Abstract
This study focused on how pulmonary function is affected by proprioceptive neuromuscular facilitation (PNF) of accessory respiratory muscles in the chronic post-stroke phase. The study involved patients who had had ischemic stroke 6 months or more before the PNF treatment investigated. The objective was to define the effect of PNF on bioelectrical resting and maximum activity of the accessory muscles. Patients were randomly assigned to PNF treatment and just positioning treatment as a reference for comparison; 30 patients each. Electromyography of accessory muscles was investigated before and after physiotherapeutic treatments. We found that there was a greater reduction in EMG activity in all muscles investigated after PNF compared to positioning treatment alone. A reduction of muscle activity due to PNF concerned both affected and unaffected body side, but it was greater on the affected side. We conclude that a reduction of the accessory respiratory muscle activity due to PNF treatment could be of benefit in chronic stoke patients in that it would help normalize breathing pattern and thereby prevent the development of hypoxia.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Accessory respiratory muscles
- Electromyography
- Proprioceptive neuromuscular facilitation
- Pulmonary function
- Respiration
- Stroke
1 Introduction
Therapists working with stroke patients increasingly use the physiotherapeutic methods that are based on the natural mechanisms of reorganization of the nervous system. Physiotherapy is used in cases of impaired automatic, deliberate, or spontaneous motion manifest by paresis, and disordered motor coordination and muscle activity control, i.e., by the typical signs of a cerebrovascular accident (Britto et al. 2011; Khedr et al. 2000; Sezer et al. 2004). Paralysis or paresis affects the quality of patient life and is a major indication for rehabilitation. Consequences of hemiplegia include irregularities in muscle activity, posture, and motor control (Teixeira-Salmela et al. 2005). Andrews and Bohannon (2000) have observed a greater decline in muscle strength in the proximal parts of the limbs, i.e., located closer to the torso, than the distal ones. That means that the muscles of the upper and lower limb girdle, and the chest and torso muscles demonstrate a greater functional impairment. In addition to impaired control of posture, paresis/hemiplegia may disturb motor control of respiratory muscles, which entails a reduction in both muscle strength and chest mobility (Voyvoda et al. 2012; de Almeida et al. 2011; Scillia et al. 2004; Lanini et al. 2003; Lanini et al. 2002; Howard et al. 2001; Similowski et al. 1996; Houston et al. 1995a, b; Cohen et al. 1994).
In the chronic post-stroke stage, physiotherapeutic rehabilitation is usually concerned with the recovery of motor agility, and breathing function often escapes notice (Teixeira-Salmela et al. 2005; Sezer et al. 2004). Therefore, the present study seeks to define how lung ventilation would be influenced by proprioceptive neuromuscular facilitation (PNF), specifically directed at accessory respiratory muscles. We addressed the issue be examining the effects of PNF on bioelectrical resting and maximum activity of the accessory muscles and comparing it with the classical positioning treatment in patients who had ischemic brain stroke at least 6 months before the therapeutic intervention investigated in this study.
2 Methods
2.1 Patients, Study Design, and Instrumentation
This study was a randomized interventional trial designed according to the guidelines of the CONsolidated Standards of Reporting Clinical Trials (CONSORT 2010). It was carried out in the External Department for Neurological Rehabilitation of the Regional Specialist Hospital in Wroclaw, Poland, from November 2013 to April 2015. We evaluated changes in the surface electromyography (EMG) activity of the accessory respiratory muscles to respiratory muscle-oriented physiotherapy in patients after ischemic brain stroke. There were 98 consecutive hospitalized patients, who had suffered a stroke at least 6 months before the study time, with resultant hemiparetic walking. On further scrutiny, 38 of the patients had to be excluded from the study due to sensory aphasia, previous cardiac events or myocardial infarction, asthma or COPD, significant abdominal obesity (BMI >28 kg/m2), previous surgery in the chest or abdominal integuments, and a reduction in daily functioning (Barthel index <60); the factors set as the exclusion criteria. The remaining 60 patients were randomly assigned to PNF treatment of respiratory muscles and to just positioning treatment taken as a reference group; 30 patients each. An interview was carried out with each participant, to collect the basic data such as age, body weight and height, smoking habits, the time of stroke occurrence, and the affected body side. The assessment of functional ability was performed using the Barthel scale (Mahoney and Barthel 1965). Next, EMG recording of accessory respiratory muscle activity was performed.
