Abstract
Removal of lacrimal glands is used as a viable model of dry eye disease in rats. However, there is no uniform agreement on the disease severity following different variants of the procedure. The interpretation of the modeled dry eye disease also is biased by the interchangeable use of male and female rats. Therefore, this study seeks to define the features of dry eye disease following removal of the extraorbital lacrimal gland, with or without excision of the infraorbital lacrimal gland in male and female rats. The experiments were performed in 12-week-old female and male Sprague-Dawley rats. The baseline blink rate and fluorescein score were assessed. Subsequently, rats underwent isolated removal of the extraorbital gland, removal of the extraorbital gland combined with excision of the infraorbital gland, or a sham surgical procedure. The assessment of blink rate and fluorescein scores was repeated 28 days following surgery. Corneas were collected for histological analysis. We found that the blink rate and fluorescein score increased in all of the experimental groups, except the control group and the male rats that underwent isolated removal of the extraorbital lacrimal gland. Histopathological analysis revealed the thinning and edema of the epithelium in all groups, except the control group. These changes were most pronounced in female rats following combined removal of extraorbital and infraorbital lacrimal glands. In conclusion, severity of dry eye disease in the rat model is influenced by both gender and the extent of surgical removal of lacrimal glands. Combined excision of lacrimal glands in female rats produced the most severe pathological changes, whereas isolated excision of the extraorbital lacrimal gland in male rats led to the least severe changes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Dry eye disease is a chronic condition caused by abnormalities of the tear film. Decreased production or increased evaporation compromises the ocular surface, leading to desiccation of epithelial cells and their apoptosis. That, in turn, triggers inflammation, which perpetuates a vicious circle of the disease (Perry 2008). The estimated prevalence of dry eye disease ranges from 5% to 50% (Stapleton et al. 2017). Although mild forms of the disease are often disregarded by patients and doctors, severe dry eye disease, due to scarring or secondary infections, is a potentially blinding condition (Deswal et al. 2017).
Considering the burden of dry eye disease and its complex pathophysiology, many experimental studies including in vitro and animal models have been developed to broaden our understanding of the disease and to evaluate potential treatments (Higuchi et al. 2011; Barabino et al. 2004; Offord et al. 1999). Although cell lines are viable for determining the safety of treatment and its anti-inflammatory properties, no clinically relevant data, i.e., a blink rate or fluorescein staining score, has by far been derived from those studies. Given a complex environment of the ocular surface, animal models are preferable to in vitro models in the studies on the pathophysiology and treatment of dry eye disease. In brief, animal models of dry eye disease emulate increased evaporation or decreased secretion of the tear film (Schrader et al. 2008). The former is achieved by cauterization of Meibomian gland orifices or genetic modification of Meibomian gland function (Ehrmann and Schneider 2016; Gilbard et al. 1989), whereas the latter by removal of lacrimal glands (Barabino and Dana 2004). Rats are equipped with the main lacrimal gland (exorbital lacrimal gland), accessory lacrimal gland (infraorbital lacrimal gland), and Harderian lacrimal glands (intraorbital lacrimal gland) (Percy et al. 1989). Given the lack of technical difficulties related to the procedure, removal of the main lacrimal gland is one of the most frequently utilized models of tear-deficient dry eye disease in rats (Fujihara et al. 2001). Although studies on tear-deficient models are popular and efficient, they might be difficult to interpret. Firstly, significant differences in the disease severity have been reported following excision of the extraorbital lacrimal glands in rats (Higuchi et al. 2010). Secondly, some studies are performed following isolated removal of the extraorbital gland and some following combined extraorbital and infraorbital lacrimal gland excision. Finally, studies, which have been published so far, utilize male and female animals interchangeably (Meng et al. 2015; Stevenson et al. 2014). Considering the influence of sex hormones on the remaining lacrimal tissue and on the Meibomian glands, comparisons between the experiments may be skewed. Therefore, the present study seeks to define the benchmark characteristics of dry eye disease in female and male rats following removal of main lacrimal gland, with or without excision of accessory lacrimal glands.
