Abstract
Glyphosate is the most used herbicide globally. It is a unique non-selective herbicide with a mode of action that is ideal for vegetation management in both agricultural and non-agricultural settings. Its use was more than doubled by the introduction of transgenic, glyphosate-resistant (GR) crops. All of its phytotoxic effects are the result of inhibition of only 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS), but inhibition of this single enzyme of the shikimate pathway results in multiple phytotoxicity effects, both upstream and downstream from EPSPS, including loss of plant defenses against pathogens. Degradation of glyphosate in plants and microbes is predominantly by a glyphosate oxidoreductase to produce aminomethylphosphonic acid and glyoxylate and to a lesser extent by a C-P lyase to produce sarcosine and phosphate. Its effects on non-target plant species are generally less than that of many other herbicides, as it is not volatile and is generally sprayed in larger droplet sizes with a relatively low propensity to drift and is inactivated by tight binding to most soils. Some microbes, including fungal plant pathogens, have glyphosate-sensitive EPSPS. Thus, glyphosate can benefit GR crops by its activity on some plant pathogens. On the other hand, glyphosate can adversely affect some microbes that are beneficial to agriculture, such as Bradyrhizobium species, although GR crop yield data indicate that such an effect has been minor. Effects of glyphosate on microbes of agricultural soils are generally minor and transient, with other agricultural practices having much stronger effects.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
After commercialization in 1974, glyphosate (N-(phosphonomethyl)glycine; CAS # 1071-83-6) became the most used herbicide worldwide. According to SciFinder®, in 2020 there were over 23,000 scientific publications, including patents, on glyphosate since 1972. Numerous general reviews (e.g., Baylis 2000; Dill et al. 2010; Duke 1988, 2018a; Duke et al. 2003a) and two entire books (Grossbard and Atkinson 1985; Franz et al. 1997) on glyphosate are available. There have been two special issues of a journal on use of glyphosate as a herbicide (Pest Management Science, April, 2008 and May, 2018) and a special issue of Critical Reviews of Toxicology (supplemental issue of 2016) on glyphosate’s toxicological properties. Additionally, there are numerous reviews on specific aspects of glyphosate, such as its metabolic degradation in plants (e.g., Duke 2011), its degradation by microbes (e.g., Zhan et al. 2018), glyphosate extraction and analysis methods (Koskinen et al. 2016), its behavior in soil (Borggaard and Gimsing 2008), human exposure to glyphosate (Solomon 2020), and its environmental toxicology (Geisy et al. 2000). This review will not deal with formulation ingredients used with glyphosate, as these can vary between different products, and can vary with a particular product name between countries and over time. Unfortunately, many published studies are designed so that the effects of glyphosate cannot be differentiated from those of formulation ingredients. Furthermore, the exact ingredients of commercial glyphosate formulations are sometimes proprietary, making it impossible to evaluate some studies done with these products. The ecotoxicology of glyphosate and its formulants are covered by Rodríguez-Gil et al. (2020) in this volume.
The selection of topics covered by this review could be considered eclectic, but they were determined by what was not covered by the three other reviews on glyphosate of this volume. The review of Green and Siehl (2020) is on glyphosate-resistant (GR) crops, that of Rodríguez-Gil et al. (2020) covers the ecotoxicology of glyphosate, its formulants, and degradation products, and Baek et al. (2020) discuss evolved GR weeds. This review covers uses of glyphosate other than on GR crops, mode of action of glyphosate, metabolic degradation of glyphosate in microbes and plants, non-target vegetation effects and indirect effects of agricultural glyphosate use on non-target organisms, and effects of glyphosate on microbes in agriculture. A significant amount of this review is germane to the environmental toxicology of glyphosate, but I have tried to avoid those aspects covered by Rodríguez-Gil et al. (2020). This review emphasizes the more recent significant literature that has not been previously reviewed and will not discuss the burgeoning literature (often questionable toxicology studies) frequently found in predatory or very low impact journals. See Mesnage and Antoniou (2017) for an analysis of some of this questionable literature and its potentially harmful effects.
Glyphosate was an important herbicide when it was introduced, as there was no previous herbicide available that was effective on all weeds (non-selective) that was also considered to have low toxicity to animals, including humans. The only highly effective, non-selective herbicide alternatives at that time were paraquat (1,1’dimethyl-4,4′’-bipyridinium dichloride; CAS # 75365-73-0) and diquat (1,1′-ethylene--2,2′-bipyridinium dibromide; CAS # 85-00-7), two pyridinium herbicides, both with high acute toxicity to animals. In the USA, paraquat use is much greater than diquat use (United States Geological Survey 2020). Paraquat is so acutely toxic to humans that it has often been used to commit suicide (Onyon and Volans 1987). Furthermore, paraquat and diquat are perhaps the fastest acting herbicides, so there is insufficient time for them to be translocated from sprayed foliage to protected plant meristems before the tissues to which they are applied are killed. Thus, after treated foliage dies, paraquat-treated plants, especially perennials, often regrow from meristems that do not come in contact with the herbicide. Glyphosate is highly systemic, translocating both acropetally and basipetally to metabolic sinks like meristems from treated parts of the plant. In most weed species, glyphosate is metabolized slowly to non-phytotoxic or very weakly phytotoxic compounds (Duke 2011), giving the herbicide time to reach critical metabolic sinks without being metabolized. It is also one of the slowest acting herbicides on most plant species, giving the plant adequate time to translocate it to meristems before translocation is adversely affected by glyphosate. This combination of attributes made it more effective than other herbicides in killing weeds with the potential to regrow, being effective on many perennial weed species.
Glyphosate was significantly more expensive than paraquat, but more effective and much safer. Even before the introduction of GR crops, its use was considerably higher than that of paraquat in agriculture (Fig. 1). The rapid increase in glyphosate use after the introduction of GR crops in the USA (Fig. 1a) did not affect the patterns of paraquat use in agriculture (Fig. 1b), and the use of paraquat went up in cotton (Gossypium hirsutum) and soybean (Glycine max) production after evolved GR weeds became a major problem in these crops (Fig. 1b). Thus, before the introduction of GR crops, glyphosate captured a strong market for vegetation management in situations, other than in most horticultural and agronomic row crops while they are growing, as all crops were susceptible to glyphosate. Before GR crops were available, most herbicide use within growing crops was with highly selective herbicides that do not substantially harm the crop, even when sprayed directly on them; e.g., diclofop-methyl ((RS)-methyl-2-[4-(2,4-dichlorophenoxy)phenoxy]propanoate; CAS # 51338-27-3) on soybean.
Glyphosate (a) and paraquat (b) use in agriculture in the USA. GRC designates the introduction of GR crops. From the United States Geological Survey (2020)
Compared to other herbicides introduced since 1974, glyphosate is a high use rate herbicide, requiring 0.5 to 2.0 kg/ha of active ingredient for management of most weeds. Most more recent herbicides, except for bioherbicides, are applied at a few hundred grams or less per hectare. Glyphosate is an anionic compound that is sold as a formulated salt (usually with potassium or isopropylamine (IPA) cations), but the glyphosate anion is the only substantially herbicidal compound in the commercialized formulations. In solution, at physiological pHs, glyphosate exists mostly as a divalent anion (Wauchope 1976). Ions of Ca, Mn, and Zn in tank mixtures of glyphosate can reduce its efficacy (Chahal et al. 2010). A glyphosate product (sometimes called sulfosate) that used a cationic sulfur counterion (trimesium or trimethylsulfonium) was sold at one time, but it was reported to have greater acute human toxicity than a commercial formulation of the IPA salt of glyphosate (Sørensen and Gregersen 1999). The trimesium salt is no longer sold.
Glyphosate’s non-selectivity significantly limited its potential market, because it could not be sprayed directly on any growing crop like a selective herbicide. This changed dramatically in the USA with the introduction of transgenic, GR crops in 1996 (Duke 2014) (Fig. 1a). Similar increases in usage occurred in other countries that adopted GR crops, such as Argentina and Brazil. Agricultural use of glyphosate use plateaued in the USA in 2012 (Fig. 1a), probably due to both GR crop market saturation and farmers turning to other herbicides due to the rapid evolution and spread of GR weeds (Heap and Duke 2018). In 2016, about 56% of all glyphosate used globally was estimated to be used on GR crops, and 72% of all glyphosate used globally in its first 40 years of sales was used in the last 10 of those years (Benbrook 2016). The topic of GR crops and glyphosate use in them has been reviewed before (e.g., Duke 2014, 2015) and will be updated in this volume by Green and Siehl (2020). Other uses of glyphosate are briefly reviewed below.
2 Uses of Glyphosate Other Than in GR Crops
Glyphosate was a very successful herbicide for more than 20 years before the introduction of GR crops. Furthermore, it is still extensively used globally for other than weed management in GR crops. Gaines (2018) reviewed the topic of glyphosate use in non-GR crop settings in the USA. Wiese et al. (2018) and Antier et al. (2020a, b) provide good analyses of glyphosate use in Europe, where GR crops are essentially not grown. Even in Europe, glyphosate is the most used herbicide, comprising more about 33% of all herbicide use by volume. Figure 2 provides a breakdown of the many uses of glyphosate in agriculture in the European Union (EU). These EU uses are similar to the non-GR crop uses of glyphosate in agriculture throughout the rest of the world.
2.1 Weed Control in Non-Agricultural Situations
In 1995, before GR crops were introduced in the USA in 1996, 31% of the glyphosate used was for non-agricultural uses (Benbrook 2016). This percentage decreased to about 10% after GR crops were introduced in 1996, but the actual amount used for non-agricultural needs had more than doubled by 2014. The main non-selective alternatives for such uses are paraquat, with its toxicity issues discussed above, and glufosinate ((RS)-2-amino-4-(hydroxy(methyl)phosphonoyl)butanoic acid; CAS # 51276-47-2), which is less effective, less non-selective, and more expensive than glyphosate in most settings. Glufosinate was first commercialized in 1993, almost 20 years after glyphosate was introduced to the market. It is structurally similar to glyphosate, but has an entirely different molecular target site, glutamine synthetase (EC 6.3.1.2), involved in amino acid metabolism (Takano and Dayan 2020). Glyphosate is an ideal herbicide for total vegetation control in non-crop settings such as roadsides, railroad sidings, and preparation of land for installation of turf. It is used in turf to spot treat weeds (e.g., Burt 1980) or when the desired turf grass is dormant in the winter to kill winter weeds (e.g., Johnson 1976; Binkholder et al. 2011). Glyphosate is virtually inactive in soil and has a relatively short half-life (5.7 to 40.9 days) in moist soil in most climates (Blake and Pallett 2018). Thus, there are no long-lasting effects of these uses, other than indirect effects of killing the unwanted vegetation.
In the USA, glyphosate is used or has been proposed to be used to manage invasive weeds in non-agricultural settings such as Bromus tectorum (Sebastian et al. 2017), Typha spp. (Linz and Homan 2011), Oxalis pes-caprae (Lazzaro et al. 2019), and Chrysanthemoides monilifera ssp. rotundata (Matarczyk et al. 2002). Glyphosate has been recommended for management of invasive weed species such as Spartina densiflora that has become a problem in tidal marshes of southwest Spain (Mateos-Naranjo et al. 2009) and Bischofia javanica, an invasive tree species in the Ogasawara Islands (Itou et al. 2015). It is effective in control of invasive Mexican petunia (Ruellia simplex) in the state of Florida of the USA (Adams et al. 2014). These are but a few of the uses and proposed uses of glyphosate to manage invasive plant species in non-agricultural ecosystems.
Glyphosate is also used for aquatic weed management (Barrett 1985). There is at least one commercial formulation of glyphosate sold in the USA exclusively for management of aquatic weeds found growing on bodies of water or along shorelines. It is used for macrophyte aquatic weeds with foliage that is not submerged such as water hyacinth (Eichhonia crassipes) (e.g., Lopez 1993) and alligator weed (Alternanthera philoxeroides) (Bowmer et al. 1993). Many such targeted weed species are invasive, exotic weeds that are harmful to native aquatic vegetation. Glyphosate formulated for aquatic vegetation is sprayed on emergent aquatic vegetation, but it can also be wiped on (e.g., Kay 1995) in order to reduce water contamination. It is not used for submerged macrophytic vegetation such as Hydrilla verticillata (Dayan and Netherland 2005) or algae control, as the concentrations required would have to be very high, with potentially harmful environmental effects. On small, floating aquatic plants that have foliage exposed to the atmosphere like duckweed (Lemna minor), glyphosate is not effective in the water in which they grow, but it is very effective when sprayed on the foliage (Lockhart et al. 1989).
2.2 Weed Control in Non-GR Crops
Negatively charged glyphosate at soil pH ranges binds soil components (especially the clay fraction and Fe and Al oxides) so tightly (Morillo et al. 2000; Borggaard and Gimsing 2008) that it has no herbicidal effects in most soils. Therefore, it is commonly used in non-GR row crops for weed control before planting. A study meant to simulate effects of the potential accumulation of glyphosate and its main degradation compound, aminomethylphosphonic acid (AMPA; CAS # 1066-51-9) in soil when used at very high rates over multiple years on the growth and development of wheat (Triticum aestivum), field peas (Pisum sativum), and canola (Brassica napus), found no effects at recommended application rates (0.5 to 2.0 kg a.e./ha) (Blackshaw and Harker 2016). They found that application rates of 17.6 to 77 kg a.e./ha would be required to add enough glyphosate to soil to cause any crop injury, depending on the crop and location. The experiment assumed that glyphosate would be retained in the top 2 cm of soil. If glyphosate was distributed throughout a deeper soil profile because of tillage or high rainfall, the application rates required to cause crop injury would be even higher. Another proof of its safety to plants in soil is that dormant turf grasses can be sprayed with glyphosate in the winter to control winter weeds without damage to the dormant grass that regrows in the spring from subterranean meristems.
However, there are a few reports of glyphosate causing crop injury by uptake from sandy soils, especially when phosphate fertilizers are used (e.g., Cornish 1992). Phosphate can displace glyphosate from its soil binding sites in some cases (Gimsing and Borggaard 2001). In sandy loam soil, glyphosate application to weeds, followed by planting of wheat immediately or 1 day after spraying the weeds sometimes reduced wheat growth (Jang et al. 2020). However, in clay loam soil, growth of wheat was sometimes increased by such treatments, perhaps because of glyphosate hormesis (see Sect. 2.5). These effects were influenced by weed densities, target weed species, and soil water conditions. Glyphosate is less commonly used to kill weeds in fields of crops (both GR and non-GR) after harvest. Despite being non-selective, glyphosate is widely used in non-GR crop agriculture, as evidenced by its heavy use in Europe, where GR crops are not grown (e.g., Weise et al. 2018. Antier et al. 2020a, b) and in the USA in non-GR crop settings (Gaines 2018). In the USA in 2014, ca. 12% of the glyphosate use in agriculture was in non-GR crops (Benbrook 2016). The analysis by Gaines (2018) of glyphosate, glufosinate, and paraquat use in various non-GR USA crops showed that glyphosate use predominated, except for peanuts (Arachis hypogaea), in which case paraquat use (percent of hectares treated) in 2013 was slightly higher than glyphosate use.
Glyphosate can be safely used in orchards and vineyards to control weeds when crop foliage is high enough to avoid significant spray reaching leaves from directed applications to lower-growing weeds among these crops. The distance between orchard and vineyard crop plants also assists in avoiding contact of the crop foliage with spray. Glyphosate was predicted to end problems with perennial weeds in tree and vine crops soon after it was introduced (Lange et al. 1975). If used properly in vineyards and other perennial, woody crops, there is no crop damage. However, used improperly, drift of glyphosate to foliage can cause crop injury (e.g., Mohseni-Moghadam et al. 2016; Schrübbers et al. 2014). Gaines (2018) reported that in the USA in 2017, glyphosate was used for weed management in 35 to 42% of such crops. Glyphosate has been used so much in some vineyards, that its use has been associated with contamination of nearby surface waters with the herbicide (Daouk et al. 2013). Another evidence of the intensive use of glyphosate in orchard crops is that one of the first cases of evolved resistance of a weed (Eleusine indica) to glyphosate was in a fruit orchard in Malaysia (Lee and Ngim 2000). Plants do not evolve resistance to glyphosate easily, as with some herbicides (e.g., the sulfonylureas), as it required more than 20 years for the first report of revolved resistance (Baek et al. 2020), despite its widespread use and resulting strong selection pressure. Glyphosate has been used extensively in conifer silvaculture (Freedman 1990), mostly in the early stages of establishment of the conifer crop. It has also been used to destroy illicit crops, including Erythroxylum coca (Solomon et al. 2007; Marshall et al. 2009), marijuana (Cannabis sativa) (Lanaro et al. 2015), and opium poppy (Papaver somniferum) (Solomon et al. 2007). Glyphosate does not have to kill the Erythroxylum coca plant in order to lower the cocaine levels in leaves to uneconomical concentrations (Casale and Lydon 2007).
Another common use of glyphosate is to kill cover crops that are used to prevent soil loss and for suppression of weeds between crops in no-tillage agriculture (e.g., Reddy and Koger 2004; Nascente et al. 2013). The most environmentally damaging weed management option is tillage, as it facilitates erosion of top soil which can take eons to replace. Reduced tillage and plant residue management provide many environmental advantages (Locke and Bryson 1997). Tillage also results in loss of soil moisture (e.g., Blevins et al. 1971). Adoption of GR crops (soybean, maize (Zea mays), cotton, canola (Brassica napus) and sugar beet (Beta vulgaris)) allowed farmers to greatly reduce tillage in these crops (Cerdeira and Duke 2006; Duke and Powles 2009; Givens et al. 2009; Morishita 2018). Use of reduced tillage and cover crops with GR crops can reduce soil erosion, moisture loss, and movement of pre-emergence herbicides from the field (Krutz et al. 2009). Even in non-GR crops, glyphosate use has reduced tillage for weed management both directly (e.g., Melander et al. 2013; Kudsk and Mathiasson 2020) and for facilitation of the use of cover crops that reduce soil erosion (e.g., Weston 1990). Glyphosate is also used extensively in wheat crops before planting and after harvesting to facilitate reduced and no-tillage agriculture (Gaines 2018). Similar practices have been used with glyphosate to facilitate reduced and no-tillage agriculture in Europe, where GR crops are not grown (Wiese et al. 2018; Antier et al. 2020a, b). Furthermore, tillage is a fossil fuel-intensive procedure. Largely due to the reduction of tillage, the use of GR crops in 2016 reduced worldwide fossil fuel use by the equivalent of removing 1.8 million family automobiles from the road for 1 year (Brookes and Barfoot 2018). This figure is only for 1 year and does not include the fuel savings by the reduction of tillage facilitated by glyphosate in non-GR crops.