The EMG of the following accessory muscles of respiration was bilaterally recorded with silver/silver-chloride self-adhesive electrodes: abdominal external oblique muscle (AEO), sternocleidomastoid (SCM), pectoralis major (PM), and the serratus anterior muscle (SA). The electrode diameter was 3.8 cm, and that of the conductive area was 1 cm. A pair of electrodes was placed on shaved skin over a muscle investigated, 2 cm apart, in parallel to the muscle fibers. The EMG was recorded with an 8-channel MyoSystem 1,400 L electromyograph (Noraxon; Scottsdale, AZ). The specifications of the EMG setup met the International Society of Electrophysiology and Kinesiology (ISEK) and surface EMG for non-invasive assessment of muscles (SENIAM) requirements (Merletti et al. 2009; Merletti and Hermens 2000; Merletti 1999). The setup consisted of the signal receiver, preamplifiers, and PC equipped with MyoResearch XP Master Edition v1.04 software. The signal was band-passed at 10–450 Hz. The characteristics of the system were as follows: mode rejection level of a minimum of 100 dB, input impedance of EMG channels higher than 100 MOhm, and sensitivity of the EMG signal of 0.1 μV.
The AEO electrodes were attached by drawing a line perpendicular to the horizontal line, starting at the anterior superior iliac spine and running to the rib line. The first electrode was placed half-way between these points. The second electrode was placed 2 cm away from the first, at a 45° angle in the caudal and medial direction, so that both electrodes ran parallel to the muscle fibers. For the SCM electrodes, a line was drawn from the mastoid process of the temporal bone to one third of the medial part of the clavicle. The first electrode was attached in the middle of the line. The second electrode was placed 2 cm above, in line with the muscle fibers. The PM electrodes were attached to the upper clavicular part of the muscle, 2 cm apart, just medial to the anterior axillary line. A fixed point for placing the electrodes on SA was determined by drawing a line at the nipple level of the 4th rib and a perpendicular anterior axillary line. The first electrode was attached at the intersection of the two lines and the second one was 2 cm from the first in the centripetal direction. The reference electrode was placed on the anterior superior iliac spine on the left side.
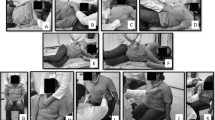
The EMG evaluation was performed with the patient in the supine position, with the forearms positioned at a slight internal rotation and the knees bent on a roller support (Fig. 1a). The pectoral girdle was well supported underneath to prevent the excessive retraction of the shoulder joints and the subclavian artery from being pressed by the clavicle. When the patient was unable to position the arm on the paralyzed side, the limb was placed on his body to maintain the maximum possible relaxation and comfort. Patients acclimated to the position assumed for 10 min before the recording started. The EMG was recorded during resting breathing and during deep inspiration and expiration breathing before and after PNF treatment. The protocol of EMG recordings was as follows:
-
rest, with the patient breathing calmly for 60 s;
-
PNF treatment directed at the diaphragm muscle and the lower rib cage intercostal muscles as described below for about 5 min;
-
during deep inspiration and expiration, with the patient inspiring deeply and exhaling as slowly as possible; the maneuver was repeated three times in 2 min.
In the positioning group, taken as a reference for comparison, the only difference in the protocol above outlined was that the PNF treatment was skipped. The rational was to observe the effect on the muscle activity of the specific body position, same as in case of PNF-treated patients, that facilitates breathing movements. The raw EMG signal was rectified and an algorithm was used to normalize the ECG artifacts, created during the collection of signal from muscles situated in the proximity of the heart.
2.2 Physiotherapeutic Interventions
The purpose of the interventions was to intensify chest respiratory movements and to normalize respiratory muscle activity on the stroke-affected and unaffected body sides. In the PNF group, the intervention consisted of two stages:
Stage I: stimulation of the diaphragm (Fig. 1b). The therapist placed his hands on the patient’s upper abdominal area. Application of the stretching stimulus at end-expiration aimed to intensify the diaphragm contraction, i.e., to initiate inspiration. During inspiration, the therapist’s hands, placed in like manner, exerted a slight pressure/resistance on the raising abdominal integuments. In that way, the resistance has a facilitating effect on the abdominal motion.
