Abstract
Altitude exposure affects hormonal homeostasis, but the adaptation of different populations is still not finely defined. This study aims to compare the mid-term effects of combining physical activity and altitude hypoxia on hormonal profiles in foreign trekkers coming from Italy versus indigenous Nepalese porters during a Himalayan trek. Participants (6 Italians and 6 Nepalese) completed a 300 km distance in 19 days of an accumulated altitude difference of 16,000 m, with an average daily walk of 6 h. The effect of high altitude on hormonal pathways was assessed by collecting blood samples the day before the expedition and the day after its completion. Foreign trekkers had an additional follow-up sample collected after 10 days. The findings revealed a different adaptation of thyroidal and gonadal axes to mid-term strenuous physical activity combined with high-altitude hypobaric hypoxia. The thyroid function shifted to the protective mechanism of low free triiodothyronine (FT3), whereas the gonadal axis was suppressed. The Italian trekkers and Nepalese porters had lower total testosterone and 17-β-estradiol levels after the expedition. At the follow-up, the Italians had increased testosterone values. Prolactin secretion decreased in the Italians but increased in the Nepalese. We conclude that exposure to high-altitude affects the hormonal axes. The effect seems notably pronounced for the hypothalamus-pituitary gonadal axis, suppressed after high-altitude exposure.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
From the pioneering work of Hans Selye on endocrinology and adaptation syndrome, the hypothalamic-pituitary axis has been recognized as the main player in the homeostatic adaptation to psychological or physical stressors (Rochette and Vergely 2017). Among the environmental stressors, high altitude has been steadily growing in interest during the last decades, considering the spread of altitude traveling. High altitude affects human systems impacting their physiological activities due to hypobaric hypoxia and climatic condition. It has been shown that central and peripheral endocrine functions are affected by high altitude, usually above 5000 m, barring persistent human colonization (von Wolff et al. 2018). The high-altitude hypoxic model is usually adopted to study the pathophysiologic effects of low oxygen tension in the aging process or cardiorespiratory disorders (Verratti et al. 2016; Cataldi and Di Giulio, 2009). Chronic conditions can also impair hormonal metabolism affecting target organs’ activities. However, findings in various studies are highly contentious, which may be caused by the wide diversity in the length and degree of hypoxic exposure, gender, age, and environmental conditions (Keenan et al. 2019; Verratti et al. 2017; Park et al. 2014).
Humans of different ethnicity, exposed from birth to different levels of hypoxia, respond and adapt to acute and chronic high-altitude exposure in diverse ways (Magliulo et al. 2020; Verratti et al. 2021). Comparisons of changes in hormonal profiles between different ethnicities, including sojourners and natives, in relation to high altitude are scarce. Researchers have focused on highland dwellers, trekkers, or climbers, somehow leaving out a population of altitude porters who have a specific geographic origin and often suffer from high altitude illness (Dawadi et al. 2020). The motivation behind the study of porters also lies in their legendary performance during uphill loaded locomotion, playing a fundamental role in the success of altitude expeditions (Minetti et al. 2006), as opposed to altitude travelers for whom the physical strain involved limits the performance and poses substantial stress. In this study, we address the effects of altitude hypoxia on hormonal profiles in foreign trekkers versus indigenous Nepalese porters during an altitude trek in the Himalayas.
2 Methods
2.1 Participants and Study Protocol
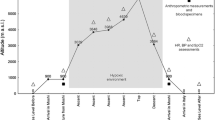
This study was conducted in accord with the STAR Data Reporting Guidelines for Clinical High Altitude Research (Brodmann Maeder et al. 2018). Six Italian trekkers and six indigenous native Nepalese porters took part in the trial, which was a separate ramification of the research project “Kanchenjunga Exploration & Physiology”. The basic demographics of the participants are presented in Table 1. Participants completed a 300 km distance in 19 days of an accumulated altitude difference of 16,000 m, with an average daily walk of 6 h. The route involved demanding ascents and descents in the Kanchenjunga region in the Himalayas in Nepal (Fig. 1). The expedition was supervised by a trained doctor who monitored symptoms, peripheral blood oxygen saturation (SpO2), arterial blood pressure, and anthropometric data throughout the whole period. None of the participants suffered acute mountain sickness.
