Abstract
Infection and the formation of biofilms have been shown to have a significant role in increased inflammation and delayed wound healing. Wound irrigation solutions are used to debride wounds, removing cell debris and infecting microorganisms, therefore preventing infection. The aim of this study was to evaluate a Polihexanide (PHMB) based wound irrigation solution, Octenidine HCl based wound irrigation solution and electrolysed water based wound care solution for antibiofilm efficacy against Staphylococcus aureus, Pseudomonas aeruginosa and a multispecies biofilm in several models to gain a broad understanding of ability. The PHMB based wound irrigation solution demonstrated broad range antibiofilm efficacy against P. aeruginosa, S. aureus and the multispecies biofilm. The Octenidine HCl based wound irrigation solution and the electrolysed water based wound care solution demonstrated potent antibiofilm efficacy against S. aureus and to a lesser extent P. aeruginosa. Overall, less efficacy was observed in the drip flow bioreactor model for all 3 test solutions, which may be attributed to the continuous flow of nutrients during treatment, which may have diluted or washed away the solution. The data presented also highlights the importance of testing antibiofilm activity in a range of biofilm models and against different bacterial strains to get an overall representation of efficacy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Multiple factors relating to a patient’s underlying physiology are thought to contribute to delayed wound healing, including age, sex hormones, stress, diabetes and nutrition (Guo and Dipietro 2010). Infection and the formation of biofilms in wounds has also been shown to have a significant role in increased inflammation and delayed wound healing (Banu et al. 2015; Percival 2017; Malone et al. 2017). Wound biofilms are formed when microbial cells adhere to a surface and each other and secrete extracellular polymeric substances (EPS), encasing themselves in a matrix (Flemming 2016). Biofilms are difficult to treat as once formed they are up to 1000x more tolerant to antimicrobials than their planktonic counterparts (Fleming et al. 2017).
Staphylococcus aureus and Pseudomonas aeruginosa are the most common microorganisms isolated from chronic wounds (Serra et al. 2015). Additionally, S. aureus and P. aeruginosa are both on the list of ESKAPE pathogens, the most common multidrug resistant (MDR) bacteria causing nosocomial infections (Esposito and De Simone 2017; Santajit and Indrawattana 2016). The ability of S. aureus and P. aeruginosa to form biofilms is also well documented (De Oliveira et al. 2016; Billings et al. 2013; Stewart et al. 2015). Although biofilms can be single species, they are often multispecies (O’Mar et al. 2017). Formation of multispecies biofilms has the potential to create a reservoir of antimicrobial resistance genes and an environment for genetic exchange, potentially contributing to MDR infections (Savage et al. 2013; Aguila-Arcos et al. 2017; Balcazar et al. 2015; Molin and Tolker-Nielsen 2003; Madsen et al. 2012; Stalder and Top 2016); therefore, demonstration of antibiofilm efficacy of an antimicrobial against P. aeruginosa and S. aureus as well as multispecies biofilms is important for a positive clinical outcome.
While there is currently no defined standard, representative of clinical treatment of a wound in vitro, there are standardised models that can be utilised for evaluating antibiofilm efficacy of wound care products. The minimum bactericidal eradication concentration (MBEC) model (ASTM E2799-17) involves growing the biofilm on a peg lid, under batch conditions, in a 96 well plate. The MBEC method is high throughput and allows simultaneous testing of multiple concentrations of the same test solution. The Centre for Disease Control (CDC) bioreactor model (ASTM E2871-19) allows growth of a biofilm in a vessel under constantly stirred conditions. The biofilm is grown under high shear conditions, which provides a more challenging biofilm to kill. The drip flow bioreactor model (ASTM E2647-20) allows growth of a biofilm close to the air/liquid interface under low shear conditions. Due to the continuous flow of proteinaceous media this model is more representative of an exuding wound environment and provides a further challenge to the efficacy of wound care products. The multispecies biofilm model involves growing the biofilm on filter discs using a hydrogel as a nutrient source to provide a dry environment for biofilm growth. Multispecies biofilms are often more challenging to eradicate, as different species in a biofilm often work synergistically together and can also provide an environment for genetic exchange of antimicrobial resistant genes (O’Mar et al. 2017; Aguila-Arcos et al. 2017). The LabTek chamber slide model involves growing the biofilm under batch conditions similar to the MBEC method; however, allows for a qualitative evaluation rather than quantitative. Qualitative evaluation is useful as it provides information on extracellular polymeric substance (EPS) breakdown/biofilm disruption and removal that may not be identified from quantitative evaluation.
Wound irrigation solutions are used to cleanse, rinse and moisturise wounds, effectively debriding wounds of cell debris, slough, eschar and microorganisms and therefore reducing wound healing time and preventing biofilm formation and infection. Addition of antimicrobial agents and surfactants in wound irrigation solutions has been shown to enhance wound healing in comparison to normal saline solution (Percival et al. 2017; Bellingeri et al. 2016). The aim of this study was to evaluate the antibiofilm efficacy of a 0.1% Polihexanide (PHMB) based wound irrigation solution (Prontosan®, B Braun Medical), Octenidine HCl based wound irrigation solution (Octenilin®, Schülke & Mayr GmbH) and electrolysed water (sodium hypochlorite and hypochlorous acid) based wound care solution (Microdacyn 60®, Sonoma™ Pharmaceuticals) in multiple in vitro models to gain a broad understanding of their antibiofilm capability.
