Abstract
Effective diphtheria, tetanus toxoids, whole-cell pertussis (wP) vaccines were used for massive immunization in the 1950s. The broad use of these vaccines significantly reduced the morbidity and mortality associated with pertussis. Because of reports on the induction of adverse reactions, less-reactogenic acellular vaccines (aP) were later developed and in many countries, especially the industrialized ones, the use of wP was changed to aP. For many years, the situation of pertussis seemed to be controlled with the use of these vaccines, however in the last decades the number of pertussis cases increased in several countries. The loss of the immunity conferred by the vaccines, which is faster in the individuals vaccinated with the acellular vaccines, and the evolution of the pathogen towards geno/phenotypes that escape more easily the immunity conferred by the vaccines were proposed as the main causes of the disease resurgence. According to their composition of few immunogens, the aP vaccines seem to be exerting a greater selection pressure on the circulating bacterial population causing the prevalence of bacterial isolates defective in the expression of vaccine antigens. Under this context, it is clear that new vaccines against pertussis should be developed. Several vaccine candidates are in preclinical development and few others have recently completed phaseI/phaseII trials. Vaccine candidate based on OMVs is a promising candidate since appeared overcoming the major weaknesses of current aP-vaccines. The most advanced development is the live attenuated-vaccine BPZE1 which has successfully completed a first-in-man clinical trial.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Current Pertussis Vaccines
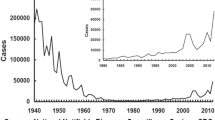
Pertussis, also known as whooping cough, is a highly contagious respiratory disease mainly caused by Bordetella pertussis, a Gram-negative bacterium. This disease that causes uncontrollable violent coughing, affects all ages, being the most vulnerable the infants under 6 months of age (Stefanelli et al. 2017). The best way to prevent pertussis is to get vaccinated. The first experimentations with vaccines began after Jules Bordet and Octave Gengou of the Pasteur Institute of Brussels identified the etiological agent in 1906; these vaccines were made from killed whole-cell B. pertussis. In ensuing years, such type of vaccine (whole-cell vaccine, wP) was used in children in different countries. Thorvald Madsen was the first to describe the use of a wP vaccine on a large scale (Madsen 1933). Madsen’s vaccine successfully controlled two outbreaks in the Faroe Islands, however some deaths within 48 h of immunization were reported (Madsen 1933). Noteworthy at that time physicians used the vaccine as either a therapeutic or a prophylactic formulation and in both cases the vaccine was given in three injections intramuscularly or subcutaneously with intervals of three to 4 days (Madsen 1933). Madsen T. in his work summarized some reports that concluded sic…if the vaccine is given early in the catarrhal stage the vaccine will have a good effect; the later the vaccine is given in the convulsive stage, the less effect can be expected. This appears from the reports of most of the Danish officers of Health and also is the consensus of the Danish pediatric society (Madsen 1933). Louis Sauer of Northwestern University Medical School, Chicago, described minor reactions to a whole-cell pertussis vaccine being used in the United States as an adjuvanted combined vaccine (Sauer 1948). Pearl Kendrick of the State of Michigan Health Department further refined wP vaccines. She and Grace Eldering combined this improved killed vaccine with diphtheria and tetanus toxoids to produce the diphtheria-tetanus-pertussis (DTP) and used it in children (Kendrick 1936). The Committee on Infectious Diseases of the American Academy of Pediatrics suggested in 1944 and recommended in 1947 the routine use of pertussis vaccine in the form of the DTP combination. The use of this vaccine was then expanded to other countries. The coverages of pertussis vaccine were improved when the Expanded Program on Immunization (EPI) was established in 1974. The mission of the EPI is to develop and expand immunization programs throughout the world. In particular, in 1977, the goal was set to make immunization against diphtheria, pertussis, tetanus, poliomyelitis, measles and tuberculosis available to every child in the world by 1990. The massive pertussis vaccination dramatically reduced the morbidity and mortality associated with the disease (Table 1). After this important achievement in the control of the disease, unfortunately, doubts about the safety of wP vaccines began to arise and this led to a decrease in the acceptance of this type of formulation by the population and even in some countries its use was rejected (Klein 2014; Romanus et al. 1987). The first published reports on irreversible brain damage after whole-cell pertussis vaccination was described by Brody and Sorley. These reports led to the first warnings that pertussis vaccine should not be administered to those with a known neurologic disorder (Brody and Sorley 1947). In Great Britain, concerns on the safety of this vaccine were widely publicized in the popular press and because of that the proportion of children vaccinated against pertussis diminished (Kulenkampff et al. 1974). The adverse reactions ranged from local reactions (redness, swelling, and pain at the injection site) to systemic reactions (fever, persistent crying and, in rare cases encephalopathy) were reported in other countries (Klein 2014; Romanus et al. 1987). Concerns about safety finally led to the development of component (acellular) pertussis vaccines that are associated with a lower frequency of adverse reactions (Sato and Sato 1985; Edwards and Karzon 1990). These second-generation of pertussis vaccines, referred to as aP vaccines, are constituted of purified B. pertussis antigens combined with diphtheria and tetanus toxoids. The first acellular vaccine that was developed in Japan in 1970 consisted of two proteins: pertussis toxin (PTx) and filamentous haemagglutinin (FHA) (Sato and Sato 1985). Field trials showed that component vaccine was as effective as and produced less side-effects than did conventional whole-cell vaccine (Sato et al. 1984). The vaccine has been used for mass immunization in Japan since 1981 and was highly effective in preventing pertussis disease. In 1994 the efficacy for two, three-component acellular, pertussis vaccines containing inactivated PTx, FHA, and pertactin (PRN), and one five-component