Abstract
Mesenchymal stem cells (MSCs) were, due to their immunomodulatory and pro-angiogenic characteristics, extensively explored as new therapeutic agents in cell-based therapy of uveitis, glaucoma, retinal and ocular surface diseases.
Since it was recently revealed that exosomes play an important role in biological functions of MSCs, herewith we summarized current knowledge about the morphology, structure, phenotype and functional characteristics of MSC-derived exosomes emphasizing their therapeutic potential in the treatment of eye diseases.
MSC-derived exosomes were as efficient as transplanted MSCs in limiting the extent of eye injury and inflammation. Immediately after intravitreal injection, MSC-derived exosomes, due to nano-dimension, diffused rapidly throughout the retina and significantly attenuated retinal damage and inflammation. MSC-derived exosomes successfully delivered trophic and immunomodulatory factors to the inner retina and efficiently promoted survival and neuritogenesis of injured retinal ganglion cells. MSC-derived exosomes efficiently suppressed migration of inflammatory cells, attenuated detrimental Th1 and Th17 cell-driven immune response and ameliorated experimental autoimmune uveitis. MSC-derived exosomes were able to fuse with the lysosomes within corneal cells, enabling delivering of MSC-derived active β-glucuronidase and consequent catabolism of accumulated glycosaminoglycans, indicating their therapeutic potential in the treatment of Mucopolysaccharidosis VII (Sly Syndrome). Importantly, beneficent effects were noticed only in animals that received MSC-derived exosomes and were not seen after therapy with fibroblasts-derived exosomes confirming specific therapeutic potential of MSCs and their products in the treatment of eye diseases.
In conclusion, MSC-derived exosomes represent potentially new therapeutic agents in the therapy of degenerative and inflammatory ocular diseases.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Due to their capacity to produce trophic and immunosuppressive factors, mesenchymal stem cells [MSCs] were extensively explored as new therapeutic agents in cell-based therapy of uveitis, glaucoma, retinal and ocular surface diseases (Joe and Gregory-Evans 2010). Although obtained results were promising, safety issues regarding MSCs-based transplantation are still a matter of debate, especially in the long-term follow up. The primary concern is unwanted differentiation of the transplanted MSCs induced by cellular and cytokine milieu of local microenvironment in which MSCs were engrafted (Volarevic et al. 2018). It was recently reported that three women suffering from macular degeneration developed complications including vision loss, detached retinas, and bleeding resulting in total blindness in stem cell-treated eyes as a consequence of unwanted differentiation of transplanted stem cells (Kuriyan et al. 2017).
As far as we know to date, beneficial effects of MSCs in cell-based therapy of degenerative and autoimmune disease are largely due to the activity of MSC-derived trophic and immunosuppressive soluble factors (Volarevic et al. 2017). Plenty of evidence indicate that MSCs conditioned medium (MSC-CM) attenuate progression of immune mediated diseases and promote regeneration of ischemic tissues in almost completely the same way as transplanted MSCs indicating that paracrine mechanisms are mainly responsible for MSC-based therapeutic effects and that therapeutic use of MSC-derived products may overcome safety concerns regarding unwanted differentiation of transplanted MSCs (Volarevic et al. 2017, 2018).
Since it was recently revealed that exosomes play an important role in biological functions of MSCs and MSC-CM (Yu et al. 2014; Rani et al. 2015; Lai et al. 2015), herewith we summarized current knowledge about the morphology, structure, phenotype and functional characteristics of MSC-derived exosomes emphasizing their therapeutic potential in the treatment of eye diseases. An extensive literature review was carried out in April 2018 across several databases (MEDLINE, EMBASE, Google Scholar), from 1990 to present. Keywords used in the selection were: “mesenchymal stem cells”, “exosomes”, “eye”, “degenerative diseases”, “inflammatory diseases”, “regeneration”, “immunosuppression”. Studies that emphasized molecular and cellular mechanisms responsible for beneficent effects of MSC-derived exosomes in the therapy of eye diseases were analyzed in this review.