2 Materials and Methods
2.1 Test Articles
A 0.1% Polihexanide (PHMB) based wound irrigation solution containing 0.1% Betaine and purified water (Prontosan®, B Braun Medical), Octenidine HCl based wound irrigation solution containing aqua valde purificata, Glycerin and Ethylhexylglycerin (Octenilin®, Schülke & Mayr GmbH) and electrolysed water (sodium hypochlorite and hypochlorous acid) based wound care solution containing super-oxidized water and 0.022% sodium chloride (Microdacyn 60®, Sonoma™ Pharmaceuticals).
A broad-spectrum neutraliser consisting of 30 g/L Tween 80, 3 g/L Lecithin, 1 g/L L-Histidine, 2 g/L L-Cysteine and 15 g/L Saponin was used to neutralise all of the wound irrigation solutions throughout this study. All reagents were purchased from Sigma-Aldrich, UK.
2.2 LabTek Chamber Slide Biofilm Model
The antibiofilm efficacy of the test solutions against P. aeruginosa ATCC 15442 and S. aureus ATCC 29213 was evaluated qualitatively by growing the biofilms in LabTek chamber slides and staining them with LIVE/DEAD™ BacLight™ bacterial viability kit (ThermoFisher Scientific, UK). The biofilms were then treated with each solution for 24 h before visualising them using confocal laser scanning microscopy (CLSM).
Overnight cultures of P. aeruginosa ATCC 15442 and S. aureus ATCC 29213 were set up by inoculating Tryptone Soya broth (TSB) with a single colony and incubating it overnight at 37 °C and 125 rpm. The following day, the overnight cultures were adjusted to 1 × 106 CFU/mL and added to LabTek chamber slides. The slides were incubated at 37 °C and 125 rpm for 24 h.
Following growth, the biofilm was washed twice with phosphate buffered saline (PBS) and stained with the LIVE/DEAD™ BacLight™ kit (ThermoFisher Scientific, UK) by preparing a 2× solution of SYTO 9 and propidium iodide fluorescent stains and adding it to the LabTek chamber slides. The slides were incubated in the dark for 15 min to allow staining of the cells, before washing the biofilms twice with PBS and adding each test solution at 100% concentration in triplicate. PBS only was added to the untreated control and the slides were incubated for 24 h.
Following incubation, each well was visualised using an LSM 780 imaging confocal microscope and Zeiss software at an excitation/emission of 480/500 nm. For each chamber, 16 images were taken and collated into 1 image to show the entire surface of each chamber. Images were processed using Image J software (National Institutes of Health, USA).
2.3 Minimum Biofilm Eradication Concentration (MBEC) Model
The antibiofilm efficacy of the 3 wound solutions was evaluated against a 24 h biofilm of P. aeruginosa ATCC 15442 and S. aureus ATCC 29213 following an adapted version of ASTM standard E2799-17 ‘Standard Test Method for Testing Disinfectant Efficacy against Pseudomonas aeruginosa Biofilm using the MBEC Assay.’
Briefly, overnight cultures of P. aeruginosa ATCC 15442 and S. aureus ATCC 29213 were set up by inoculating TSB with a single colony and incubating at 37 °C and 125 rpm in an orbital shaking incubator. The following day the overnight cultures were adjusted to 1 × 105 CFU/mL in TSB and used to inoculate a 96 well plate. A peg lid was placed onto the 96 well plate which was incubated at 37 °C and 110 rpm for 24 h.
The challenge plates for each test solution were set up by adding the solution to a new 96 well plate and serial diluting it 1:2 in PBS. A neutraliser effectiveness control was set up by adding each solution to the neutraliser at a ratio of 1:1. A neutraliser toxicity control was set up by adding the neutraliser only and an untreated control was set up by adding PBS only to the plate.
The peg lid containing biofilm was transferred to a rinse plate containing PBS before transferring it to the challenge plate. The challenge plates containing P. aeruginosa were incubated at room temperature for 24 h. The challenge plates containing S. aureus were incubated at 37 °C for 24 h.
Following 24 h treatment, the peg lids were transferred to recovery plates containing neutraliser and sonicated on full power (100 W) in an Ultrawave water bath for 30 min. Samples were transferred to new 96 well plates and serial diluted 1:10. Serial dilutions were spot plated onto Tryptone Soya agar (TSA) and incubated overnight at 37 °C. The following day counts were enumerated.
2.4 Centers for Disease Control (CDC) Bioreactor Model
The antibiofilm efficacy of the 3 wound solutions was also evaluated in the CDC bioreactor model against 24 h biofilms of P. aeruginosa ATCC 15442 and S. aureus ATCC 29213.
Overnight cultures of P. aeruginosa ATCC 15442 and S. aureus ATCC 29213 were set up by inoculating TSB with a single colony and incubating at 37 °C and 125 rpm. The overnight culture of P. aeruginosa was adjusted to 1 × 108 CFU/mL in TSB and used to inoculate the CDC bioreactor. The CDC bioreactor was then incubated at room temperature and 125 rpm for 24 h. The overnight culture of S. aureus was pelleted by centrifugation, resuspended in TSB and used to inoculate the CDC bioreactor. The CDC bioreactor was incubated for 24 h on at 37 °C and 100 rpm.