Stage II: stimulation of the costal inferolateral regions (lower costal respiratory pattern) (Fig. 1c). The therapist’s hands were placed with fingers in the rib line. At end-expiration, pressure (stretching) was applied in the caudal and centripetal direction, at the same time instructing the subject to take a deep breath. Akin to the diaphragm stimulation, the therapist exerted a slight resistance on the ribs expanding during inspiration. Stage I and II interventions were performed three times at 1-min intervals each, with a 3-min break between the two stages.
In the positioning reference group, patients lay in the supine position with the knees bent, in which the diaphragm is in the optimum high position and the bent knees facilitate relaxation of abdominal integuments, which yields a greater inspiratory effectiveness.
2.3 Statistical Analysis
Quantitative data were presented as means ±SD. The Shapiro-Wilk test was used to establish data distribution. A two-tailed unpaired t-test, the Wilcoxon test, and the Mann-Whitney U test were used, as required, for the assessment of differences between the PNF and positioning groups. Qualitative data were presented as percentages and were analyzed using a chi-squared test. The statistical significance level was set at α = 0.05. The analysis was performed using a commercial statistical package of Statistica v10 (StatSoft Inc., Tulsa, OK).
3 Results
Patient demographic and clinical characteristics are shown in Table 1. There were 12 women and 18 men, aged 57–75 (mean 64 ± 5.2 years) in the PNF group and 8 women and 22 men, aged 57–77 (mean 64 ± 7 years) in the positioning group. The demographic characteristics were matched in both groups. Ten patients in the PNF group and 14 in the positioning group admitted to smoking cigarettes. Paresis was on the left body side in 13 and 15 patients, respectively.
The effects of physiotherapeutic maneuvers on bioelectrical activity of accessory respiratory muscles on both stroke affected and unaffected sides in both groups of patients are compared in Tables 2, 3, 4 and 5.
3.1 Abdominal External Oblique (AEO) Muscle
AEO muscle activity decreased in stroke patients after PNF–treatment in all conditions investigated, i.e., during resting breathing and deep inspiratory and expiratory breathing on both affected and unaffected body sides (Table 2). The decrease was significant and amounted to about 25–30%, and it was greater on the affected side. Interestingly, the activity also decreased in patients not subjected to PNF treatment after the breathing maneuvers above outlined. However, the decrease was noticed only on the affected side and was about half of that present after PNF. There was no decrease in AEO activity on the unaffected side without PNF treatment.
3.2 Sternocleidomastoid Muscle (SCM)
SCM muscle activity demonstrated significantly lower values in PNF-treated patients in all breathing conditions investigated (Table 3). The decreases mostly concerned the stroke affected body side. Percentagewise, the muscle activity decrease was many-fold greater than the tendency for a decrease in the corresponding activities in the PNF-untreated stroke patients. The muscle activity on the unaffected side, except during deep inspiration remained grossly unchanged, as did also here activity in the PNF-untreated patients.
3.3 Pectoralis Major (PM) Muscle
PM muscle activity significantly decreased on the stroke affected body side after PNF-treatment. The decrease was strongest during resting breathing and during deep expirations, in a range of 32% and 19% off the corresponding baseline levels, respectively (Table 4). The activity decreased less appreciably on the unaffected side and it failed to change in PNF-untreated patients where it demonstrated just a slight downward trend (Table 4).
3.4 Serratus Anterior (SA) Muscle
SA muscle activity demonstrated significant decreases by about 10–13% off the baseline levels on both stroke affected and unaffected body sides after PNF treatment. The decreases, however, reached statistical significance only on the affected side (Table 5). In the PNF-untreated patients, the decrease in the SA muscle activity was much meager on the affected side, reaching just a few percentage points off the baseline level present before the breathing maneuvers. In this group of patients, activity changes on the unaffected side were close to null.
For all accessory respiratory muscles investigated, baseline bioelectrical activity before breathing maneuvers somehow differed between the PNF-treated and PNF-untreated groups of patients. The activity, in most cases, tended to be lower, sometimes significantly so, in the former group, which likely reflects inter-individual differences, all other study factors being equal. This difference was accentuated after breathing maneuvers since the decrease in muscle activity was ubiquitously greater in the PNF-treated patients.