Blood samples were collected from the antecubital vein, stored in vacutainer serum tubes, and immediately centrifuged at 3000 rpm × 10 min in the Bir Hospital (Kathmandu, Nepal, 1340 m), the day before and the day after the Himalayan trek. The serum was stored frozen at −5 °C and transported to Italy for later analyses in the Laboratory of Clinical Pathology of Teramo Hospital in the city of Teramo. Additionally, Italian participants’ serum was collected and analyzed at a 10-day follow-up once they returned to Italy.
The blood content of hormones was determined using the immuno-chemiluminescence assay in the ADVIA Centaur XP Immunoassay System (Siemens Healthcare; Erlangen Germany). We assayed thyroid function – free triiodothyronine (FT3), free thyroxine (FT4), thyroid-stimulating hormone (TSH), the hypothalamus-pituitary-gonadal axis – total testosterone, follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol, and the prolactin cortisol pathways.
2.2 Statistical Analysis
Normality of data distribution was assessed with the Shapiro-Wilk, the equality of variances with the Levene test, and whether the two data sets came from populations with a common distribution was determined with the quantile-quantile (Q-Q) plots. Considering the presence of missing points, we run a mixed-model analysis for all the parameters (Armstrong 2017). Specifically, we used the general linear mixed model (GLMM), with restricted maximum likelihood (REML) estimation and likelihood ratio testing (LRT) for random effects, time × ethnicity comparisons, and participants as the random variable. In the case of foreign participants, this analysis concerned pre vs. post vs. follow-up comparisons. The post hoc tests were conducted with the Bonferroni correction for multiple comparisons. The Satterthwaite method for degrees of freedom was used, and partial eta squared (η2p) and partial omega squared (ω2p) were calculated as measures of effect size (Fritz et al. 2012). The analysis was performed using R-based open-source Jamovi v1.2.5.0 software (https://www.jamovi.org).
3 Results
3.1 Thyroid Function
Thyroid-Stimulating Hormone
Changes in TSH in both Italian and Nepalese participants after altitude exposure were unremarkable (Table 2).
Free Triiodothyronine (FT3)
The investigation of thyroid function showed a reduction in FT3 concentration from pre-to-post altitude exposure (p = 0.012, η2p = 0.525, ω2p = 0.448). The time × ethnicity comparison revealed a tendency for a difference (p = 0.078, η2p = 0.306, ω2p = 0.212), with the Italian participants showing a greater reduction (from 2.75 ± 0.15 to 2.20 ± 0.46 pg/mL; p = 0.016) than the Nepalese (from 2.76 ± 0.31 to 2.63 ± 0.23 pg/mL) in post hoc analysis. At a 10-day follow up, FT3 reverted to the baseline level in the Italians (2.87 ± 0.35 pg/mL), as revealed by the pre vs. post vs. follow-up comparison (p = 0.011, η2p = 0.703, ω2p = 0.599), and significant post hoc differences were found (pre vs. post: p = 0.037 and post vs. follow-up: p = 0.020). The random effect of participants was not significant, and the effect sizes for the overall model were as follows: R2 marginal = 0.352, R2 conditional = 0.569.
Free Thyroxine (FT4)
In the Italian participants, FT4 content increased from pre-to-post altitude exposure, the increase persisted at a 10-day follow-up (1.07 ± 0.20 pg/mL, 1.21 ± 0.08 pg/mL, and 1.20 ± 0.09 pg/mL, respectively). In the Nepalese, we found an opposite trend, with FT4 reduction after altitude exposure (1.27 ± 0.14 pg/mL and 1.15 ± 0.08 pg/mL, respectively). Moreover, time × ethnicity comparison revealed a significant difference (p = 0.025, η2p = 0.443, ω2p = 0.359). The random effect of participants was not significant, and the effect sizes for the overall model were as follows: R2 marginal = 0.248, R2 conditional = 0.425.