Following 24 h incubation, coupons were washed twice in PBS and placed into 6 well plates. Each neat wound solution was added to wells in triplicate (4 mL/well). A set of untreated coupons were also added to the wells for a comparison growth control. The plates were then incubated for 24 h at room temperature.
The following day, coupons were removed from wells, added to neutraliser and sonicated on full power for 30 min. Samples were vortexed briefly, serial diluted 1:10 in PBS and plated onto TSA. Plates were incubated at 37 °C overnight and the following day colony counts were enumerated.
2.5 Drip Flow Bioreactor Model
The antibiofilm efficacy of the test solutions was evaluated in the drip flow bioreactor model following ASTM E2647-13 Quantification of Pseudomonas aeruginosa Biofilm Grown Using Drip Flow Biofilm Reactor with Low Shear and Continuous Flow.
The drip flow bioreactor was prepared by adding a clean borosilicate microscope slide to each channel and autoclaving at 121 °C for 15 min. An overnight inoculum was set up by inoculating TSB with a single colony of P. aeruginosa ATCC 700888 and incubating at 37 °C and 125 rpm. The following day, the overnight culture was adjusted to 1 × 108 CFU/mL and used to inoculate the drip flow bioreactor chambers. The drip flow bioreactor was incubated for 6 h in batch phase before connecting it to a nutrient carboy containing 270 mg/L TSB operated continuously at a flow rate of 50 mL/h/channel.
After 24 h biofilm growth, sterile gauze was soaked in the test solutions for 30 min and added to microscope slides in triplicate. The biofilms were incubated in continuous phase for a further 24 h. After the challenge period, each microscope slide was scraped into 45 mL of neutraliser washed with 5 mL neutraliser. Each sample was homogenised for 30 s before serial diluting it 1:10 in PBS and plating it out in duplicate onto TSA. The plates were incubated overnight at 37 °C and the following day counts were enumerated.
2.6 Multispecies Biofilm Model
The antibiofilm efficacy of the 3 wound solutions was evaluated against a 24 h multispecies biofilm of P. aeruginosa ATCC 15442, S. aureus ATCC 29213 and E. faecalis ATCC 29212. The biofilm was grown on filter discs using a hydrogel as a nutrient source.
The hydrogel was prepared by dissolving 3-sulfopropyl acrylate potassium salt (polymer) in PBS and then adding PEG dissolved in PBS, foetal bovine serum (FBS) and 1% 1-hydroxy cyclohexyl phenol ketone prepared in 70% ethanol (photo-initiator) to it. The mixture was added to a 12 well plate (2 mL/well) and set by exposing the hydrogel to 366 nm UV light.
Overnight cultures of P. aeruginosa ATCC 15442, S. aureus ATCC 29213 and E. faecalis ATCC 29212 were set up by inoculating TSB with a single colony and incubating at 37 °C and 125 rpm. Overnight cultures were adjusted to 1 × 108 CFU/mL before adding all 3 strains together in TSB at a final concentration of 1 × 106 CFU/mL. Durapore 13 mm (1 μM) membrane filter discs (Merck, UK) were incubated with the culture for 2 h at 37 °C and 125 rpm. Following this, the filters were transferred to a 12 well plate containing the hydrogel and incubated at 37 °C for 24 h.
Following 24 h biofilm growth, the filter discs were transferred to fresh 12 well plates and treated with each solution in triplicate. PBS was added to the untreated control in triplicate. Biofilms were treated for 24 h at 37 °C. Following 24 h treatment, filter discs were transferred to neutraliser and sonicated on full power for 30 min. Samples were vortexed briefly, serial diluted 1:10 in PBS and plated onto TSA. The plates were incubated overnight at 37 °C and the following day counts were enumerated.
2.7 Statistical Analysis
Raw data was entered into Microsoft Excel and average CFU/mL was calculated. To determine if there was a statistical difference between the untreated control and the treated biofilms one-way ANOVA using Dunnett’s multiple comparison test was carried out using Prism 7 software.
3 Results
3.1 Antibiofilm Efficacy in the LabTek Chamber Slide Model
The antibiofilm efficacy of the test solutions was evaluated qualitatively against 24 h biofilms of S. aureus ATCC 29213 and P. aeruginosa ATCC 15442.
All 3 test solutions demonstrated efficacy against P. aeruginosa (Fig. 1) and S. aureus (Fig. 2) biofilms, with evidence of biofilm disruption and removal of the biofilms being observed in all the treated wells in comparison to the untreated control.
Representative images of P. aeruginosa biofilm on the surface of the Lab Tek chamber slide. Biofilms were stained with LIVE/DEAD BacLight stain. The images show the untreated biofilm (a) and biofilm following treatment with the PHMB wound irrigation solution (b), the Octenidine HCl based wound irrigation solution (c) and the electrolysed water based wound care solution (d)
Representative images of S. aureus biofilm on the surface of the Lab Tek chamber slide. Biofilms were stained with LIVE/DEAD BacLight stain. The images show the untreated biofilm (a) and biofilm following treatment with (b), the Octenidine HCl based wound irrigation solution (c) and the electrolysed water based wound care solution (d)
3.2 Antibiofilm Efficacy in the MBEC Model
Following treatment of a 24 h P. aeruginosa biofilm and S. aureus biofilm with the PHMB based wound irrigation solution at 100% concentration, complete eradication of the biofilms were found (Fig. 3). In comparison the untreated growth controls had a bacterial cell density of 2.84 × 104 CFU/mm2 (p < 0.0001) and 7.29 × 104 CFU/mm2 (p 0.0003), respectively showing a 4 log reduction in both biofilms. The PHMB based wound irrigation solution also demonstrated efficacy against the P. aeruginosa biofilm at 50%, 25% and 12.5% concentration, showing a 2–4 log reduction in bacterial cell density (p ≤ 0.0308) and at 50%, 25%, 12.5% and 6.25% concentration against the S. aureus biofilm, showing a 1.5–3 log reduction in bacterial cell density (p ≤ 0.0321).