2 MSC Derived Exosomes: Morphology and Structure
MSC-derived exosomes are nano-sized extracellular vesicles that originate via the inward budding of the late endosome membranes called multivesicular bodies (MVBs). Upon the fusion of MVBs with the plasma membrane, MSC-derived exosomes are released into the extracellular milieu and can be either taken up by target cells residing in the microenvironment of engrafted MSCs or may be carried to distant sites via biological fluids where, in endocrine manner, modulate function of immune cells, endothelial cells (ECs), pericytes and other tissue-resident cells (Hyenne et al. 2015)
MSC derived exosomes have a narrow diameter range of 40–100 nm and a density of 1.13–1.19 gml−1 in sucrose solution. Their membranes are enriched in cholesterol sphingomyelin ceramide and lipid raft proteins (de Gassart et al. 2003) which enable protection of exosome’s cargo from degradative enzymes or chemicals and facilitates uptake of exosome’s content into target cells through endocytosis or membrane fusion regardless of biological barriers (Lai et al. 2011). MSC-derived exosomes express evolutionary conserved set of proteins including tetraspanins (CD81 CD63 CD9-involved in MSC proliferation and signaling) heat-shock proteins (HSP60 HSP70 HSP90-involved in MSC respond to stress) ALG-2-interacting protein X (Alix-apoptosis regulating protein) tumor susceptibility gene 101 (TSG101-having role in cell growth and proliferation) and adhesion molecules (CD29 CD44,CD73- enabling migration of exosomes to the inflamed and injured tissues) (Fig. 1). The incorporation of all these proteins in exosomes is thought to be controlled by lipid-dependent mechanisms primarily by the activity of the endosomal sorting complex required for transport (ESCRT) (Colombo et al. 2014).
MSC-derived exosomes: morphology and structure. MSC-derived exosomes are nano-sized extracellular vesicles which are released from MSCs into the extracellular milieu and taken up by target cells. Their membranes are enriched in cholesterol, sphingomyelin, ceramide and lipid raft proteins which enable protection of exosome’s cargo from degradative enzymes or chemicals and facilitates uptake of exosome’s content into target cells through endocytosis or membrane fusion, regardless of biological barriers. MSC-derived exosomes express evolutionary conserved set of proteins, including tetraspanins (CD81, CD63, CD9), heat-shock proteins (HSP60, HSP70, HSP90), and adhesion molecules (CD29, CD44,CD73) and carry nucleic acids, proteins (cytokines, chemokines) and lipids having important role in immunomodulation and tissue regeneration
Exosome content may vary according to the physiological and pathological conditions of the tissue microenvironment in which engrafted MSC is exposed. In this regard, the exosomal cargo can reveal the state of the donor MSC and can also influence in a paracrine and/or endocrine manner the fate of the recipient cell (Schey et al. 2015; Villarroya-Beltri et al. 2014). The reliability of intercellular communication between MSC and target cell is maintained and translated by specific components within the MSC-derived exosomes. These components are generally made of proteins, lipids, DNA fragments, mRNA and small RNA species (Villarroya-Beltri et al. 2014; Vlassov et al. 2012). The cargo is not randomly distributed into exosomes: strictly regulated mechanisms determine the “information” that will be distributed from MSC to the recipient cell by exosome (Villarroya-Beltri et al. 2014).
MSC derived exosomes carry nucleic acids proteins (cytokines chemokines) and lipids. In cargo of MSC-derived exosomes 4850 unique gene products and 4150 miRNAs have been detected and identified by mass spectrometry antibody array and microarray analysis (Lai et al. 2012; Chen et al. 2010). Furthermore, proteasome subunits were observed in MSC-derived exosomes (Carayon et al. 2011). It has been revealed that the 20S proteasome is responsible for degradation of intracellular oxidative damaged proteins which may partly contribute to the cardioprotective activity of MSC-derived exosomes (Lai et al. 2012). Through the activation of phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/Akt pathway MSC-derived exosomes increased levels of adenosine triphosphate (ATP) reduced oxidative stress attenuated myocardial ischemic injury and promoted myocardial viability and cardiac function (Lai et al. 2010; Li et al. 2013), indicating their potential therapeutic use in the treatment of myocardial ischemia (Arslan et al. 2013)
The presence of nucleic acids inside the exosomes had the crucial role in altering the fate of recipient cells. Within the nucleic acids spectrum, miRNA sequences become the most intensively investigated (Zaharie et al. 2015; Berindan-Neagoe and Calin 2014). Several miRNAs, detected in MSC-derived exosomes, including miR-191, miR-222, miR-21, miR-222, and miR-6087 were responsible for increased differentiation of ECs enabling modulation of angiogenesis (Merino-González et al. 2016). Similarly, through the activity of miR-494, MSC-derived exosomes accelerate muscle regeneration and promote myogenesis and angiogenesis (Nakamura et al. 2015).
