Abstract
Atopic, allergic, and especially asthmatic subjects might be particularly susceptible to sensory irritation induced by airborne chemicals compared to healthy individuals. Therefore, a good characterization of subjects is essential in inhalation exposure studies on sensory irritants. A total of 105 volunteers, 87% of whom reported to be non-allergic, participated in a medical examination that included skin prick test (SPT), measurements of total IgE, specific IgE (sIgE) to an ubiquitous allergen mix (sx1), and fractionated exhaled nitric oxide (FeNO), as well as pulmonary function and methacholine test. The median value of sIgE to sx1 was 0.20 kU/L (0.07–91.3 kU/L) and correlated significantly with total IgE (28.8 kU/L (2–756 kU/L)) and FeNO (14 ppb (5–100 ppb)). Forty-three subjects (41%) had sIgE to sx1 ≥ 0.35 kU/L and were classified as atopic. Thirty-five subjects, all also sx1-positive, were positive in SPT. Obstruction, small airway disease, and/or bronchial hyperreactivity were diagnosed in 18 subjects. Receiver operating characteristics (ROC) were performed to check whether signs of sensitization are useful to discriminate subjects with and without airway diseases. However, sx1, total IgE, FeNO, and SPT reached only low areas under the curve (AUC: 0.57–0.66). Although predominantly young and, according to their own statements, mostly non-allergic subjects participated in the study, almost half of them were atopic, and 10% had airway disease or bronchial hyperreactivity. This indicates that the validity of self-reported data might be inaccurate. In summary, diversified investigations of the allergy-related health status appear necessary for a thorough characterization of subjects for exposure studies on sensory irritants.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In human risk assessment, animal data are the major source of evidence when evaluating the effects on the upper respiratory tract of sensory irritation. Nevertheless, due to major differences in anatomy, physiology, and airflow dynamics between rodents and humans, controlled human inhalation exposure studies are considered the gold standard for assessing acute and reversible sensory irritant effects (Brüning et al. 2014). Human studies are often criticized for the inherent variability of subjective and objective sensory irritation effects. Therefore, the characterization of a study group and the recruitment of either healthy volunteers or carefully selected subjects with predispositions to measurable reversible physiologic or biomarker responses are of particular importance. During the recruitment process, participants who are at risk of adverse outcome are excluded (Rosenkranz et al. 2020).
Since allergic inflammation results from a specific pattern of cellular and humoral responses leading to the activation of the innate and adaptive immune system, allergic subjects and those who are predisposed to developing an allergy (atopics) are likely to respond differently or more sensitively than non-allergic ones to sensory irritants. In a study of nasal sensory function, 31 subjects with seasonal allergic rhinitis (SAR) have shown a significantly lower perceptual threshold to propanol than 29 subjects without SAR (p < 0.05) (Shusterman et al. 2003). In another study, inhalation of 15 ppm acetic acid for 15 min has resulted in a significantly higher nasal airflow obstruction in 8 subjects with SAR compared to 8 subjects without SAR (Shusterman et al. 2005). Furthermore, in a controlled human exposure study with formic acid (Kleinbeck et al. 2018) or with ammonia (Pacharra et al. 2017), subjects with SAR have a higher blink rate than controls. Likewise, atopic subjects have a higher blink rate compared to non-atopic ones in a controlled exposure study with ethyl acrylate performed by Sucker et al. (2019). A systematic review of experimental studies on health effects of short-term exposure to airborne chemicals has confirmed that asthmatics are more sensitive to sulfuric acid and sulfur dioxide, but not to ozone and nitrogen dioxide (Johansson et al. 2016). In addition, studies on inhalation exposure show that deposition of ultrafine carbon particles in distal airways and alveoli is greater in patients with asthma than in healthy subjects (Chalupa et al. 2004; Daigle et al. 2003), which may be caused by enhanced breathing in the former due to increased dead space ventilation. Therefore, a good characterization of subjects regarding atopy, allergy, or asthma is essential in inhalation exposure studies on sensory irritants, which requires time-consuming and expensive multistage recruiting procedures. The aim of the present study was to retrospectively verify whether all tests carried out were necessary for a successful characterization of a study group. In addition, the study addressed the issue of a match between the subjective (health questionnaire) and objective (measured) data collected on atopy, allergy, and asthma.