Some effort has been made to use glyphosate in glyphosate-sensitive row crops by using devices to wipe glyphosate on weeds that are taller than the crop (McWhorter and Derting 1985; Derting 1987; Harrington and Ghanizadeh 2017) and by using shielded or hooded sprayers between rows (e.g., Westerman and Murray 1994). Such methods greatly reduce the amount of herbicide needed per unit area. These approaches have been used with tractor-mounted booms over several crop rows and with hand-held devices for spot treatments. Even with these devices to reduce contact of the crop by glyphosate, crop injury is common. Contact with even one leaf of a plant can cause significant injury or plant death because of glyphosate’s ability to translocate well (see Sect. 3.2) once it enters the plant. Although these application technologies were largely developed in the USA, this type of glyphosate application in the USA became rare after the introduction for GR crops. However, methods are being developed to apply herbicides with robotic systems that can differentiate between crops and weeds, applying the herbicide only to the weeds (e.g., Rajaa et al. 2020). Because glyphosate is non-selective, it is ideal for this technology, as the robot would only have to determine if the detected plant is the crop or not. Such technology used with glyphosate would change it from a high use rate herbicide to a very low use rate herbicide.
2.3 Use as a Crop Harvest Aid
After the harvested portion of annual crops are mature, there is an advantage to killing the crop and letting it desiccate so that it can be harvested efficiently with mechanical equipment. Living, green shoots of crops can interfere with harvesting equipment. Also, waiting for the annual crop to die naturally and desiccate so that it can be harvested can delay harvesting until times of the year that are too wet for harvesting (e.g., cotton in the southeast USA). Several herbicides have been used as crop harvest aids to rapidly kill the crop, and glyphosate has become the most commonly used herbicide for this purpose (Griffin et al. 2010). An additional benefit of this practice is that seed-producing weeds that are in the field at the time of application are killed, preventing them from contributing viable seeds to the weed seed bank for future cropping seasons. For example, late season application of glyphosate after seed set of the crop reduced seed production of the weeds Sesbania herbacea and Senna obtusifolia by 85%, and the S. herbacea seeds produced had only 6% viability (Clay and Griffin 2000).
Glyphosate-based herbicides are recommended to be used as a harvest aid at least a week before harvest during the ripe stage of physiological seed maturity. When so used, some shikimic acid ((3R,4S,5R)-(−)-3,4,5-trihydroxy-1-cyclohexenecarboxylic acid; CAS 138-59-0) can accumulate in the grain (see Sect. 3), indicating that some glyphosate translocates to the grain, but no impact on amino acid composition or gluten protein composition is seen, unless glyphosate is applied too early (Malalgoda et al. 2020). Glyphosate applied too early as a harvest aid can result in translocation of enough glyphosate to developing seeds to cause developmental problems. If this occurs, the germination vigor of some or all of these seeds may be compromised (e.g., Jeffery et al. 1981; Whigham and Stoller 1979), and residues of glyphosate and AMPA in the harvested food product will be increased (e.g., Cessna et al. 2002). However, when properly used as a harvest aid in wheat, most of the glyphosate ends up in the straw, with very little in the seed, and relatively little AMPA, the main metabolite of glyphosate, is found (Cessna et al. 1994). Even if there is no translocation, glyphosate residues, but not AMPA, can contaminate harvested food products from use of glyphosate as a harvest aid.
Reports of a few ppm of glyphosate contamination of cereal grain-based foods (e.g., Harris and Gaston 2004) such as beer (e.g., Jansons et al. 2018) and grain-based breakfast foods (e.g., Zoller et al. 2018) are almost certainly due to contamination from use as a harvest aid. How much of the glyphosate is due to translocation to the seed vs contamination from sprayed surfaces is unknown. Residues of glyphosate in these food products are generally below what is permitted by regulatory agencies and are thus not considered to be a health concern by these agencies. In a recent review of the topic, Xu et al. (2019) found that the reported glyphosate levels in grains and other foods were below the residue limits of all regulatory authorities listed in the paper. For example, the maximum residue levels (MRL – called tolerances by the USEPA) for glyphosate in wheat are 30 ppm in the USA and for FAO/WHO, 10 ppm in the EU, and 5 pm in Canada (Xu et al. 2019). The highest level reported by Xu et al. (2019) was 11.1 ppm by Gélinas et al. (2018), but the sample from this study was not from the commercial food supply. This was far higher than most of the other reports that found most wheat-based foods to have glyphosate residues of less than 1 ppm. AMPA was found in some of the samples of the papers reviewed by Xu et al. (2019), indicating that translocated glyphosate was degraded in the grain or at some point in the food supply chain. Similar results were reported by Kolakowski et al. (2020) who found glyphosate residues in a wide range of foods in Canada, but the levels in 99.4% of the almost 8,000 samples tested were lower than Canadian MRLs. No glyphosate was found in dairy and meat samples, and the highest amounts tended to be in grain-derived foods, especially wheat products, likely to be due to glyphosate use as a harvest aid. A recent review by Solomon (2020) of glyphosate levels found in urine of the general public (e.g., in California from 1993–2016 that are assumed to be mostly from dietary exposure (Mills et al. 2017)), concluded that the exposure from this source poses a de minimis risk. The results of Mills et al. (2017) indicated an increasing exposure during the time period of the study (1993–2016), a time span when the use of glyphosate in agriculture in the USA grew rapidly until 2012 (Fig. 1a).
2.4 Use as a Sugarcane Ripener
Low application rates (0.16 to 0.47 kg a.i./ha) of glyphosate applied to sugarcane (Saccharum officinarum) at 8 weeks before harvest enhances the yield of sucrose (Dalley and Richard 2010; Dusky et al. 1986; Legendre and Finger 1987; Nguyen et al. 2019; Velini et al. 2010). Used in this way, glyphosate is called a ripener. These glyphosate rates are lower than those recommended to kill weeds and are sublethal to sugarcane at the growth stage at which it is treated, yet glyphosate use at these low application rates causes marked increases in shikimic acid (up to 12-fold increases, reaching concentrations of up to 120 ppm) (Carbonari et al. 2014; Viana et al. 2019; Pincelli-Souza et al. 2020), the best biomarker for glyphosate reaching its molecular target site as a herbicide (see Sect. 3). The sucrose yield increase resulting from glyphosate treatment can be more than 10%, depending on the cultivar, weather, treatment timing, application rate of glyphosate, and timing of harvest after treatment (Dalley and Richard 2010). In addition to increasing sucrose yield, low application rates of glyphosate can enhance other growth parameters, such as leaf area and internode numbers (Pincelli-Souza et al. 2020). The low glyphosate application rates used may be sufficient to reduce enough metabolic activity in metabolic sink tissues such as meristems and developing leaves, so that less sucrose is translocated to them. These low application rates, however, do not affect photosynthesis and transport of sucrose from mature leaves to stem internodes. Thus, sucrose accumulates to higher than normal levels in the harvested part of the plant. Some other herbicides with different modes of action (e.g., fluazifop-butyl; butyl-(R)-2-(4-{[trifluoromethyl)-2-pyridyl]oxy}phenoxy)propionate; CAS # 79241-46-6) cause similar effects, but they are not permitted for this use in the USA. GR sugarcane, as proposed by several groups (e.g., Wang et al. 2017), would render glyphosate ineffective as a sugarcane ripener.
Because glyphosate and sucrose translocate similarly (see Sect. 3.2), glyphosate contamination of sugars from glyphosate-treated sugarcane and GR sugar beet might be expected. However, Barker and Dayan (2019) found that, even with the high application rates of glyphosate for weed control in GR sugar beet (Morishita 2018), processing of the sugar reduced glyphosate levels to below the limit of detection in the refined, crystalline sugar. Similar results should be expected with refined sugarcane sugar, especially since the application rate of glyphosate used as a ripener is much less than that used for weed management in GR sugar beet. A recent study found ca. 1 ppm of glyphosate in a crude extract of juice of sugarcane which had been treated with glyphosate to enhance sugar yields in Vietnam (Nguyen et al. 2019). This level was stated to be below the MRL of 2 ppm allowed by the Vietnamese Ministry of Health.
2.5 Potential Use as a Plant Growth Regulator
Low application rates of glyphosate have been proposed to slow turf growth without unacceptable injury (e.g., Johnson 1990; Fry 1991; Dias et al. 2019). However, glyphosate is not used for this purpose, as the risk of injuring or killing the turf instead of stunting its growth is too great. Transgenic GR turf grasses have been developed (e.g., Blume et al. 2010; Wang and Brummer 2012), and glyphosate-tolerant fescue (Festuca arundinacea) has been developed through conventional breeding (Rose-Fricker 2002), although such products have not yet reached the commercial market. Low application rates of glyphosate (up to 0.7 kg/ha) can provide good weed control with some available fineleaf fescue varieties without damage to the turf (Askew et al. 2019). There is concern that glyphosate resistance genes could move from GR or glyphosate-tolerant turf grasses, creating major GR weeds in GR crops (Zapiola and Mallory-Smith 2012). As mentioned earlier, glyphosate can be used in winter to kill weeds without injury to dormant turf grass. Low application rates of glyphosate have been proposed as a plant growth regulator for tomato (Solanum lycopersicum) production (Pombo et al. 1985), but this use has not materialized. Later work showed that low application rates of glyphosate can enhance tomato plant photosynthetic rates and growth (Khan et al. 2020).
Hormesis is the stimulatory effect of a subtoxic dose of a toxin (Calabrese et al. 2007). Such an effect is not always beneficial. Very low, subtoxic application rates of herbicides often enhance plant growth (Belz and Duke 2014), but glyphosate is unique, in that its stimulatory effects are the strongest and most consistent among herbicides (Belz and Duke 2017; Brito et al. 2018). Application rates of glyphosate that are effective in stimulation of growth usually range from 1.8 to 32 g/ha (compared to the 500–2,000 g/ha used to kill most weeds) for glyphosate-susceptible plants. Hormetic application rates of glyphosate can increase growth, photosynthesis, seed production, and other developmental parameters. Increases in growth for herbaceous plants are generally 10 to 30% (e.g., Wagner et al. 2003) and sometimes greater (e.g., Sammons et al. 2018), whereas for some woody plants, such as Eucalyptus spp., the increase can be 50 to more than 100% increase over untreated plants, depending on the plant part measured (e.g., Velini et al. 2008) (Fig. 3).
Effects of different doses of glyphosate on Eucalyptus 60 days after spraying. From Velini et al. (2008) with permission
The physiological mechanism of glyphosate-caused hormesis is unknown, but the fact that hormesis is not seen in GR crops at glyphosate application rates that cause hormesis in non-GR crops (Velini et al. 2008) indicates that the effect is tied to the herbicidal mode of action of glyphosate. Sammons et al. (2018) found that glyphosate hormesis of GR Arabidopsis thaliana lines with one, two, or four copies of a transgene for GR 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS, EC 2.5.1.19), glyphosate’s molecular target, occurred at the same and higher glyphosate application rates than hormesis of susceptible plants. Application rates that were very toxic to the wild type were hormetic to the transformants, and the more resistant the transformant, the higher the maximum hormetic application rate. Thus, hormesis might be seen in GR crops at much higher glyphosate application rates than in non-GR crops, because the dose-response curves are shifted to higher application rates by a factor of about fifty (Nandula et al. 2007). Thus, it is possible that the weed-killing rates of glyphosate used on GR crops might sometimes stimulate their growth. I am unaware of any published studies designed to specifically test this hypothesis. However, in a multi-year study with GR maize, ear number, green ear mass, and kernel mass were increased by a recommended glyphosate rate (1.7 kg/ha) for weed management compared to maize kept weed-free without glyphosate use (Williams et al. 2015). Likewise, 1 and 3.33 kg a.e./ha of glyphosate stimulated early growth of GR canola in greenhouse studies in which the plants were not grown full term to harvest (Corrêa et al. 2016).
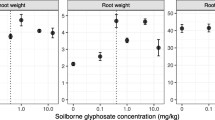
The hormetic effect of glyphosate has led some to propose that ultralow application rates of glyphosate could be used commercially to increase crop yield (e.g., Abbas et al. 2015, 2016). However, the stimulatory effects on growth are generally transitory and seldom lead to yield increases (Cedergreen 2008; Brito et al. 2018). Nevertheless, the hormetic effects (greater tiller numbers, culm length and dry mass, leaf dry mass, internode numbers, leaf area, and sugar yield) of a low glyphosate dose on sugarcane are sustained until harvest (Pincelli-Souza et al. 2020). For other crops, getting reproducible and predictable results in the field is difficult, as the hormetic dose range is affected by environmental conditions and plant developmental stage, as well as the time between application and harvest. For example, hormetic effects can be reduced by water stress in the weed Echinochloa colona (Mollaee et al. 2020). However, in safflower (Carthamus tinctorius), a drought-tolerant crop, a glyphosate application rate of 36 g a.e./ha caused hormesis under water stress (dos Santos et al. 2021). The makeup of the microbiome associated with the plant root can have a strong effect on glyphosate hormesis. Ramirez-Villacis et al. (2020) found the presence of a few root growth-inhibiting microbial strains (e.g., Firmicutes spp. and Burkholdia spp.) could eliminate the hormetic effect of glyphosate on A. thaliana. The presence of these soil microbiota could account for the fewer reports of glyphosate hormesis in the field than one would expect. However, the study of Ramirez-Villacis et al. (2020) was not done in soil and, thus, may not extrapolate to the field. Furthermore, in this study, glyphosate was applied to the roots in agar rather than as a foliar application, as it is used as a herbicide in the field.
As far as I know, glyphosate is not being used as a yield enhancer, except for sugarcane. The ripener effect of glyphosate on sugar yield of sugarcane is an atypical example of hormesis. Whether the stimulatory effects of glyphosate on growth of woody plants is a benefit of using the herbicide at the early stage of tree establishment is unclear. For example, the use of glyphosate for weed management in early cultivated Pinus taeda forest establishment results in larger tree seedlings (Pehl and Shelnutt 1990), and glyphosate use during the establishment of several tree species resulted in larger trees (Fu et al. 2008). Whether these effects are due to elimination of competition with other, more glyphosate-sensitive vegetation, to hormesis, or to both was not determined in these studies.
Glyphosate-associated hormesis has recently been proposed to facilitate evolution of GR weeds (Belz and Duke 2017; Brito et al. 2018). In the field, drift concentrations of glyphosate can stimulate the growth of glyphosate-susceptible weeds, such as Urochloa decumbens (de Moraes et al. 2020). Hormesis can be more pronounced in GR weeds, giving them a growth advantage in a competitive environment (Belz 2014). Furthermore, low application rates of glyphosate can be more advantageous to certain subpopulations of a single plant species than another, altering the makeup of the population (Belz and Sinkkonen 2019) in a way that favors survival of tolerant members of the population.
2.6 Glyphosate Effects on Non-Plant Pests
Phytotoxicity of glyphosate to non-target plant species outside of fields can influence ecosystems, especially if it changes the species composition of an ecosystem. For example, glyphosate could have a harmful effect on an animal species that depends on a plant species that is adversely impacted by glyphosate. This is likely if both species are native to a region in which glyphosate is heavily used. In some cases, glyphosate is used to influence unwanted non-plant species. For example, glyphosate management of invasive cattail (Typha spp.) has also had the benefits of reducing the sanctuary of cattail stands for blackbird (Icteridae) pests (Linz and Homan 2011). This program reduced blackbird damage to sunflower (Helianthus annuus) crops in North and South Dakota of the USA.
Glyphosate elimination of most weeds in agroecosystems should reduce the incidence of pests that use weeds as a food source and/or breeding habitat, but very little has been done to verify this. Elimination of all vegetation, other than the crop, in a GM crop field can also result in disruption of some pest biocontrol technologies, as vegetational diversity is needed for many biological control organisms as a source of habitat and nutritional resources (Lundgren et al. 2009). A few studies have correlated patterns of decline of certain arthropods with glyphosate-killed weeds (e.g., Haughton et al. 2001). There is much more literature on the direct effects of glyphosate (usually as a formulated product) on insects (e.g., Bernal and Dussán 2020) than on the much more severe and long-lasting effects of killing their food sources and habitat.