MSC based modulation of vascular endothelial growth factor (VEGF)-driven angiogenesis is mediated by miR contained within MSC-derived exosomes (Merino-González et al. 2016; Nakamura et al. 2015). Lee and coworkers (Lee et al. 2013) revealed that MSC-derived exosomes enriched with miR-16 suppress tumor progression and angiogenesis via down-regulation of the VEGF expression in tumor cells. Opposite results were reported by Zhu and colleagues (Zhu et al. 2012) who showed that in vivo application of MSC-derived exosomes activated extracellular signal-regulated kinase1/2 (ERK1/2) pathway in tumor cells that resulted with the enhanced expression of VEGF, increased neo-angiogenesis and accelerated tumor growth.
Intravenous transplantation of MSC-derived exosomes improves neurogenesis, neurite remodeling and angiogenesis after ischemic brain injury (Xin et al. 2013). Therapy based on the delivery of MSC-derived exosomes promoted axonal growth and significantly increased the number of neuroblasts and ECs in ischemic and injured regions of central nervous system (CNS) (Xin et al. 2013). MSCs communicate with brain parenchymal cells and regulate neurites outgrowth by transferring miR-133b in neurons and astrocytes via exosomes (Xin et al. 2012) which could be a promising therapeutic strategy in the treatment of spinal cord injury.
3 Modulation of Immune Response and Inflammation in the Eye by MSC-Derived Exosomes
MSCs have capacity to synthesize and secrete a broad spectrum of exosomes, significantly more than other exosome producing cells of mesodermal origin (Yeo et al. 2013). MSC-derived exosomes are involved in important physiological and pathological processes such as disposal of unwanted proteins, genetic exchange, modulation of immune response and inflammation (Théry et al. 2009; Zöller 2009).
Immediately after engraftment, MSCs through the release of exosomes interact with multiple cell types to elicit appropriate cellular responses: affect the support of stromal cells enabling maintenance of dynamic and homeostatic tissue microenvironment (Lai et al. 2015) and modulate immune response by delivering immunosuppressive factors to the effector immune cells (Lai et al. 2010; Li et al. 2013; van Koppen et al. 2012; Zhang et al. 2014; Kordelas et al. 2014).
It was recently revealed that exosomes derived from amniotic fluid derived MSCs (AF-MSCs) contain immunosuppressive factors TGF-β and HGF. TGF-β suppresses activation of Jak-Stat signaling pathway in T cells, causing the G1 cell cycle arrest (Volarevic et al. 2017; Bright et al. 1997) while HGF acts synergistically with TGF-β enabling suppression of T cell proliferation and attenuation of T cell-mediated inflammation (Volarevic et al. 2017; Di Nicola et al. 2002). In line with these findings, when phytohemagglutinin-stimulated peripheral blood mononuclear cells [PB-MNCs] were cultured in the presence of TGF-β and HGF-containing AF-MSCs-derived exosomes, proliferation of PB-MNCs was notably reduced and their apoptosis was significantly enhanced (Balbi et al. 2017). Similarly, maturation and proliferation of B cells was reduced and their capacity for production of antibodies was suppressed, indicating strong immunosuppressive potential of AF-MSCs-derived exosomes (Balbi et al. 2017). Interestingly, among PB-MNCs, AF-MSC-derived exosomes did not attenuate proliferation of immunosuppressive CD4 + CD25 + FoxP3+ T regulatory cells, confirming significance of AF-MSC-derived exosomes as potentially new therapeutic agents in the therapy of inflammatory diseases.
In line with these findings, we recently developed immunomodulatory ophthalmic solution (“Derived Multiple Allogeneic Proteins Paracrine Signaling “D-MAPPS”) the activity of which is based on the activity of AF-MSC-derived exosomes, cytokines and growth factors capable to attenuate inflammation in the eye: IL-1 receptor antagonist [IL-1Ra], indoleamine 2,3-dioxygenase (IDO) and growth related oncogene (GRO). Based on our preliminary results, this product had beneficent effects in treatment of corneal injuries and dry eye syndrome (DED).
Corneal injuries are usually complicated with the influx of immune cells and consequently developed inflammation (Dana et al. 2000). During the early stage of corneal damage, injured epithelial cells secrete the inflammatory cytokine IL-1, which is stored in epithelial cells and released when the cell membrane is damaged by external insults (Yamada et al. 2003). IL-1Ra has an important anti-inflammatory role in corneal protection and regeneration. When IL-1Ra binds to the IL-1 receptor (IL-1R), interaction between inflammatory IL-1 and IL-1R is prevented. Accordingly, various pro-inflammatory events, initiated by IL-1:IL-1R interaction, including the synthesis and release of chemokines, and enhanced influx of neutrophils, macrophages, and lymphocytes in injured corneas are inhibited (Balbi et al. 2017). In line with these observations, our preliminary findings suggest that IL-1Ra containing AF-MSC-derived ophthalmic solution significantly attenuated inflammation in patients suffering from corneal injury.