2 Methods
2.1 Study Design
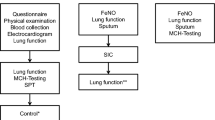
A total of 105 volunteers, 87% of whom reported to be non-allergic, participated in the study. All of them were investigated at the Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr University (IPA) in Bochum, Germany, during the recruitment process for controlled human exposure studies on the effects of sensory irritation. Healthy adults were invited to participate in the study using information sheets published via electronic job advertisements or on the homepage of the institute. The volunteers interested in participation filled a standardized online health questionnaire assessing demographic data, smoking habits, and chronic diseases, including asthma and allergies. Inclusion criteria were age 18–40 years and never smoked tobacco or quit smoking at least 1 year before the study. Subjects who reported to be pregnant, having asthma, another chronic illness, or on prescription drugs were excluded. Since it was planned to include atopic subjects in upcoming studies, a number of subjects also were involved who reported a previous positive allergy test. Some of them reported allergic symptoms such as rhinitis and conjunctivitis, but never asthma. Subjects who met the inclusion criteria in the health questionnaire were invited to a medical examination, consisting of an interview by a physician and clinical tests. Smoking habit was assessed by the questionnaire and verified by urine cotinine content. Arterial blood pressure was measured and a resting 12-lead ECG was performed. Blood samples were taken and investigated for standard laboratory values. In the case of clinically relevant findings or a positive cotinine test, the examination was terminated, and the subject was excluded from further analysis.
2.2 Atopy
Atopy was assessed by skin prick testing (SPT) and measurement of specific IgE antibodies (sIgE). A panel of nine common inhalant allergens (cat dander, trees, grass, latex, Dermatophagoides farinae, Dermatophagoides pteronyssinus, Alternaria alternata, Aspergillus fumigatus, and Ambrosia elatior) and histamine 10 mg/mL (positive) and saline (negative control) solutions were used for SPT. Total IgE as well as sIgE to a mixture of common environmental allergens (Dermatophagoides pteronyssinus, cat and dog danders, Cladosporium herbarum, and pollen of timothy, rye, birch, and mugwort) (sx1 Phadiatop; ThermoFisher Phadia AB; Uppsala, Sweden) were measured with the ImmunoCAP 250 system. A positive atopic status was assumed in case of a wheal diameter greater than 3 mm in SPT to one of the allergens listed above or sIgE concentrations to sx1 ≥ 0.35 kU/L.
2.3 Lung Function, Methacholine Test, and Exhaled Nitric Oxide
Pulmonary function was assessed with spirometry and body plethysmography (CareFusion, Würzburg, Germany) as described earlier (Miller et al. 2005; Wanger et al. 2005). Reference values were chosen according to the Global Lung Function Initiative (Quanjer et al. 2012). By convention, the lower limit of normal (LLN) for forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), FEV1/FVC, mean expiratory flow at 25% of the FVC (MEF25), and maximal mid-expiratory flow (MMEF) is designed to be the fifth percentile. Cases with FEV1/FVC < LLN were classified as obstructive, those with normal FEV1/FVC but MEF25 or MMEF < LLN as small airway disease. Methacholine test was performed as previously described with a reservoir method (Merget et al. 2005). Bronchial hyperresponsiveness was assumed when FEV1 decreased ≥20% from baseline or specific airway resistance (sRt) doubled and increased to ≥2 kPa × s with a cumulative dose of ≤300 μg methacholine. Fractional exhaled nitric oxide (FeNO) measurement was performed using a Niox Mino device (Aerocrine, Solna, Sweden) according to the recommendations of the American Thoracic Society and the European Respiratory Society (ATS/ERS 2005).
2.4 Statistical Analysis
Results were reported as medians with ranges or counts and percentages and were compared using the Mann-Whitney U test for continuous variables and Fisher’s exact test for dichotomous variables. Spearman’s correlation coefficient (r) was used to assess the strength of relationship between pairs of variables. A p-value <0.05 defined statistically significant differences.
Receiver operating characteristic (ROC) plots are one possible graphical presentation for describing and comparing diagnostic tests. The area under the curve (AUC) is a global measure for the diagnostic performance of a test. The AUC ranges from 0 to 1. ROC plots were performed using the clinical diagnoses of obstruction, small airways diseases, and bronchial hyperreactivity based on spirometry and body plethysmography. AUCs were calculated to compare the ability of sIgE to sx1, total IgE, FeNO, and SPT to discriminate between subjects with and without airway disease/bronchial hyperreactivity. The analysis was performed using a commercial GraphPad Prism v7.04 statistical package (GraphPad Software; La Jolla, CA).