Desirable insects can be indirectly adversely affected by killing weeds on which they rely on or very near agricultural fields where glyphosate is used. For example, both the monarch butterfly (Danaus plexippus) and certain Asclepias species upon which this butterfly exclusively depends are found in the parts of North America where glyphosate is heavily used because of GR crop adoption. The decline of this butterfly has been largely attributed to glyphosate use by some (e.g., Pleasants and Oberhauser 2013; Thogmartin et al. 2017). However, an analysis by Boyle et al. (2019) reported that the beginning of the decline of the monarch butterfly predates the adoption of GR crops. Their analysis shows that the decline of both Asclepias species and the monarch butterfly in North America began at close to the same time, when there was a widespread shift to synthetic herbicide-based weed management in the middle of the twentieth century. The use of synthetic insecticides also increased dramatically at approximately the same time. With the widespread adoption of GR crops, there was no inflection in the decline plot of either the butterfly nor its host plant (Boyle et al. 2019). Hartzler (2010) found little effect of adoption of GR crops in Iowa (USA) on Asclepias syriaca, the main milkweed species host of the monarch butterfly outside of agricultural fields in this area, where insecticides are generally not used. However, in agricultural fields, where insecticides are often sprayed, A. syriaca populations were reduced after the introduction of GR crops. Asclepias spp. in fields where insecticides are used could be considered an attract and kill situation for the monarch butterfly. Thus, as long as insecticides are sprayed in crops, Asclepias spp. growing in such crops could be more of a risk than a benefit to the monarch butterfly. Therefore, glyphosate reducing the milkweed in GR crops, while having almost no effect on this plant species outside of fields where insecticides are not sprayed, might benefit the butterfly. Clearly, more study of the roles of these factors in the decline of the monarch butterfly is warranted. This example illustrates that cause and effect conclusions based on incomplete knowledge of all factors affecting an ecosystem or a species in it can be erroneous.
3 Mode of Action of Glyphosate
3.1 Effect of Glyphosate on 5-Enolpyruvylshikimate-3-Phosphate Synthase
The only molecular target site of glyphosate as a herbicide is EPSPS, an enzyme of the shikimate pathway that produces the three aromatic amino acids (phenylalanine (CAS 63-91-2), tyrosine (CAS 60-18-4), and tryptophan (CAS 73-22-3) required for protein synthesis and for production of compounds required for plant growth and development such as the plant hormone indole acetic-3-acid (IAA, CAS 87-51-4 and plastoquinone (PQ, CAS 4299-57-4) that is essential for photosynthesis and carotenoid synthesis (Fig. 4). Plants, fungi, and bacteria, but not animals, possess EPSPS (Kishore and Shah 1988; Dill et al. 2010). The only exceptions are most of the Apicomplexan parasitic parasites, such as those that cause malaria, which all contain a vestigial plastid, the apicoplast, which is considered to be the result of endosymbiosis of a red alga by a heterotopic, unicellular eukaryote (Arisue and Hashimoto 2015). Even though the apicoplast is not photosynthetic, it contains much of the biosynthetic capability of a plant plastid, including EPSPS that is sensitive to glyphosate (Roberts et al. 1998; McConkey et al. 2004). Glyphosate was once proposed as an antimalarial pharmaceutical with inhibition of EPSPS as its mode of action (Roberts et al. 2002). This has not occurred, but environmentally realistic exposure of mosquito larvae to glyphosate can reduce their infection with Plasmodium relictum, a prevalent avian malaria in Europe (Bataillard et al. 2020).
The shikimate pathway with some of its products. The bold arrow indicates the target of glyphosate (6). Numbered enzymes of the pathway are: (1) 3-deoxy-D-arabinoheptulosonate-7-phosphate synthase (DAHPS); (2) 3-dehydroquinate synthase (DQS); (3) 3-dehydroquinate dehydratase; (4) shikimate dehydrogenase; (5) shikimate kinase; (6) 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS); (7) chorismate synthase; (8) chorismate mutase; (9) prephenate amino transferase; (10) arogenate dehydratase; (11) arogenate dehydrogenase; (12) anthranilate synthase
The percent of the carbon in terrestrial plants that passes through the shikimate pathway is estimated to range from 20 to 50% (Tohge et al. 2013), varying largely with the amount of lignin synthesized. Therefore, blocking this pathway has profound effects on plant metabolism. There has been speculation about some of the toxic effects of glyphosate on plants being due to effects unrelated to the shikimate pathway, but the finding that transgenes encoding GR EPSPS render plants approximately 50-fold less sensitive to foliar-applied glyphosate (application rates for 50% growth reduction were 0.47 and 22.8 kg a.e./ha for sensitive and GR soybean, respectively) (Fig. 5) (Nandula et al. 2007) proves EPSPS to be the only herbicide target for glyphosate at the range of recommended application rates used for weed management (0.5–2.0 kg/ha). This supports the view that none of the molecular targets held in common between plants and animals are likely to be affected by the much lower concentrations of glyphosate to which animals are exposed than to which target plants are exposed. For example, some have claimed that because glyphosate can be an in vitro inhibitor of some P450 monooxygenase enzymes (e.g., Xiang et al. 2005), they could cause human toxicity by such a mechanism in gut microbes (e.g., Samsel and Seneff 2013). Because P450 monooxygenases are essential to plants, and GR crops are completely resistant to much higher concentrations (more than 10 kg a.e./ha) of glyphosate than to which they are exposed in the field, such enzymes are highly unlikely to be affected by glyphosate in vivo at recommended application rates for weed management (0.5 to 2.0 kg a.e./ha). Thus, the much lower concentrations of glyphosate to which animals are normally exposed (Solomon 2020), compared to those used for weed control, are highly unlikely to affect any P450 monooxygenases of gut microbes of animals, including humans, at concentrations found in the food supply.
Growth response of GR soybean (Agrow 460RR) and non-GR soybean (HBKC 5025) 3 weeks after treatment with glyphosate applications to 22-day-old (one- to two-trifoliolate leaf stage) seedlings. The I50 values for the GR and non-GR varieties were 22.8 and 0.47 kg a.e./ha, respectively. From Nandula et al. (2007) with permission
Early glyphosate mode of action research findings indicated that it inhibited synthesis of aromatic amino acids (Jaworski 1972). The main clue that led to the discovery of EPSPS as the target of glyphosate (Steinrücken and Amrhein 1980) was the large increase in shikimic acid seen soon after plant exposure to glyphosate (Amrhein et al. 1980). The shikimic acid concentrations in most plant species are exceedingly low and sometimes undetectable. For example, Lydon and Duke (1988) found 0, 0, 0, 5, and 31 nmoles/g dry weight in leaf tissues of Amaranthus retroflexus, Abutilon theophrasti, soybean, Lolium perenne, and Cyperus esculentus that had not been treated with glyphosate. Six days after treatment with 10 mM glyphosate, the concentrations were 65, 211, 120, 190, and 135 nmoles/g dry weight, respectively. This rapid, pronounced, and easily measured response to glyphosate is the best biomarker for glyphosate exposure or injury to almost all plants (Harring et al. 1998; Singh and Shaner 1998; Shaner et al. 2005). Even glyphosate exposures which cause no injury or even promote growth (see Sect. 2.5 for discussion of hormesis) can result in shikimate increases (Velini et al. 2008). Hydroxybenzoic acids, such as gallic (CAS 149-91-7), protocatechuic (CAS 99-50-3), and 4-hydroxybenzoic (CAS 99-96-7) acids can also accumulate in glyphosate-treated plants (Lydon and Duke 1988; Becerril et al. 1989) and glyphosate-sensitive microbes (Moorman et al. 1992), apparently derived from shikimate. For example, 6 days of treatment with 10 mM glyphosate caused increases in gallate, protocatechuate, and hydroxybenzoate in soybean leaves from 0.7, 5.9, and 2.3 nmoles/g dry weight to 3.9, 44.6, and 4.8 nmoles/g dry weight, respectively (Lydon and Duke 1988). These biomarkers for glyphosate exposure are not as pronounced as that of shikimate accumulation.
EPSPS is a nuclear-coded enzyme that is located in the plastid. All plant cells contain plastids (green chloroplasts in leaves and other green tissues, chromoplasts (plastids without chlorophyll, but with carotenoids), and leucoplasts (with neither carotenoids nor chlorophyll) such as amyloplasts and etioplasts in roots and other non-green tissues) that are involved in many aspects of plant metabolism other than photosynthesis. Like all other nuclear-coded, plastid enzymes, EPSPS is synthesized in the cytoplasm and enters the plastid by cleavage of a terminal transit peptide in the process of crossing the plastid envelope (della-Cioppa et al. 1986). Unlike other nuclear-coded plastid enzymes, EPSPS is catalytically active in the cytoplasm with its transit peptide (preEPSPS). Furthermore, EPSPS and preEPSPS bind glyphosate with the same affinity. Glyphosate-bound preEPSPS is not processed to EPSPS or taken up by plastids (della-Cioppa and Kishore 1988). Glyphosate has no direct effect on the import of other nuclear-coded enzymes into the plastid.
EPSPS transfers the enolpyruvyl moiety of phosphoenolpyruvate (PEP; CAS 138-08-9) to the 5-hydroxyl of shikimate-3-phosphate (S3P; CAS 63959-45-5) to produce 5-enolpyruvylshikimate-3-phosphate (EPSP; CAS 9068-73-9). The active catalytic site of the enzyme is highly conserved (CaJacob et al. 2003). Glyphosate forms a tight ternary complex with EPSPS and S3P and is competitive with respect to PEP, with a Ki of 1.1 μM, and is an uncompetitive inhibitor with respect to S3P (Boocock and Coggins 1983; Sammons et al. 1995). S3P must bind the enzyme first, followed by either PEP or glyphosate (Anderson et al. 1988; Boocock and Coggins 1983). However, the inhibition is reversible (Boocock and Coggins 1983; Steinrücken and Amrhein 1984). Binding of S3P ligand-free EPSPS causes a large conformational change in the enzyme (Fig. 6a), after which either PEP or glyphosate can bind (Fig. 6b) (Pollegioni et al. 2011). The EPSPS reaction occurs through a tetrahedral intermediate formed between S3P and the carbonation state of PEP, after which inorganic phosphate is released (Anderson and Johnson 1990a, b). The binding interactions of glyphosate and PEP to the same binding site are similar (Eschenburg et al. 2003). The complete enzyme kinetics for each step in the enzymatic production of EPSP from PEP and S3P are discussed in Anderson et al. (1988) and Anderson and Johnson (1990a). The 12 rate constants for EPSPS for the six steps of the EPSPS reaction are provided in Fig. 7. These constants were obtained by analysis of data from a large number of experiments with a computer simulation (modification of KINSIM). The overall equilibrium constant calculated by [EPSP][Pi]/[S3P][PEP] was calculated to be 180 (Anderson and Johnson 1990a).
Molecular binding of glyphosate to EPSPS (a) In its ligand-free state, EPSPS exists in the open conformation (left; Protein Data Bank (PDB): 1eps). Binding of S3P induces a large conformational change in the enzyme to the closed state, to which glyphosate or the substrate PEP bind (PDB: 1g6s). The respective crystal structures of the E. coli enzyme are shown, with the N-terminal globular domain colored pale green and the C-terminal domain colored brown. The helix containing Pro101 is colored magenta, and the S3P and glyphosate molecules are colored green and yellow, respectively. (b) Schematic representation of potential hydrogen-bonding and electrostatic interactions between glyphosate and active site residues including bridging water molecules in EPSPS from E. coli (PDB: 1g6s). Adapted from Pollegioni et al. (2011)
The six steps of the conversion of S3P and PEP to EPSP and inorganic phosphate by EPSPS (E). The tetrahedral intermediate is created by step 3. The equilibrium constants are from Anderson and Johnson (1990a)
One of the commercial advantages of glyphosate is that no other inhibitor of EPSPS has been found that is a good herbicide. This is unusual for herbicide target sites, as there are several commercial herbicides targeting most other herbicide targets (Herbicide Resistance Action Committee 2020). Considering the enormous commercial success of glyphosate, it is reasonable to assume that there has been considerable effort to find other herbicides that target EPSPS. Some of these discovery efforts have been published (e.g., Funke et al. 2007; Marzabadi et al. 1996), but none have resulted in a commercial herbicide. Good inhibitors of EPSPS such as N-amino-glyphosate (Knowles et al. 1993) have been found, but they have not been commercialized. Good in vitro activity on a molecular target of a herbicide is only one of many characteristics required for commercial viability.
3.2 Glyphosate Uptake and Translocation
To have its desired effects, glyphosate must be taken up by the plant and moved to the plastid (in both green and non-green cells), where EPSPS resides in plant cells. Caseley and Coupland (1985) and Duke (1988) reviewed the uptake and translocation of glyphosate decades ago, and little of significance has been added to the literature since then. Of 147 commercial herbicides used in postemergence applications, glyphosate is second to glufosinate as the most hydrophilic (Dayan 2018). Without the help of adjuvants in the solution to be sprayed, glyphosate is poorly taken up by plants compared to the uptake of most other foliar-applied herbicides. A problem with early formulations of glyphosate was that rain within a day or two after application would prevent enough glyphosate from being absorbed by foliage to act effectively as a herbicide. The most efficient formulation (the IPA salt of glyphosate with cationic surfactants, including polyethoxylated tallow amines) studied by Feng et al. (2000) on the common weed A. theophrasti resulted in about 15 and 30% of the glyphosate on the leaf being taken up by the plant within 6 and 24 h, respectively. Other less efficient commercial formulations of glyphosate took 24 h for 15% uptake. About 2 and 6% of the glyphosate applied to the foliage had been translocated to the root after 6 and 24 h, respectively, with the most efficient formulation, whereas about 1 and 3.5% was translocated to the root after 6 and 24 h, respectively, with the less efficient formulations. After simulated rainfall at 0.5, 1, and 2 h after application, growth inhibition was doubled by the use of the most efficient formulation over that of the others. The authors concluded that “rainfastness” of the formulation correlates more with the speed (% of glyphosate retained by the leaf after application that is taken up per unit time before a rainfall event) and the quantity of uptake by the foliage than with how much is retained by the leaf surface after a rainfall event. Considerable effort has been exerted in improving the earlier, less rainfast formulations of glyphosate. Unfortunately, some formulation ingredients have proven more toxic than glyphosate (Rodríguez-Gil et al. 2020).
The movement across the cuticle and cell wall is passive, with the rate of diffusion being dependent on many factors such as cuticle composition and thickness, temperature, concentration gradient, and formulation ingredients. With the help of formulation ingredients, sufficient glyphosate for herbicidal effect moves through the leaf cuticle and cell wall to reach the epidermal cell plasma membrane relatively rapidly (e.g., 6 h or less (Feng et al. 2000)). Glyphosate salts (K, Na, NH4, IPA, and trimethylsulfonium) move across the cuticle better than the free acid of glyphosate, moving in a first order process (Schönherr 2002). For example, at 90% relative humidity (RH), the time for 50% uptake of the free acid was 866 h, whereas that for the IPA salt was ca. 10 h. The time for 50% penetration of the cuticle increased with lower humidity, being ca. 10, 21, and 37 h for the IPA salt at 90, 80, and 70% RH, respectively. The tolerance (not evolved resistance) of some plant species is at least partly due to reduced glyphosate uptake due to low levels of movement from the leaf surface into the plant cells (absorption). For example, Norsworthy et al. (2001) found glyphosate-tolerant Ipomoea lacunosa to take up only about 5% of radiolabeled glyphosate in a 0.28 kg a.e./ha glyphosate application 48 h after application. In the same experiment, they found the uptake of glyphosate to be 15 to 62% in three more glyphosate-sensitive species.
With the help of effective formulation ingredients, glyphosate is more readily taken up from sprayed foliage. After traversing the non-living cuticle and cell wall, the herbicide must enter living cells of the leaf and the phloem by crossing the plasma membrane. Early work by Gougler and Geiger (1981) indicated that glyphosate crosses the plasma membrane by passive diffusion, with dependency on glyphosate concentration. After 3 h of exposure, they found a linear relationship between cellular uptake and external glyphosate concentration up to 10 mM with sugar beet leaf discs. However, a later study found uptake through the plasma membrane is first order with respect to extracellular glyphosate concentration, independent of pH and dependent on ATP (Ge et al. 2014). Also, glyphosate does not passively diffuse across semi-permeable membranes such as the plant plasma and vacuolar membranes (Takano et al. 2019). Evidence exists to support the view that phosphate transporters are involved in cellular uptake of glyphosate (Morin et al. 1997; Pereira et al. 2019).
One of the reasons that glyphosate is so effective is that it is a slow-acting herbicide, usually taking several days to kill a plant. It thus has time to be translocated to metabolic sinks such as young, developing leaves and meristems, to which it is translocated in hours (e.g., Gougler and Geiger 1981). Glyphosate moves in both the phloem (symplastic) and xylem (apoplastic) of plants, but its movement in phloem is much greater than xylem movement. Its phloem movement in plants is very much like that of sucrose, with a linear relationship between movement of radiolabeled sucrose and glyphosate from a treated leaf to other parts of a sugar beet plant (Gougler and Geiger 1981; Duke 1988). Gougler and Geiger (1981) found that glyphosate is taken up slowly and released slowly by plant cells, with a plasma membrane permeability of 1.7 × 10−10 m per second, allowing it to accumulate in and be transported by the phloem to plant tissues far from the tissues to which it is applied and taken up before exiting the phloem cell. In a later study from Geiger’s lab, glyphosate and CO2 assimilate accumulated similarly in rhizomes of the perennial weed Elytrigia repens (Shieh et al. 1993). McAllister and Haderlie (1985) also found phloem movement of glyphosate and photoassimilate to translocate similarly in Cirsium arvense, but they found glyphosate to translocate a little better to roots than photoassimilates. In an analysis of the phloem mobility of all herbicides, based on their pKa and log Kow values, in Chap. 5 of Devine et al. (1993), glyphosate ranks among the most phloem mobile. Phytotoxic effects on cells that take up glyphosate can limit its movement to phloem cells and translocation in hypersensitive plant species like sugar beet (Geiger and Bestman 1990), but its action in most species is so slow that translocation is initially very good, even at eventually lethal application rates. Some weeds have evolved glyphosate resistance mechanisms based on reduced translocation. This uncommon mechanism of evolved glyphosate resistance is dealt with in the chapter in this volume by Baek et al. (2020).
Vacuolar uptake of glyphosate competes with movement into the phloem and perhaps into the plastid (Ge et al. 2013). In some cases, enhanced vacuolar uptake of glyphosate results in reduced translocation and glyphosate resistance. Grown under similar conditions and treated with the same amount of glyphosate, the fraction of glyphosate that is found in the vacuole varies considerably between species (Ge et al. 2013). Those species with relatively high vacuole content were less sensitive to glyphosate, as vacuolar sequestration removes the herbicide from the translocatable pool, as well as from glyphosate in the plastid.