2 Methods
The experiments were performed in 12-week-old male (n = 24) and female (n = 24) Sprague-Dawley rats. Throughout the study, animals stayed in their housing cages with access to water and chow ad libitum. Air temperature was set at 23.8 °C and humidity at 54.4%. All female rats were put in one room at birth, which enabled the synchronization of menstrual cycles and similar exposure to female sex hormones.
2.1 Experimental Protocol
On Day 1, baseline blink rate (BR) and fluorescein score (FS) were assessed in all animals. Subsequently, rats underwent surgical removal of the extraorbital lacrimal gland (main lacrimal gland) (n = 16; female = 8, male = 8), removal of the extraorbital gland with removal of the infraorbital lacrimal gland (accessory lacrimal gland) (n = 16; female = 8, male = 8), or sham surgery (n = 16, female = 8, male = 8). On Day 28, BR and FS were reassessed.
2.2 Surgery
All procedures were performed unilaterally and were as follows.
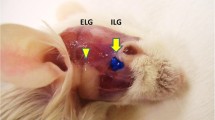
Isolated Removal of the Extraorbital Lacrimal Gland
The skin was incised from the lateral canthus of the eye toward the auricle. The connective tissue was dissected, and the extraorbital lacrimal gland was visualized just anterior to the auricle. All relevant vessels were closed with 4-0 nylon sutures. Subsequently, the extraorbital lacrimal gland was released from the connective tissue and removed. The skin was closed with 4-0 nylon sutures.
Combined Removal of the Extraorbital Lacrimal and Infraorbital Lacrimal Glands
Following removal of the extraorbital lacrimal gland, the superficial temporal artery was visualized and dissected rostrally. Infraorbital lacrimal gland, which lies over the temporal artery and posterior to the lateral canthus, was removed. The skin was closed with 4-0 nylon sutures.
Sham Surgery
The skin was incised and connective tissue dissected as described above. The extraorbital lacrimal gland and the infraorbital lacrimal gland were visualized. No lacrimal tissue was removed. The skin was closed with 4-0 nylon sutures.
2.3 Grading Scores
Fluorescein Score (FS)
was measured under short anesthesia (ketamine 100 mg/kg and xylazine 10 mg/kg, i.p.). One drop of fluorescein was instilled into both eyes and rinsed with 1 ml of 0.9% NaCl 2 min later. Photographs of the corneas were taken under cobalt blue light and 14x magnification. Subsequently, FS (range, 1–5) was assessed by two blinded observers, and it was averaged for the final result.
Blink Rate (BR)
was measured by two independent observers for 2 min. During the count, rats were housed in their living cages. To minimize a possible error, the cover of the cage was removed. Two counts were averaged for the final result. BR was expressed as the number of blinks per minute.
2.4 Histopathology
Tissues sections were fixed in 10% buffered formalin and embedded in paraffin wax. Sections of 3–4 μm thick were stained with hematoxylin and eosin. A general histopathological examination was performed under the magnifications of 10x, 40x, and 100x (objective lens) and 10x (eyepiece). Photographic documentation was performed. Additionally, the following morphometric measurements were made: width of the cornea, height of the anterior epithelium of cornea, and number of cell layers in the epithelium at magnification of 40x (objective lens). The ratio of the height of the epithelium to the number of cell layers was calculated. All histological procedures were performed using a standard light microscope Olympus BX41 and cellSens software (Olympus Corporation, Tokyo, Japan).
2.5 Statistical Analysis
Normality of data distribution was tested with the Kolmogorov-Smirnov test. Continuous variables and discrete variables were expressed as means ±SE and as medians ±median absolute deviation (MAD), respectively. Normally distributed data were compared with Student’s t-test, one-way analysis of variance (ANOVA), or one-way analysis of variance for repeated measurements (RM-ANOVA). Discrete variables were analyzed with the Mann-Whitney U and Kruskal-Wallis tests, whereas categorical data with chi-squared test. Significance was set at the level of p<0.05.