acellular pertussis vaccine containing the same components plus fimbriae 2 and 3 was compared with a UK whole-cell vaccine (Olin et al. 1997). This study demonstrated that the wP vaccine and the five-component aP vaccine had similar efficacy against culture-confirmed typical pertussis, defined by at least 21 days of paroxysmal cough. The authors also found that the three-component acellular vaccine was less effective than the five-component-vaccine and the whole-cell vaccines against culture-confirmed pertussis when all cases irrespective of the duration of severity of cough, were included in the analysis (Olin et al. 1997). Thus, though there was no compelling evidence to support that wP vaccines should not be used, the aP vaccines began to be broadly accepted because of their lower reactogenicity, especially in industrialized countries where wP vaccines of the primary series (3 doses in infancy) was replaced by aP vaccine (Table 1). Currently, US and most of the EU countries use only aP vaccines (Table 1). The aP formulations restored people’s confidence in pertussis-containing vaccines, and the infection was controlled for several years. Notwithstanding, during the last decades the epidemiology of pertussis has changed (Clark 2014; Tan et al. 2015) with several major outbreaks occurring, the incidence of which not only indicated a waning immunity but also demonstrated that the wP vaccines gave children a longer lasting immunity than aP (Klein et al. 2013; Witt et al. 2012; Sheridan et al. 2012). Furthermore, the risk of pertussis was increased in schoolchildren and adolescents vaccinated exclusively with aP compared to those receiving at least one wP dose (Witt et al. 2013; Sheridan et al. 2012). This difference could result from the weaker immune response induced by aP vaccines (Mills et al. 2014): while aP vaccines mainly induce a Th2-skewed response (Ryan et al. 1998), wP vaccines induce a robust Th1 profile and the proliferation of respiratory tissue-resident memory CD4 T cells (Brummelman et al. 2015; Wilk and Mills 2018). Therefore, the aP vaccine induced immunity shows a more rapid decay and possibly a reduced impact on transmission compared with currently available wP vaccines (Tartof et al. 2013; McGirr et al. 2013). In addition to the waning of immunity induced by vaccination, in particular with aP vaccines (Koepke et al. 2014; McGirr and Fisman 2015), pathogen adaptation to escape vaccine induced immunity (King et al. 2001; Mooi et al. 2001; Mäkelä 2000; David et al. 2004; He et al. 2003; Bottero et al. 2007; Gzyl et al. 2004; Bowden et al. 2016), and the failure of pertussis vaccines, in particular aP vaccines, to prevent infection and spread of B. pertussis were also proposed to explain the resurgence of the disease. Regarding pathogen evolution, the first reports were related to polymorphism in genes coding for proteins included in the vaccine (PRN and PTx among others) (Mooi et al. 1998) and later in the pertussis toxin promoter (ptxP) (Advani et al. 2011; Kallonen et al. 2012). Recently, there has been an increase in B. pertussis isolates that do not produce some of the vaccine antigens (Lam et al. 2014; Barkoff et al. 2019). It has been proposed that the loss of this vaccine antigen probably provides a selective advantage for bacterial survival in populations vaccinated with aP vaccines (Martin et al. 2015). Commercial aP vaccines containing PTx, PRN and FHA are not as effective as expected in controlling the infection caused by the recent circulating bacteria that do not express PRN (Hegerle et al. 2014). Moreover, recently it was demonstrated in a mixed infection mouse model that PRN deficient B. pertussis strain colonizes the respiratory tract of aP immunized mice more effectively than the PRN positive strain (Safarchi et al. 2015).
Under this context, in 2015 the Strategic Advisory Group of Experts on immunization expressed concerns regarding the resurgence of pertussis in certain industrialized countries despite high aP-vaccine coverage (Meeting of the Strategic Advisory Group of Experts on immunization 2015). The switch from wP to aP for primary infant immunization was proposed as, at least partially responsible for that resurgence (Table 1, see reported cases of European Region). The World Health Organization (WHO) therefore recommended that the switch be considered only if, in the national immunization schedules, large numbers of doses including several boosters can be assured. Countries currently using aP vaccines may continue using them, but should consider the need for additional booster doses and strategies to prevent early-childhood mortality upon pertussis resurgence. In fact, the WHO published a position paper on this subject and wrote the following:
A switch from wP to aP vaccines for primary infant immunization should only be considered if the inclusion in the national immunization schedules of additional periodic booster or maternal immunization can be assured and sustained (Pertussis vaccines: WHO position paper, August 2015—Recommendations 2016).
National programmes currently using aP vaccine may continue using this vaccine but should consider the need for additional booster doses and strategies to prevent early childhood mortality such as maternal immunization in case of resurgence of pertussis (Pertussis vaccines: WHO position paper, August 2015—Recommendations 2016).
2 New Pertussis Vaccines
Pertussis vaccines are currently on the agenda due to the worrying increase of pertussis cases detected in different countries. There are an estimated 24.1 million cases of the disease and approximately 160,700 deaths occurring worldwide every year in children younger than 5 years of age (Yeung et al. 2017). It is very clear that the non-use of the current pertussis vaccines would lead to an even more challenging epidemiological scenario and for this reason the current vaccine administration and surveillance of the disease should be improved while new vaccines are being developed. The development of a new pertussis vaccine is a difficult task to achieve since no absolute correlate for protection exists, however there are enough data from animal models and human studies showing that although antibodies may mediate protection, Th1 and Th17 cellular responses and tissue resident memory (TRM) response are responsible for long-lasting protection (Mills et al. 2014). To induce or drive a Th1, Th17 and TRM response, different approaches have already been proposed (Allen and Mills 2014; Mielcarek et al. 2006; Dias et al. 2013). In the next section, the main approaches used so far for the development of new vaccines are discussed.