The shikimate pathway and EPSPS reside in the plant plastid stroma, where the pathway is required for cell maintenance, whether the cell is green or not. As mentioned earlier, glyphosate binds preEPSPS (della-Cioppa and Kishore 1988), so it does not necessarily have to be taken up by the plastid to kill the cell if the EPSPS is poisoned entering the plastid. The relative amount of binding of glyphosate to preEPSPS versus EPSPS in plant cells has not been determined. If glyphosate does enter the plastid, it is probably transported by either a phosphate or an amino acid transporter. Apparently, there is more than one type of glyphosate transporter, as overexpression of one associated with the tonoplast can cause glyphosate resistance, based on sequestration of glyphosate in the plant vacuole (reviewed by Sammons and Gaines 2014). A glutamate/aspartate transporter has recently been reported to also be a glyphosate transporter in the soil bacterium Bacillus subtilis (Wicke et al. 2019). The same transporter is also involved in glufosinate transport. Plants also have glutamate/aspartate transporters, but the glutamate transporter of the plastid transports the amino acid from the plastid to the cytosol (Renné et al. 2003), so it may not transport glyphosate into the plastid where EPSPS functions in the shikimate pathway. How much of the glyphosate taken up by the cell that enters the plastid and the mechanism of plastid uptake of glyphosate are still unknown.
3.3 How Inhibition of EPSPS Kills Plants
Only inhibition of EPSPS by glyphosate leads to the processes that eventually kill the plant. Thousands of papers have been published on secondary and tertiary biochemical and physiological effects of glyphosate on plants that provide little insight into its “mode of action.” In some cases, people mistake indirect effects for direct effects of glyphosate. For example, many papers describe elevated levels of reactive oxygen species (ROS) in response to glyphosate and insinuate that this effect is somehow unrelated to inhibition of EPSPS (e.g., Gomes et al. 2016). ROS generation is a general effect of stress in plants (Suzuki et al. 2012). Thus, elevation of ROS is a tertiary effect of all herbicides that is not directly related to the target site, except for herbicides that have more direct effects on photosynthesis (photosystem II inhibitors, such as atrazine (2-chloro-4-ethylamino-6-isopropylamino-1,3,5-triazine; CAS # 1912-24-9) and photosystem I energy diverters such as paraquat) (Dayan et al. 2019) and chlorophyll synthesis inhibitors that cause the photodynamic compound protoporphyrin IX (CAS 553-12-8) to accumulate (Dayan and Duke 2003). The latter are all protoporphyrinogen oxidase (PPO, EC 1.3.3.4) inhibitors, such as acifluorfen (5-[2-chloro-4-(trifluoromethyl)phenoxy]-2-nitrobenzoic acid; CAS # 50594-66-6).
Inhibition of EPSPS causes at least three linked effects that can contribute to phytotoxicity. See Fig. 4 for some of the compounds mentioned in this discussion. The most obvious is loss of aromatic amino acids and compounds derived from them, such as IAA and PQ that are essential for plant metabolism. Aromatic amino acids are required for protein synthesis. IAA, derived from tryptophan, is required for many aspects of plant growth and development. PQ, derived from tyrosine, is required for photosynthetic electron transport and is a co-factor phytoene desaturase (EC 1.3.99.31) (PDS) (Breitenbach et al. 2001), an enzyme required for synthesis of carotenoids. PQ is also required for proper functioning of PPO, which is required for chlorophyll synthesis (Brzezowski et al. 2019). At one time, the strong effect of glyphosate on chlorophyll accumulation in plants under some conditions led some to think that it was more than a secondary effect (Kitchen et al. 1981). Many phenylpropanoids (e.g., certain phytoalexins and all flavonoids) and lignin are derived from phenylalanine and tyrosine. Lignin accounts for a major fraction of the carbon passing through the shikimate pathway, especially in woody plants. Blocking production of only these latter products of the shikimate pathway might not kill a plant, at least not quickly enough to be considered as a herbicide. For example, blocking production of phenylalanine-derived secondary compound production in soybean by inhibiting phenylalanine ammonia-lyase (PAL) (EC 4.3.1.24) with the specific inhibitor L-α-aminooxy-β-phenylpropionic acid (AOPP; CAS # - 42990-62-5) does not cause herbicide-like effects (Duke et al. 1980), but, like glyphosate, it reduces production of compounds needed for pathogen resistance, thereby causing greater susceptibility to plant pathogens (e.g., Moerschbacher et al. 1990; Carver et al. 1992) (see Sect. 3.4). An interesting aspect of the paper by Duke et al. (1980) is that both glyphosate and AOPP induced high levels of extractable PAL activity, indicating the phenylalanine pools in glyphosate-treated plants are not only not replenished, but are also probably further reduced by enhanced in vivo PAL activity. The same phenomenon was found in maize treated with glyphosate (Duke and Hoagland 1978). A problem with loss of aromatic amino acids and essential compounds made from them as the only mechanism of action is that, even though glyphosate clearly causes depletion of free pools of aromatic amino acids, providing glyphosate-treated plants with supplementary aromatic amino acids does always not provide strong rescue of glyphosate-treated plant cells or tissues (e.g., Haderlie et al. 1977; Jenson 1985) or bacteria (Fischer et al. 1986). Other, more indirect effects of inhibition of EPSPS may contribute to the herbicidal effects of glyphosate.
There is evidence that prephenate (1-(2-carboxy-2-oxoethyl)-4-hydroxycyclohexa-2,5-dienecarboxylic acid; CAS # 126-49-8) and/or arogenate (1-[(2S)-2-azaniumyl-2-carboxylatoethyl]-4-hydroxycyclohexa-2,5-diene-1-carboxylate; CAS # 53078-86-7) may be feedback inhibitors of the shikimate pathway at the level of 3-deoxy-D-arabinoheptulosonate-7-phosphate synthase (EC 2.5.1.54) (DAHPS) (Fischer et al. 1986; Jenson 1985; Herrmann 1995). In a few plant species, one or more aromatic amino acids may act as a feedback inhibitor at the DAHPS level (Maeda and Dudareva 2012; Zulet-González et al. 2020). The relative gene expression of several enzymes of the shikimate pathway in Amaranthus palmeri leaf discs was elevated by exposure to glyphosate, and a mixture of the aromatic amino acids reduced this effect (Zulet-González et al. 2020). Arogenate and prephenate levels will still be depleted in glyphosate-treated plants cells provided exogenous aromatic amino acids, so deregulation of the shikimate pathway may not be completely corrected. Reduced products of the shikimate pathway will result in elevated DAHPS activity, subsequently causing consumption of erythrose-4-phosphate (E4P; CAS 585-18-2), PEP and ATP, to produce uncontrolled production of shikimate and other derivatives of intermediates of the shikimate pathway that occur before EPSPS, depleting carbon fixation pathways of key intermediates (e.g., PEP and erythrose-4-phosphate) and ATP. Glyphosate reduces carbon flow to the carotenoid pathway (Corniani et al. 2014), but part of this reduction could be due to reduced PDS activity because of reductions of PQ synthesis from tyrosine. If depletion of carbon fixation intermediates is sufficient, greatly reduced carbon fixation would be relatively rapid (<2 h), as seen in glyphosate-treated sugar beets (Geiger et al. 1986; Servaites et al. 1987). Cessation of carbon fixation in strong sunlight will result in energy dissipation through destructive oxidative processes. The symptoms of glyphosate toxicity in most species are not consistent with this mechanism. However, these symptoms are seen in sugar beets (Geiger and Bestman 1990; Madsen et al. 1986) and in GR Ambrosia trifida in which the effects are so rapid that the sprayed foliage dies very rapidly like that of glyphosate-sensitive sugar beet (van Horn et al. 2018; Moretti et al. 2018). In the case of GR A. trifida, the foliage dies before glyphosate can be translocated to meristems from which the plant regrows (similar to what is seen with paraquat treatment). In the few plant species like sugar beet and GR A. trifida, the drain of intermediates and ATP caused by deregulation of the shikimate pathway by glyphosate may be rapid, causing strong inhibition of carbon fixation, resulting in photosystem energy dissipation via ROS, a rapid process. This process probably occurs to a lesser degree in other plant species under certain environmental situations (e.g., strong sunlight).
The herbicide efficacy of glyphosate on some weeds species diminishes with increases in atmospheric CO2 concentrations (Ziska and Teasdale 1999, 2000; Ziska et al. 2004; Ziska and Goins 2006). The enzyme responsible for most carbon fixation by plants is ribulose-1,5-bisphosphate carboxylase (4.1.1.39) (RUBISO). RUBISCO is a very inefficient enzyme because of its low affinity for CO2 and the competition of CO2 and O2 for the same binding site. Photorespiration occurs when RUBISCO uses O2 instead of CO2, resulting in adding oxygen to ribulose-1,5-bisphosphate to produce 3-phosphoglycerate ((2R)-2-hydroxy-3-phosphonooxypropanoic acid; CAS 820-11-1)) (PGA) and 2-phosphoglycolate (2-phosphonatooxyacetate; CAS 13147-57-4) (2PG). 2PG inhibits some enzymes involved in carbon fixation. Thus, photorespiration not only wastes energy produced by the photosystems of photosynthesis, but also inhibits carbohydrate production from fixed CO2. Plants that rely on RUBISCO for carbon fixation are termed C3 plants because RUBISCO produces PGA, a three-carbon compound, by combining ribulose-1,5-bisphosphate and CO2. Elevated CO2 levels increase the enzymatic efficiency of RUBISCO, enhancing photosynthesis in C3 plants. Some plants, such as most grasses (Poaceae), have a more efficient means of carbon fixation, in which CO2 is first fixed by the enzyme PEP carboxylase (EC 4.1.1.31) to produce oxaloacetate (2-oxobutanoic acid; CAS 328-42-7), a four-carbon compound. Thus, these plants are termed C4 plants. With PEP carboxylase, CO2 does not compete significantly with O2, and CO2 levels are not as limiting for C4 plants as with C3 plants. The anatomy of the leaves of C4 plants is usually characterized by an inner ring of cells (bundle sheath cells) that fix carbon with RUBISCO, surrounded by mesophyll cells that fix carbon with PEP carboxylase. The mesophyll cells provide high concentrations of CO2 to bundle sheath cells, so that their RUBISCO is more efficient.
The reduction of glyphosate activity by elevated CO2 (up to 250 ppm above ambient 360 ppm) levels is more pronounced and consistent in C3 than in C4 plants (Ziska and Goins 2006; Fernando et al. 2016) (Table 1), as might be expected because C3 plants do not have a means of concentrating CO2 to enhance carbon fixation as C4 plants do. In fact, C4 plant growth is saturated at 360 ppm atmospheric CO2 (Leegood 2002), a level slightly lower than current atmospheric CO2 concentration (410 ppm), making C4 plants less likely to respond positively to CO2 above current levels. Glyphosate efficacy is compromised in a few C4 plants by elevated CO2 concentrations and not others (Table 1). In the case of Parthenium hysterophorus, different tissues and different developmental stages can be C3 or C4. Accordingly, researchers have reported elevated CO2 (600 to 800 ppm) to have both no effect (Bajwa et al. 2019) or a reduction (Cowie et al. 2020) on glyphosate efficacy on P. hysterophorus, but how much of the tissues were C3 and C4 in the two studies was not reported.
The clear decrease in glyphosate efficacy in C3 plants could be due to two causes. The additional growth of C3 plants at elevated CO2 concentrations will dilute a glyphosate concentration, reducing the amount per unit of fresh weight. Furthermore, the additional fixation of CO2 in C3 plants at high CO2 concentrations should reduce the effect of glyphosate in draining metabolic intermediates from carbon fixation pathways. Thus, the reduced effect of glyphosate on C3 plants at high CO2 concentrations supports the view that part of the mode of action of glyphosate is deregulation of the shikimate pathway to drain intermediates from metabolic pathways. These findings suggest that future glyphosate use will increasingly favor C3 weeds (e.g., Chenopodium album, A. theophrasti, and Convolvulus arvensis) as atmospheric CO2 levels increase.
A third part of the mode action of glyphosate may be accumulation of toxic derivatives of the shikimic acid pathway (Dayan and Duke 2020). Most plant species have very low levels of S3P (a substrate of EPSPS) or shikimate (the substrate of shikimate kinase (EC 2.7.1.71), the enzyme just before EPSPS) (Fig. 4), but treatment with glyphosate causes high levels of accumulation of shikimate and to a lesser extent hydroxybenzoic acids (e.g., protocatechuate) and quinate (CAS 77-95-2), another product of a shikimate pathway intermediate (3-dehydroquinate; CAS 10534-44-8) (Fig. 4). Quinate can also be generated from shikimate by quinate hydrolyase (EC 4.2.1.10) (Bentley 1990). Plants treated with acetolactate synthase (ALS; EC 2.2.1.6; also called acetohydroxy acid synthase – AHAS) inhibitor herbicides also accumulate high levels of quinate (Orcaray et al. 2010). The mechanism of this effect of ALS inhibitors is unknown.
The levels of shikimate that accumulate in response to glyphosate treatment generally dwarf those of quinate and hydroxybenzoates. Although no data on the phytotoxicity of shikimate could be found, there are reports that shikimate inhibits PEP carboxylase at high concentrations (I50 = 71 μM for leaf and ca. 5 mM for nodular PEP carboxylase) (Colombo et al. 1998; de María et al. 2006). There is an additive effect of shikimate and protocatechuate as PEP carboxylase inhibitors (de María et al. 2006), so that the combined concentrations of these inhibitors could be sufficient in some tissues of glyphosate-treated plants to significantly inhibit PEP carboxylase. As mentioned above, this enzyme is a key enzyme in carbon fixation in C4 plants. It also has an important role in C/N metabolism in C3 plants (Chollet et al. 1996). It is amazing that there has been no further research to determine whether shikimate itself is causing metabolic disruption through inhibition of PEP carboxylase. A more indirect contribution of toxicity by glyphosate-caused shikimate accumulation may be through shikimate-caused induction of genes of the shikimate pathway (Zulet-González et al. 2020), further deregulating the pathway to cause metabolic disruption.
Quinate is moderately phytotoxic, causing some of the effects of glyphosate (Orcaray et al. 2010; Zabalza et al. 2017, 2020; Zulet et al. 2013), and, as mentioned above, shikimate can be converted to quinate in vivo. Therefore, at least part of the effects of glyphosate in some plant species may be due to high levels of quinate. A non-phytotoxic application concentration (400 mM) of quinate applied with a mildly phytotoxic application rate (0.21 kg a.e./ha) of glyphosate-killed Amaranthus palmeri plants (Zulet-González et al. 2019). Treatment with quinate did not increase the shikimate levels in the plants over that caused by glyphosate alone. Neither glyphosate nor quinate alone caused increases in the extractable activity of the shikimate pathway enzyme anthranilate synthase (EC 4.1.3.27), but glyphosate with quinate caused a four-fold increase in the enzyme. In quinate-sensitive Papaver rhoeas, the mode of action of quinate as a herbicide appeared to be related to general perturbations in carbon/nitrogen metabolism, rather than to specific effects on the shikimate pathway (Zabalza et al. 2020).
Shikimate and quinate are both usually found at very low levels (undetectable to a few ppm of dry weight) in plant tissues of the majority of plant species, making their accumulation an excellent biomarker for glyphosate exposure. However, a few plant species accumulate high levels of shikimate (e.g., star anise (Illicium verum) and sweetgum (Liquidambar styraciflua)) (Enrich et al. 2008; Ghosh et al. 2012) and quinate (e.g., Cinchona officianalis) (Eliel and Ramirez 1997) without exposure to glyphosate. In order to avoid autotoxicity, these plants probably have a means of compartmentalizing these compounds away from cells involved in normal growth and development, as is commonly found with many other compounds that can cause autotoxicity to plants (reviewed by Duke et al. 1999). Interestingly, both shikimate and quinate can be starting compounds for synthesis of the anti-influenza pharmaceutical oseltamivir (ethyl (3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)-cyclohex-1-ene-1-carboxylate; CAS # – 196618-13-0) (Ghosh et al. 2012; Federspiel et al. 1999), and glyphosate-treated plants have been proposed as a source of these oseltamivir precursors (Matallo et al. 2014; Hobbie et al. 2017).
In summary, glyphosate probably adversely affects plants by more than just reducing levels of aromatic amino acids and necessary compounds derived from these three amino acids. The role of deregulation of the shikimate pathway with ensuing disruption of carbon assimilation and of phytotoxic quinate accumulation probably varies between species and within a species, depending on the developmental and environmental factors. Variations in the roles of these processes between different tissues and cell types in a plant are also likely. Thus, the mode of action of glyphosate is apparently more complex than might be expected. Nonetheless, all of the effects are ultimately due to the inhibition of EPSPS.
The fact that there are no commercial herbicides that target other enzymes of the shikimate pathway may reflect that other targets may not cause all of the metabolic dysfunctions caused by glyphosate, even though they block the shikimate pathway. For example, the natural cyanobacterial compound 7-deoxy-sedoheptulose, an inhibitor of 3-dehydroquinate synthase (DQS; EC 4.2.3.4), an early step of the shikimate pathway (Fig. 4), is phytotoxic and has been proposed as a herbicide (Brilisauer et al. 2019). Inhibition of this enzyme does not cause quinate or shikimate to accumulate; however, it does cause accumulation of the substrate of DQS, 3-deoxy-D-arabino-heptulosonate-7-phosphate (CAS # 2627-73-8). Thus, it should cause deregulation of the shikimate pathway.