3 Results
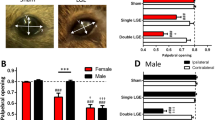
BR and FS were similar between the groups at the beginning of the experiment. Both increased significantly in all groups 1 month after surgical procedures (p<0.05), except sham controls and the male rats that underwent isolated removal of the extraorbital lacrimal gland. The highest BR and FS were observed in female rats following the combined extraorbital and infraorbital lacrimal gland excision (p<0.05). BR and FS were lower, to a similar extent, in female rats after the extraorbital lacrimal gland excision and in male rats after the combined procedure (p<0.05) (Figs. 1 and 2).
Blink rate, expressed as means ±SE. Combined removal of both extraorbital and infraorbital lacrimal gland, isolated removal of extraorbital lacrimal gland alone, control sham procedure; *p<0.05 for 30 days after surgery vs. baseline; † p<0.05 combined vs. isolated vs. control; # p<0.05 male vs. female
Fluorescein score, expressed as medians ±MAD. Combined removal of both extraorbital and infraorbital lacrimal glands, isolated removal of extraorbital lacrimal gland alone, control sham procedure; *p<0.05 for 30 days after surgery vs. baseline; † p<0.05 combined vs. isolated; # p<0.05 male vs. female
3.1 Histopathology
In all of the studied groups, the number of epithelial layers was lower than that in sham controls 1 month after surgery (p<0.05). The lowest number was found in female rats which underwent the combined extraorbital and infraorbital lacrimal gland excision (p<0.05). That was followed by female rats following the isolated extraorbital lacrimal gland removal and male rats after the combined extraorbital and infraorbital lacrimal gland removal (p<0.05). The least significant changes were noted in male rats after the isolated extraorbital lacrimal gland excision. The A/B index, i.e., ratio of epithelial thickness to the number of epithelial layers, followed a reverse pattern among the studied groups (Table 1) (Fig. 3).
4 Discussion
The major finding of this study was that combined and isolated excisions of the extraorbital lacrimal gland in female and male rats produced a model dry eye disease of diversified severity. Combined excision of the extraorbital and infraorbital lacrimal glands in female rats led to the most severe form of the disease. Less severe changes were induced by isolated extraorbital lacrimal gland removal in female rats and by combined excision in male rats. The least severe changes were observed following isolated extraorbital lacrimal gland excision in male rats.
Several symptomatic discrepancies in the models of dry eye disease have previously been reported. Some studies have shown that clinically significant symptoms, i.e., increased BR and FS, are manifest after isolated excision of the extraorbital lacrimal gland (Higuchi et al. 2010; Fujihara et al. 2001). On the other hand, Meng et al. (2015) have shown that only subtle symptoms may be elicited by such excision, and it takes a combined gland removal to obtain more pronounced symptoms in male Sprague-Dawley rats. In line with the study above outlined, in the current study we found that although isolated removal of the extraorbital lacrimal gland produced only subclinical symptoms of dry eye disease, a combined procedure led to clinically significant symptoms in male rats. However, both procedures produced significant symptoms in female rats. Likewise, histopathology revealed more significant changes in female rats. It has been previously shown that female gender is related to a greater prevalence of dry eye disease. Although various hypotheses have been coined to explain this observation, e.g., increased prevalence of Sjogren disease in females or the effect of estrogens on lacrimal and Meibomian glands, no uniform theory has been established (Sullivan et al. 2017). We believe that a greater severity of dry eye disease in female rats could be attributed to the effect of estrogens on the lacrimal tissue, remaining after surgery, conjunctival goblet cells, or meibomian glands.
The current study is subject to some limitations. It cannot be excluded that a longer than the 4-week-long observation period employed in this study after isolated removal of extraorbital lacrimal glands would reveal more significant symptoms in male rats. Other authors, however, have reported that symptoms of dry eye disease are noticeable as soon as a week following removal of lacrimal glands (Meng et al. 2015). Despite the limitations we believe we have convincingly demonstrated that combined and isolated removal of the extraorbital lacrimal glands in female and male rats might be utilized as a reproducible model of dry eye disease, with the provision of gender-related differences in disease severity.