Similarly, as for progression of corneal injury, inflammation has crucial role in the pathogenesis of DED, multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability (Gayton 2009). It is well known that Th17 cell-driven inflammation plays important role in the pathogenesis of DED (Théry et al. 2009). Inflammatory dendritic cells (DCs), in IL-1, IL-6, and IL-23 dependent manner induce differentiation of naïve T cells into Th17 cells which reduce tear production and promote progression of DED in IL-17 dependent manner (Gayton 2009; De Paiva et al. 2009). AF-MSCs, through the production of immunomodulatory GRO, attenuate maturation and antigen-presenting function of inflammatory DCs and suppress Th17 immune response. At the same time, AF-MSC-derived GRO promote generation of regulatory DCs capable to produce high levels of anti-inflammatory IL-10 (Merino-González et al. 2016; Nakamura et al. 2015; Yi and Song 2012) creating immunosuppressive microenvironment. Similarly, MSC-derived IDO acts as a critical molecular switch that simultaneously blocks re-programming of Tregs into inflammatory, IL-17 producing effector Th17 cells having important role in Treg-based immunosuppression of Th17 driven inflammation (Volarevic et al. 2017). In line with these observations, our preliminary results showed that AF-MSC-derived ophthalmic solution, which contains a high concentration of immunosuppressive GRO and IDO, significantly attenuated dryness, grittiness, scratchiness, soreness, irritation, burning, watering, and eye fatigue in patients suffering from DED, indicating therapeutic potential of AF-MSC-derived secretomes in the treatment of DED.
4 MSC Derived Exosomes in the Therapy of Retinal Injury
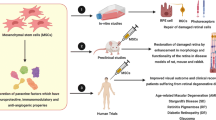
Damage of retinal cells caused by injury, infection or ischemia triggers degeneration in neighboring neural cells, resulting with the spread of morphological and functional retinal damage and irreversible visual impairment (Yoles and Schwartz 1998). Till now, there is no effective neuroprotection therapy currently available for retinal injury and, accordingly, transplantation of stem cells and their products have been extensively tested as new therapeutic approach for retinal regeneration. By using animal model of laser-induced retinal injury, Yu and coworkers recently demonstrated therapeutic potential of MSC-derived exosomes in attenuation of retinal damage and inflammation (Fig. 2) (Yu et al. 2016). One hour after their intravitreal injection, MSC-derived exosomes diffused rapidly throughout the neural retina, retinal pigment epithelium and gradually spread to the outer layers. Importantly, MSC-derived exosomes were as efficient as transplanted MSCs in limiting the extent of retinal damage. MSC-exosome-treated and MSC-treated eyes showed equivalent attenuation of laser-induced retinal injury with milder disorganization of the tissue, more residual photoreceptor cells, smaller retinal disordered areas, and reduced loss of nuclei in the outer nuclear layers compared with the eyes that were treated with vehicle only. Furthermore, application of MSC-derived exosomes significantly reduced infiltration of immune cells, particularly CD68+ macrophages and attenuate consequent apoptosis of retinal cells when compared to vehicle-treated group. MSC-derived exosomes managed to significantly alleviate expression of inflammatory mediators in the injured retinas involved in migration of monocytes in the eye: cytokine (TNF-α), chemokine (monocyte chemoattractant protein-1, MCP-1) and adhesion molecule (intercellular Adhesion Molecule 1, ICAM-1). Application of MCP-1 abolished effects of MSC-derived exosomes suggesting that they reduce retinal injury and inflammation mainly by targeting MCP-1-dependent migration of monocytes (Yu et al. 2016). In accordance to the attenuated retinal injury and inflammation, the significant improvement of dark- and light-adapted electroretinogram response in laser-injured mice treated with MSC-derived exosomes was observed, indicating functional recovery of retinal cells (Yu et al. 2016).