Some have claimed that glyphosate causes either direct effects on plants due to its ability to chelate divalent metal cations, and they have claimed such an effect occurs when glyphosate is applied to GR crops (e.g., Yamada et al. 2009; Zobiole et al. 2010; Martinez et al. 2018; Mertins et al. 2018). This purported effect on plant mineral nutrition was proposed to be linked to greater plant disease in GR crops treated with glyphosate (e.g., Johal and Huber 2009; Kremer and Means 2009). Glyphosate does reduce the ability of non-GR plants to fight plant disease, but this phenomenon is related to reduced levels of defense compounds (see Sect. 3.4) and not to effects on mineral nutrition. The topic of mineral chelation in plants and its potential role in the mode of action of glyphosate was reviewed by Duke et al. (2012), who concluded that the data debunking this hypothesis are much stronger than those supporting it. Since this review was published, additional support has accumulated in support of the view that none of glyphosate’s mode of action is associated with effects on plant mineral nutrition (e.g., Costa et al. 2018; Duke et al. 2018b; Kandel et al. 2015; Reddy et al. 2018). These papers found no effects of glyphosate applications on mineral content of GR maize and GR soybean treated with recommended glyphosate application rates in replicated field experiments over more than 1 year at sites in different states of the USA, one Canadian province, and Brazil. The generally steady increase in yields of cotton, maize, and soybean USA, after more than 90% adoption of GR varieties, argues against there being any significant phytotoxicity issues with glyphosate in these crops. A recent short review summarized the state of the current knowledge of this topic (Duke and Reddy 2018).
There have been exceedingly few recent papers that meaningfully probe the mode of action of glyphosate, but there have been many papers describing secondary and tertiary effects. Modern metabolomic, transcriptomic, proteomic, and other methods (e.g., Maroli et al. 2016, 2018a, b; Patterson et al. 2020) provide tools for a better understanding of the more direct effects of inhibiting the shikimate pathway at the EPSPS site. However, interpretation of massive amounts of metabolomic or transcriptomic data to gain insight into a herbicide mode of action can be challenging (Duke et al. 2013, 2018a).
3.4 Role of Microbes in Glyphosate Efficacy
An important part of the mode of action of glyphosate in the field is the role of reduction of plant defenses to plant pathogens. A sublethal application of some, but not all, herbicides can predispose a plant to greater susceptibility of a herbicide (see review by Altman and Campbell (1977)), but this effect is more pronounced with glyphosate (Hammerschmidt 2018). Glyphosate is a more effective herbicide in soil containing microbes than in sterilized soil because of the reduction in plant defenses to soil-borne pathogens by glyphosate (Lévesque and Rahe 1992; Schafer et al. 2012, 2013) (Fig. 8). The reduction in shikimate pathway-derived plant defense compounds (e.g., phytotalexins and lignin) against plant pathogens by glyphosate has been used to enhance and synergize the efficacy of microbial bioherbicides (Christy et al. 1993; Duke et al. 2007; Hoagland et al. 2018; Gressel 2010). For example, Sharon et al. (1992) found a concentration of glyphosate (50 μM) that caused no visible phytotoxicity to the weed Cassia obtusifolia (now Senna obtusifolia) to almost completely block synthesis of the shikimic pathway-derived phytoalexin 2-(p-hydroxyphenyloxy)-5,7-dihydroxychromene. This glyphosate concentration, combined with a dose of the mycoherbicide derived from Alternaria cassia conidia that caused only a few necrotic spots on the foliage when used alone, completely killed the weed. This is but one of many examples of the lowering of plant defenses to pathogens by glyphosate. The topic of glyphosate’s effects on plant disease via inhibition of shikimate pathway-derived defenses is reviewed in detail by Hammerschmidt (2018) and Duke et al. (2018b).
Ambrosia trifida grown in sterile (a) and unsterile (b) soil sprayed with different rates (kg ae/ha) of glyphosate at 21 days after spraying. From Schafer et al. (2012) with permission
Although the contribution of soil pathogens to glyphosate efficacy has been clearly demonstrated under controlled conditions, little is known of this effect in the field. The magnitude of this augmentation to glyphosate efficacy by the activity by pathogens would be dependent on several factors, such as both the types and amounts of pathogens in the soil and environmental conditions. We know that glyphosate acts as a fungicide on some plant pathogens (see Sect. 6), so the balance between direct effects of glyphosate on the pathogen and indirect effects from reducing the plant’s capacity to produce pathogen defenses could be complicated, depending on many factors.
4 Metabolic Degradation of Glyphosate in Microbes and Plants
There are non-enzymatic means by which glyphosate can degrade by breakage of the C-P bond. For example, Barrett and McBride (2005) reported that both glyphosate and AMPA are degraded by breaking the C-P bond by Mn oxide in aqueous media. Glyphosate degraded faster than AMPA by this mechanism. Because AMPA has a longer half-life than glyphosate in soil (e.g., Simonsen et al. 2008), this mechanism could contribute to glyphosate metabolism in soil. Metal ions in solution have also been implicated in abiotic degradation of glyphosate to AMPA (Yael et al. 2014).
Biological degradation in nature is clearly the predominant mechanism of breakdown of glyphosate, because degradation in sterile soil is nil (e.g, Torstensson and Aamisepp 1977). Soil type can influence the rate of degradation (e.g., Qiao et al. 2020), but how much of this variation is due to differences in bioavailability and microbial differences has not be determined. There are two means of metabolic degradation of glyphosate (Borggaard and Gimsing 2008; Duke 2011; Nandula et al. 2019; Zhan et al. 2018). The predominant route is via a glyphosate oxidoreductase (GOX; EC 1.5.3.23) that converts glyphosate to AMPA and glyoxylate (CAS # 563-96-2) (see Fig. 3 of Green and Siehl 2020), a common, natural metabolite. AMPA is also a degradation product of some detergents, so some of the AMPA found in the environment is from this source (e.g., Botta et al. 2009). A gene (goxv247) that encodes GOX from the soil microbe Ochrobactrum anthropi was identified, cloned, and used as a glyphosate resistance transgene in the first commercialized GR canola cultivars (Green 2009). An apparently less common means of glyphosate metabolic degradation is by a C-P lyase that converts the herbicide to the sarcosine (CAS # 107-97-1), a natural product, and inorganic phosphate (e.g., Kishore and Jacob 1987; Jacob et al. 1988) (see Fig. 3 of Green and Siehl 2020). The reviews of Zhan et al. (2018) and Singh et al. (2020) provide similar lists of microbes that degrade glyphosate. Most of those listed degrade it with a GOX enzyme and most are bacteria, although some fungi, such as Penicillium citrinum (Zboińska et al. 1992), Alternaria sp., and Trichoderma spp. (Krzyśko-Łupicka and Orlik 1997) also degrade glyphosate. Sarcosine is seldom found or found in very small amounts in studies on the degradation of glyphosate in soils (e.g., Al-Rajab et al. 2008) and plants (Duke 2011). However, being a natural metabolite, it may have a shorter half-life than AMPA, which might mask the importance of this metabolic degradation route. Also, sarcosine if not always looked for in studies of the degradation of glyphosate (e.g., Arregui et al. 2003).
AMPA, which is more environmentally persistent than glyphosate, requires a C-P lyase enzyme to be degraded. Microbes that break down glyphosate with a C-P lyase can also metabolize AMPA, using both glyphosate and AMPA as a sole source of phosphorus (Selvapanidiyan and Bhatnagar 1994), although some of these microbes apparently have both a GOX and a C-P lyase (Lerbs et al. 1990; Obojska et al. 2002). The greater persistence of AMPA than glyphosate in soils may indicate that microbes that use this degradation pathway are less common than those with GOX. The biochemistry and genetics of C-P lyases that metabolize glyphosate and AMPA are reviewed by Hove-Jensen et al. (2014). The finding that glyphosate is readily broken down by many different microbes has led to numerous papers and patents on use of such microbes to remove glyphosate from soil (e.g., Ermakova et al. 2010) and water (e.g., Hallas et al. 1992). The need for such bioremediation is unlikely in normal use of glyphosate for weed management because of its relatively short half-life in soil and water (Blake and Pallett 2018; Rodríguez-Gil et al. 2020).
A number of publications exist on other microbial enzymes that will transform glyphosate to non-herbicidal compounds. These other glyphosate-degrading enzymes include glyphosate N-acetyltransferase (GAT) (Castle et al. 2004), a bacterial glycine oxidase (Nicolia et al. 2014), and a glyphosate decarboxylase (Hammer et al. 2007). There are no data indicating that any of these routes of degradation of glyphosate are significant in the environment. In the case of GAT, the enzymatic activity with glyphosate is so low for the enzyme obtained from Bacillus licheniformis that several rounds of gene shuffling with selection for the best enzymatic activity were needed to generate a gene encoding a GAT that could be used to produce a GR crop (Castle et al. 2004). Although most of the genes for these glyphosate-transforming enzymes were proposed for producing GR crops, only a gene for GOX was used in one GR crop (Green 2009), and it is no longer used (see discussion below). GR crops with the highly engineered GAT gene reached a high level of development (Green et al. 2008), but were never commercialized.
Glyphosate degradation in the environment can be enhanced by certain animals in soil and water. For example, glyphosate and AMPA degradation in soil containing earthworms (Eisenia fetida) is faster than the same soil without earthworms (Lescano et al. 2020). In this study, the earthworms were not harmed by glyphosate. Degradation of glyphosate in water is enhanced by the presence of the golden mussel (Limnoperna fortunei) (Gattás et al. 2020). However, there is no evidence in either of these papers that the animals themselves degrade glyphosate.
Plants also degrade glyphosate, predominantly by a GOX-type activity, although evidence of metabolism by a C-P lyase has been reported in a few species of higher plants (Duke 2011). The amount of GOX activity varies considerably, from little or no metabolism in some grasses to much higher levels in some legumes, including soybeans (Reddy et al. 2008; Duke 2011). Vemanna et al. (2017) showed that rice (Oryza spp.) has an aldo-keto reductase (AKR) that acts as a GOX and that, when used as an overexpressed transgene, can provide glyphosate resistance to tobacco (Nicotiana tabacum). There are many AKRs in plants with a wide spectrum of substrates, and some of these monomeric enzymes are associated with abiotic stress (Sengupta et al. 2015). Pan et al. (2019) reported that the evolved glyphosate resistance of a GR weed (E. colona) is due to elevated AKR activity due to two upregulated AKR genes. This is the most clearly confirmed case of evolved resistance to glyphosate by enhanced metabolic degradation (Duke 2019), although a few other cases have been reported (summarized by Baek et al. (2020)). However, this GR E. colona with enhanced AKR-mediated degradation of glyphosate was later shown to also have a GR EPSPS (McElroy and Hall 2020). The relative contributions of the two resistance mechanisms have not been determined. Whether AKR is the only enzyme responsible for GOX type of glyphosate metabolism to AMPA in plants is unknown. We also do not know if all plants have an AKR with GOX activity. In plants in which low levels of glyphosate metabolism occurs, finding AMPA in glyphosate-treated plants is difficult. In such plants, metabolism could be due in part or wholly to endophyte metabolism of the herbicide, as endophyte-mediated metabolism of other herbicides has been documented (Tétard-Jones and Edwards 2016), and some endophyte-type microbes can metabolize glyphosate (discussed in Sect. 6).
The amount of AMPA found in glyphosate-treated plants will be both a function of the amount of glyphosate applied to the plant and the sensitivity of the plant to that application rate, as a very toxic amount of glyphosate will reduce the ability of the plant to metabolize it. This was considered in the study of Reddy et al. (2008), in which the ratios of glyphosate to AMPA in non-GR plant species treated with an application rate of glyphosate that inhibits growth by 50% were compared at 7 days after treatment in a variety of plant species. This ratio will be affected by many factors, such as time after treatment, species, and degradation of AMPA. AMPA was found in most species, and the ratio of glyphosate to AMPA was less than 10 in three species, indicating strong metabolism of glyphosate. No AMPA was detected in four species, including both GR and non-GR maize. Nevertheless, in a later field study using higher application rates of glyphosate, the same scientists, using the same analytical methods, found low levels of AMPA in glyphosate-treated GR maize leaves in one field site (Mississippi, USA) two years in a row, but not in another site (Illinois, USA) with different GR cultivars (Reddy et al. 2018). However, the harvested seeds at the Mississippi site had no AMPA, whereas a very low AMPA level (ca. 30 ng/g dry wt) was found in seeds in one of 2 years in Illinois. Bernal et al. (2012) also reported levels of AMPA in glyphosate-treated (1.6 kg/ha) GR maize leaves that were 65-fold less than the glyphosate levels at a week after treatment. Both glyphosate and AMPA concentrations decreased with time after spraying, but the ratio of glyphosate to AMPA decreased with time, indicating that AMPA degrades and/or translocates more slowly than glyphosate in vivo. AMPA and glyphosate compete for movement into the vacuole and the cell and perhaps the plastid (Ge et al. 2013), so these processes will also influence the ratio. Hearon et al. (2021) reported AMPA to be readily taken up by GR maize from soil treated with either AMPA or glyphosate, so all AMPA found in GR maize is not necessarily from degradation of glyphosate in the plant. They also claimed conversion of glyphosate to AMPA in planta, but this was not rigorously proven. The finding that microbe-free cell cultures of maize can metabolize glyphosate to AMPA (Komoßa et al. 1992) proves that maize has an enzyme that can act as a GOX at relatively low in vivo activity levels compared to some other species. In general, however, members of the Poaceae (Gramineae) like maize have very little capability for degrading glyphosate (Duke 2011).
The question of how much glyphosate and AMPA ends up in harvested GR crops is of great interest because of the current human toxicology controversy. Because glyphosate preferentially translocates from sprayed foliage to metabolic sinks such as developing seeds and storage organs (e.g., sugar beet roots) (Duke et al. 2003a; Gougler and Geiger 1981) and GR crops are not impaired in any significant way by the application rates of glyphosate used for weed management (Nandula et al. 2007), one would expect high levels of glyphosate and/or AMPA in harvested parts of GR crops. The only GR crops for which glyphosate and AMPA residue data from peer-reviewed papers exist are GR soybean, sugar beet, and maize. As mentioned above, neither glyphosate nor AMPA is found in processed sugar from GR sugar beets (Barker and Dayan 2019). In a field study, only trace amounts (<0.1 ppm) of glyphosate were found in GR sugar beet roots 2 weeks after spraying glyphosate either 0.825 kg a.e./ha once or 1.26 kg a.e./ha twice approximately 6 weeks apart. At harvest, the glyphosate concentrations in the fresh sugar beet root from different fields ranged from 1.5 (one glyphosate application) to 32 ppb (two applications). The USEPA MRL for fresh sugar beet is 10 ppm. Glyphosate is exuded from roots of glyphosate-treated non-GR plants (e.g., Coupland and Caseley 1979; Rodrigues et al. 1982; Kremer et al. 2005; Laitenen et al. 2007; Barker and Dayan 2019) provided evidence that rapid loss of glyphosate in sugar beet roots is due to root exudation. There are more reports of glyphosate exudation from plant roots than for any other herbicide (Ghanizadeh and Harrington 2020), but there are no reports that this means of glyphosate loss from the plant contributes to glyphosate resistance (Duke 2019; Baek et al. 2020). Roots of some species can retain glyphosate for long periods, as glyphosate and AMPA were found in the roots of perennial, herbaceous plants where glyphosate was applied at a rate of 2.16 kg a.e./ha a year earlier, with tissue glyphosate concentrations ranging from 77 to 1,050 ppb and AMPA from 16 to 48 ppb (Wood 2019). AMPA was not found in all species and, when found, shoot concentrations of both were much lower than root concentrations.
Both glyphosate and AMPA accumulate in the harvested seed of GM soybean (Arregui et al. 2003; Duke et al. 2003b, Bohm et al. 2014, Bøhn et al. 2014), but reported residues are generally well within the maximum tolerance level. For example, the MRL for glyphosate content for soybean seed in the USA is 20 ppm (United States Electronic Code of Federal Regulations 2020), and concentrations as high as 10 ppm have been reported in a survey of GR soybeans grown in the USA (Bøhn et al. 2014). The yearly analysis of soybean samples in the USA by the U.S. Department of Agriculture, Agricultural Marking Service (USDA AMS 2020) reports no presumptive tolerance violations. Only trace amounts of glyphosate or AMPA are sometimes found in GR maize grain (Reddy et al. 2018; Costa et al. 2018). No glyphosate has been reported in maize in the annual USDA AMS (2020) survey. Very low levels or no glyphosate in seeds of glyphosate-treated GR maize is surprising because, just as developing soybean seeds are metabolic sinks that accumulate glyphosate along with photosynthate, developing maize seeds should also accumulate glyphosate along with sucrose from sprayed leaves. Because at least three labs have found either trace amounts or no glyphosate in seed of glyphosate-treated GR maize from different locations and in multiple years, maize seeds of glyphosate-treated GR maize apparently do not accumulate significant glyphosate or AMPA residues.
The first commercial varieties of GR canola contained transgenes for both a bacterial GOX and a GR form of EPSPS (Green 2009). These two transgenes provide a resistance factor of about 50-fold (Nandula et al. 2007). Only one paper has examined AMPA formation in one of these GR varieties in detail (Corrêa et al. 2016). In a laboratory study, at a very low application rate of radiolabeled glyphosate, virtually all of the glyphosate applied to the plants was converted to AMPA within 7 days, whereas very little AMPA was produced in a conventional, non-GR, isogenic variety. Only AMPA and no glyphosate was found in untreated leaves of GR canola. Whether this AMPA was translocated from treated leaves or was formed by oxidation of translocated glyphosate in the untreated leaves was not determined. In a greenhouse study, plants treated with 3.3 kg a.e./ha converted about a third of the glyphosate taken up to AMPA within 2 weeks. How much the added GOX activity contributed to glyphosate resistance in this GR crop is unknown because there are no publicly available data comparing glyphosate resistance imparted by only the GOX gene, only the GR EPSPS gene, and the two genes together in the same canola germplasm or even different germplasms. However, later varieties of GR canola have only a transgene for GR EPSPS, so the contribution of the GOX was apparently not necessary unless the level of expression of the GR EPSPS gene in these first canola varieties was insufficient for robust resistance. Why the GOX transgene was used with a GR EPSPS in the first GR canola varieties was never disclosed.