4 Discussion
The goal of this study was to evaluate the influence of PNF physiotherapy directed at the diaphragm and intercostal muscles on bioelectric activity of accessory respiratory muscles. The activity was assessed noninvasively from the EMG recordings, symmetrically on both paretic and unaffected sides, in patients after a distant time from ischemic cerebral stroke. The activity was collected during two conditions of the respiratory effort: resting breathing and strained deep inspiration/expiration. Physiotherapeutic treatment was performed in the supine body position with support-raised and bent knees, considered conducive to the optimum function of the respiratory muscle pump. The PNF-driven changes in respiratory muscle activity were contrasted with those occurring in patients without any physiotherapeutic intervention other than being in the same specific positioning.
The major finding was that PNF treatment resulted, except a single instance of SCM in deep inspiration, in an appreciable decrease in bioelectrical activity of all investigated accessory muscles of respiration. The decrease concerned both disease affected and unaffected sides, but it was clearly accentuated, occasionally to a several–fold extent, as in case of AEO, or many–fold, as in case of SCM, on the affected side. In contradistinction, the body positioning alone showed, in the majority of cases, a smaller decrease in the muscle bioactivity or just a trend for a decrease after the maximum inspiration/expiration maneuver, and the potentiation of the activity decrease on the affected side seen in the PNF group was blurred. The outstanding example of differences in muscle activity with and without PNF stimulation was the PM muscle, whose activity strongly decreased after PNF treatment on the stroke affected side after inspiration/expiration maneuvers, but only showed a decreasing trend in the PNF-untreated group. Roh et al. (2013) have shown that PM activity predominates in respiratory muscle synergies after stroke, although it may change differently on the affected and unaffected sides. Hwang et al. (2005) and Morris et al. (2004) have found an increase in PM activity on the affected side while performing a flexion movement of the contralateral limb, which may have to do with the role this muscle plays in stabilizing the shoulder girdle. PNF stimulation of respiratory muscles, such as that performed in the present study, is supposed to activate the entire chest, torso, and the shoulder girdle by providing muscle relaxation and reduce muscle spasticity. A reduction in PM and other accessory muscle activity, may thus underlie the PNF-related benefits in respiratory function. Likewise, we noticed a much greater reduction in SCM activity on the affected than unaffected body side. This muscle also was activated to a greater extent on both sides during deep breathing, which could be related to its role in taking a deep breath. Tomich et al. (2007) have reported a predominant increase in EMG activity of SCM during the exercise consisting of deep and slow inspiration using a device for controlled diaphragm-driven deep inspiration.
There are no studies in the literature, akin to the present one, concerning the EMG changes while applying PNF to the chest and abdominal muscles in this specific body position. Other studies that assess EMG of upper and lower limbs, for instance, during various dynamic and static muscle stretch are unsuitable for a meaningful comparison with the present results (Konrad et al. 2015; Patten et al. 2013; Reis Eda et al. 2013; Guissard and Duchateau 2004; Badics et al. 2002). Some authors put in doubt the use of electrophysiological methods for investigating muscle function due to the uncertainty of scientific verification of results (Hindle et al. 2012; Arya et al. 2011; Chan et al. 2006). Nonetheless, it is a general finding that cerebral stroke patients have a reduction in EMG activity of the respiratory muscles contralateral to the damage, which underlies disorders of motor control of synergistic work of respiratory muscles (Tomczak et al. 2008; Laghi and Tobin 2003; Khedr et al. 2000). Ratnovsky et al. (2008) have reported in stroke patients that accessory respiratory muscles, such as SCM, rectus abdominis, and the external intercostal muscles, are more vulnerable to fatigue than lower limb muscles. Further, Ezeugwu et al. (2013) have reported that stroke survivors suffer from excessive fatigue and dyspnea during physical exercise. Consequently, a decrease in respiratory capacity may underlie difficulties in daily functioning of patients (Britto et al. 2011).
A method to prevent a deterioration of respiratory function after stroke is the inspiratory muscles training. It involves training of the diaphragm and external intercostal muscles. The method consists of generating resistance to inspiration, which bears semblance to the PNF technique used in the present study. The literature shows that the inspiratory muscles training improves the muscle strength and endurance. Britto et al. (2011) have shown that the inspiratory muscles training significantly improves the maximum inspiratory pressure and also activities of daily living and quality of life in patients with stroke in stroke survivors.