Mechanisms responsible for therapeutic effects of MSC-derived exosomes in the therapy of autoimmune uveitis and retinal injury. MSC-derived exosomes deliver immunomodulatory enabling suppression of detrimental immune response in autoimmune uveitis. By providing trophic support, MSC-derived exosome attenuate apoptosis of retinal cells and promote survival of retinal ganglion cells (RGCs) enabling regeneration of injured retinal tissue
In line with result obtained by Yu and colleagues (Yu et al. 2016) are findings recently reported by Mead and Tomarev (Mead and Tomarev 2017) who demonstrated therapeutic potential of bone marrow MSCs [BM-MSCs]-derived exosomes in the regeneration of injured retinal ganglion cells [RGCs]. RGCs are the sole projection neurons and their axons make up the optic nerve, making them susceptible to traumatic (optic nerve crush; ONC) and degenerative (glaucoma) diseases. Since RGC are CNS neurons, they are neither replaceable nor capable of axon regeneration and their loss or dysfunction results with irreversible blindness. BM-MSC-derived exosomes efficiently promoted survival and neuritogenesis of RGCs in vitro and in vivo, in ONC experimental model. In compared to untreated animals where, 3 weeks after ONC, more than 80% of RGCs are lost, cell death of RGCs was reduced to 30% in rats treated with BM-MSC-derived exosomes. Moreover, in BM-MSC exosome-treated retinas, over 50% of RGC function was maintained, suggesting that exosomes managed not only to protect RGC from death but also to preserve their function. Importantly, this was significantly higher neuroprotection of RGCs than those observed after transplantation of BM-MSCs. BM-MSCs lack the capacity to integrate into the retina and they remain in the vitreous after application. On contrary, within 1 hour after intavitreal injection, MSC-derived exosomes diffused rapidly and successfully delivered their cargo to the inner retina, including the RGCs where they elicited their therapeutic effects through miRNA dependent mechanisms (particularly through miR-17-92 and miR21 that regulate phosphatase and tensin homolog (PTEN) expression which is an important suppressor of RGC axonal growth and survival and through miR-146a that targets expression of epidermal growth factor receptor (EGFR) involved in inhibition of axon regeneration. Importantly, beneficial effects of MSC-derived exosomes in the treatment of retinal injury and in protection of RGCs were noticed only in animals that received MSC-derived exosomes and were not seen after therapy with fibroblasts-derived exosomes confirming specific therapeutic potential of MSCs and their products in retinal regeneration (Yu et al. 2016; Mead and Tomarev 2017).
5 Therapeutic Potential of MSC-Derived Exosomes in the Treatment of Autoimmune Uveitis
Autoimmune uveitis represents one of leading causes of visual disability. Since long-term use of immunosuppressive drugs and corticosteroids is limited due to the serious side effects and possible development of glaucoma and cataract, new therapeutic approaches for attenuation of autoimmune reaction in the eye are urgently needed. Most recently, Bai and colleagues demonstrated that MSC-derived exosomes efficiently attenuated experimental autoimmune uveitis (EAU), well established murine model of autoimmune uveitis (Bai et al. 2017), indicating their potential therapeutic use in the treatment of this disease (Fig. 2). Both clinical and histological analysis revealed that periocular injection of MSC-derived exosomes significantly ameliorated EAU, protect retinal structure and rescue retinal function in experimental rats. This was followed with notably reduced number of Gr-1+ granulocytes, CD161+ natural killer (NK) cells, CD68+ macrophages and CD4+ T cells in the inflamed retinas. Application of MSC-derived exosomes inhibited influx of leukocytes in the eye by suppressing effects of CCL2 and CCL21 chemokines which are involved in chemotaxis of inflammatory cells in the injured eyes. Interestingly, MSC-exosomes did not affect proliferation of activated T cells but managed to remarkably down-regulate presence of inflammatory, IFN-γ producing Th1 and IL-17 producing Th17 cells in the retinas, without affecting total number of immunosuppressive CD4 + CD25 + FoxP3+ T regulatory cells. Similar to these results are findings obtained by Shigemoto-Kuroda and colleagues (Shigemoto-Kuroda et al. 2017) who demonstrated that intravenous injection of MSC-derived exosomes, given immediately after immunization, prevented development of EAU in the same way as intravenously transplanted MSCs. Little structural damage of retinas with few inflammatory infiltrates were noticed in the eyes of EAU mice that received MSCs or MSC-derived exosomes while EAU mice that received vehicle showed severe disruption of the retinal photoreceptor layer accompanied with massive infiltration of inflammatory cells. Total number of retinal-infiltrating CD3+ T lymphocytes was significantly reduced both in MSCs and exosome-treated EAU mice when compare to vehicle-treated animals with EAU. In similar manner as it was observed by Bai and colleagues (Bai et al. 2017), MSC-derived exosome efficiently attenuated Th1 and Th17 immune response in the eye, without affecting total cell number, phenotype and function of immunosuppressive T regulatory cells (Shigemoto-Kuroda et al. 2017). The transcript levels of Th1 and Th17 related inflammatory cytokines (IFN-γ, IL-17A, IL-2, IL-1β, IL-6, and IL-12) and total number of eye-infiltrated Th1 and Th17 cells were significantly lower in the eyes of MSCs- and exosome-treated mice when compared with the vehicle-treated controls, while there was no significant difference in total number of T regulatory cells and immunosuppressive IL-10 (Shigemoto-Kuroda et al. 2017). Mixed lymphocyte reaction and flow cytometry analysis of DCs revealed that MSC-derived exosomes attenuated Th1 and Th17 immune response directly, by attenuating proliferation, effector function and cytokine production of CD4+ T lymphocytes and indirectly, by suppressing expression of costimulatory molecules (CD40, CD80 and CD86) and major histocompatibility complex (MHC) class II molecules on DCs, inhibiting their capacity for antigen presentation (Shigemoto-Kuroda et al. 2017). Results obtained by Bai and colleagues (Bai et al. 2017) and Shigemoto-Kuroda and coworkers strongly suggest that MSC-derived exosomes efficiently suppress migration of inflammatory cells in inflamed retinas, attenuate detrimental Th1 and Th17 cell-driven immune response and, accordingly, should be further explored as novel therapeutic agents for the treatment of human autoimmune uveitis, for which local non-corticosteroid therapy is urgently needed.