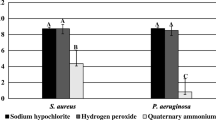
MBEC of the PHMB based wound irrigation solution (a), the Octenidine HCl based wound irrigation solution (b) and the electrolysed water based wound care solution (c) against a 24 h biofilm of P. aeruginosa ATCC 15442 (left) and S. aureus ATCC 29213 (right). Error bars represent the standard error of the mean. * = a statistically significant reduction in biofilm in comparison to the untreated control (p ≤ 0.0423)
Following treatment with the Octenidine HCl based wound irrigation solution at 100%, 50%, 25%, 12.5%, 6.25% and 3.13% antibiofilm efficacy was observed against a 24 h biofilm of P. aeruginosa, with a bacterial cell density ranging from 5.83 x 103 to 1.36 × 104 CFU/mm2. In comparison the untreated growth control had a bacterial cell density of 1.66 × 105 CFU/mm2 showing a 1–1.5 log reduction in biofilm (p ≤ 0.0376). Following treatment with the Octenidine HCl based wound irrigation solution at 100% and 50% complete eradication of a 24 h S. aureus biofilm was found. In comparison the untreated biofilm growth control had a bacterial cell density of 2.90 × 105 CFU/mm2 showing a 5 log reduction (p < 0.0001). At 25%, 12.5% and 6.25% concentration, the Octenidine HCl based wound irrigation solution also demonstrated antibiofilm efficacy showing a 2–5 log reduction in bacterial cell density (p ≤ 0.0423).
Following treatment of a 24 h P. aeruginosa biofilm with the electrolysed water based wound care solution at 100% and 50% concentration, complete eradication of the biofilm was found. In comparison the untreated biofilm growth control had a bacterial cell density of 4.02 × 104 CFU/mm2 showing a 4 log reduction following treatment (p 0.0005). The electrolysed water based wound care solution at 25% and 12.5% concentration also showed antibiofilm efficacy with a 3–4 log reduction (p ≤ 0.0221). Following treatment of a 24 h S. aureus biofilm with the electrolysed water based wound care solution at 100% concentration, complete eradication of the biofilm was found. In comparison the untreated biofilm growth control had a bacterial cell density of 1.98 × 105 CFU/mm2 showing a 5 log reduction (p < 0.0001). The electrolysed water based wound care solution at 50% and 25% concentration also showed antibiofilm efficacy against S. aureus, with a 1 log reduction being found following treatment (p ≤ 0.0058).
The neutraliser effectiveness and neutraliser toxicity controls in this model demonstrated that it neutralised all 3 of the wound irrigation solutions and that it was also non-toxic to the bacteria.
3.3 Antibiofilm Efficacy in the CDC Bioreactor Model
The antibiofilm efficacy of the 3 test solutions was also evaluated in the CDC bioreactor model and all showed antibiofilm efficacy against 24 h biofilms of P. aeruginosa ATCC 15442 (Fig. 4) and S. aureus ATCC 29213 (Fig. 5) to different extents.
Log10 bacterial cell density of P. aeruginosa ATCC 15442 24 h biofilm following treatment with the PHMB based wound irrigation solution, the Octenidine HCl based wound irrigation solution and the electrolysed water based wound care solution. Error bars represent the standard error of the mean. * = a statistically significant reduction in biofilm in comparison to the untreated control (p ≤ 0.0009)
Log10 bacterial cell density of S. aureus ATCC 29213 24 h biofilm following treatment with the PHMB based wound irrigation solution, the Octenidine HCl based wound irrigation solution and the electrolysed water based wound care solution. Error bars represent the standard error of the mean. * = a statistically significant reduction in biofilm in comparison to the untreated control (p 0.0001)
Following 24 h growth of P. aeruginosa ATCC 15442 biofilm in the CDC bioreactor model, the untreated growth control had a bacterial cell density of 4.53 × 107 CFU/mL. The biofilm treated with the PHMB based solution, Octenillin solution and electrolysed water solution had a bacteria cell density of 3.00 x 102 CFU/mL, 2.80 × 104 CFU/mL and 3.33 × 106 CFU/mL, showing a 5 (p 0.0005), 3 (p 0.0005) and 1 (p 0.0009) log reduction in biofilm compared to the untreated control, respectively.
Following 24 h treatment of the S. aureus ATCC 29213 biofilm, the growth control had a bacterial cell density of 4.93 × 106 CFU/mL. Treatment with each solution resulted in complete eradication of the biofilm, showing a 6 log reduction in bacterial cell density in comparison to the untreated control (p 0.0001).
3.4 Antibiofilm Efficacy in the Drip Flow Bioreactor Model
The antibiofilm efficacy of the test solutions was evaluated against a 24 h biofilm of P. aeruginosa ATCC 700888 in the drip flow bioreactor model, by growing the biofilm and applying the treatment with a continuous supply of nutrients (Fig. 6).
