:1033.e1031–1033.e1039
Saylor DM, Craven BA, Chandrasekar V et al (2018) Predicting patient exposure to nickel released from cardiovascular devices using multi-scale modeling. Acta Biomater 70:304–314
Scalise F, Auguadro C, Sorropago G et al (2016) Long-term contrast echocardiography and clinical follow-up after percutaneous closure of patent foramen Ovale using two different atrial septal Occluder devices. J Interv Cardiol 29:406–413
Schwartz RS, Holmes DR, Van Tassel RA et al (2010) Left atrial appendage obliteration: mechanisms of healing and intracardiac integration. JACC Cardiovasc Interv 3:870–877
Sellers SL, Blanke P, Leipsic JA (2019) Bioprosthetic heart valve degeneration and dysfunction: focus on mechanisms and multidisciplinary imaging considerations. Radiol Cardiothorac Imaging 1:e190004
Shayan M, Chun Y (2015) An overview of thin film nitinol endovascular devices. Acta Biomater 21:20–34. https://doi.org/10.1016/j.actbio.2015.03.025. Epub 2015 Mar 31
Shukla R, Bansal V, Chaudhary M et al (2005) Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: a microscopic overview. Langmuir 21:10644–10654
Sievert H, Babic UU, Hausdorf G et al (1998) Transcatheter closure of atrial septal defect and patent foramen ovale with ASDOS device (a multi-institutional European trial). Am J Cardiol 82:1405–1413
Sigler M, Bartmus D, Paul T (2005a) Histology of a surgically removed stenotic modified Blalock-Taussig shunt after previous endovascular stenting. Heart 91:1097
Sigler M, Paul T, Grabitz RG (2005b) Biocompatibility screening in cardiovascular implants. Z Kardiol 94:383–391
Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ (2009) HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol 28:367–388
Singh M, Saini HK (2003) Resident cardiac mast cells and ischemia-reperfusion injury. J Cardiovasc Pharmacol Ther 8:135–148
Smiley ST, King JA, Hancock WW (2001) Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol 167:2887–2894
Soehnlein O, Lindbom L (2010) Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol 10:427–439
Søndergaard L, Kasner SE, Rhodes JF et al (2017) Patent foramen Ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med 377:1033–1042
Stoeckel D, Pelton A, Duerig T (2004) Self-expanding nitinol stents: material and design considerations. Eur Radiol 14:292–301
Stokes KB, Bird T, Gunderson B (1991) The mythology of threshold variations as a function of electrode surface area. Pacing Clin Electrophysiol 14:1748–1751
Sun JS, Lin FH, Tsuang YH et al (2000) Effect of anti-inflammatory medication on monocyte response to titanium particles. J Biomed Mater Res 52:509–516
Talman V, Ruskoaho H (2016) Cardiac fibrosis in myocardial infarction—from repair and remodeling to regeneration. Cell Tissue Res 365:563–581
Tang L, Jennings TA, Eaton JW (1998) Mast cells mediate acute inflammatory responses to implanted biomaterials. Proc Natl Acad Sci 95:8841–8846
Thannickal VJ, Fanburg BL (2000) Reactive oxygen species in cell signaling. Am J Phys Lung Cell Mol Phys 279:L1005–L1028
Timmers L, Sluijter JP, Van Keulen JK, Hoefer IE, Nederhoff MG, Goumans M-J, Doevendans PA, Van Echteld CJ, Joles JA, Quax PH (2008) Toll-like receptor 4 mediates maladaptive left ventricular remodeling and impairs cardiac function after myocardial infarction. Circ Res 102:257–264
Tjong FV, Stam OC, Van Der Wal AC et al (2015) Postmortem histopathological examination of a leadless pacemaker shows partial encapsulation after 19 months. Circ Arrhythm Electrophysiol 8:1293–1295
Tramontana M, Bianchi L, Hansel K et al (2020) Nickel allergy: epidemiology, Pathomechanism, clinical patterns, treatment and prevention programs. Endocr Metab Immune Disord Drug Targets 20:992–1002
Ungersboeck A, Geret V, Pohler O et al (1995) Tissue reaction to bone plates made of pure titanium: a prospective, quantitative clinical study. J Mater Sci Mater Med 6:223–229
Wan E, Yeap XY, Dehn S, Terry R, Novak M, Zhang S, Iwata S, Han X, Homma S, Drosatos K (2013) Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res 113:1004–1012
Wang R, Kawashima H, Mylotte D, Rosseel L, Gao C, Aben J-P, Abdelshafy M, Onuma Y, Yang J, Soliman O (2021) Quantitative angiographic assessment of aortic regurgitation after transcatheter implantation of the Venus A-valve: comparison with other self-expanding valves and impact of a learning curve in a single Chinese center. Glob Heart 16(1):20
Weirather J, Hofmann UD, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T, Frantz S (2014) Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res 115:55–67
Welt FG, Rogers C (2002) Inflammation and restenosis in the stent era. Arterioscler Thromb Vasc Biol 22:1769–1776
Witten ML, Sheppard PR, Witten BL (2012) Tungsten toxicity. Chem Biol Interact 196:87–88
Yao B, La LB, Chen YC, Chang LJ, Chan EK (2012) Defining a new role of GW182 in maintaining miRNA stability. EMBO Rep 13:1102–1108
Yona S, Kim K-W, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A (2013) Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38:79–91
Youker K, Smith CW, Anderson DC, Miller D, Michael LH, Rossen RD, Entman ML (1992) Neutrophil adherence to isolated adult cardiac myocytes. Induction by cardiac lymph collected during ischemia and reperfusion. J Clin Invest 89:602–609
Zahn EM, Wilson N, Cutright W et al (2001) Development and testing of the Helex septal occluder, a new expanded polytetrafluoroethylene atrial septal defect occlusion system. Circulation 104:711–716
Zdolsek J, Eaton JW, Tang L (2007) Histamine release and fibrinogen adsorption mediate acute inflammatory responses to biomaterial implants in humans. J Transl Med 5:1–6
Zhang Q, Hitchins VM, Schrand AM et al (2011) Uptake of gold nanoparticles in murine macrophage cells without cytotoxicity or production of pro-inflammatory mediators. Nanotoxicology 5:284–295
Zouggari Y, Ait-Oufella H, Waeckel L, Vilar J, Loinard C, Cochain C, Recalde A, Duriez M, Levy BI, Lutgens E (2009) Regulatory T cells modulate postischemic neovascularization. Circulation 120:1415
Zymek P, Bujak M, Chatila K, Cieslak A, Thakker G, Entman ML, Frangogiannis NG (2006) The role of platelet-derived growth factor signaling in healing myocardial infarcts. J Am Coll Cardiol 48:2315–2323
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. We would like to express our gratitude to the companies mentioned in the journal for permission to reprint the product images.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Edlinger, C. et al. (2022). Endothelialization and Inflammatory Reactions After Intracardiac Device Implantation. In: Turksen, K. (eds) Cell Biology and Translational Medicine, Volume 17. Advances in Experimental Medicine and Biology(), vol 1401. Springer, Cham. https://doi.org/10.1007/5584_2022_712
Download citation
DOI: https://doi.org/10.1007/5584_2022_712
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-20513-2
Online ISBN: 978-3-031-20514-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)