6 Effects of MSC-Derived Exosomes in the Treatment of Sly Syndrome
Mucopolysaccharidosis (MPS) is a group of seven related disorders caused by a mutation in one of the lysosomal exoglycosidases required for the sequential degradation of glycosaminoglycans (GAGs), resulting with lysosomal storage in several organs, including eyes. MPS VII, also known as Sly syndrome, manifested by corneal clouding, hepatomegaly, skeletal dysplasia, short stature, and delayed development is caused by a mutation of β-glucuronidase which leads to the accumulation of heparin sulfate, dermatan sulfate and chondroitin-4- and -6-sulfate. Current treatment for MPS VII is transplantation of bone marrow-derived stem cells or enzyme substitution therapy, but neither of these two therapeutic approaches are effective for ameliorating the corneal clouding due to corneal avascularity. Therefore, corneal clouding is ultimately treated by corneal transplantation (keratoplasty) which requires general anesthetic which is usually not possible in MPS VII patients suffering from respiratory dysfunction and/or severe cardiomyopathy. Accordingly, new therapeutic approaches are urgently needed for these patients. Most recently, Coulson-Thomas and colleagues proposed MSC-derived exosomes as potentially new agents for the treatment of MPS VII patients (Coulson-Thomas et al. 2013). Results obtained in this study suggest that β-glucuronidase-containing exosomes released from umbilical cord derived MSCs (UC-MSCs) are able to successfully enter into host corneal keratocytes and ECs of MPS VII mice (Coulson-Thomas et al. 2013). Furthermore, UC-MSC-derived exosomes managed to fuse with the lysosomes within recipient cells, enabling delivering of MSC-derived active β-glucuronidase and consequent catabolism of accumulated GAGs in MPS VII mice. These findings strongly support the hypothesis that UC-MSCs-derived exosomes have great potential for being successful in treating MPS VII and other human corneal congenital metabolic diseases.
7 Conclusion
Although transplantation of MSCs has enormous perspective in regenerative medicine, accumulating evidence indicates that treatment using MSC-derived exosomes have multiple advantages over MSC-based therapy. The risks of allogeneic immunological rejection, unwanted differentiation, and obstruction of small vessels by intravenously injected MSCs might be avoided by therapeutic application of MSC-derived exosomes that have similar effects and migration potential as MSCs. Additionally, exosomes, due to their nano-dimension, can easily pass through biological barriers and enter all target organs (Yu et al. 2016).
Nevertheless, there are still some challenges that need to be addressed in order to develop MSC-derived exosomes as an effective therapeutic agent in the treatment of eye diseases. Further studies are needed to optimize the injection frequency and dose to maintain the long-lasting effects of MSC-derived exosomes. Moreover, having in mind that exosomes are highly heterogeneous depending on the tissue origin of MSCs from which they were derived, pre-selection of the most effective tissue source of MSCs-derived exosomes is of crucial importance for their further successful use in ophthalmology. Finally, precise exosome-containing factors responsible for therapeutic effects of MSC-derived exosomes should be defined for each eye disease. In this way, defined therapeutic factor could be overexpressed in MSCs-derived exosomes prior to application in patients maximizing their therapeutic potential and efficacy.