Following 24 h treatment of the P. aeruginosa biofilm with the PHMB based wound irrigation solution, a bacterial cell density of 2.54 × 106 CFU/mL was found. In comparison the untreated biofilm had a bacterial cell density of 2.15 × 109 CFU/mL, showing a 3 log reduction. Treatment with the Octenidine HCl based wound irrigation solution and the electrolysed water based wound care solution resulted in a bacterial cell density of 5.40 × 105 CFU/mL and 9.93 × 107 CFU/mL, showing a 3.5 log and 1.5 log reduction in comparison to the untreated control, respectively.
3.5 Antibiofilm Efficacy in the Multispecies Biofilm Model
The antibiofilm efficacy of the test solutions was evaluated against a 24 h multispecies biofilm of P. aeruginosa, S. aureus and E. faecalis by growing the biofilm on filter discs with a hydrogel as a nutrient supply.
Following 24 h treatment with the PHMB based wound irrigation solution and the electrolysed water based wound care solution no colonies were observed, showing complete eradication of the biofilm (p 0.0016; Fig. 7). In comparison the untreated biofilm had a bacterial cell density of 1.07 × 106 CFU/mL showing a 6 log reduction with the treatments. Following treatment with the Octenidine HCl based wound irrigation solution a bacterial cell density of 3.10 × 103 CFU/mL was found, showing a 3 log reduction in biofilm in comparison to the untreated control (p 0.0016).
Log10 bacterial cell density of a 24 h multispecies biofilm following treatment with the PHMB based wound irrigation solution, the Octenidine HCl based wound irrigation solution and the electrolysed water based wound care solution. Error bars represent the standard error of the mean. * = a statistically significant reduction in biofilm in comparison to the untreated control (p 0.0016)
4 Discussion
In this study, the antibiofilm efficacy of a PHMB based wound irrigation solution in comparison to an Octenidine HCl based wound irrigation solution and the electrolysed water based wound care solution was evaluated in several different models against P. aeruginosa, S. aureus and a multispecies biofilm of P. aeruginosa, S. aureus and E. faecalis. The solutions were applied at 100% concentration and at diluted concentrations for a contact time of 24 h to represent clinical application of wound irrigation solutions when they are used longer term in comparison to short contact times such at 15 min.
The PHMB based wound irrigation solution contains the PHMB as an antimicrobial preservative and the surfactant Betaine. PHMB has been shown to have a broad spectrum of activity against bacteria, yeast and fungi through cell membrane disruption; although the ability to selectively enter bacterial cells and bind chromosomes has also been shown (Ali and Wilson 2017; Chindera et al. 2016; Kamaruzzaman et al. 2016; Rembe et al. 2016). Treatment of wounds with PHMB has been associated with a positive clinical outcome (Webster et al. 2017; To et al. 2016; Villela-Castro et al. 2018). Surfactants, such as Betaine, are widely used in wound care to aid cleaning and debridement of wounds (Percival et al. 2017; Bellingeri et al. 2016). The PHMB based wound irrigation solution has shown the ability to break down dried human plasma, representative of wound coatings in vitro and also clean, moisten and decontaminate encrusted chronic wounds in a small clinical study of 10 patients (Horrocks 2006; Kaehn and Eberlein 2009). In a previous study, the PHMB based wound irrigation solution demonstrated antimicrobial activity against S. aureus (Hirsch et al. 2011). Additionally, treatment of chronic wounds, such as venous leg ulcers, with the PHMB based wound irrigation solution has been associated with an improved clinical outcome, with wounds healing faster and more wounds completely healing (Andriessen and Eberlein 2008).
Octenidine has demonstrated broad spectrum antimicrobial activity in vitro (Alvarez-Marin et al. 2017; Assadian 2016). Additionally, following central venous catheter insertion in a double-blind randomized controlled trial, the Octenidine HCl based wound irrigation solution significantly reduced insertion site skin colonisation and colonisation of the catheter tip (Dettenkofer et al. 2010). The Octenidine HCl based wound irrigation solution has also been shown to effectively reduce MRSA colonisation in observational studies (Krishna and Gibb 2010). The electrolysed water based wound care solution contains 0.004% sodium hypochlorite and 0.004% hypochlorous acid as preservative agents. The electrolysed water based wound care solution primarily debrides wounds, decreasing infection rates and improving wound healing. A randomised single-blind clinical control study of patients with diabetic foot ulcers demonstrated the electrolysed water based wound care solution to be more efficacious in infection control, odour reduction and erythema reduction than conventional disinfectants (Martinez-De Jesus et al. 2007). However, some studies have demonstrated that sodium hypochlorite/hypochlorous acid wound care solutions with low total chlorine have low antimicrobial and antibiofilm efficacy (Severing et al. 2019; Krasowski et al. 2021).
In this study, the antibiofilm efficacy of all 3 solutions was evaluated in multiple models against S. aureus and P. aeruginosa, which are commonly associated with wound infections (Serra et al. 2015). The LabTek chamber slide model involves growing the biofilm in batch phase and staining the biofilm with SYTO 9 and propidium iodide fluorescent stains, to allow visualisation of the biofilm and qualitative analysis following treatment with test solutions. Using this method, disruption and removal of both P. aeruginosa and S. aureus biofilms could be observed following treatment with all 3 test solutions. It has been shown previously that in models such as the LabTek chamber slide model, pipette-based wash steps can cause random holes and alterations within the biofilms as it is quite an aggressive method (Tasse et al. 2018). To account for possible biofilm removal through use of the pipette washing technique, an untreated biofilm control that was subject to the same washing and staining steps was used as a comparison when visually analysing the effects of each treatment. Additionally, each group was tested in triplicate to allow consistent results to be drawn from each treatment group compared to the untreated group. Although these measures were taken to account for any biofilm disruption caused by the methodology rather than the treatment, the limitations of this model should be taken into consideration when reviewing the data.