AMPA is weakly phytotoxic (Hoagland 1980; Gomes et al. 2014), and GR crops are not resistant to AMPA (Reddy et al. 2004; Ding et al. 2011), indicating that AMPA has one or more molecular targets other than EPSPS. Amounts of AMPA or glyphosate applied to GR soybeans that result in the same levels of AMPA within the plant tissues result in similar phytotoxicity symptoms (Reddy et al. 2004). Under rare environmental conditions, glyphosate-treated GR soybean accumulates enough AMPA to cause chlorosis (called “yellow flash” by farmers). This effect is not seen in all GR soybean varieties (Cerny et al. 2014). These differences could be due to different AMPA levels accumulating in the different varieties, but differences in glyphosate degradation between varieties have not been determined under the same conditions. The yellow flash effect is temporary and has not been found to affect yield of the crop. Yellow flash is not seen in GR maize, which produces little or no AMPA when treated with glyphosate. Reddy et al. (2004) concluded that yellow flash in glyphosate-treated soybean is due to the phytotoxicity of accumulated AMPA. As noted above, glyphosate-treated canola with the GOX gene accumulates high levels of AMPA, but yellow flash has not been reported in canola. The reason(s) for this difference is unclear, especially since the phytotoxicity effects of treating GR soybean and GR canola with AMPA are similar (Nandula et al. 2007).
5 Non-target Vegetation Effects
Glyphosate is non-selective, so it can be harmful to almost all plant species if the dose is high enough. Non-target vegetation can be exposed to glyphosate by exposure to the root or foliage. Although some have discussed the potential effects of glyphosate on non-target vegetation by root exposure (e.g., Saunders and Pezeshki 2015), this type of exposure is almost irrelevant because, as discussed above, glyphosate is virtually inactive in most soils. Even if glyphosate were significantly bioavailable to plants in soil, glyphosate is not effectively taken up and translocated acropetally from the roots, and the concentrations found in ground and surface waters are generally lower than amounts needed for a significant physiological effect. Glyphosate drift from sprayed fields to foliage of plants outside the field is the main source of exposure of non-target plants. The amount of glyphosate needed to cause phytotoxicity varies between species. Drift levels of glyphosate can also vary considerably, and even the amount of a herbicide reaching a plant within a sprayed field can be highly variable (Velini et al. 2017). Because glyphosate translocates readily from foliage to growing parts of the plant, good coverage of the target weed is not needed for efficacy. Thus, large spray droplets, without good coverage of the weed, can be effective in delivering lethal glyphosate quantities to target plants. The larger the spray droplet, the less the drift problem, especially for an essentially non-volatile compound like glyphosate. Even with aerial spraying of glyphosate, plant injury is usually minimal at distances of >20 m downwind from sprayed fields (Marrs et al. 1993; Reddy et al. 2010). For mature plants of many species, there is minimal damage at distances of less than 20 m. There are reports of significant effects of very high simulated glyphosate drift levels on non-GR crops. For example, a simulated drift level of 100 g/ha was found to adversely affect nitrogen metabolism in non-GR soybeans (Bellaloui et al. 2006). However, there was no effect on yield, seed protein, or seed oil content by this relatively high “drift” level. Wild plant species are generally less sensitive to glyphosate than domesticated plant species (Cederland 2017). An analysis by Cederland (2017) found that drift of 5 g a.e./ha of glyphosate would not result in even minor adverse effects of drift on 95% of plant species, and that drift levels of 1 to 2 g a.e./ha of glyphosate would essentially cause no harm to any vascular plants. However, there can be stimulatory effects of glyphosate on plant growth at such low application rates (hormesis – as discussed in Sect. 2.5). Nevertheless, there has been a report of injury to an endangered plant species (Pimelea spicata) from glyphosate drift from a non-agricultural use (Matarczyk et al. 2002), but the “drift” concentration of glyphosate was not provided.
As mentioned above and below, glyphosate can influence plant disease by directly inhibiting the pathogen or reducing plant defenses against plant disease, and this effect could cause effects on plant communities subjected to glyphosate drift. Such potential effects have not been studied, other than the beneficial effects of simulated glyphosate drift in Eucalyptus grandis, due to its fungicidal effects on rust (dos Santos et al. 2019) – see Sect. 6.
As with any postemergence herbicide, the effects of glyphosate on non-target vegetation vary with the amount of drift, plant species, environmental conditions, and other factors. Although more plant species might be expected to be affected by glyphosate drift than by drift of any single selective herbicide, in most cases, especially in GR crops, glyphosate replaced several selective herbicides. Thus, the effects of glyphosate on non-target vegetation should be contrasted with the combined effects of the herbicides that it replaced. The relatively short environmental half-life of glyphosate and its lower drift potential than many of the herbicides that it replaced could mean adverse effects on non-target vegetation are likely to be less or, at the most, similar. However, with the increasing evolution and spread of GR weeds (Heap and Duke 2018; Baek et al. 2020), some of the herbicides that glyphosate replaced are now being sprayed again, along with glyphosate (e.g., Gage et al. 2019), reducing the early environmental benefits glyphosate used in GR crops (Cerdeira and Duke 2006; Cerdeira et al. 2007; Duke and Powles 2009).
6 Effects of Glyphosate on Microbes in Agriculture
Fungi and bacteria, as well as members or the phylum Apicomplexa, contain EPSPS that is sensitive to glyphosate (Dill et al. 2010; Roberts et al. 1998). However, there is considerable variation in the EPSPS among microbes, with some having glyphosate-sensitive, class I EPSPS (similar to that in higher plants) and others having relatively insensitive class II enzyme (Funke et al. 2007; Mir et al. 2015). Glyphosate can act as a fungicide and a bactericide on microbes with class I EPSPS. Duke et al. (2018b) recently reviewed much of the literature on this topic. Table 2 provides examples of the effects of glyphosate on variety of microbes as reported in the literature. The concentrations in the papers listed in Table 2 were given as molarity. The molarity of 1 kg a.e./ha of glyphosate ranges from ca. 2.4 to 15 mM with glyphosate manufacturer recommended spray volumes of 40 to 250 L/ha (Monsanto 2020). Thus, the actual concentration of glyphosate that is applied to plants in the field is often sufficiently high to adversely affect many microbes. However, the concentrations of glyphosate in plants and soils will be lower than the concentration in the spray solution, reducing the possibility of there being an antimicrobial or antifungal effect of glyphosate.
Unfortunately, direct comparisons of effects between species from the data in Table 2 are not possible because of the different methods in the different papers. Furthermore, much of the literature is on the effects of formulated glyphosate, which does not differentiate between effects of formulation ingredients and that of glyphosate (Duke 2018b). Hormesis is common with fungitoxic compounds (Pradhan et al. 2017), so this phenomenon may occur with numerous plant pathogens at low glyphosate concentrations. For example, a sub-millimolar concentration (ca. 0.33 mM) of glyphosate stimulated mycelial dry weight accumulation of the plant pathogens Fusarium solani f. sp. pisi and Pythium ultimum (Kawate et al. 1992). The fact that glyphosate can serve as a source of phosphorus for some fungi (e.g., Adelowo et al. 2014) could contribute to hormesis.
Glyphosate is a relatively weak fungicide on most fungi in in vitro assays (Dill et al. 2010), and, as discussed above, glyphosate in non-GR plants reduces the shikimic acid pathway-based plant defenses, giving microbial plant pathogens an advantage, even though glyphosate could be toxic to the pathogen at the right dose. The bioavailable concentration of glyphosate in soil of sprayed weeds in the field may be insufficient to directly affect these pathogens, although the concentration for inhibition of the growth of some fungal plant pathogens is less than 1 μM in an in vivo assay (Puccinia spp. in wheat; Dill et al. 2010). This was a thousand times time less than glyphosate’s activity in an in vitro assay (Table 2). Dill et al. (2010) attributed this discrepancy to the fact that Puccinia species are obligate pathogens that may not be amenable to in vitro screens.
The high application rates of glyphosate used on GR crops can thus have beneficial effects for the crop by their fungicidal effects on plant pathogens. This is particularly true for rusts. For example, a weed-killing application rate of glyphosate (0.84 kg/ha) applied to GR wheat 1 day before inoculation with wheat leaf rust (Puccinia triticina) prevented significant infection compared to plants that were not treated with glyphosate (Feng et al. 2005) (Fig. 9). This application rate in a typical carrier volume of 100 L/ha has a glyphosate concentration of ca. 5 mM, a concentration found to be fungitoxic to several fungi with in vitro assays (Table 2). Anderson and Kolmer (2005) reported similar results with glyphosate on wheat leaf rust and wheat stem rust (P. graminis f. sp. tritici) in GR wheat, obtaining good disease prevention with applications 22 days before inoculation that was evident 20 days after inoculation. Feng et al. (2005, 2008) also found preventative and curative effects of glyphosate on P. striiformis f.sp. tritici in GR wheat and suppression of Asian soybean rust (Phakopsora pachyrhizi) in GR soybeans. Glyphosate is inhibitory to some other cereal fungal pathogens, including Septoria nodorum (leaf blotch) (Harris and Grossbard 1979), Pyrenophora tritici-repentis (tan spot) (Sharma et al. 1989), Gaeumannomyces graminis (take-all) (Wong et al. 1993), and Rhizoctonia solani (Rhizoctonia root rot) (Wong et al. 1993) in studies not involving spraying infected live plants. These results suggest that glyphosate would have a beneficial effect on controlling these diseases in GR crops.
The effect of glyphosate treatment on severity of wheat leaf rust (Puccinia triticina) in GR wheat leaves 13 days after inoculation with the rust. Treatment A, no spray; treatment B, glyphosate formulation (0.84 kg ae/ha in a commercial formulation) 14 days before inoculation; treatment C, glyphosate formulation at 1 day before inoculation. From Feng et al. (2005). Copyright (2005) National Academy of Sciences, U.S.A
Rust infections in non-cereal GR crops are also reduced by glyphosate. Alfalfa rust (Uromyces striatus) was controlled in GR alfalfa by glyphosate (Samac and Foster-Hartnett 2012). It had both preventive and curative effects. Although phytotoxic to glyphosate-susceptible Eucalyptus grandis, glyphosate reduced rust infection by Austropuccinia psidii at sublethal doses to the tree (dos Santos et al. 2019; Tuffi-Santos et al. 2011). Glyphosate at 0.84 kg a.e./ha has been reported to reduce disease symptoms of Rhizoctonia solani in GR cotton (Pankey et al. 2005).
Examples of no effect of glyphosate on a plant disease in a GR crop include a multi-year, multisite study of the influence of glyphosate on Goss’s wilt (Clavibacter michiganensis ssp. nebraskensis) in GR maize (Williams et al. 2015), and a massive, multi-year study in five US states and one Canadian province on the effect of glyphosate on sudden death syndrome (Fusarium virguliforme) in GR soybean (Kandel et al. 2015). Earlier work (Njiti et al. 2003; Sanogo et al. 2001) found no influence of glyphosate on sudden death syndrome in GR soybeans. In a two-year field study, Harikrishnan and Yang (2002) found no effect of glyphosate on root rot and damping off caused by Rhizoctonia solani in GR soybeans. Likewise, there was no effect of glyphosate on R. solani virulence in GR sugar beet (Barnett et al. 2012) and GR cotton (Baird et al. 2004). Another example is the negative findings of Lee et al. (2000, 2003) and Nelson et al. (2002) on the effects of glyphosate on white mold (Sclerotinia sclerotiorum) in GR soybean. Baley et al. (2009a) found no effect of glyphosate on virulence of several pathogens (Gaeumannomyces graminis var. tritici, Pythium ultimum, Rhizoctonia oryzae and R. solani) to GR wheat.
As discussed above, glyphosate makes glyphosate-sensitive plants more susceptible to plant pathogens by reducing synthesis of shikimate pathway-derived defense compounds. However, there is no viable rationale for why GR crops would be more susceptible to plants pathogens, as claimed by some (e.g., Johal and Huber 2009; Yamada et al. 2009). GR crops are about 50-fold more resistant to glyphosate than isogenic lines of the same crops (Nandula et al. 2007), and the lack of shikimate accumulation when these crops are treated with glyphosate (e.g., Velini et al. 2008) indicates that shikimate pathway-based pathogen defenses should not be impaired by glyphosate treatment. As mentioned above, a connection between mineral nutrition of GR crops and disease susceptibility has not been proven (Duke et al. 2012). The preponderance of well-replicated field studies in many geographically diverse locations has found either reduction of or no effect on plant disease in glyphosate-treated GR crops. In his extensive review, Hammerschmidt (2018) concludes that neither the glyphosate resistance gene (discussed in Green and Siehl (2020)) nor glyphosate applied to GR crops makes these crops more susceptible to plant pathogens. His only caveat is that treatment of glyphosate-susceptible plants in the near vicinity of GR crops could cause a temporary increase in inoculum of soil-borne plant pathogens that could increase GR crop disease. However, evidence for this being a significant problem in field situations is lacking.
In summary, glyphosate can act as a fungicide on some plant pathogens in GR crops, and it has been patented for this use (Baley et al. 2009b; Kohn and South 2020). The latter patent claims suppression of the non-rust diseases Fusarium virguliforme, Phialophora gregata, Diaporthe phaseolorum, and Macrophomina phaseolina in GR soybeans, generally increasing yields. However, use of glyphosate as a fungicide is not on the glyphosate label, probably partly because it is not as good as most commercial fungicides (e.g., for fungal disease management), having little or no effects on many such microbes. Also, the timing for application of glyphosate for weed management and that for disease control are unlikely to coincide. Peer-reviewed comparisons of glyphosate with commercial fungicides in field settings are not available. Nevertheless, the fungicidal effect of glyphosate on some plant pathogens is an unrecognized benefit of unknown magnitude in GR crops. However, it has little or no effect on many plant pathogens in these crops. Evidence of enhanced plant disease caused by glyphosate in GR crops is weak and, in some cases, may be the result of indirect effects of glyphosate such as increases in pathogen inoculum coming from nearby glyphosate-susceptible plants. However, such an effect must be rare, as the yields of maize, soybean, and cotton in the USA after there was more than 90% of adoption of GR cultivars of these crops has continued to rise at the same rate as before GR crops were introduced (Duke and Reddy 2018).
A virtually unexplored area of research is the effect of glyphosate on diseases of GR weeds. GR hairy fleabane (Conyza bonariensis) is more susceptible to powdery mildew caused by Podosphaera erigerontis-canadensis than a susceptible biotype (Pazdiora et al. 2019). However, in weeds that have evolved very high levels of glyphosate resistance such as Amaranthus palmeri with multiple copies of EPSPS (Gaines et al. 2010, 2011) (more than 20-fold resistant, requiring more than 7 kg a.e./ha to get the level of control that 0.2 kg ae/ha provides with susceptible biotypes), or in E. indica with a two codon change (Yu et al. 2015) (threonine to isoleucine at codon 102 and proline to serine at codon 106 – known as the TIPS mutation in EPSPS, requiring more than 30 kg a.e./ha to achieve the effect of 0.3 kg a.e/ha), recommended field rates (0.5–2 kg/ha) of glyphosate could increase their fitness by providing protection from some plant pathogens, in addition to the potential benefits of hormesis as discussed in Sect. 2.5.
Some non-pathogenic microbes interfere with plant pathogens, giving the host plant some protection (e.g., Haidar et al. 2016). For example, some endoyphytic bacteria can suppress plant diseases (Sturtz et al. 2000). If the concentrations of glyphosate reaching these microbes were more toxic to them than to the plant pathogen, glyphosate could enhance the success of the pathogen in GR crops. One study (Kuklinsky-Sobral et al. 2005) found the endophyte species of soybeans grown in soil treated with glyphosate were different than those in soil without a glyphosate treatment. No mention was made of whether the soybean varieties used were GR or not. The total population density of endophytes in the stem and roots (ca. 1,000 and 40,000 CFU (colony-forming units)/g fresh tissue, respectively) was unaffected by growing plants in glyphosate-treated soil, and was reduced from ca. 300 to 100 CFU/g fresh tissue in leaves. A later study found GR soybean cultivars treated with glyphosate to generally have a greater abundance of endophytic bacterial communities (de Almeida Lopes et al. 2016). The endophyte species in GR soybean were different from those of the non-GR cultivars. The experiments were not designed to determine whether the differences were due to glyphosate application or to the genetics of the different cultivars.
Some plant growth-promoting endophytes might be benefitted by glyphosate if they can use it as a source of phosphorous with a C-P lyase, as found in the endophyte Enterobacter cloacae (Kryuchkova et al. 2014). Such endophytes might be involved in glyphosate metabolism attributed to the plant, but those metabolizing it with a C-P lase are unlikely to be significantly involved in plant metabolism of glyphosate, because, as previously discussed, sarcosine is rarely reported as a glyphosate metabolite in plants. As far as I can determine, no publications have demonstrated any effects glyphosate on plant disease due to adverse effects on endophytes. Publications that show no effects of glyphosate on endophytes may be rare because “no effect” publications are considered low priority and rejected by the “so what?” rationale of many journals. Thus, unpublished studies such as that by Nolan (2016), who found no effect of glyphosate application on endophytic bacteria associated with roots of GR maize, whereas tillage practices and maize cultivars had effects, are less likely to appear in refereed journals.