3.2 Hypothalamus-Pituitary-Gonadal and Hypothalamus-Pituitary-Adrenal Axes
Gonadotropins
Changes in FSH and LH in both Italian and Nepalese participants after altitude exposure were unremarkable (Table 2).
Testosterone
The content of total testosterone decreased from pre-to-post altitude exposure, albeit the changes were of borderline significance (p = 0.109, η2p = 0.260, ω2p = 0.164), with the Nepalese participants having a greater reduction (from 4.56 ± 0.82 ng/mL to 3.40 ± 1.30 ng/mL) when compared with the Italians (from 4.86 ± 1.68 ng/mL to 4.35 ± 0.95 ng/mL); the difference between the two ethnic groups was insignificant (Fig. 2). At a 10-day follow up in the Italian participants, the baseline testosterone significantly increased to 6.57 ± 1.38 ng/mL, as revealed by the pre vs. post vs. follow-up comparison (p = 0.038, η2p = 0.675, ω2p = 0.515), and post-hoc differences were found (pre vs. post: p = 0.161 and post vs. follow-up: p = 0.043). The random effect of participants was not significant, and the effect sizes for the overall model were as follows: R2 marginal = 0.181, R2 conditional = 0.335.
Hormonal adaptations during exposure to high altitude in the Italian trekkers and Nepalese porters in Kanchenjunga Exploration & Physiology” expedition. Measurements were done a day before (pre) and a day after (post) the trek in the blood serum. An additional measurement was done at a 10-day follow-up (FU) in Italians only
17-β-Estradiol
In the Italian participants, 17-β-estradiol content decreased from the pre-altitude exposure level of 30.98 ± 3.20 pg/mL to 11.80 pg/mL post-exposure, which was the lower detection limit, and reverted at a 10-day follow-up to 30.52 ± 2.73 pg/mL. In the Nepalese, a reduction in 17-β-estradiol after exposure was variably expressed; three of them had a massive decrease from 19.60 ± 3.36 pg/mL to below the detection limit of 11.80 pg/mL, another two had a modest decrease, on average, from 24.05 pg/mL to 21.68 pg/mL, and it tended to increase in one subject from 11.80 pg/mL to 12.80 pg/mL.
Stress Hormones
Changes in cortisol in both Italian and Nepalese participants after altitude exposure were unremarkable (Table 2).
Considering prolactin, it decreased from pre-to-post altitude exposure in the Italian participants to bounce back, exceeding the baseline level at a 10-day follow-up (5.63 ± 1.39 ng/mL, 4.28 ± 1.34 ng/mL, and 6.11 ± 0.19 ng/mL, respectively), as revealed by the pre vs. post vs. follow-up comparison (p = 0.009, η2p = 0.741, ω2p = 0.642), and post-hoc differences were found (pre vs. post: p = 0.068 and post vs. follow-up: p = 0.010). Contrarily, in the Nepalese, prolactin increased from pre-to-post exposure (6.53 ± 2.38 ng/mL and 7.52 ± 2.22 ng/mL, respectively); time × ethnicity comparison revealed a borderline difference (p = 0.148, η2p = 0.218, ω2p = 0.120). The random effect of participants was not significant, and the effect sizes for the overall model were as follows: R2 marginal = 0.281, R2 conditional = 0.432.
4 Discussion
High altitude affects human systems influencing their physiological activities due to hypobaric hypoxia and climatic environment. Hypoxia per se influences oxidative metabolism, stimulating compensatory mechanisms to ensure proper oxygenation of tissues (Di Giulio et al. 2006). Central and peripheral endocrine functions, such as hormonal pathways, are affected by altitude above 5000 m, preventing persistent human colonization (von Wolff et al. 2018). In the present chapter, we investigated whether a combination of physical effort and hypobaric hypoxia was associated with a perturbation of hormonal profile in a group of foreign trekkers coming from Italy when compared with indigenous Nepalese porters.