The lack of the evaluation of maximum inspiratory and expiratory pressures, body side-related respiratory movements, and chest expansion by means of inductive plethysmography, and of the effects of a longer term PNF treatment is what makes limitations of this study. Nonetheless, we believe we have shown that PNF of the diaphragm and rib cage intercostal muscles clearly contributes to a reduction in bioelectric activity of accessory respiratory muscles of the paretic side in patients during the chronic stage of recovery after ischemic cerebral stroke. Reduced muscle activity may enhance their contractility, particularly during deep breathing, and thus may counteract disordered respiratory muscle-pump function. The findings of this study suggest the potential usefulness of PNF treatment directed at accessory respiratory muscles for improved lung ventilation and thus tissue oxygenation in stroke survivors.
References
Andrews AW, Bohannon RW (2000) Distribution of muscle strength impairments following stroke. Clin Rehabil 14(1):79–87
Arya KN, Pandian S, Verma R, Garg RK (2011) Movement therapy induced neural reorganization and motor recovery in stroke: a review. J Bodyw Mov Ther 15(4):528–537
Badics E, Wittmann A, Rupp M, Stabauer B, Zifko UA (2002) Systematic muscle building exercises in the rehabilitation of stroke patients. NeuroRehabilitation 17(3):211–214
Britto RR, Rezende NR, Marinho KC, Torres JL, Parreira VF, Teixeira-Salmela LF (2011) Inspiratory muscular training in chronic stroke survivors: a randomized controlled trial. Arch Phys Med Rehabil 92(2):184–190
Chan DY, Chan CC, Au DK (2006) Motor relearning programme for stroke patients: a randomized controlled trial. Clin Rehabil 20(3):191–200
Cohen E, Mier A, Heywood P, Murphy K, Boultbee J, Guz A (1994) Diaphragmatic movement in hemiplegic patients measured by ultrasonography. Thorax 49(9):890–895
CONSORT (2010) The CONSORT 2010 statement. https://www.consort-statement.org/consort-2010. Accessed on 1 Nov 2018
de Almeida IC, Clementino AC, Rocha EH, Brandão DC, Dornelas de Andrade A (2011) Effects of hemiplegy on pulmonary function and diaphragmatic dome displacement. Respir Physiol Neurobiol 178(2):196–201
Ezeugwu VE, Olaogun M, Mbada CE, Adedoyin R (2013) Comparative lung function performance of stroke survivors and age-matched and sex-matched controls. Physiother Res Int 18(4):212–219
Guissard N, Duchateau J (2004) Effect of static stretch training on neural and mechanical properties of the human plantar-flexor muscles. Muscle Nerve 29(2):248–255
Hindle KB, Whitcomb TJ, Briggs WO, Hong J (2012) Proprioceptive neuromuscular facilitation (PNF): its mechanisms and effects on range of motion and muscular function. J Hum Kinet 31:105–113
Houston JG, Fleet M, Cowan MD, McMillan NC (1995a) Comparison of ultrasound with fluoroscopy in the assessment of suspected hemidiaphragmatic movement abnormality. Clin Radiol 50(2):95–98
Houston JG, Morris AD, Grosset DG, Lees KR, McMillan N, Bone I (1995b) Ultrasonic evaluation of movement of the diaphragm after acute cerebral infarction. J Neurol Neurosurg Psychiatry 58(6):738–741
Howard RS, Rudd AG, Wolfe CD, Williams AJ (2001) Pathophysiological and clinical aspects of breathing after stroke. Postgrad Med J 77(913):700–702
Hwang IS, Tung LC, Yang JF, Chen YC, Yeh CY, Wang CH (2005) Electromyographic analyses of global synkinesis in the paretic upper limb after stroke. Phys Ther 85(8):755–765
Khedr EM, El Shinawy O, Khedr T, Aziz Ali YA, Awad EM (2000) Assessment of corticodiaphragmatic pathway and pulmonary function in acute ischemic stroke patients. Eur J Neurol 7(5):509–516
Konrad A, Gad M, Tilp M (2015) Effect of PNF stretching training on the properties of human muscle and tendon structures. Scand J Med Sci Sports 25(3):346–355
Laghi F, Tobin MJ (2003) Disorders of the respiratory muscles. Am J Respir Crit Care Med 168(1):10–48
Lanini B, Gigliotti F, Coli C, Bianchi R, Pizzi A, Romagnoli I, Grazzini M, Stendardi L, Scano G (2002) Dissociation between respiratory effort and dyspnoea in a subset of patients with stroke. Clin Sci (Lond) 103(5):467–473