3 Live Attenuated Vaccine
The most advanced novel pertussis vaccine candidate is that developed by Locht et al. in Lille, France (Thorstensson et al. 2014; Mielcarek et al. 2010; Feunou et al. 2010; Skerry et al. 2009). This vaccine candidate, referred as BPZE1, and consisting in a live attenuated bacterial strain, (Locht 2014) was shown to be immunogenic and protective in mice and baboons after intranasal administration (Locht 2016, 2017). In mice a single nasal administration of BPZE1, but not a high dose of current commercial aP vaccine, induced B. pertussis-specific secretory IgA in the nasal cavity, and transfer of the nasal IgA was able to protect recipient mice against nasal colonization after B. pertussis challenge (Solans and Locht 2018). Though no protection experiments have yet been performed with BPZE1 against circulating bacteria, other interesting findings have already reported. It was detected that BPZE1 vaccine was able to induce CD4+CD69+CD103+ TRM cells in the nasal mucosa of mice, and these cells produced high levels of IL-17 and appreciable levels of IFN-γ. Thus, BPZE1 protects mice against nasal infection by virulent B. pertussis via an IL-17-dependent and sIgA-mediated mechanism (Solans and Locht 2018; Fedele et al. 2011). Moreover, recently a double-blind, placebo-controlled, dose-escalating study of BPZE1 given intranasally for the first time to human volunteers was performed as the first trial of a live attenuated bacterial vaccine against pertussis. In this study, 12 subjects per dose group received different quantities of colony-forming units as droplets with half of the dose in each nostril and 12 subjects received the diluent (control group) (Thorstensson et al. 2014). Local and systemic safety and immune responses were assessed during 6 months, and nasopharyngeal colonization with BPZE1 was determined with repeated cultures during the first 4 weeks after vaccination. In this trial, the vaccine candidate was found safe in young human adults, able to transiently colonize the human nasopharynx, and to induce antibodies to PTx, FHA, PRN and fimbriae after a single nasal administration (Thorstensson et al. 2014). This vaccine candidate is currently entering a clinical phase II trial.
4 Less Reactogenic Whole Cell Vaccine
The major cause of wP vaccine reactions is associated to the endotoxin which is a lipo-oligosaccharide (LOS) and because of that attempts were made to detoxify wP vaccines. Researchers at the Institute Butantan in São Paulo, Brazil, diminished the endotoxicity of the wP vaccine by performing a chemical extraction of LOS from the outer membrane (Dias et al. 2013). Chemical extraction of LOS resulted in a significant decrease in endotoxin content without affecting the integrity of the product. This development, however, raises doubts because with the LOS extraction the adjuvant capacity associated with this molecule would also be decreasing. Other alternative strategies to LOS removal are being sought, specifically a consortium of researchers proposed to work on structural changes of the molecule (on the LipidA) in order to retain de beneficial effects induced by the molecule but eliminating its reactogenicity. The results on this strategy have not yet been disclosed.
5 Acellular Pertussis Vaccines Containing Recombinant Inactivated Pertussis Toxin
The safety and superior immunogenicity of 9 K/129G genetically detoxified PTx (rPT) was demonstrated long time ago (Rappuoli 1999; Podda et al. 1993). Under this context, BioNet-Asia developed a new rPT-expressing B. pertussis strain (Buasri et al. 2012). This strain generated increased amounts of rPT compared to wild type strain and strains used in vaccine production and the purified rPT did not show any toxicity (Buasri et al. 2012). Thus, Bionet formulated a new acellular vaccine containing the recombinant genetically detoxified Pertussis Toxin (PTgen), FHA and PRN and presented the results of the first clinical study of this recombinant aP vaccine formulated alone or in combination with tetanus and diphtheria toxoids. For the phase I/II trial, 60 subjects (20 per each vaccine group) were enrolled and included in the safety analysis. This first-in-human study showed that BioNet’s PTgen-containing vaccine has a similar reactogenicity and safety profile than the Adacel® acellular vaccine. Moreover, the high immunogenicity of PTgen in adults was demonstrated Sirivichayakul et al. (2016). The results were consistent with previous studies that demonstrated high and sustained efficacy of rPT-containing aP vaccines in infants (Seubert et al. 2014). Recent findings on the ability of rPT-containing acellular vaccine to induce memory response make a significant difference with current acellular vaccines that include chemically detoxified components in terms of long-term protection. Specifically, the authors reported that the boosting of aP-primed adolescents with recombinant-aP induced higher anti-PTx and PTx-neutralizing responses than the current aP vaccine and increased PTx-specific memory B cells (Blanchard Rohner et al. 2018). These new acellular vaccines can thus overcome one of the weaknesses of current acellular vaccines: the rapid loss of induced immunity. However, it remains to study the protection capacity of this vaccine against current circulating bacteria and the selection pressure that this type of vaccine would exert on the circulating bacterial population. This last aspect, in principle, would not be solved with the recombinant acellular vaccine, since it is constituted by the same few immunogens as the current acellular vaccines.
6 New Antigens and Adjuvants for aP Formulations
The incorporation of novel antigens derived from B. pertussis to improve the current aP vaccines has also been explored. The B. pertussis adenylate cyclase toxin (Cheung et al. 2006), the serum-resistance autotransporter protein BrkA (Marr et al. 2008) and the iron-regulated B. pertussis proteins (Alvarez Hayes et al. 2013) among others, have been proposed as a protective antigen. Though none of these antigens alone offered significant protection against B. pertussis infection in an intranasal challenge model, when combined with acellular pertussis vaccine, they conferred improved protection over the acellular vaccine alone. The combination of all these immunogens together with the current acellular vaccines could be an attractive proposal to reduce the selection pressure of the current acellular vaccines by offering a greater number of epitopes.