References
Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP (2013) Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res 10:301–312
Bai L, Shao H, Wang H, Zhang Z, Su C, Dong L, Yu B, Chen X, Li X, Zhang X (2017) Effects of mesenchymal stem cell-derived exosomes on experimental autoimmune uveitis. Sci Rep 7:4323
Balbi C, Piccoli M, Barile L, Papait A, Armirotti A, Principi E, Reverberi D, Pascucci L, Becherini P, Varesio L, Mogni M, Coviello D, Bandiera T, Pozzobon M, Cancedda R, Bollini S (2017) First characterization of human amniotic fluid stem cell extracellular vesicles as a powerful paracrine tool endowed with regenerative potential. Stem Cells Transl Med 6:1340–1355
Berindan-Neagoe I, Calin GA (2014) Molecular pathways: microRNAs, cancer cells, and microenvironment. Clin Cancer Res 20:6247–6253
Bright JJ, Kerr LD, Sriram S (1997) TGF-beta inhibits IL-2-induced tyrosine phosphorylation and activation of Jak-1 and Stat 5 in T lymphocytes. J Immunol 159:175–183
Carayon K, Chaoui K, Ronzier E, Lazar I, Bertrand-Michel J, Roques V, Balor S, Terce F, Lopez A, Salomé L, Joly E (2011) Proteolipidic composition of exosomes changes during reticulocyte maturation. J Biol Chem 286:34426–34439
Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK (2010) Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res 38:215–224
Colombo M, Raposo G, Théry C (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30:255–289
Coulson-Thomas VJ, Caterson B, Kao WW (2013) Transplantation of human umbilical mesenchymal stem cells cures the corneal defects of mucopolysaccharidosis VII mice. Stem Cells 31:2116–2126
Dana MR, Qian Y, Hamrah P (2000) Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea 19:625–643
de Gassart A, Geminard C, Fevrier B, Raposo G, Vidal M (2003) Lipid raft-associated protein sorting in exosomes. Blood 102:4336–4344
De Paiva CS, Chotikavanich S, Pangelinan SB, Pitcher JD 3rd, Fang B, Zheng X, Ma P, Farley WJ, Siemasko KF, Niederkorn JY, Stern ME, Li DQ, Pflugfelder SC (2009) IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol 2:243–253
Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM (2002) Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99:3838–3843
Gayton JL (2009) Etiology, prevalence, and treatment of dry eye disease. Clin Ophthalmol 3:405–412
Hyenne V, Apaydin A, Rodriguez D, Spiegelhalter C, Hoff-Yoessle S, Diem M, Tak S, Lefebvre O, Schwab Y, Goetz JG, Labouesse M (2015) RAL-1 controls multivesicular body biogenesis and exosome secretion. J Cell Biol 211:27–37
Joe AW, Gregory-Evans K (2010) Mesenchymal stem cells and potential applications in treating ocular disease. Curr Eye Res 35:941–952
Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, Epple M, Horn PA, Beelen DW, Giebel B (2014) MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 28:970–973
Kuriyan AE, Albini TA, Townsend JH, Rodriguez M, Pandya HK, Leonard RE 2nd, Parrott MB, Rosenfeld PJ, Flynn HW Jr, Goldberg JL (2017) Vision loss after intravitreal injection of autologous “stem cells” for AMD. N Engl J Med 376:1047–1053
Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK (2010) Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 4:214–222
Lai RC, Chen TS, Lim SK (2011) Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med 6:481–492
Lai RC, Tan SS, Teh BJ, Sze SK, Arslan F, de Kleijn DP, Choo A, Lim SK (2012) Proteolytic potential of the MSC exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome. Int J Proteomics 2012:971907
Lai RC, Yeo RW, Lim SK (2015) Mesenchymal stem cell exosomes. Semin Cell Dev Biol 40:82–88
Lee JK, Park SR, Jung BK, Jeon YK, Lee YS, Kim MK, Kim YG, Jang JY, Kim CW (2013) Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One 8:e84256
Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, Wang M, Zhou Y, Zhu W, Li W, Xu W (2013) Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev 22:845–854
Mead B, Tomarev S (2017) Bone marrow-derived mesenchymal stem cells-derived exosomes promote survival of retinal ganglion cells through miRNA-dependent mechanisms. Stem Cells Transl Med 6:1273–1285
Merino-González C, Zuñiga FA, Escudero C, Ormazabal V, Reyes C, Nova-Lamperti E, Salomón C, Aguayo C (2016) Mesenchymal stem cell-derived extracellular vesicles promote angiogenesis: potencial clinical application. Front Physiol 7:24