The thyroidal axis showed an adaptive mechanism characterized by a reduction in the serum level of FT3 both in foreign and Nepalese trekkers. The FT4 level showed ethnically reversing features, increasing in the foreigners and decreasing in the Nepalese. The TSH level remained unchanged. The FT3 and FT4 levels reverted to the baseline pre-altitude exposure value in the foreign trekkers at a 10-day follow-up. Richalet et al. (2010) have reported a 16% increase in both total and free T3 after 3–4 days of stay at 4350 m. Contrarily, we found a reduction in T3 after the altitude trek, which was more accentuated in the foreign trekkers than the Nepalese porters. A reduction in T3 is typical of non-thyroidal illness syndrome (NTIS), which occurs in various stressful non-thyroidal illnesses and during starvation. Under such conditions, peripheral conversion of T4 to T3 is reduced in the presence of normal thyroid hormone secretion resulting in an FT3 decrease, while FT4 may be unchanged, decreased, or increased. The NTIS is underlain by impaired conversion of T4 to T3 due to enzymatic dysfunction of deiodinases. The low-T3 syndrome caused by reduced peripheral conversion of the prohormone T4 is also present in chronic inflammatory diseases. As a result, inflammation disrupts redox homeostasis, inhibiting the hypothalamic-pituitary-thyroid axis. Hypothyroidism that follows exacerbates the redox homeostasis disruption, further worsening the activity of deiodinase. Our findings suggest that a reduction in T3 might involve the same inflammatory redox pathway that drives the low-T3 syndrome (Mancini et al. 2016). Further, there is a biological plausibility that indigenous porters in Nepal have an innate protective mechanism sparing the function of deiodinases.
We found in this study that the gonadal hormone axis was partially suppressed as confirmed by a non-significant reduction in testosterone levels and a significant decrease in 17-β-estradiol after the expedition in both foreign and Nepalese participants. These findings are in line with those of Benso et al. (2007), who found reductions in serum testosterone in eight mountaineers at the base camp located at 5200 m during a Mount Everest expedition. Marinelli et al. (1994) have reported a prompt decrease in serum testosterone occurring within 24 h after exposure to high altitude. Thus, the possibility arises that an inappreciable reduction in testosterone we observed after days of trekking during the expedition could be due to a delayed blood sampling which enabled the rebound of testosterone level, likely triggered by increased LH. Hypobaric hypoxia, rather than physical effort, seems the main reason for a transient reduction in testosterone as prolonged strenuous physical activity in endurance athletes or marathon runners at sea level fails to show appreciable effects on circulating reproductive hormones (Lucía et al. 1996; Jensen et al. 1995; Bagatell and Bremner 1990). Noteworthy, we found a significant increase in serum testosterone in foreign trekkers at follow-up, the finding in line with a previous study in which testosterone increased 10 days after the end of prolonged trekking at 5900 m in seven male mountaineers, which could be related to changes in the body composition (Pelliccione et al. 2011).
We also found that serum prolactin decreased in the Italian trekkers at high altitude with a reversal to the baseline level at the 10-day follow-up. It is a reasonable assumption that altitude hypoxia mitigates the activation of lactotroph cells in the anterior pituitary resulting in less prolactin secretion, which may be a sign of general bodily suffering of foreign sojourners at altitude. Likewise, Benso et al. (2007) have found a significant increase in serum prolactin in Caucasian mountaineers after climbing Mount Everest. The authors suggest changes in prolactin reciprocate those in testosterone. Somewhat in line with this suggestion, we found that prolactin increased from the pre-to-post trek in the Nepalese porters, a reverse reflection of those in testosterone. The ingenious high-altitude settlers perform physically better compared to foreigners even though they usually are exposed to a greater physical load.
The present study also shows that serum cortisol was not appreciably affected by high-altitude trekking. The lack of effect of cortisol content is in line with the findings of Wolff et al. (2018), who have conducted a major study on cerebral, cardiovascular, pulmonary, and humoral adaptations to prolonged hypobaric hypoxia during an ascent to Mt. Himlung Himal (7126 m) in Nepal, involving forty healthy sojourners, 21 men and 19 women aged 18–70. In that study, serum cortisol was slightly modulated with increasing altitude, changes were unrelated to sex or arterial oxygen saturation, and the level of cortisol after the expedition was akin to that before it. That study, in line with our present findings, also shows that prolactin secretion and thyroid function are affected by increasing altitude and the gonadal hormonal axis is suppressed, with reversal of changes to the baseline levels at a follow-up.