The MBEC model involves growing the biofilm under batch conditions, so there is no flow of nutrients and allows high throughput testing so that multiple concentrations of test solution can be evaluated simultaneously. In this model, the PHMB based wound irrigation solution showed complete eradication of the P. aeruginosa and S. aureus biofilms at 100% concentration. Complete eradication of the P. aeruginosa biofilm was also found with the electrolysed water based wound care solution at 50% and complete eradication of the S. aureus biofilm was found with the Octenidine HCl based wound irrigation solution at 50% and the electrolysed water based wound care solution at 100%. The Octenidine HCl based wound irrigation solution was less efficacious in this model against S. aureus but showed some activity with a 1.5 log reduction in biofilm in comparison to the untreated control. The MBEC of the wound irrigation solutions has been evaluated elsewhere recently using a 96-well plate method and 1% tetrazolium chloride staining method (Krasowski et al. 2021). Krasowski et al. showed the PHMB based wound irrigation solution eradicated the S. aureus and P. aeruginosa biofilms at approx. 20% and 35% concentration, respectively, whilst the Octenidine solution eradicated them at approx. 10% and 40%, respectively. The electrolysed water based wound care solution did not eradicate the biofilm at up to 50% concentration. The differences in concentration required to fully eradicate the biofilms between the 2 studies may be as a result of differences in the methodology, such as the sensitivity of bacterial detection methods and that different bacterial strains used.
The CDC bioreactor model involves growing a biofilm under high shear conditions and adding the test samples in a dry environment to the biofilm containing coupons. In this model, the PHMB based wound irrigation solution showed the greatest antibiofilm efficacy against a P. aeruginosa biofilm, reducing it by 5 log in comparison to the untreated control. The Octenidine HCl based wound irrigation solution and the electrolysed water based wound care solution showed some antibiofilm activity with a 3 log and 1 log reduction, respectively. All 3 test solutions demonstrated greater efficacy against S. aureus in this model, with complete eradication of the biofilm being found following treatment.
The drip flow bioreactor model involves growing a P. aeruginosa biofilm close to the air/liquid interface in an environment with continuous nutrient flow under low shear conditions. The nutrient flow is continued during treatment application and is designed to represent a highly exudative wound environment. In this model, the PHMB based wound irrigation solution and the Octenidine HCl based wound irrigation solution both showed antibiofilm efficacy, reducing the bacterial cell density by 3 log and 3.5 log, respectively. The electrolysed water based wound care solution was less efficacious, showing a 1 log reduction in the P. aeruginosa biofilm.
The test solutions were also evaluated against a multispecies biofilm model of P. aeruginosa, S. aureus and E. faecalis, as clinical studies have shown that biofilms are often multispecies rather than single species (Alexiou et al. 2017). Additionally, all 3 strains are commonly associated with nosocomial infections and wound biofilms (Krishna and Gibb 2010; Banu et al. 2015; Serra et al. 2015; Obermeier et al. 2018; Faron et al. 2016). Therefore, the biofilm in this model may be more representative of a wound environment. In this model, the PHMB based wound irrigation solution and the electrolysed water based wound care solution eradicated the multispecies biofilm showing potent antibiofilm efficacy. The Octenidine HCl based wound irrigation solution also demonstrated antibiofilm efficacy, with treatment resulting in a 3 log reduction of the biofilm.
Overall, the PHMB based wound irrigation solution demonstrated potent antibiofilm efficacy across most of the biofilm models used in this study, with treatment resulting in complete eradication of the biofilm and a 5 log reduction of a P. aeruginosa biofilm grown in the CDC bioreactor model. The Octenidine HCl based wound irrigation solution showed potent antibiofilm efficacy against S. aureus, completely eradicating the biofilm in both the MBEC and CDC bioreactor model. Although some antibiofilm activity was found against P. aeruginosa and the multispecies biofilm, the Octenidine HCl based wound irrigation solution was less efficacious against these biofilms than the S. aureus one. The electrolysed water based wound care solution completely eradicated the S. aureus biofilm in the MBEC and CDC bioreactor models and the multispecies biofilm, showing potent efficacy against these biofilms; however, less efficacy was observed against P. aeruginosa in the CDC bioreactor model and the drip flow bioreactor model. Overall, less efficacy was observed in the drip flow bioreactor model for all 3 test solutions, which may be attributed to the continuous flow of proteinaceous media during treatment, which may have diluted or washed away the solution.
The data presented in this study shows the PHMB based wound irrigation solution to have the greatest broad range antibiofilm activity against both P. aeruginosa, S. aureus and a multispecies biofilm in comparison to the other solutions tested. The Octenidine HCl based wound irrigation solution demonstrated potent antibiofilm activity against S. aureus, but to a lesser extent against P. aeruginosa and the multispecies biofilm and the electrolysed water based wound care solution demonstrated potent antibiofilm activity against S. aureus and the multispecies biofilm, but to a lesser extent against P. aeruginosa. The data presented also highlights the importance of testing antibiofilm activity in a range of biofilm models and against different bacterial strains to get an overall representation of efficacy.