Nakamura Y, Miyaki S, Ishitobi H, Matsuyama S, Nakasa T, Kamei N, Akimoto T, Higashi Y, Ochi M (2015) Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett 589:1257–1265
Rani S, Ryan AE, Griffin MD, Ritter T (2015) Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther 23:812–823
Schey KL, Luther JM, Rose KL (2015) Proteomics characterization of exosome cargo. Methods 87:75–82
Shigemoto-Kuroda T, Oh JY, Kim DK, Jeong HJ, Park SY, Lee HJ, Park JW, Kim TW, An SY, Prockop DJ, Lee RH (2017) MSC-derived extracellular vesicles attenuate immune responses in two autoimmune murine models: type 1 diabetes and Uveoretinitis. Stem Cell Rep 8:1214–1225
Théry C, Ostrowski M, Segura E (2009) Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 9:581–593
van Koppen A, Joles JA, van Balkom BW, Lim SK, de Kleijn D, Giles RH, Verhaar MC (2012) Human embryonic mesenchymal stem cell-derived conditioned medium rescues kidney function in rats with established chronic kidney disease. PLoS One 7:e38746
Villarroya-Beltri C, Baixauli F, Gutiérrez-Vázquez C, Sánchez-Madrid F, Mittelbrunn M (2014) Sorting it out: regulation of exosome loading. Semin Cancer Biol 28:3–13
Vlassov AV, Magdaleno S, Setterquist R, Conrad R (2012) Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta 1820:940–948
Volarevic V, Gazdic M, Simovic Markovic B, Jovicic N, Djonov V, Arsenijevic N (2017) Mesenchymal stem cell-derived factors: Immuno-modulatory effects and therapeutic potential. Biofactors 43:633–644
Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, Armstrong L, Djonov V, Lako M, Stojkovic M (2018) Ethical and safety issues of stem cell-based therapy. Int J Med Sci 15:36–45
Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M (2012) Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 30:1556–1564
Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M (2013) Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab 33:1711–1715
Yamada J, Dana MR, Sotozono C, Kinoshita S (2003) Local suppression of IL-1 by receptor antagonist in the rat model of corneal alkali injury. Exp Eye Res 76:161–167
Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, Lim SK (2013) Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev 65:336–341
Yi T, Song SU (2012) Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch Pharm Res 35:213–221
Yoles E, Schwartz M (1998) Degeneration of spared axons following partial white matter lesion: implications for optic nerve neuropathies. Exp Neurol 153:1–7
Yu B, Zhang X, Li X (2014) Exosomes derived from mesenchymal stem cells. Int J Mol Sci 15:4142–4157
Yu B, Shao H, Su C, Jiang Y, Chen X, Bai L, Zhang Y, Li Q, Zhang X, Li X (2016) Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Sci Rep 6:34562
Zaharie F, Muresan MS, Petrushev B, Berce C, Gafencu GA, Selicean S, Jurj A, Cojocneanu-Petric R, Lisencu CI, Pop LA, Pileczki V, Eniu D, Muresan MA, Zaharie R, Berindan-Neagoe I, Tomuleasa C, Irimie A (2015) Exosome-carried microRNA-375 inhibits cell progression and dissemination via Bcl-2 blocking in colon cancer. J Gastrointestin Liver Dis 24:435–443
Zhang B, Yin Y, Lai RC, Tan SS, Choo AB, Lim SK (2014) Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev 23:1233–1244
Zhu W, Huang L, Li Y, Zhang X, Gu J, Yan Y, Xu X, Wang M, Qian H, Xu W (2012) Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett 315:28–37
Zöller M (2009) Tetraspanins: push and pull in suppressing and promoting metastasis. Nat Rev Cancer 9:40–55
Acknowledgment
Study was supported by Swiss National Science Foundation project (SCOPES IZ73Z0_152454/1), Novartis foundation for medical-biological research (Grant No.16C197), Serbian Ministry of Science (ON175069, ON175103), and Faculty of Medical Sciences University of Kragujevac (JP02/09).
Conflict of Interest The authors declare that there is no conflict of interests regarding the publication of this paper.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Harrell, C.R. et al. (2018). Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes in the Treatment of Eye Diseases. In: Turksen, K. (eds) Cell Biology and Translational Medicine, Volume 2. Advances in Experimental Medicine and Biology(), vol 1089. Springer, Cham. https://doi.org/10.1007/5584_2018_219
Download citation
DOI: https://doi.org/10.1007/5584_2018_219
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-04169-4
Online ISBN: 978-3-030-04170-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)