Improvements of the acellular vaccines could also be achieved by using novel adjuvants for pertussis. Combination of aP vaccine with adjuvants that are able to drive Th1 and Th17 responses would be expected to enhance protection. Cyclic di-GMP, MF59 emulsions, the combination of aluminium hydroxide with the TLR-4 agonist monophosphoryl lipid A, have been shown to enhance Th1 type immune responses however the impact in protection of these adjuvants was not deeply investigated (Geurtsen et al. 2007; Allen et al. 2018). The B. pertussis lipoprotein BP1569, a TLR-2 agonist that activates murine dendritic cells and macrophages has recently been shown to possess adjuvant properties (Dunne et al. 2015). Recently it was reported that this protein in combination with c-di-GMP synergistically induces the production of IFN-β, IL-12 and IL-23, and maturation of dendritic cells (Allen et al. 2018). Parenteral immunization of mice with an experimental aP vaccine formulated with this combined adjuvant promoted Th1 and Th17 responses and conferred protection against lung infection with B. pertussis. Interestingly, intranasal immunization with this vaccine induced potent B. pertussis-specific Th17 responses and IL-17-secreting respiratory tissue-resident memory (TRM) CD4 T cells, and conferred a high level of protection against nasal colonization (sterilizing immunity) as well as lung infection. Furthermore, long-term protection against nasal colonization with B. pertussis was observed. This formulation would thus prolong the duration of the protective response but it is not clear that it is capable of overcoming the deficiencies of the current acellular vaccines against the circulating bacterial population. More research must be done in this regard.
7 Outer Membrane Vesicles as Vaccine Candidates Against B. pertussis Infections
All Gram-negative bacteria that have been investigated so far are able to naturally release spherical structures originated from the outer membrane (referred to as outer membrane vesicles, OMVs). Although OMVs formation seems to be a common feature of Gram-negative bacteria, the knowledge of their biogenesis and biological roles remains limited. OMVs naturally contain multiple native surface-exposed antigens as well as immunostimulatory molecules. Based on their aforementioned immunogenic potency and on positive examples of the OMV-derived vaccines against Neisseria meningitides serogroup B, we initiated several studies over the last years to analyze the potential of OMVs derived from Bordetella pertussis as vaccine candidates (Hozbor et al. 1999; Roberts et al. 2008; Asensio et al. 2011). We characterized the composition of the pertussis nanoparticles at >200 protein components—including the virulence factors PT, PRN, fimbriae, FHA, and adenylate-cyclase (Hozbor 2016). The presence of a high number of immunogens in the vaccine formulation is essential since they may avoid the high selective pressure conferred by a single or a few protective-vaccine antigens. To date, we have obtained almost 50 batches of B. pertussis–derived OMVs with robust results. Our OMV-based vaccine is safe and exhibits an adequate protection capacity against different B. pertussis genetic backgrounds, including those not expressing the vaccine antigen PRN (Gaillard et al. 2014).
The OMVs derived from B. pertussis represent an attractive acellular pertussis vaccine candidate (Hozbor 2016; Ormazabal et al. 2014; Asensio et al. 2011; Roberts et al. 2008) not only because of its safety and ability to induce protective Th1, Th17 cells (Mills et al. 1993; Ryan et al. 1997; Raeven et al. 2014; Warfel and Merkel 2013; Ross et al. 2013) and TRM cells, but because it contains a greater number of immunogens in conformations close to those found in pathogen, when compared with the current aP vaccines (Hozbor 2016; Advani et al. 2011). Consistent with previous reports (Hegerle et al. 2014; Safarchi et al. 2015), we found that immunization with commercial aP vaccine does not protect against PRN deficient isolate as effectively as against B. pertussis Tohama strain (PRN+). Since the PRN deficient isolate is not isogenic to B. pertussis Tohama strain (PRN+) and contains polymorphisms at other loci that may affect the fitness of these bacteria, we have also examined the protection of the OMV based vaccine against a PRN defective mutant derived from B. pertussis Tohama strain. We found that the commercial aP vaccine but not the OMV based vaccine exhibits lower level of protection against the PRN deficient strain when compared with the parental PRN(+) positive strain. These results clearly showed the impact of the absence of PRN expression in the effectiveness of aP vaccine against B. pertussis when comparisons are made on strains that contain the same genetic background (submitted manuscript).
The results obtained here clearly showed that the OMVs vaccine is more effective than a current commercial aP vaccine against PRN deficient strains. Therefore, the OMV formulation appears as an attractive vaccine candidate that could replace the current aP without causing concern on the reactogenicity associated with wP vaccines because of the proven safety of the OMVs vaccines (Bottero et al. 2016). Since major limitations of the current aP are their strong selection pressure exerted on the circulating bacterial population and their failure to induce sustained protective immunity, the OMV-based vaccine, that contains high number of antigens and that induces INF-γ and IL17-secreting TRM cells, has the potential to replace the current aP vaccine.