References
Aguila-Arcos S, Alvarez-Rodriguez I, Garaiyurrebaso O, Garbisu C, Grohmann E, Alkorta I (2017) Biofilm-forming clinical staphylococcus isolates harbor horizontal transfer and antibiotic resistance genes. Front Microbiol 8:2018
Alexiou K, Drikos I, Terzopoulou M, Sikalias N, Ioannidis A, Economou N (2017) A prospective randomised trial of isolated pathogens of surgical site infections (SSI). Ann Med Surg (Lond) 21:25–29
Ali S, Wilson APR (2017) Effect of poly-hexamethylene biguanide hydrochloride (PHMB) treated non-sterile medical gloves upon the transmission of Streptococcus pyogenes, carbapenem-resistant E. coli, MRSA and Klebsiella pneumoniae from contact surfaces. BMC Infect Dis 17:574
Alvarez-Marin R, Aires-De-Sousa M, Nordmann P, Kieffer N, Poirel L (2017) Antimicrobial activity of octenidine against multidrug-resistant gram-negative pathogens. Eur J Clin Microbiol Infect Dis 36:2379–2383
Andriessen AE, Eberlein T (2008) Assessment of a wound cleansing solution in the treatment of problem wounds. Wounds Compendium Clin Res Pract 20:171–175
Assadian O (2016) Octenidine dihydrochloride: chemical characteristics and antimicrobial properties. J Wound Care 25:S3–S6
Balcazar JL, Subirats J, Borrego CM (2015) The role of biofilms as environmental reservoirs of antibiotic resistance. Front Microbiol 6:1216
Banu A, Noorul Hassan MM, Rajkumar J, Srinivasa S (2015) Spectrum of bacteria associated with diabetic foot ulcer and biofilm formation: a prospective study. Australas Med J 8:280–285
Bellingeri A, Falciani F, Traspedini P, Moscatelli A, Russo A, Tino G, Chiari P, Peghetti A (2016) Effect of a wound cleansing solution on wound bed preparation and inflammation in chronic wounds: a single-blind RCT. J Wound Care 25:160
Billings N, Millan M, Caldara M, Rusconi R, Tarasova Y, Stocker R, Ribbeck K (2013) The extracellular matrix component Psl provides fast-acting antibiotic defense in Pseudomonas aeruginosa biofilms. PLoS Pathog 9:e1003526
Chindera K, Mahato M, Sharma AK, Horsley H, Kloc-Muniak K, Kamaruzzaman NF, Kumar S, Mcfarlane A, Stach J, Bentin T, Good L (2016) The antimicrobial polymer PHMB enters cells and selectively condenses bacterial chromosomes. Sci Rep 6:23121
DE OLIVEIRA A, Cataneli Pereira V, Pinheiro L, Moraes Riboli DF, Benini Martins K, Ribeiro De Souza Da Cunha Mde L (2016) Antimicrobial resistance profile of planktonic and biofilm cells of staphylococcus aureus and coagulase-negative staphylococci. Int J Mol Sci 17:1423
Dettenkofer M, Wilson C, Gratwohl A, Schmoor C, Bertz H, Frei R, Heim D, Luft D, Schulz S, Widmer AF (2010) Skin disinfection with octenidine dihydrochloride for central venous catheter site care: a double-blind, randomized, controlled trial. Clin Microbiol Infect 16:600–606
Esposito S, De Simone G (2017) Update on the main MDR pathogens: prevalence and treatment options. Infez Med 25:301–310
Faron ML, Ledeboer NA, Buchan BW (2016) Resistance mechanisms, epidemiology, and approaches to screening for vancomycin-resistant Enterococcus in the health care setting. J Clin Microbiol 54:2436–2447
Fleming D, Chahin L, Rumbaugh K (2017) Glycoside hydrolases degrade polymicrobial bacterial biofilms in wounds. Antimicrob Agents Chemother 61:e01998
Flemming HC (2016) EPS-then and now. Microorganisms 4:41
Guo S, Dipietro LA (2010) Factors affecting wound healing. J Dent Res 89:219–229
Hirsch T, Limoochi-Deli S, Lahmer A, Jacobsen F, Goertz O, Steinau HU, Seipp HM, Steinstraesser L (2011) Antimicrobial activity of clinically used antiseptics and wound irrigating agents in combination with wound dressings. Plast Reconstr Surg 127:1539–1545
Horrocks A (2006) Prontosan wound irrigation and gel: management of chronic wounds. Br J Nurs 15(1222):1224–1228
Kaehn K, Eberlein T (2009) In-vitro test for comparing the efficacy of wound rinsing solutions. Br J Nurs 18(S4):S6–S8, S10
Kamaruzzaman NF, Firdessa R, Good L (2016) Bactericidal effects of polyhexamethylene biguanide against intracellular Staphylococcus aureus EMRSA-15 and USA 300. J Antimicrob Chemother 71:1252–1259
Krasowski G, Junka A, Paleczny J, Czajkowska J, Makomaska-Szaroszyk E, Chodaczek G, Majkowski M, Migdal P, Fijalkowski K, Kowalska-Krochmal B, Bartoszewicz M (2021) In vitro evaluation of polihexanide, octenidine and NaClO/HClO-based antiseptics against biofilm formed by wound pathogens. Membranes (Basel) 11:62
Krishna BV, Gibb AP (2010) Use of octenidine dihydrochloride in meticillin-resistant Staphylococcus aureus decolonisation regimens: a literature review. J Hosp Infect 74:199–203