Lanini B, Bianchi R, Romagnoli I, Coli C, Binazzi B, Gigliotti F, Pizzi A, Grippo A, Scano G (2003) Chest wall kinematics in patients with hemiplegia. Am J Respir Crit Care Med 168(1):109–113
Mahoney FI, Barthel D (1965) Functional evaluation: the Barthel index. Md State Med J 14:56–61
Merletti R (1999) Standards for Reporting EMG Data. J Electromyogr Kinesiol 9(1):III–IIV
Merletti R, Hermens H (2000) Introduction to the special issue on the SENIAM European concerted action. J Electromyogr Kinesiol 10(5):283–286
Merletti R, Botter A, Troiano A, Merlo E, Minetto MA (2009) Technology and instrumentation for detection and conditioning of the surface electromyographic signal: state of the art. Clin Biomech (Bristol, Avon) 24(2):122–134
Morris SL, Dodd KJ, Morris ME (2004) Outcomes of progressive resistance strength training following stroke: a systematic review. Clin Rehabil 18(1):27–39
Patten C, Condliffe EG, Dairaghi CA, Lum PS (2013) Concurrent neuromechanical and functional gains following upper-extremity power training post-stroke. J Neuroeng Rehabil 10:1
Ratnovsky A, Elad D, Halpern P (2008) Mechanics of respiratory muscles. Respir Physiol Neurobiol 163(1–3):82–89
Reis Eda F, Pereira GB, de Sousa NM, Tibana RA, Silva MF, Araujo M, Gomes I, Prestes J (2013) Acute effects of proprioceptive neuromuscular facilitation and static stretching on maximal voluntary contraction and muscle electromyographical activity in indoor soccer players. Clin Physiol Funct Imaging 33(6):418–422
Roh J, Rymer WZ, Perreault EJ, Yoo SB, Beer RF (2013) Alterations in upper limb muscle synergy structure in chronic stroke survivors. J Neurophysiol 109(3):768–781
Scillia P, Cappello M, De Troyer A (2004) Determinants of diaphragm motion in unilateral diaphragmatic paralysis. J Appl Physiol 96(1):96–100
Sezer N, Ordu NK, Sutbeyaz ST, Koseoglu BF (2004) Cardiopulmonary and metabolic responses to maximum exercise and aerobic capacity in hemiplegic patients. Funct Neurol 19(4):233–238
Similowski T, Catala M, Rancurel G, Derenne JP (1996) Impairment of central motor conduction to the diaphragm in stroke. Am J Respir Crit Care Med 154(2. Pt 1):436–441
Teixeira-Salmela LF, Parreira VF, Britto RR, Brant TC, Inácio EP, Alcântara TO, Carvalho IF (2005) Respiratory pressures and thoracoabdominal motion in community-dwelling chronic stroke survivors. Arch Phys Med Rehabil 86(10):1974–1978
Tomczak CR, Jelani A, Haennel RG, Haykowsky MJ, Welsh R, Manns PJ (2008) Cardiac reserve and pulmonary gas exchange kinetics in patients with stroke. Stroke 39(11):3102–3106
Tomich GM, França DC, Diório AC, Britto RR, Sampaio RF, Parreira VF (2007) Breathing pattern, thoracoabdominal motion and muscular activity during three breathing exercises. Braz J Med Biol Res 40(10):1409–1417
Voyvoda N, Yücel C, Karataş G, Oğuzülgen İ, Oktar S (2012) An evaluation of diaphragmatic movements in hemiplegic patients. Br J Radiol 85(1012):411–414
Acknowledgements
The study was registered with the international clinical trials platform of the Australian and New Zealand Clinical Trials Registry (ACTRN 12613001315707). Funded in part by grant ST.E060.16.059 of Wroclaw Medical University in Wroclaw, Poland.
Conflicts of Interest
The authors report no conflicts of interest in relation to this article.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Bioethics Committee of the Medical University in Wroclaw, Poland (permit no. KB867/2012).
Informed Consent
Written informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Slupska, L. et al. (2019). Proprioceptive Neuromuscular Facilitation for Accessory Respiratory Muscles Training in Patients After Ischemic Stroke. In: Pokorski, M. (eds) Advances in Pulmonary Medicine: Research and Innovations. Advances in Experimental Medicine and Biology(), vol 1160. Springer, Cham. https://doi.org/10.1007/5584_2018_325
Download citation
DOI: https://doi.org/10.1007/5584_2018_325
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-21098-4
Online ISBN: 978-3-030-21099-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)