References
Advani A, Gustafsson L, Ahren C, Mooi FR, Hallander HO (2011) Appearance of Fim3 and ptxP3-Bordetella pertussis strains, in two regions of Sweden with different vaccination programs. Vaccine 29(18):3438–3442. https://doi.org/10.1016/j.vaccine.2011.02.070
Allen AC, Mills KH (2014) Improved pertussis vaccines based on adjuvants that induce cell-mediated immunity. Expert Rev Vaccines 13(10):1253–1264. https://doi.org/10.1586/14760584.2014.936391
Allen AC, Wilk MM, Misiak A, Borkner L, Murphy D, Mills KHG (2018) Sustained protective immunity against Bordetella pertussis nasal colonization by intranasal immunization with a vaccine-adjuvant combination that induces IL-17-secreting TRM cells. Mucosal Immunol 11:1763–1776. https://doi.org/10.1038/s41385-018-0080-x
Alvarez Hayes J, Erben E, Lamberti Y, Principi G, Maschi F, Ayala M, Rodriguez ME (2013) Bordetella pertussis iron regulated proteins as potential vaccine components. Vaccine 31(35):3543–3548. https://doi.org/10.1016/j.vaccine.2013.05.072
Asensio CJ, Gaillard ME, Moreno G, Bottero D, Zurita E, Rumbo M, van der Ley P, van der Ark A, Hozbor D (2011) Outer membrane vesicles obtained from Bordetella pertussis Tohama expressing the lipid A deacylase PagL as a novel acellular vaccine candidate. Vaccine 29(8):1649–1656. https://doi.org/10.1016/j.vaccine.2010.12.068
Barkoff AM, Mertsola J, Pierard D, Dalby T, Hoegh SV, Guillot S, Stefanelli P, van Gent M, Berbers G, Vestrheim D, Greve-Isdahl M, Wehlin L, Ljungman M, Fry NK, Markey K, He Q (2019) Pertactin-deficient Bordetella pertussis isolates: evidence of increased circulation in Europe, 1998 to 2015. Euro Surveill 24(7). https://doi.org/10.2807/1560-7917.ES.2019.24.7.1700832
Blanchard Rohner G, Chatzis O, Chinwangso P, Rohr M, Grillet S, Salomon C, Lemaitre B, Boonrak P, Lawpoolsri S, Clutterbuck E, Poredi IK, Wijagkanalan W, Spiegel J, Pham HT, Viviani S, Siegrist CA (2018) Boosting teenagers with acellular pertussis vaccines containing recombinant or chemically inactivated pertussis toxin: a randomized clinical trial. Clin Infect Dis 68:1213–1222. https://doi.org/10.1093/cid/ciy594
Bottero D, Gaillard ME, Fingermann M, Weltman G, Fernandez J, Sisti F, Graieb A, Roberts R, Rico O, Rios G, Regueira M, Binsztein N, Hozbor D (2007) Pulsed-field gel electrophoresis, pertactin, pertussis toxin S1 subunit polymorphisms, and surfaceome analysis of vaccine and clinical Bordetella pertussis strains. Clin Vaccine Immunol 14(11):1490–1498. https://doi.org/10.1128/CVI.00177-07
Bottero D, Gaillard ME, Zurita E, Moreno G, Martinez DS, Bartel E, Bravo S, Carriquiriborde F, Errea A, Castuma C, Rumbo M, Hozbor D (2016) Characterization of the immune response induced by pertussis OMVs-based vaccine. Vaccine 34(28):3303–3309. https://doi.org/10.1016/j.vaccine.2016.04.079
Bowden KE, Weigand MR, Peng Y, Cassiday PK, Sammons S, Knipe K, Rowe LA, Loparev V, Sheth M, Weening K, Tondella ML, Williams MM (2016) Genome structural diversity among 31 Bordetella pertussis isolates from two recent U.S. Whooping Cough Statewide Epidemics. mSphere 1(3). https://doi.org/10.1128/mSphere.00036-16
Brody M, Sorley RG (1947) Neurologic complications following the administration of pertussis vaccine. N Y State J Med 47(9):1016
Brummelman J, Wilk MM, Han WG, van Els CA, Mills KH (2015) Roads to the development of improved pertussis vaccines paved by immunology. Pathog Dis 73(8):ftv067. https://doi.org/10.1093/femspd/ftv067
Buasri W, Impoolsup A, Boonchird C, Luengchaichawange A, Prompiboon P, Petre J, Panbangred W (2012) Construction of Bordetella pertussis strains with enhanced production of genetically-inactivated Pertussis Toxin and Pertactin by unmarked allelic exchange. BMC Microbiol 12:61. https://doi.org/10.1186/1471-2180-12-61
Cheung GY, Xing D, Prior S, Corbel MJ, Parton R, Coote JG (2006) Effect of different forms of adenylate cyclase toxin of Bordetella pertussis on protection afforded by an acellular pertussis vaccine in a murine model. Infect Immun 74(12):6797–6805. https://doi.org/10.1128/IAI.01104-06
Clark TA (2014) Changing pertussis epidemiology: everything old is new again. J Infect Dis 209(7):978–981. https://doi.org/10.1093/infdis/jiu001
David S, van Furth R, Mooi FR (2004) Efficacies of whole cell and acellular pertussis vaccines against Bordetella parapertussis in a mouse model. Vaccine 22(15–16):1892–1898. https://doi.org/10.1016/j.vaccine.2003.11.005
Dias WO, van der Ark AA, Sakauchi MA, Kubrusly FS, Prestes AF, Borges MM, Furuyama N, Horton DS, Quintilio W, Antoniazi M, Kuipers B, van der Zeijst BA, Raw I (2013) An improved whole cell pertussis vaccine with reduced content of endotoxin. Hum Vaccin Immunother 9(2):339–348
Dunne A, Mielke LA, Allen AC, Sutton CE, Higgs R, Cunningham CC, Higgins SC, Mills KH (2015) A novel TLR2 agonist from Bordetella pertussis is a potent adjuvant that promotes protective immunity with an acellular pertussis vaccine. Mucosal Immunol 8(3):607–617. https://doi.org/10.1038/mi.2014.93
Edwards KM, Karzon DT (1990) Pertussis vaccines. Pediatr Clin N Am 37(3):549–566