Madsen JS, Burmolle M, Hansen LH, Sorensen SJ (2012) The interconnection between biofilm formation and horizontal gene transfer. FEMS Immunol Med Microbiol 65:183–195
Malone M, Bjarnsholt T, Mcbain AJ, James GA, Stoodley P, Leaper D, Tachi M, Schultz G, Swanson T, Wolcott RD (2017) The prevalence of biofilms in chronic wounds: a systematic review and meta-analysis of published data. J Wound Care 26:20–25
Martinez-De Jesus FR, Ramos-De La Medina A, Remes-Troche JM, Armstrong DG, Wu SC, Lazaro Martinez JL, Beneit-Montesinos JV (2007) Efficacy and safety of neutral pH superoxidised solution in severe diabetic foot infections. Int Wound J 4:353–362
Molin S, Tolker-Nielsen T (2003) Gene transfer occurs with enhanced efficiency in biofilms and induces enhanced stabilisation of the biofilm structure. Curr Opin Biotechnol 14:255–261
O’Mar A, Wright JB, Schultz G, Burrell R, Nadworny P (2017) Microbial biofilms and chronic wounds. Microorganisms 5:9
Obermeier A, Schneider J, Harrasser N, Tubel J, Muhlhofer H, Pforringer D, Deimling CV, Foehr P, Kiefel B, Kramer C, Stemberger A, Schieker M, Burgkart R, Von Eisenhart-Rothe R (2018) Viable adhered Staphylococcus aureus highly reduced on novel antimicrobial sutures using chlorhexidine and octenidine to avoid surgical site infection (SSI). PLoS One 13:e0190912
Percival SL (2017) Importance of biofilm formation in surgical infection. Br J Surg 104:e85–e94
Percival SL, Mayer D, Malone M, Swanson T, Gibson D, Schultz G (2017) Surfactants and their role in wound cleansing and biofilm management. J Wound Care 26:680–690
Rembe JD, Fromm-Dornieden C, Schafer N, Bohm JK, Stuermer EK (2016) Comparing two polymeric biguanides: chemical distinction, antiseptic efficacy and cytotoxicity of polyaminopropyl biguanide and polyhexamethylene biguanide. J Med Microbiol 65:867–876
Santajit S, Indrawattana N (2016) Mechanisms of antimicrobial resistance in ESKAPE pathogens. Biomed Res Int 2016:2475067
Savage VJ, Chopra I, O’Neill AJ (2013) Staphylococcus aureus biofilms promote horizontal transfer of antibiotic resistance. Antimicrob Agents Chemother 57:1968–1970
Serra R, Grande R, Butrico L, Rossi A, Settimio UF, Caroleo B, Amato B, Gallelli L, De Franciscis S (2015) Chronic wound infections: the role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev Anti-Infect Ther 13:605–613
Severing AL, Rembe JD, Koester V, Stuermer EK (2019) Safety and efficacy profiles of different commercial sodium hypochlorite/hypochlorous acid solutions (NaClO/HClO): antimicrobial efficacy, cytotoxic impact and physicochemical parameters in vitro. J Antimicrob Chemother 74:365–372
Stalder T, Top E (2016) Plasmid transfer in biofilms: a perspective on limitations and opportunities. NPJ Biofilms Microbiomes 2:16022
Stewart PS, Franklin MJ, Williamson KS, Folsom JP, Boegli L, James GA (2015) Contribution of stress responses to antibiotic tolerance in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 59:3838–3847
Tasse J, Cara A, Saglio M, Villet R, Laurent F (2018) A steam-based method to investigate biofilm. Sci Rep 8:13040
To E, Dyck R, Gerber S, Kadavil S, Woo KY (2016) The effectiveness of topical Polyhexamethylene Biguanide (PHMB) agents for the treatment of chronic wounds: a systematic review. Surg Technol Int 29:45–51
Villela-Castro DL, Santos V, Woo K (2018) Polihexanide versus metronidazole for odor management in malignant (Fungating) wounds: a double-blinded, randomized, clinical trial. J Wound Ostomy Continence Nurs 45(5):413–418
Webster J, Larsen E, Marsh N, Choudhury A, Harris P, Rickard CM (2017) Chlorhexidine gluconate or polyhexamethylene biguanide disc dressing to reduce the incidence of central-line-associated bloodstream infection: a feasibility randomized controlled trial (the CLABSI trial). J Hosp Infect 96:223–228
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Salisbury, AM., Mullin, M., Chen, R., Percival, S.L. (2021). Antibiofilm Efficacy of Polihexanide, Octenidine and Sodium Hypochlorite/Hypochlorous Acid Based Wound Irrigation Solutions against Staphylococcus aureus, Pseudomonas aeruginosa and a Multispecies Biofilm. In: Donelli, G. (eds) Advances in Microbiology, Infectious Diseases and Public Health. Advances in Experimental Medicine and Biology(), vol 1369. Springer, Cham. https://doi.org/10.1007/5584_2021_645
Download citation
DOI: https://doi.org/10.1007/5584_2021_645
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-01994-4
Online ISBN: 978-3-031-01995-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)