Mycorrhizae are much like fungal endophytes, but they form obvious physical interactions with plants which provide benefits to the plant, such as increasing root surface area and enhancing water and nutrient uptake. Arbuscular mycorrhizal fungi (AMF) form structures in cortical roots cells called arbuscles that are involved in exchange of phosphorous, carbon, water, and other nutrients. Glyphosate (2.25 kg a.e./ha) applied to soil reduces root colonization by AMF in glyphosate-susceptible plants, with the effect being influenced by tillage and presence of endophytes (Helander et al. 2018). For example, in Festuca pratensis, there was less effect of glyphosate on the number of arbuscles with tillage or endophytes than without. Whether the glyphosate effects were due to effects on the plant, the AMF, or both was not determined. Four treatments of a high glyphosate rate (3 kg a.e./ha X 4, for total of 12 kg/ha) in a single year for 4 years in succession reduced root colonization of meadow grass (Lolium arundinaceum) by AMF and certain endophytes (Druille et al. 2016). There was no effect at an application rate that kills most weeds (0.8 kg a.e./ha per treatment X 4, for a total of 3.2 kg a.e./ha). The high rates used to get such an effect are unrealistic, as such high rates (12 kg/ha/year) are not needed to kill almost all unwanted vegetation, and the combined costs of the glyphosate and its application would be economically prohibitive. In a field study such as this, whether the effects are direct effects on the microbes or indirect effects from killing almost all of the plant life is unclear.
Before GR soybeans were commercially introduced, glyphosate was found to be toxic to Bradyrhizobium japonicum grown in vitro (Moorman et al. 1992) (Table 2), the microbe responsible for nitrogen fixation in soybean nodules. In this study, there was some variation in the sensitivity of different strains of the microbe to the herbicide. Variation in sensitivity could be due to differences in degradation of glyphosate by B. japonicum, as this microbe has the genetics for a C-P lyase (Hove-Jensen et al. 2014). Hydroxybenzoic acids, upstream by-products of shikimic acid (Lydon and Duke 1988), accumulated in the treated microbes (Moorman et al. 1992), indicating that the toxicity is due to inhibition of EPSPS. Glyphosate causes accumulation of shikimate and protocatechuate in Bradyrhizobium sp. nodules also (de María et al. 2006). Moorman et al. (1992) reasoned that since soybean nodules are metabolic sinks and because glyphosate preferentially translocates to metabolic sinks, there could be problems with glyphosate translocating to nodules in GR soybeans, where it could adversely affect B. japonicum, thereby reducing nitrogen fixation. Reddy et al. (2001) later found no effects of 1.12 kg a.e./ha glyphosate on nodule number or biomass in GR soybean, but 2.24 kg a.e./ha reduced both of these parameters and also reduced leghemoglobin by 6 to 18%. They stated that the adverse effects of the higher rate of glyphosate were of minimal consequence due to the potential of soybean to compensate for short durations of stress. King et al. (2001) found that glyphosate (1.68 kg a.e./ha) applied to twice GR soybeans interfered with nitrogen fixation, but the effect varied with cultivar and location. The effects were not long lived where there was adequate soil moisture throughout the growing season.
Subsequently, Reddy and Zablotowicz (2003) found that glyphosate accumulated in the nodules of glyphosate-treated (0.84 kg/ha) GR soybeans, up to ca. 150 ng/g dry weight. This concentration is similar to that reported in seeds (ca. 200 ng/g dry weight) of glyphosate-treated (0.84 kg/ha) GR soybeans (Duke et al. 2003b). Nodule biomass was reduced ca. 25%, and leghemoglobin was reduced as much as 10%. However, the crop recovered from these effects of glyphosate. A more comprehensive study found nitrogen fixation and/or assimilation in GR soybean to be only slightly affected at glyphosate label use rates (0.84 and 1.68 kg a.e./ha), whereas applications above label use rates (2.52 kg/ha applied twice) consistently reduced nitrogen assimilation, and reduced yield slightly in 1 year out of three (Zablotowicz and Reddy 2007). Bohm et al. (2014) found no effects of glyphosate at 0.96 kg a.e./ha applied twice (1.92 kg a.e./ha total) on nitrogen fixation in field-grown GR soybean. The composition and amounts of both free and protein amino acids of harvested seeds of GR soybean were unaffected by glyphosate treatment (applied at 0.87 kg/ha at both 5 and 7 weeks after planting) (Duke et al. 2018b), indicating no significant effects on nitrogen metabolism of the plant. In summary, glyphosate is unlikely to significantly affect nitrogen metabolism in GR crops when applied at recommended application rates, however, there is recent evidence that some farmers are using significantly higher than recommended rates (Miyazaki et al. 2019) that could adversely affect nitrogen fixation in nodules, thereby affecting yields and quality of harvested seed. Nevertheless, the facts that over 90% of the soybeans grown in the USA are GR (Duke 2018a) and that yields of soybeans have risen in a close to linear fashion since the introduction of GR soybean (Duke and Reddy 2018) support the view that such adverse effects are thus far uncommon.
Some have claimed that glyphosate applied for weed management disrupts soil microflora (e.g., Kremer and Means 2009; Druille et al. 2016; van Bruggen et al. 2018), whereas others have found little effect of single season use (e.g., Hart et al. 2009; Weaver et al. 2007) or repeated use of glyphosate in cropping situations (e.g., Barriuso et al. 2011; Schlatter et al. 2017; Kepler et al. 2020). To put effects of glyphosate on soil microbial communities in perspective, several factors must be considered. As discussed earlier, glyphosate is biologically unavailable to plants in most soils because it binds so tightly to certain soil components and rarely moves farther than a few centimeters into soil. Furthermore, it has a relatively short half-life in most soils in most climates, due to microbial metabolism, mostly to AMPA (Borggaard and Gimsing 2008; Blake and Pallett 2018; Zhan et al. 2018), so even though largely unavailable to plants in soil, it is available to at least some soil microbes. Some microbes can use glyphosate as a sole source of phosphorus, due to a microbial C-P lyase (Selvapanidiyan and Bhatnagar 1994), and, as discussed above, some bacteria and fungi are adversely affected by glyphosate because of glyphosate effects on their EPSPS. Finally, outside of areas sprayed to kill weeds with glyphosate, the concentrations would be expected to be much lower. All of these factors argue against glyphosate having a long-lasting effect on soil microflora, especially outside of sprayed fields. However, glyphosate might be expected to cause soil microflora perturbations in soils of sprayed fields, especially those treated with higher than label rates (>2.0 kg a.e./ha). For example, those microflora that can utilize glyphosate as a phosphorus source might increase, while those adversely affected would decrease in abundance. Nevertheless, as found by Kepler et al. (2020), agronomic practices other than glyphosate use are much more likely to influence soil microbial communities in agricultural fields. Another factor to consider is the effects of glyphosate formulation ingredients. For example, Mendonca et al. (2019) found the polyethoxylated tallow amine used in some glyphosate formulations is toxic to plant-beneficial soil Pseudomonas species and that addition of glyphosate did not add toxicity with two of the species, but it did significantly add toxicity with a third species. Unfortunately, the study was not done in soil, which probably would have reduced or eliminated any glyphosate toxicity, and there was no treatment with glyphosate alone, making the results difficult to interpret. Another example of potential effects of glyphosate on microbes being confounded by use of a formulated product is a study in which a glyphosate formulation was applied to soil in which potatoes were later grown (Gómez-Gallego et al. 2020). The plants were then infested with Colorado potato beetle (Leptinotarsa decemlineata) larvae, and the microbes found in resulting adult insects was altered, compared to those from plants grown without glyphosate in the soil. There were no treatments with glyphosate alone, making the role of glyphosate impossible to determine.
Most of the one-year studies have found small, but transient effects of glyphosate on soil microflora. For example, Weaver et al. (2007), in a laboratory study, found three-fold a recommended rate of glyphosate (i.e., 0.84 kg a.e./ha X 3) for weed management to cause only a small and brief (<7 days) effect on soil microflora. Similar results were obtained by Ratcliff et al. (2006), who found that glyphosate applied to forest soils caused few and transient changes in bacterial and fungal communities. They concluded that application of recommended rates of glyphosate to these soils has a benign effect on microbial community structure. Another example is that of Zabaloy et al. (2016), who found in a two-year study that glyphosate had negligible effects on eubacteria and ammonia-oxidizing bacteria and concluded that glyphosate use at recommended rates poses low risk to soil microbiota. Their highest application rate was 1.2 kg a.e./ha. In a three-year study, Bohm et al. (2014) found no effects of the yearly use of two applications of 0.96 kg a.e./ha of glyphosate on soil microbe populations. Two studies have examined the effects of long-term use of glyphosate in field situations on soil microbia. In the first of these studies, Schlatter et al. (2017) compared the effects of 20 years of glyphosate use on bacterial populations in wheat soils with wheat soils where glyphosate had never been used. Glyphosate use was related to only 2 to 5% of the variation in bacterial communities, whereas most of the variation was associated with cropping history, year, location, and proximity to roots. Less than 1% of the taxa were affected by glyphosate use, and most of these were increased. In a well-replicated study repeated in 2 years in two widely separated states (Maryland and Mississippi) of the USA and with two GR crops (maize and soybean), glyphosate application had almost no effects on soil fungal and prokaryote communities, whereas geography, farming systems, and seasons had profound effects (Kepler et al. 2020). Glyphosate had been used with GR crops for 15 previous concurrent years in one of the study sites.
There are many generations for a microbial species during a crop growing season. Furthermore, the number of individual microbes of a microbial species in a field is many orders of magnitude greater than that of weeds. Thus, if glyphosate is toxic to a microbe, the probability of evolved resistance to it is theoretically very high, although I am unaware of documentation of this in an agricultural field. However, in the laboratory, GR microbes can easily be selected for in glyphosate-containing growth media. For example, Amrhein et al. (1983) produced GR Aerobacter aerogenes by repeated (9X) transfer of a cultures to 5 mM glyphosate. The mechanism of resistance was a 10- to 30-fold increase in EPSPS activity, a mechanism that has also evolved in some plant species (Baek et al. 2020). Microbes with glyphosate-insensitive class II EPSPS (Funke et al. 2007; Mir et al. 2015) isolated from fields with a history of extensive glyphosate use have been reported (Firdous et al. 2018), but there was no determination as to whether the microbe was simply enriched in the field or evolved a highly GR EPSPS from a less resistant EPSPS.
7 Conclusions
Glyphosate is a remarkably successful herbicide that has dominated the herbicide market for decades. Its many attributes include its non-selectivity, high level of efficacy on most species, excellent translocation, relatively slow action on most weed species, and its relative safety to non-target organisms. It was a major herbicide before the introduction of GR crops in 1996, and became the clearly dominant herbicide worldwide after their introduction. Glyphosate has been an important tool in managing cover crops and has promoted reduced and no-tillage agriculture in both non-GR and GR crops, thereby reducing soil erosion, moisture loss, and use of fossil fuels. It is used extensively in non-GR crops for preplant and postharvest weed management, as well as in orchards, vineyards, and silviculture. It is widely used for weed management in non-agricultural settings such as turf, roadsides, and aquatic weed management. In sugarcane it is used at low application rates to enhance sucrose yields, and in some agronomic crops it is used as a harvest aid to quickly kill the crop to facilitate mechanized harvesting. It is the only commercial herbicide that acts by inhibition of EPSPS or any other enzyme of the shikimate pathway. It has no other molecular target as a herbicide, however, inhibition of EPSPS causes several effects that contribute to its adverse effects on plants. These include: 1) depletion of aromatic amino acids needed for synthesis of proteins, IAA, PQ, and secondary products required for plant defense; 2) deregulation of the shikimate pathway, leading to loss of intermediates in carbon fixation and other biosynthetic pathways; and 3) accumulation of the phytotoxic shikimate pathway intermediate, quinic acid. The importance of each of these aspects of glyphosate’s mode of action varies between species and within a species with biotic and abiotic environmental factors and plant growth stage. Plants and microbes can metabolically degrade glyphosate by converting it to AMPA and glyoxylate, and to a lesser extent by breaking the C-P bond, creating sarcosine and inorganic phosphate. Many fungi and bacteria are sensitive to glyphosate, and glyphosate can act as a fungicide against some plant pathogens in GR crops. Bradyrhizobium spp. are sensitive to glyphosate, and glyphosate can inhibit nitrogen fixation in GR soybeans, although this effect has not been found to significantly influence soybean yields at recommended glyphosate application rates. The effects of glyphosate on microflora of crop soils is generally low and transient, with weather and other agricultural practices such as tillage having much stronger effects. Glyphosate has been a valuable tool in economically managing weeds in many settings for the past 45 years.
Abbreviations
- 2PG:
-
2-Phosphoglycolate
- AKR:
-
Aldo-keto reductase
- ALA:
-
Acetolactate synthase
- AMF:
-
Arbuscular mycorrhizal fungi
- AMPA:
-
Aminomethylphosphonic acid
- AOPP:
-
L-α-aminooxy-β-phenylpropionic acid
- CFU:
-
Colony-forming unit
- DAHPS:
-
3-Deoxy-D-arabinoheptulosonate-7-phosphate synthase
- DQS:
-
3-Dehydroquinate synthase
- E4P:
-
Erythrose-4-phosphate
- EPSPS:
-
5-Enolpyruvylshikimate-3-phosphate synthase
- EU:
-
European Union
- GAT:
-
Glyphosate acyltransferase
- GOX:
-
Glyphosate oxidoreductase
- GR:
-
Glyphosate-resistant
- IAA:
-
Indole acetic-3-acid
- IPA:
-
Isopropylamine
- MRL:
-
Minimum residue level
- NT:
-
No-tillage
- PAL:
-
Phenylalanine ammonia-lyase
- PDS:
-
Phytoene desaturase
- PEP:
-
Phosphoenolpyruvate
- PGA:
-
3-Phosphoglycerate
- PPO:
-
Protoporphyrinogen oxygenase
- RH:
-
Relative humidity
- ROS:
-
Reactive oxygen species
- RUBISCO:
-
Ribulose-1,5-bisphosphate carboxylase
- S3P:
-
Shikimate-3-phosphate
- USA:
-
United States of America
References
Abbas T, Nadeem MA, Tanveer A, Zohaib A, Rasool T (2015) Glyphosate hormesis increases growth and yield of chickpea (Cicer arietinum L.). Pak J Weed Sci Res 21:533–542
Abbas T, Nadeem MA, Tanveer A, Maqbool R, Shehzad A, Farooq N (2016) Glyphosate herbicide causes hormesis in wheat. Pak J Weed Sci Res 22:575–586
Adams CR, Weise C, Cobb LC (2014) Effect of season and number of glyphosate applications on control of invasive Mexican petunia (Ruellia simplex). Ecol Restorations 32:133–137
Adelowo FE, Olu-Arotiowa OA, Amuda OS (2014) Biodegradation of glyphosate by fungal species. Adv Biosci Bioeng 2:104–118
Al-Rajab AJ, Amellal S, Shiavon M (2008) Sorption and leaching of 14glyphosate in agricultural soils. Agron Sustain Dev 28:419–428
Altman J, Campbell CL (1977) Effects of herbicides on plant diseases. Annu Rev Phytopathol 15:361–385
Amrhein N, Deus B, Gehrke P, Steinrücken HC (1980) The site of the inhibition of the shikimate pathway by glyphosate. II. Interference of glyphosate with chorismate formation in vivo and in vitro. Plant Physiol 66:830–844
Amrhein N, Johänning D, Schab J, Schulz A (1983) Biochemical basis for glyphosate-tolerance in a bacterium and a plant tissue culture. FEBS Lett 157:191–196
Anderson KS, Johnson KA (1990a) Kinetic and structural analysis of enzyme intermediates: lessons from EPSP synthase. Chem Rev 90:1131–1149
Anderson KS, Johnson KA (1990b) “Kinetic competence” of the 5-enolpyruvylshikikimate-3-phosphate synthase tetrahedral intermediate. J Biol Chem 265:5567–5572