Fedele G, Bianco M, Debrie AS, Locht C, Ausiello CM (2011) Attenuated Bordetella pertussis vaccine candidate BPZE1 promotes human dendritic cell CCL21-induced migration and drives a Th1/Th17 response. J Immunol 186(9):5388–5396. https://doi.org/10.4049/jimmunol.1003765
Feunou PF, Kammoun H, Debrie AS, Mielcarek N, Locht C (2010) Long-term immunity against pertussis induced by a single nasal administration of live attenuated B. pertussis BPZE1. Vaccine 28(43):7047–7053. https://doi.org/10.1016/j.vaccine.2010.08.017
Gaillard ME, Bottero D, Errea A, Ormazabal M, Zurita ME, Moreno G, Rumbo M, Castuma C, Bartel E, Flores D, van der Ley P, van der Ark A, FH D (2014) Acellular pertussis vaccine based on outer membrane vesicles capable of conferring both long-lasting immunity and protection against different strain genotypes. Vaccine 32(8):931–937. https://doi.org/10.1016/j.vaccine.2013.12.048
Geurtsen J, Banus HA, Gremmer ER, Ferguson H, de la Fonteyne-Blankestijn LJ, Vermeulen JP, Dormans JA, Tommassen J, van der Ley P, Mooi FR, Vandebriel RJ (2007) Lipopolysaccharide analogs improve efficacy of acellular pertussis vaccine and reduce type I hypersensitivity in mice. Clin Vaccine Immunol 14(7):821–829. https://doi.org/10.1128/CVI.00074-07
Gzyl A, Augustynowicz E, Gniadek G, Rabczenko D, Dulny G, Slusarczyk J (2004) Sequence variation in pertussis S1 subunit toxin and pertussis genes in Bordetella pertussis strains used for the whole-cell pertussis vaccine produced in Poland since 1960: efficiency of the DTwP vaccine-induced immunity against currently circulating B. pertussis isolates. Vaccine 22(17–18):2122–2128. https://doi.org/10.1016/j.vaccine.2003.12.006
He Q, Makinen J, Berbers G, Mooi FR, Viljanen MK, Arvilommi H, Mertsola J (2003) Bordetella pertussis protein pertactin induces type-specific antibodies: one possible explanation for the emergence of antigenic variants? J Infect Dis 187(8):1200–1205. https://doi.org/10.1086/368412
Hegerle N, Dore G, Guiso N (2014) Pertactin deficient Bordetella pertussis present a better fitness in mice immunized with an acellular pertussis vaccine. Vaccine 32(49):6597–6600. https://doi.org/10.1016/j.vaccine.2014.09.068
Hozbor DF (2016) Outer membrane vesicles: an attractive candidate for pertussis vaccines. Expert Rev Vaccines 16:1–4. https://doi.org/10.1080/14760584.2017.1276832
Hozbor D, Rodriguez ME, Fernandez J, Lagares A, Guiso N, Yantorno O (1999) Release of outer membrane vesicles from Bordetella pertussis. Curr Microbiol 38(5):273–278
Kallonen T, Mertsola J, Mooi FR, He Q (2012) Rapid detection of the recently emerged Bordetella pertussis strains with the ptxP3 pertussis toxin promoter allele by real-time PCR. Clin Microbiol Infect 18(10):E377–E379. https://doi.org/10.1111/j.1469-0691.2012.04000.x
Kendrick P (1936) Progress report on pertussis immunization. Am J Public Health Nations Health 26:8–12
King AJ, Berbers G, van Oirschot HF, Hoogerhout P, Knipping K, Mooi FR (2001) Role of the polymorphic region 1 of the Bordetella pertussis protein pertactin in immunity. Microbiology 147. (Pt 11:2885–2895
Klein NP (2014) Licensed pertussis vaccines in the United States. History and current state. Hum Vaccin Immunother 10(9):2684–2690. https://doi.org/10.4161/hv.29576
Klein NP, Bartlett J, Fireman B, Rowhani-Rahbar A, Baxter R (2013) Comparative effectiveness of acellular versus whole-cell pertussis vaccines in teenagers. Pediatrics 131(6):e1716–e1722. https://doi.org/10.1542/peds.2012-3836
Koepke R, Eickhoff JC, Ayele RA, Petit AB, Schauer SL, Hopfensperger DJ, Conway JH, Davis JP (2014) Estimating the Effectiveness of Tdap Vaccine for Preventing Pertussis: Evidence of Rapidly Waning Immunity and Differences in Effectiveness by Tdap Brand. J Infect Dis 210:942–953. https://doi.org/10.1093/infdis/jiu322
Kulenkampff M, Schwartzman JS, Wilson J (1974) Neurological complications of pertussis inoculation. Arch Dis Child 49(1):46–49
Lam C, Octavia S, Ricafort L, Sintchenko V, Gilbert GL, Wood N, McIntyre P, Marshall H, Guiso N, Keil AD, Lawrence A, Robson J, Hogg G, Lan R (2014) Rapid Increase in Pertactin-deficient Bordetella pertussis Isolates, Australia. Emerg Infect Dis 20(4):626–633. https://doi.org/10.3201/eid2004.131478
Locht CMN (2014) Live attenuated vaccines against pertussis. Expert Rev Vaccines 13(9):1147–1158. https://doi.org/10.1586/14760584.2014.942222
Locht C (2016) Live pertussis vaccines: will they protect against carriage and spread of pertussis? Clin Microbiol Infect 22(Suppl 5):S96–S102. https://doi.org/10.1016/j.cmi.2016.05.029
Locht C, Papin JF, Lecher S, Debrie AS, Thalen M, Solovay K, Rubin K, Mielcarek N (2017) Live Attenuated Pertussis Vaccine BPZE1 Protects Baboons Against Bordetella pertussis Disease and Infection. J Infect Dis 216(1):117–124. https://doi.org/10.1093/infdis/jix254
Madsen T (1933) Vaccination against whooping cough. JAMA 101(3):187–188
Mäkelä PH (2000) Vaccines, coming of age after 200 years. FEMS Microbiol Rev 24(1):9–20