In conclusion, we believe we have shown that brain-driven humoral responses are modulated by a prolonged strenuous effort during a Himalayan high-altitude trek. The accompanying hypobaric hypoxia appeared to notably affect the hypothalamus-pituitary gonadal axis suppressing serum testosterone and 17-β-estradiol contents; the effects were more pronounced in indigenous Nepalese porters than in foreign Caucasian sojourners. Hypobaric hypoxia also shifted the thyroid metabolic function to the protective way of low free triiodothyronine level, the effect was more pronounced in Caucasian sojourners. There is a degree of ethnic diversity in specific humoral changes toward the stronger adaptive downregulation of humoral function in high-altitude native Nepalese. This adaptation may underlie their superior performance in the physically loaded condition, when compared to foreign sojourners, due possibly to the ensuing lower cellular ATP demand mitigating the effects of oxygen depletion at high altitude. Generally, however, the ethnic differences in humoral responses were modest and reversible on return from the trek in both Caucasians and native Nepalese. The exact molecular mechanisms of humoral changes underlying the effects of high-altitude hypobaric hypoxia could not be resolved in this observational study. Further research, particularly considering the diurnal response, in addition to sequential assessments during altitude exposure, is needed to provide insights into ethnicity-dependent differential responses to physical effort at high altitude.
References
Armstrong RA (2017) Recommendations for analysis of repeated-measures designs: testing and correcting for sphericity and use of MANOVA and mixed model analysis. Ophthalmic Physiol Opt 37(5):585–593
Bagatell CJ, Bremner WJ (1990) Sperm counts and reproductive hormones in male marathoners and lean controls. Fertil Steril 53(4):688–692
Benso A, Broglio F, Aimaretti G, Lucatello B, Lanfranco F, Ghigo E, Grottoli S (2007) Endocrine and metabolic responses to extreme altitude and physical exercise in climbers. Eur J Endocrinol 157(6):733–740
Brodmann Maeder M, Brugger H, Pun M, Strapazzon G, Dal Cappello T, Maggiorini M, Hackett P, Bärtsch P, Swenson ER, Zafren K (2018) The STAR data reporting guidelines for clinical high altitude research. High Alt Med Biol 19(1):7–14
Cataldi A, Di Giulio C (2009) Oxygen supply as modulator of aging processes: hypoxia and hyperoxia models for aging studies. Curr Aging Sci 2(2):95–102
Dawadi S, Basnyat B, Adhikari S (2020) A review of medical problems in Himalayan porters. High Alt Med Biol 21(2):109–113
Di Giulio C, Bianchi G, Cacchio M, Artese L, Piccirilli M, Verratti V, Valerio R, Iturriaga R (2006) Neuroglobin, a new oxygen binding protein is present in the carotid body and increases after chronic intermittent hypoxia. Adv Exp Med Biol 580:15–19
Fritz CO, Morris PE, Richler JJ (2012) Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen 141(1):2–18
Jensen CE, Wiswedel K, McLoughlin J, van der Spuy Z (1995) Prospective study of hormonal and semen profiles in marathon runners. Fertil Steril 64(6):1189–1196
Keenan DM, Pichler Hefti J, Veldhuis JD, Von Wolff M (2019) Regulation and adaptation of endocrine axes at high altitude. Am J Physiol Endocrinol Metab 318(2):E297–E309
Lucía A, Chicharro JL, Pérez M, Serratosa L, Bandrés F, Legido JC (1996) Reproductive function in male endurance athletes: sperm analysis and hormonal profile. J Appl Physiol 81(6):2627–2636
Magliulo L, Bondi D, Pietrangelo T, Fulle S, Piccinelli R, Jandova T, Blasio GD, Taraborrelli M, Verratti V (2020) Serum ferritin and vitamin D evaluation in response to high altitude comparing Italians trekkers vs Nepalese porters. Eur J Sport Sci:1–9. https://doi.org/10.1080/17461391.2020.1792559