3 Results
Among the 105 subjects who completed medical examination and clinical testing, there were 14 (13%) who reported a previous positive allergy test, with 10 of them having allergic symptoms such as rhinitis and/or conjunctivitis. No subject showed symptoms at the time of the examination. With the exception of methacholine test in four cases, there were no other missing values. Personal and clinical data of the whole study group are summarized in Table 1 (left column). The group consisted mainly of young subjects and slightly more women than men (median age: 24 (19–37) years; 56% female). Forty-three subjects (41%) had sIgE to sx1 ≥ 0.35 kU/L, and 35 of them (81%) also showed at least 1 positive SPT. There was no positive SPT without elevated sIgE to sx1. About half of the atopics reacted in SPT to grass pollen (58%) and/or the house dust mite Dermatophagoides pteronyssinus (49%). Besides the atopy markers (sIgE and SPT results), the main differences in clinical data between the 43 atopic (Table 1, middle column) and 62 non-atopic subjects (right column) were higher total IgE (p < 0.0001), higher FeNO (p = 0.01), and lower FEV1/FVC (p = 0.01) in atopics. However, there was no significant difference in the proportion of subjects with obstruction (p = 0.57), small airways disease (p = 0.99), and bronchial hyperreactivity (p = 0.12) between the two groups. When comparing atopics who reported allergic symptoms (n = 10) with atopics without symptoms (n = 33), there was no significant difference in any of the parameters listed in Table 1 (data not shown).
In addition to the finding that all SPT-positive subjects were also sIgE-positive to sx1, the number of positive SPT results correlated with the sx1-sIgE values (r = 0.81; p < 0.0001) (Fig. 1a). Although less pronounced, the correlation between the sx1-sIgE values and the levels of FeNO (r = 0.24; p = 0.014) was significant (Fig. 1b).
Thirteen (93%) out of the 14 subjects who had reported a previous positive allergy test were positive in both sIgE and SPT. Of the 91 subjects who reported being non-allergic, 30 (33%) were atopic, 22 (24%) with positive results in both sIgE and SPT (Fig. 2).
The Venn diagram showing the concordance of positive sIgE results (against sx1) and SPT results (at least one positive SPT). In addition to the number of subjects indicated by numerics, the subjects who reported a previous positive allergy test (n = 14) are depicted as dots in the grey oval (filled dots: subjects reporting allergic symptoms). The dot outside the bottom intersection (circular dotted outline) represents a subject who had reported a previous positive allergy test but who did react neither in sIgE test nor in SPT
In 18 (17%) subjects, airway obstruction or small airway disease and/or bronchial hyperreactivity were diagnosed. Out of the 14 subjects, who reported a previous positive allergy test, 1 showed features of obstruction and bronchial hyperreactivity. To check whether signs of sensitization are useful to discriminate subjects with and without obstruction, small airway disease, and bronchial hyperreactivity, ROC curves were performed (Fig. 3). However, AUC reached rather low values for sIgE to sx1 (AUC = 0.57), total IgE (AUC = 0.66), FeNO (AUC = 0.60), and SPT (as a number of positive reactions; AUC = 0.59), which indicates that no variable standing alone would be useful to correctly discriminate the subjects.
Receiver operating characteristic (ROC) plots. The content of sIgE to sx1, total IgE, FeNO, and the number of positive SPT reactions were evaluated in 105 subjects under the angle of discriminating power for the diagnosis of obstruction, small airway disease, and/or bronchial hyperreactivity. AUC area under the curve, sIgE specific IgE antibodies, FeNO fractional exhaled nitric oxide, SPT skin prick test
4 Discussion
Since individuals with atopy, allergy, and/or asthma are suspected to be particularly susceptible to sensory irritants, extensive examinations were carried out to thoroughly characterize the study group. For this, a time-consuming and expensive multistage recruiting procedure was used. Out of the 105 young subjects who participated in the medical examination, 41% were atopic. Although this percentage is in line with the most recent “German Health Interview and Examination Survey for Adults (DEGS)” in which 45% of 18 to 29-year-olds were atopic (positive sIgE to sx1) (Haftenberger et al. 2013), it must be taken into account that 87% of our study group stated in the health questionnaire that they were non-allergic. That the outcome of a questionnaire could be inaccurate is in agreement with a literature review showing that self-reports of different types of health-risk behaviors are affected by both cognitive and situational factors (Brener et al. 2003). In fact, studies investigating the congruence between measured and reported values for height and weight of subjects found an underreporting for weight and over-reporting for height (Neermark et al. 2019; Gorber et al. 2007). In a recent study on young and according to the initial health questionnaire for healthy volunteers, 20% showed signs of a heart, liver, or airway disease in medical examinations (Rosenkranz et al. 2020). In addition, the accuracy of self-reported information depends on the knowledge of health status. For example, in our study the subjects may have not known that they had an atopy/allergy or they may have not been willing to report it. Since 13 (93%) out of the 14 subjects, who reported a previous positive allergy test, and 30 (33%) out of the 91 subjects, who reported being non-allergic, were diagnosed as atopic, positive information in the questionnaire appears more reliable than negative information. Therefore, although the questionnaire method is relatively fast and cheap in comparison to physical examinations and clinical measurements, the validity of self-reported data, particularly on atopy and allergy, might be inaccurate.