Marr N, Oliver DC, Laurent V, Poolman J, Denoel P, Fernandez RC (2008) Protective activity of the Bordetella pertussis BrkA autotransporter in the murine lung colonization model. Vaccine 26(34):4306–4311. https://doi.org/10.1016/j.vaccine.2008.06.017
Martin SW, Pawloski L, Williams M, Weening K, DeBolt C, Qin X, Reynolds L, Kenyon C, Giambrone G, Kudish K, Miller L, Selvage D, Lee A, Skoff TH, Kamiya H, Cassiday PK, Tondella ML, Clark TA (2015) Pertactin-negative Bordetella pertussis strains: evidence for a possible selective advantage. Clin Infect Dis 60(2):223–227. https://doi.org/10.1093/cid/ciu788
McGirr A, Fisman DN (2015) Duration of pertussis immunity after DTaP immunization: a meta-analysis. Pediatrics 135:331–343. https://doi.org/10.1542/peds.2014-1729
McGirr AA, Tuite AR, Fisman DN (2013) Estimation of the underlying burden of pertussis in adolescents and adults in Southern Ontario, Canada. PLoS One 8(12):e83850. https://doi.org/10.1371/journal.pone.0083850
Meeting of the Strategic Advisory Group of Experts on immunization, April 2015: conclusions and recommendations (2015) Releve epidemiologique hebdomadaire / Section d’hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record / Health Section of the Secretariat of the League of Nations 90 (22):261–278
Mielcarek N, Debrie AS, Raze D, Bertout J, Rouanet C, Younes AB, Creusy C, Engle J, Goldman WE, Locht C (2006) Live attenuated B. pertussis as a single-dose nasal vaccine against whooping cough. PLoS Pathog 2(7):e65. https://doi.org/10.1371/journal.ppat.0020065
Mielcarek N, Debrie AS, Mahieux S, Locht C (2010) Dose response of attenuated Bordetella pertussis BPZE1-induced protection in mice. Clin Vaccine Immunol 17(3):317–324. https://doi.org/10.1128/CVI.00322-09
Mills KH, Barnard A, Watkins J, Redhead K (1993) Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect Immun 61(2):399–410
Mills KH, Ross PJ, Allen AC, Wilk MM (2014) Do we need a new vaccine to control the re-emergence of pertussis? Trends Microbiol 22(2):49–52. https://doi.org/10.1016/j.tim.2013.11.007
Mooi FR, van Oirschot H, Heuvelman K, van der Heide HG, Gaastra W, Willems RJ (1998) Polymorphism in the Bordetella pertussis virulence factors P.69/pertactin and pertussis toxin in The Netherlands: temporal trends and evidence for vaccine-driven evolution. Infect Immun 66(2):670–675
Mooi FR, van Loo IH, King AJ (2001) Adaptation of Bordetella pertussis to vaccination: a cause for its reemergence? Emerg Infect Dis 7(3 Suppl):526–528
Olin P, Rasmussen F, Gustafsson L, Hallander HO, Heijbel H (1997) Randomised controlled trial of two-component, three-component, and five-component acellular pertussis vaccines compared with whole-cell pertussis vaccine. Ad Hoc Group for the Study of Pertussis Vaccines. Lancet 350(9091):1569–1577
Ormazabal M, Bartel E, Gaillard ME, Bottero D, Errea A, Zurita ME, Moreno G, Rumbo M, Castuma C, Flores D, Martin MJ, Hozbor D (2014) Characterization of the key antigenic components of pertussis vaccine based on outer membrane vesicles. Vaccine 32(46):6084–6090. https://doi.org/10.1016/j.vaccine.2014.08.084
Podda A, Carapella De Luca E, Titone L, Casadei AM, Cascio A, Bartalini M, Volpini G, Peppoloni S, Marsili I, Nencioni L et al (1993) Immunogenicity of an acellular pertussis vaccine composed of genetically inactivated pertussis toxin combined with filamentous hemagglutinin and pertactin in infants and children. J Pediatr 123(1):81–84
Raeven RH, Brummelman J, Pennings JL, Nijst OE, Kuipers B, Blok LE, Helm K, van Riet E, Jiskoot W, van Els CA, Han WG, Kersten GF, Metz B (2014) Molecular signatures of the evolving immune response in mice following a Bordetella pertussis infection. PLoS One 9(8):e104548. https://doi.org/10.1371/journal.pone.0104548
Rappuoli R (1999) The vaccine containing recombinant pertussis toxin induces early and long-lasting protection. Biologicals 27(2):99–102. https://doi.org/10.1006/biol.1999.0189
Roberts R, Moreno G, Bottero D, Gaillard ME, Fingermann M, Graieb A, Rumbo M, Hozbor D (2008) Outer membrane vesicles as acellular vaccine against pertussis. Vaccine 26(36):4639–4646. https://doi.org/10.1016/j.vaccine.2008.07.004
Romanus V, Jonsell R, Bergquist SO (1987) Pertussis in Sweden after the cessation of general immunization in 1979. Pediatr Infect Dis J 6(4):364–371
Ross PJ, Sutton CE, Higgins S, Allen AC, Walsh K, Misiak A, Lavelle EC, McLoughlin RM, Mills KH (2013) Relative contribution of Th1 and Th17 cells in adaptive immunity to Bordetella pertussis: towards the rational design of an improved acellular pertussis vaccine. PLoS Pathog 9(4):e1003264. https://doi.org/10.1371/journal.ppat.1003264
Ryan M, Murphy G, Gothefors L, Nilsson L, Storsaeter J, Mills KH (1997) Bordetella pertussis respiratory infection in children is associated with preferential activation of type 1 T helper cells. J Infect Dis 175(5):1246–1250
Ryan M, Murphy G, Ryan E, Nilsson L, Shackley F, Gothefors L, Oymar K, Miller E, Storsaeter J, Mills KH (1998) Distinct T-cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunology 93(1):1–10