References
Barabino S, Dana MR (2004) Animal models of dry-eye: a critical assessment of opportunities and limitations. Invest Ophthalmol Vis Sci 45:1641–1646
Barabino S, Chen W, Dana MR (2004) Tear film and ocular surface tests in animal models of dry-eye: uses and limitations. Exp Eye Res 79:613–621
Deswal J, Arya SK, Raj A, Bhatti A (2017) A case of bilateral corneal perforation in a patient with severe dry-eye. J Clin Diagn Res 11:ND01–ND02
Ehrmann C, Schneider MR (2016) Genetically modified laboratory mice with sebaceous glands abnormalities. Cell Mol Life Sci 73:4623–4642
Fujihara T, Murakami T, Fujita H, Nakamura M, Nakata K (2001) Improvement of corneal barrier function by the P2Y(2) agonist INS365 in a rat dry-eye model. Invest Ophthalmol Vis Sci 42:96–100
Gilbard JP, Rossi SR, Heyda KG (1989) Tear film and ocular surface changes after closure of the meibomian gland orifices in the rabbit. Ophthalmology 96:1180–1186
Higuchi A, Takahashi K, Hirashima M, Kawakita T, Tsubota K (2010) Selenoprotein P controls oxidative stress in cornea. PLoS One 5:e9911
Higuchi A, Kawakita T, Tsubota K (2011) IL-6 induction in desiccated corneal epithelium in vitro and in vivo. Mol Vis 17:2400–2406
Meng ID, Barton ST, Mecum NE, Kurose M (2015) Corneal sensitivity following lacrimal gland excision in the rat. Invest Ophthalmol Vis Sci 56:3347–3354
Offord EA, Sharif NA, Macé K, Tromvoukis Y, Spillare EA, Avanti O, Howe WE, Pfeifer AM (1999) Immortalized human corneal epithelial cells for ocular toxicity and inflammation studies. Invest Ophthalmol Vis Sci 40:1091–1101
Percy DH, Wojcinski ZW, Schunk MK (1989) Sequential changes in the harderian and exorbital lacrimal glands in Wistar rats infected with sialodacryoadenitis virus. Vet Pathol 26:238–245
Perry HD (2008) Dry-eye disease: pathophysiology, classification, and diagnosis. Am J Manag Care 14:S79–S87
Schrader S, Mircheff AK, Geerling G (2008) Animal models of dry-eye. Dev Ophthalmol 41:298–312
Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, Na KS, Schaumberg D, Uchino M, Vehof J, Viso E, Vitale S, Jones L (2017) TFOS DEWS II epidemiology report. Ocul Surf 15:334–365
Stevenson W, Chen Y, Lee SM, Hua J, Dohlman T, Shiang T, Dana R (2014) Extraorbital lacrimal gland excision: a reproducible model of severe aqueous tear-deficient dry-eye disease. Cornea 33:1336–1341
Sullivan DA, Rocha EM, Aragona P, Clayton JA, Ding J, Golebiowski B, Hampel U, McDermott AM, Schaumberg DA, Srinivasan S, Versura P, Willcox MDP (2017) TFOS DEWS II sex, gender, and hormones report. Ocul Surf 15:284–333
Conflicts of Interest
The authors report no conflicts of interest in relation to this article.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. The study was approved by a local Bioethics Committee.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Skrzypecki, J., Tomasz, H., Karolina, C. (2019). Variability of Dry Eye Disease Following Removal of Lacrimal Glands in Rats. In: Pokorski, M. (eds) Medical Science and Research. Advances in Experimental Medicine and Biology(), vol 1153. Springer, Cham. https://doi.org/10.1007/5584_2019_348
Download citation
DOI: https://doi.org/10.1007/5584_2019_348
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-19058-3
Online ISBN: 978-3-030-19059-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)