Another question was whether all medical examinations of our study were relevant or whether the identification of subjects with atopy/allergy identified all subjects with respiratory problems. In this context it is known that for some allergens there is a close correlation between the results of sIgE and SPT. Similar to our findings, a recent study has shown a concordance between sIgE and SPT for ten tested allergens of 74–88% (Knight et al. 2018). In view of these results, the SPT might be redundant in our recruitment scheme. However, since SPT results are immediately available and in contrast to sIgE to the mixture sx1 also provide information on sensitization to perennial or seasonal allergens, it seems appropriate to test both SPT and sIgE to sx1. Taking into account the FeNO, a study on 28 asthmatic subjects has found a positive correlation between FeNO level and the number as well as diameter of positive SPT results (Ho et al. 2000). Also in our study, significantly higher FeNO levels in atopic than in non-atopic subjects were found.
In some studies, signs for eosinophilic inflammation, such as a high level of sIgE or FeNO, have appeared useful predictors for allergic asthma. The ROC analysis of the data of 96 preschool children has shown that FeNO is a good discriminator (AUC = 0.91) between children with probable asthma and healthy controls (Malmberg et al. 2003). In a group of 107 symptomatic bakers, high content of rye flour-sIgE (AUC = 0.83) and clear SPT results (AUC = 0.81) have been good predictors for an asthmatic reaction during a challenge test (van Kampen et al. 2008). However, in the present study, neither sIgE to sx1 nor total IgE, FeNO, or SPT were identified as useful tools to discriminate subjects with and without obstruction, small airway disease, or bronchial hyperreactivity (AUCs: 0.57–0.66).
Although young and according to their own statements mostly non-allergic subjects participated in the medical examination, almost half of them showed signs of atopy, and 10% had airway disease/bronchial hyperreactivity. The accuracy of self-reported data on a lack of atopy and allergy was questionable. In contradistinction, positive statements on the presence of allergy seemed mostly reliable.
In conclusion, we have shown that multiple medical investigations, even though involving enhanced effort and cost, should be included into the recruitment scheme for a successful discrimination between atopic, allergic, and asthmatic study participants.
References
ATS/ERS (2005) ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am J Respir Crit Care Med 171:912–930
Brener ND, Billy JO, Grady WR (2003) Assessment of factors affecting the validity of self–reported health–risk behavior among adolescents: evidence from the scientific literature. J Adolesc Health 33(6):436–457
Brüning T, Bartsch R, Bolt HM, Desel H, Drexler H, Gundert–Remy U, Hartwig A, Jäckh R, Leibold E, Pallapies D, Rettenmeier AW, Schlüter G, Stropp G, Sucker K, Triebig G, Westphal G, van Thriel C (2014) Sensory irritation as a basis for setting occupational exposure limits. Arch Toxicol 88(10):1855–1879
Chalupa DC, Morrow PE, Oberdörster G, Utell MJ, Frampton MW (2004) Ultrafine particle deposition in subjects with asthma. Environ Health Perspect 112(8):879–882
Daigle CC, Chalupa DC, Gibb FR, Morrow PE, Oberdörster G, Utell MJ, Frampton MW (2003) Ultrafine particle deposition in humans during rest and exercise. Inhal Toxicol 15(6):539–552
Gorber CS, Tremblay M, Moher D, Gorber B (2007) A comparison of direct vs. self–report measures for assessing height, weight and body mass index: a systematic review. Obes Rev 8(4):307–326
Haftenberger M, Laußmann D, Ellert U, Kalcklösch M, Langen U, Schlaud M, Schmitz R, Thamm M (2013) Prävalenz von Sensibilisierungen gegen Inhalations– und Nahrungsmittelallergene. Ergebnisse der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1). Bundesgesundheitsblatt 56:687–697. (Article in German)
Ho LP, Wood FT, Robson A, Innes JA, Greening AP (2000) Atopy influences exhaled nitric oxide levels in adult asthmatics. Chest 118:1327–1331
Johansson MK, Johanson G, Öberg M (2016) Evaluation of the experimental basis for assessment factors to protect individuals with asthma from health effects during short–term exposure to airborne chemicals. Crit Rev Toxicol 46(3):241–260