Safarchi A, Octavia S, Luu LD, Tay CY, Sintchenko V, Wood N, Marshall H, McIntyre P, Lan R (2015) Pertactin negative Bordetella pertussis demonstrates higher fitness under vaccine selection pressure in a mixed infection model. Vaccine 33(46):6277–6281. https://doi.org/10.1016/j.vaccine.2015.09.064
Sato H, Sato Y (1985) Protective antigens of Bordetella pertussis mouse-protection test against intracerebral and aerosol challenge of B. pertussis. Dev Biol Stand 61:461–467
Sato Y, Kimura M, Fukumi H (1984) Development of a pertussis component vaccine in Japan. Lancet 1(8369):122–126
Sauer LW (1948) Simultaneous immunization against diphtheria, tetanus and pertussis; a preliminary report. Q Bull Northwest Univ Med Sch 22(3):281–285
Seubert A, D'Oro U, Scarselli M, Pizza M (2014) Genetically detoxified pertussis toxin (PT-9K/129G): implications for immunization and vaccines. Expert Rev Vaccines 13(10):1191–1204. https://doi.org/10.1586/14760584.2014.942641
Sheridan SL, Ware RS, Grimwood K, Lambert SB (2012) Number and order of whole cell pertussis vaccines in infancy and disease protection. JAMA 308(5):454–456. https://doi.org/10.1001/jama.2012.6364
Sirivichayakul C, Chanthavanich P, Limkittikul K, Siegrist CA, Wijagkanalan W, Chinwangso P, Petre J, Hong Thai P, Chauhan M, Viviani S (2016) Safety and immunogenicity of a combined Tetanus, Diphtheria, recombinant acellular Pertussis vaccine (TdaP) in healthy Thai adults. Hum Vaccin Immunother 13(1):36–143
Skerry CM, Cassidy JP, English K, Feunou-Feunou P, Locht C, Mahon BP (2009) A live attenuated Bordetella pertussis candidate vaccine does not cause disseminating infection in gamma interferon receptor knockout mice. Clin Vaccine Immunol 16(9):1344–1351. https://doi.org/10.1128/CVI.00082-09
Solans L, Locht C (2018) The Role of Mucosal Immunity in Pertussis. Front Immunol 9:3068. https://doi.org/10.3389/fimmu.2018.03068
Stefanelli P, Buttinelli G, Vacca P, Tozzi AE, Midulla F, Carsetti R, Fedele G, Villani A, Concato C (2017) Severe pertussis infection in infants less than 6 months of age: Clinical manifestations and molecular characterization. Hum Vaccin Immunother 13(5):1073–1077. https://doi.org/10.1080/21645515.2016.1276139
Tan T, Dalby T, Forsyth K, Halperin SA, Heininger U, Hozbor D, Plotkin S, Ulloa-Gutierrez R, von Konig CH (2015) Pertussis across the globe: recent epidemiologic trends from 2000–2013. Pediatr Infect Dis J 34(9):e222–e232. https://doi.org/10.1097/INF.0000000000000795
Tartof SY, Lewis M, Kenyon C, White K, Osborn A, Liko J, Zell E, Martin S, Messonnier NE, Clark TA, Skoff TH (2013) Waning immunity to pertussis following 5 doses of DTaP. Pediatrics 131(4):e1047–e1052. https://doi.org/10.1542/peds.2012-1928
Thorstensson R, Trollfors B, Al-Tawil N, Jahnmatz M, Bergstrom J, Ljungman M, Torner A, Wehlin L, Van Broekhoven A, Bosman F, Debrie AS, Mielcarek N, Locht C (2014) A phase I clinical study of a live attenuated Bordetella pertussis vaccine--BPZE1; a single centre, double-blind, placebo-controlled, dose-escalating study of BPZE1 given intranasally to healthy adult male volunteers. PLoS One 9(1):e83449. https://doi.org/10.1371/journal.pone.0083449
Warfel JM, Merkel TJ (2013) Bordetella pertussis infection induces a mucosal IL-17 response and long-lived Th17 and Th1 immune memory cells in nonhuman primates. Mucosal Immunol 6(4):787–796. https://doi.org/10.1038/mi.2012.117
WHO (2016) Pertussis vaccines: WHO position paper, August 2015—Recommendations. Vaccine 34(12):1423–1425. https://doi.org/10.1016/j.vaccine.2015.10.136
Wilk MM, Mills KHG (2018) CD4 TRM Cells Following Infection and Immunization: Implications for More Effective Vaccine Design. Front Immunol 9:1860. https://doi.org/10.3389/fimmu.2018.01860
Witt MA, Katz PH, Witt DJ (2012) Unexpectedly limited durability of immunity following acellular pertussis vaccination in preadolescents in a North American outbreak. Clin Infect Dis 54(12):1730–1735. https://doi.org/10.1093/cid/cis287
Witt MA, Arias L, Katz PH, Truong ET, Witt DJ (2013) Reduced risk of pertussis among persons ever vaccinated with whole cell pertussis vaccine compared to recipients of acellular pertussis vaccines in a large US cohort. Clin Infect Dis 56(9):1248–1254. https://doi.org/10.1093/cid/cit046
Yeung KHT, Duclos P, Nelson EAS, Hutubessy RCW (2017) An update of the global burden of pertussis in children younger than 5 years: a modelling study. Lancet Infect Dis 17(9):974–980. https://doi.org/10.1016/S1473-3099(17)30390-0
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This study was funded by a grant from the ANCPyT (PICT 2014-3617, PICT 2012- 2719), CONICET and FCE-UNLP (Argentina) grants to DFH. DFH is member of the Scientific Career of CONICET. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Hozbor, D. (2019). New Pertussis Vaccines: A Need and a Challenge. In: Fedele, G., Ausiello, C. (eds) Pertussis Infection and Vaccines. Advances in Experimental Medicine and Biology(), vol 1183. Springer, Cham. https://doi.org/10.1007/5584_2019_407
Download citation
DOI: https://doi.org/10.1007/5584_2019_407
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-33248-8
Online ISBN: 978-3-030-33249-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)