Mancini A, Di Segni C, Raimondo S, Olivieri G, Silvestrini A, Meucci E, Currò D (2016) Thyroid hormones, oxidative stress, and inflammation. Mediat Inflamm 2016:6757154
Marinelli M, Roi GS, Giacometti M, Bonini P, Banfi G (1994) Cortisol, testosterone, and free testosterone in athletes performing a marathon at 4,000 m altitude. Horm Res 41(5–6):225–229
Minetti AE, Formenti F, Ardigò LP (2006) Himalayan porter’s specialization: metabolic power, economy, efficiency and skill. Proc Biol Sci 273(1602):2791–2797
Park JY, Hwang TK, Park HK, Ahn RS (2014) Differences in cardiovascular and hypothalamic-pituitary-adrenal axis functions between high altitude visitors and natives during a trek on the Annapurna circuit. Neuroendocrinology 99(2):130–138
Pelliccione F, Verratti V, D’Angeli A, Micillo A, Doria C, Pezzella A, Iacutone G, Francavilla F, Di Giulio C, Francavilla S (2011) Physical exercise at high altitude is associated with a testicular dysfunction leading to reduced sperm concentration but healthy sperm quality. Fertil Steril 96(1):28–33
Richalet JP, Letournel M, Souberbielle JC (2010) Effects of high altitude hypoxia on the hormonal response to hypothalamic factors. Am J Physiol Regul Integr Comp Physiol 299(6):R1685–R1692
Rochette L, Vergely C (2017) Hans Selye and the stress response: 80 years after his “letter” to the Editor of Nature. Ann Cardiol Angéiologie 66(4):181–183
Verratti V, Di Giulio C, D’Angeli A, Tafuri A, Francavilla S, Pelliccione F (2016) Sperm forward motility is negatively affected by short-term exposure to altitude hypoxia. Andrologia 48(7):800–806
Verratti V, Ietta F, Paulesu L, Romagnoli R, Ceccarelli I, Doria C, Fanò Illic G, Di Giulio C, Aloisi AM (2017) Physiological effects of high altitude trekking on gonadal, thyroid hormones and macrophage migration inhibitory factor (MIF) responses in young lowlander women. Physiol Rep 5(20):e13400
Verratti V, Bondi D, Shakir A, Pietrangelo T, Piccinelli R, Altieri VM, Migliorelli D, Tafuri A (2021) Uroflowmetry and altitude hypoxia: a report from healthy Italian trekkers and Nepalese porters during Himalayan expedition. Adv Exp Med Biol 1289:99–105
von Wolff M, Nakas CT, Tobler M, Merz TM, Hilty MP, Veldhuis JD, Huber AR, Pichler Hefti J (2018) Adrenal, thyroid and gonadal axes are affected at high altitude. Endocr Connect 7(10):1081–1089
Acknowledgments
Supported by a grant from the Department of Psychological, Health and Territorial Sciences of “G. d’Annunzio” University of Chieti-Pescara, Italy. Our thanks for the logistic support go to the Mission Nepal Holidays Pvt. Ltd. in Kathmandu, Nepal, the Laboratory of Clinical Pathology of Teramo Hospital, the Clinical Biochemistry Unit in the Bir Hospital in Kathmandu, and the Unique Diagnostic Polyclinic Laboratory in Kathmandu. We also thank all participating trekkers.
Competing Interests
The authors declare no competing interests in relation to this chapter.
Ethical Approval
All procedures performed in studies involving human participants were in accord with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This project was approved by the Ethics Review Board of the Nepal Health Research Council (NHRC).
Informed Consent
Written informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tafuri, A. et al. (2021). Effects of Physical Activity at High Altitude on Hormonal Profiles in Foreign Trekkers and Indigenous Nepalese Porters. In: Pokorski, M. (eds) Best Practice in Health Care. Advances in Experimental Medicine and Biology(), vol 1335. Springer, Cham. https://doi.org/10.1007/5584_2021_627
Download citation
DOI: https://doi.org/10.1007/5584_2021_627
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-77741-8
Online ISBN: 978-3-030-77742-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)