Kleinbeck S, Pacharra M, Schäper M, Blaszkewicz M, Golka K, Brüning T, van Thriel C. (2018) Sensorische Irritationen durch Ameisensäure: Reagieren allergische Probanden stärker auf kontrollierte Expositionen? (Sensory irritations due to formic acid: Do allergic subjects react more strongly to controlled exposures?) In: Deutschen Gesellschaft für Arbeitsmedizin und Umweltmedizin e.V. (Hrsg.): Dokumentation der 58. Jahrestagung der DGAUM, 7–9 März 2018 in München, p 50 (Article in German)
Knight V, Wolf ML, Trikha A, Curran–Everett D, Hiserote M, Harbeck RJ (2018) A comparison of specific IgE and skin prick test results to common environmental allergens using the HYTEC™ 288. J Immunol Methods 462:9–12
Malmberg LP, Pelkonen AS, Haahtela T, Turpeinen M (2003) Exhaled nitric oxide rather than lung function distinguishes preschool children with probable asthma. Thorax 58:494–499
Merget R, Heinze E, Neumann L, Taeger D, Brüning T (2005) Comparison of the PARI Provotest II reservoir method with the ATS dosimeter method to assess bronchial hyperresponsiveness to methacholine. In: Proceedings of the 45th annual conference of the German Society for Occupational and Environmental Medicine (DGAUM) 624–625 (Article in German)
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J (2005) Standardisation of spirometry. Eur Respir J 26:319–338
Neermark S, Holst C, Bisgaard T, Bay–Nielsen M, Becker U, Tolstrup JS (2019) Validation and calibration of self–reported height and weight in the Danish health examination survey. Eur J Pub Health 29(2):291–296
Pacharra M, Kleinbeck S, Schäper M, Blaszkewicz M, Golka K, van Thriel C (2017) Does seasonal allergic rhinitis increase sensitivity to ammonia exposure? Int J Hyg Environ Health 220(5):840–848
Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MS, Zheng J, Stocks J, ERS Global Lung Function Initiative (2012) Multi–ethnic reference values for spirometry for the 3–95 yr age range: the global lung function 2012 equations. Eur Respir J 40(6):1324–1343
Rosenkranz D, Bünger J, Hoffmeyer F, Monsé C, van Kampen V, Raulf M, Brüning T, Sucker K (2020) How healthy is healthy? Comparison between self–reported symptoms and clinical outcomes in connection with the enrollment of volunteers for human exposure studies on sensory irritation effects. Adv Exp Med Biol. https://doi.org/10.1007/5584_2019_472. (Epub ahead of print)
Shusterman D, Murphy MA, Balmes J (2003) Differences in nasal irritant sensitivity by age, gender, and allergic rhinitis status. Int Arch Occup Environ Health 76(8):577–583
Shusterman D, Tarun A, Murphy MA, Morris J (2005) Seasonal allergic rhinitic and normal subjects respond differentially to nasal provocation with acetic acid vapor. Inhal Toxicol 17(3):147–152
Sucker K, Hoffmeyer F, Monsé C, Jettkant B, Berresheim H, Rosenkranz N, Raulf M, Bünger J, Brüning T (2019) Ethyl acrylate: influence of sex or atopy on perceptual ratings and eye blink frequency. Arch Toxicol 93(10):2913–2926
van Kampen V, Rabstein S, Sander I, Merget R, Brüning T, Broding HC, Keller C, Müsken H, Overlack A, Schultze–Werninghaus G, Walusiak J, Raulf–Heimsoth M (2008) Prediction of challenge test results by flour–specific IgE and skin prick test in symptomatic bakers. Int Arch Occup Environ Health 63(7):897–902
Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson D, Macintyre N, McKay R, Miller MR, Navajas D, Pellegrino R, Viegi G (2005) Standardisation of the measurement of lung volumes. Eur Respir J 26(3):511–522
Acknowledgments
We thank the volunteers for their participation and all the involved technical assistants from our institute for their excellent assistance. We gratefully acknowledge Anja Deckert for support in recruitment.
Conflicts of Interest
The authors declare no conflicts of interest in relation to this article.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The recruitment procedure and the exposure studies were approved by the Ethic Committee of the Ruhr-University Bochum in Germany.
Informed Consent
All participants provided written informed consent and received financial compensation for participation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
van Kampen, V. et al. (2020). Discrimination Between Atopic, Allergic, and Asthmatic Volunteers for Human Exposure Studies on Sensory Irritation. In: Pokorski, M. (eds) Health and Medicine. Advances in Experimental Medicine and Biology(), vol 1279. Springer, Cham. https://doi.org/10.1007/5584_2020_520
Download citation
DOI: https://doi.org/10.1007/5584_2020_520
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-51120-3
Online ISBN: 978-3-030-51121-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)