Anderson JA, Kolmer JA (2005) Rust control in glyphosate tolerant wheat following application of the herbicide glyphosate. Plant Dis 89:1136–1142
Anderson KS, Sikorski JA, Johnson KA (1988) A tetrahedral intermediate in the EPSP synthase reaction observed by rapid quench kinetics. Biochemistry 27:7395–7406
Antier C, Kudsk P, Reboud X, Ulber L, Baret PV, Messéan A (2020a) Glyphosate use in the European sector and framework for its further monitoring. Sustainability 12:5682. https://doi.org/10.3390/su12145682
Antier C, Andersson R, Auskalniene O, Barić K, Baret P, Bessenhofer G, Calha I, Carrola Dos Santos S, De Cauwer B, Chachalis D, Dorner Z, Follak S, Forristal D, Gaskov S, Gonzalez Andujar JL, Hull R, Jalli H, Kierzek R et al (2020b) A survey on the uses of glyphosate in European countries. INRAE. https://doi.org/10.15454/A30K-D531
Arisue N, Hashimoto T (2015) Phylogeny and evolution of apicoplasts and apicomplexan parasites. Parasitol Int 64:254–259
Arregui MC, Lenardón A, Sanchez D, Maitre MI, Scotta R, Enrique S (2003) Monitoring glyphosate residues in transgenic glyphosate-resistant soybean. Pest Manag Sci 60:163–166
Askew SD, Askew WB, Goatley JM (2019) Fineleaf fescue species and variety tolerance to glyphosate. Weed Technol 33:185–191
Baek, Y, Bobadilla LK, Giacomini DA, Montgomery JS, Murphy BP, Tranel PJ (2020) Evolution of glyphosate-resistant weeds. Rev Environ Contam Toxicol. (This volume)
Baird R, Batson W, Watson C, Hightower P (2004) Evaluation of transgenic cotton varieties and a glyphosate application of seedling disease incidence. Mycopathologia 158:363–368
Bajwa AA, Wang H, Chouhan BS, Adkins SW (2019) Effect of elevated carbon dioxide concentration on growth, productivity and glyphosate response of parthenium weed (Parthenium hysterophorus L.). Pest Manag Sci 75:2934–2941
Baley GJ, Campbell KG, Yenish J, Kidwell KK, Paulitz TC (2009a) Influence of glyphosate, crop volunteer and root pathogens on glyphosate-resistant wheat under controlled environmental conditions. Pest Manag Sci 65:288–299
Baley GJ, Kohen FC, Pitkin JW, Rinehart J, Taylor JH (2009b) Method for disease control in MON89788. US7608761 B1, 2009
Barker AL, Dayan FE (2019) Fate of glyphosate during production and processing of glyphosate-resistant sugar beet (Beta vulgaris). J Agric Food Chem 67:2061–2065
Barnett KA, Sprague CL, Kirk WW, Hanson LE (2012) Influence of glyphosate on Rhizoctonia crown and root rot (Rhizoctonia solani) in glyphosate-resistant sugar beet. Weed Sci 60:113–120
Barrett P (1985) Efficacy of glyphosate in the control of aquatic weeds. In: Grossbard E, Atkinson D (eds) The herbicide glyphosate. Butterworths, London, pp 365–374
Barrett KA, McBride MB (2005) Oxidative degradation of glyphosate and aminomethylphosphonate by manganese oxide. Environ Sci Technol 39:9223–9228
Barriuso J, Marin S, Mellado RP (2011) Potential accumulative effects of the herbicide glyphosate on glyphosate-tolerant maize rhizobacterial communities over a three-year cultivation period. PLoS One 6:e27558
Bataillard D, Christe P, Pigeault R (2020) Impact of field-realistic doses of glyphosate and nutritional stress on mosquito life history traits and susceptibility to malaria parasite infection. Ecol Evol 10:5079–5088
Baylis AD (2000) Why glyphosate is a global herbicide: strengths, weaknesses and prospects. Pest Manag Sci 56:299–308
Becerril JM, Duke SO, Lydon J (1989) Glyphosate effects on shikimate pathway products in leaves and flowers of velvetleaf. Phytochemistry 28:695–699
Bellaloui N, Reddy KN, Zablotowicz RM, Mengistu A (2006) Simulated glyphosate drift influence nitrate assimulation and nitgrogen fixation in non-glyphosate-resistant soybean. J Agric Food Chem 54:3357–3364
Belz RG (2014) Is hormesis an underestimated factor in the development of herbicide resistance. Julius-Kühn-Arch 443:81–91
Belz RG, Duke SO (2014) Herbicides and hormesis. Pest Manag Sci 70:698–707
Belz RG, Duke SO (2017) Herbicide-mediated hormesis. Amer Chem Soc Symp Ser 1249:135–148
Belz RG, Sinkkonen A (2019) Low toxin doses change plant size distribution in dense populations – glyphosate exposed Hordeum vulgare in a greenhouse study. Envron Internat 132. https://doi.org/10.1016/j.envint.2019.105072
Benbrook CM (2016) Trends in glyphosate herbicide use in the United States and globally. Environ Sci Eur 28:1–15
Bentley R (1990) The shikimate pathway – a metabolic tree with many branches. Crit Rev Biochem Mol Biol 24:307–384
Bernal L, Dussán J (2020) Synergistic effect of Lysinibacillus sphaericus and glyphosate on temephos-resistant larvae of Aedes aegypti. Parasites Vectors 13:Article no. 13
Bernal J, Martin MT, Soto ME, Nozal MJ, Marotti I, Dinelli G, Bernal JL (2012) Development and application of a liquid chromatography-mass spectrometry method to evaluate the glyphosate and aminomethylphosphonic acid dissipation in maize plants after foliar treatment. J Agric Food Chem 60:4017–4025
Binkholder KM, Fresenburg BS, Teuton TC, Xiong X, Smeda RJ (2011) Selection of glyphosate-resistant annual bluegrass (Poa annua) on a golf course. Weed Sci 59:286–289
Blackshaw RE, Harker KN (2016) Wheat, field pea, and canola response to glyphosate and AMPA soil residues. Weed Technol 30:985–991
Blake R, Pallett K (2018) The environmental fate and ecotoxicology of glyphosate. Outlooks Pest Manag 29:266–269
Blevins RL, Cook D, Phillips SH, Phillips RE (1971) Influence of no-tillage on soil moisture. Agron J 63:593–596
Blume CJ, Fei S-Z, Christians NE (2010) Field evaluation of reduced-growth, glyphosate-resistant Kentucky bluegrass in a noncompetitive setting. Crop Sci 50:1048–1056
Bohm GMB, Rombaldi CV, Genovese MI, Castilhos D, Alves BJR, Rumjanek NG (2014) Glyphosate effects on yield, nitrogen fixation, and seed quality in glyphosate-resistant soybean. Crop Sci 54:1737–1743
Bøhn T, Cuhra M, Traavik T, Sanden M, Fagan J, Primicerio R (2014) Compositional differences in soybeans on the market: glyphosate accumulates roundup ready GM soybeans. Food Chem 153:207–215
Boocock MR, Coggins JR (1983) Kinetics of 5-enolpyruvylshikimate-3-phosphate synthase inhibition by glyphosate. FEBS Lett 154:127–133
Borggaard OK, Gimsing AL (2008) Fate of glyphosate in soil and the possibility of leaching to ground and surface waters: a review. Pest Manag Sci 64:441–456
Botta F, Lavison G, Couturier G, Alliot F, Moreau-Guigon E, Fauchon N, Guery B, Chevreuil M, Blanchoud H (2009) Transfer of glyphosate and its degradate AMPA to surface waters through urban sewerage systems. Chemosphere 77:133–139
Bowmer KH, Eberbach PL, McCorkkelle G (1993) Uptake and translocation of 14C-glyphosate in Alternanthera philoxeeroides (Mart.) Griseb. (alligator weed) I. Rhizome concentrations required for inhibition. Weed Res 33:53–57
Boyle JH, Dalgleish HJ, Puzey JR (2019) Monarch butterfly and milkweed declines substantially predate the use of genetically modified crops. Proc Natl Acad Sci U S A 116:3006–3011
Breitenbach J, Zhu C, Sandmann G (2001) Bleaching herbicide norflurazon inhibits phytoene desaturase by competition with cofactors. J Agric Food Chem 49:5270–5272
Brilisauer K, Rapp J, Rath P, Schöllhorn A, Bleul L, Weiss E, Stahl M, Grond S, Frochhammer K (2019) Cyanobacterial antimetabolite 7-deoxy-sedoheptulose blocks the shikimate pathway to inhibit the growth of phototrophic organisms. Nat Commun 10:545. https://doi.org/10.1038/s41467-019-08476-8
Brito IPFS, Tropaldi L, Carbonari CA, Velini ED (2018) Hormetic effects of glyphosate on plants. Pest Manag Sci 74:1064–1070
Brookes G, Barfoot P (2018) Environmental impacts of genetically modified (GM) crop use 1996-2016: impacts on pesticide use and carbon emission. GM Crops Food 8:216–228
Brzezowski P, Ksas B, Havaux M, Grimm B, Chazaux M, Peltier G, Johnson X, Alric J (2019) The function of protoporphyrinogen IX oxidase in chlorophyll biosynthesis requires oxidized plastoquinone in Chlamydomonas reinhardtii. Commun Biol 2:159. https://doi.org/10.1038/s42003-019-039505
Burt EO (1980) Glyphosate for torpedograss and bermudagrass control. In: Proc 3rd Int Turfgrass Res Conf, vol 3. pp. 257–262
CaJacob CA, Feng PCC, Heck GR, Murtaza FA, Sammons RD, Padgett SR (2003) Engineering resistance to herbicides. In: Christou P, Klee H (eds) Handbook of biotechnology. Wiley, Chichester, pp 353–372
Calabrese EJ, Bachmann KA, Bailer AJ, Bolger PM, Borak J, Cai L Cedergreen N, Cherian MG, Chiueh CC, Clarkson TW et al (2007) Biological stress terminology: integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol 222:122–128
Carbonari CA, Gomes GLGC, Velini ED, Machado RF, Simões PS, Macedo GD (2014) Glyphosate effects on sugarcane metabolism and growth. Am J Plant Sci 5:3585–3593
Carver TLW, Robbins MP, Zeyen RJ, Dearne GA (1992) Effects of PAL-specific inhibition on suppression of activated defence and quantitative susceptibility of oats to Erysiphe graminis. Physiol Molec Plant Pathol 41:149–163
Casale J, Lydon J (2007) Apparent effects of glyphosate on alkaloid production in coca plants grown in Columbia. J Forensic Sci 52:573–578
Caseley JC, Coupland D (1985) Environmental and plant factors affecting glyphosate uptake, movement and activity. In: Grossbard E, Atkinson D (eds) The herbicide glyphosate. Butterworths, London, pp 92–123
Castle LA, Siehl DL, Gorton R, Patten PA, Chen YH, Bertain S, Cho H-J, Duck N, Wong J, Liu D, Lassner MW (2004) Discovery and directed evolution of a glyphosate tolerance gene. Science 304:1151–1154
Cedergreen N (2008) Is the growth simulation by low doses of glyphosate sustained over time? Envir Pollut 156:1099–1104
Cederland H (2017) Effects of spray drift of glyphosate on nontarget terrestrial plants – a critical review. Environ Toxicol Chem 36:2879–2886
Cerdeira AL, Duke SO (2006) The current status and environmental impacts of glyphosate-resistant crops: a review. J Environ Qual 35:1633–1658
Cerdeira AL, Gazziero DLP, Duke SI, Matallo MB, Spadoto CA (2007) Review of potential environmental impacts of transgenic glyphosate-resistant soybean in Brazil. J Environ Sci Health Part B 42:539–549
Cerny L, Wu K, Floyd T (2014) Molecular markers associated with yellow flash in glyphosate tolerant soybeans. US Patent 9,617,605 B2. April 11, 2017
Cessna AJ, Darwent AL, Kirkland KJ, Townley-Smith L, Harker KN, Lefkovitch LP (1994) Residues of glyphosate and its metabolite AMPA in wheat seed and foliage following preharvest applications. Can J Plant Sci 74:653–661
Cessna AJ, Darwent AL, Townley-Smith L, Harker KN, Kirkland KJ (2002) Residues of glyphosate and its metabolite AMPA in field pea, barley and flax seed following preharvest applications. Can J Plant Sci 82:485–489
Chahal GS, Jordan DL, Burton JD, Danehower D, York AC, Eure PM, Clewis B (2010) Influence of water quality and coapplied agrochemicals on efficacy of glyphosate. Weed Technol 26:167–176
Chollet R, Vidal J, O’Leary MH (1996) Phosphoenolpyruvate carboxylase: a ubiquitous, highly regulated enzyme in plants. Annu Rev Plant Physiol Plant Mol Biol 47:273–298
Christy AL, Herbst KA, Kostka SJ, Mullen JP, Carlson PS (1993) Synergizing weed biocontrol agents with chemical herbicides. Am Chem Soc Symp Ser 524:87–100
Clay PA, Griffin JL (2000) Weed seed production and seedling emergence responses to late-season glyphosate applications. Weed Sci 48:481–486
Colombo SL, Andreo CS, Chollet R (1998) The interaction of shikimic acid and protein phosphorylation with PEP carboxylase from the C4 dicot Amaranthus viridis. Phytochemistry 48:55–59
Corniani N, Velini ED, Silva FML, Nanayakkara NPD, Witschel M, Dayan FE (2014) Novel bioassay for the discover of inhibitors of the 2-C-methyl-D-erythritol 4-phosphate (MEP) and terpenoid pathways leading to carotenoid biosynthesis. PLoS One 9(7):e103704
Cornish PS (1992) Glyphosate residues in a sandy soil affect tomato transplants. Aust J Exp Agric 32:395–399
Corrêa EL, Dayan FE, Owen DL, Rimando AM, Duke SO (2016) Glyphosate-resistant and conventional canola (Brassica napus L.) responses to glyphosate and aminomethylphosphonic acid (AMPA) treatment. J Agric Food Chem 64:3508–3513
Costa FR, Rech R, Duke SO, Carvalho LB (2018) Lack of effects of glyphosate and glufosinate on growth, mineral content, and yield of glyphosate- and glufosinate-resistant maize. GM Crops Food 9. https://doi.org/10.1080/21645698.2018.1511204
Coupland D, Caseley JC (1979) Presence of 14C activity in root exudates and guttation fluid from Agropyron repens treated with 14C-labelled glyphosate. New Phytol 83:17–22
Cowie BW, Venter N, Witkowski ETF, Byrne MJ (2020) Implications of elevated carbon dioxide on the susceptibility of the globally invasive weed, Parthenium hysterophorus to glyphosate herbicide. Pest Manag Sci 76:2324–2332
Dalley CD, Richard EP (2010) Herbicides as ripeners for sugarcane. Weed Sci 58:329–333
Daouk S, De Alencastro LF, Pfeifer H-R (2013) The herbicide glyphosate and its metabolite AMPA in the Lavaux vineyard area, western Switzerland: proof of widespread export to surface waters. Part II: the role of infiltration and surface runoff. J Environ Sci Health Part B 48:725–736
Dayan FE (2018) Is there a natural route to the next generation of herbicides? Outlooks Pest Manag 29:54–57
Dayan FE, Duke SO (2003) Herbicides: protoporphyrinogen oxidase inhibitors. In: Plimmer JR, Gammon DW, Ragsdale NN (eds) Encyclopedia of agrochemicals, vol 2. Wiley, New York, pp 850–863
Dayan FE, Duke SO (2020) Discovery for new herbicide sites of action by quantification of plant primary metabolite and enzyme pools. Engineering 6:509–514
Dayan FE, Netherland MD (2005) Hydrilla, the perfect aquatic weed, become more noxious than ever. Outlooks Pest Manag 16:277–282
Dayan FE, Barker A, Bough R, Ortiz M, Takano H, Duke SO (2019) Herbicide mechanisms of action and resistance. In: Moo-Young M (ed) Comprehensive biotechnology, vol 4, 3nd edn. Elsevier, Permagon, pp 36–48. https://doi.org/10.1016/B978-0-444-64046-8.00211-1
de Almeida Lopes KB, Carpentieri-Pipolo V, Oro TH, Pagliosa ES, Degrassi G (2016) Culturable endophytic bacterial communities associated with field-grown soybean. J Appl Microbiol 120:740–755
de María N, Becerril JM, García-Plazaola HA, de Felipe MR, Fernández M (2006) New insights on glyphosate mode of action in nodular metabolism: role of shikimate accumulation. J Agric Food Chem 54:2621–2628
de Moraes CP, de Brito IPFS, Tropaldi L, Carbonari CA, Velini ED (2020) Hormetic effect of glyphosate on Urochloa decumbens plants. J Envron Sci Health Part B 55:376–381
della-Cioppa G, Kishore GM (1988) Import of a precursor protein into chloroplasts is inhibited by the herbicide glyphosate. The EMBO J 7:1299–1305
della-Cioppa G, Bauer SC, Klein BK, Shah DM, Fraley RT, Kishore G (1986) Translocation of the precursor of 5-enolpyruvylshikimate-3-phosphate synthase into chloroplasts of higher plants in vitro. Proc Natl Acad Sci U S A 83:6873–6877
Derting CW (1987) Wiper application. In: McWhorter CG, Gebhardt MR (eds) Methods of applying herbicides, Weed Science Society of America Monograph 4, Champaign. pp. 207–229
Devine MD, Duke SO, Fedtke C (1993) Physiology of herbicide action. Prentice Hall, Englewood Cliffs, p 441
Dias RC, Dadazio TS, Tropaldi L, Carbonari CA, Velini ED (2019) Glyphosate as growth regulator for bahiagrass and broadleaf carpetgrass. Planta Daninha 37:e019213829. https://doi.org/10.1590/S0100-83582019370100144
Dill GM, Sammons RD, Feng PCC, Kohn F, Kretzmer K, Mehrsheikh A, Bleeke M, Honegger JL, Farmer D, Wright D, Haupfear (2010) Glyphosate: discovery development, applications, and properties. In: Nandula VK (ed) Glyphosate resistance in crops and weeds, Wiley, Hoboken, pp. 1–33
Ding W, Reddy KN, Zablotowicz RM, Bellaloui N, Bruns HA (2011) Physiological responses of glyphosate-resistant and glyphosate-sensitive soybean to aminomethylphosphonic acid, a metabolite of glyphosate. Chemosphere 83:593–598
dos Santos SA, Tuffi-Santos LD, Tanaka FAO, Sant’Anna-Santos BF, Rodrigues FA, Alfenas AC (2019) Carfentrazone-ethyl and glyphosate drift inhibits uredinial formation of Austropuccinia psidii on Eucalyptus grandis leaves. Pest Manag Sci 75:53–62
dos Santos JCC, da Silva DMR, Amorim DJ, Sab MPV, Silva MA (2021) Glyphosate hormesis mitigates the effect of water deficit in safflower (Carthamus tintoris L.). Pest Manag Sci 77. https://doi.org/10.1002/ps.6231
Druille M, Garćia-Parisi PA, Golluscio RA, Cavagnaro FP, Omacini M (2016) Repeated annual glyphosate applications may impair beneficial soil microorganisms in temperate grassland. Agric Ecosyst Environ 230:184–190
Duke SO (1988) Glyphosate. In: Kearney PC, Kaufman DD (eds) Herbicides: chemistry degradation, and mode of action, vol 3. Marcel Dekker, New York, pp 1–70
Duke SO (2011) Glyphosate degradation in glyphosate-resistant and -susceptible crops and weeds. J Agric Food Chem 59:5835–5841
Duke SO (2014) Biotechnology: herbicide-resistant crops. In: van Alfen M (ed) Encyclopedia of agriculture and food systems, vol 2. Elsevier, San Diego, pp 94–116
Duke SO (2015) Perspectives on transgenic, herbicide-resistant crops in the USA almost 20 years after introduction. Pest Manag Sci 71:652–657
Duke SO (2018a) The history and current status of glyphosate. Pest Manag Sci 74:1027–1034
Duke SO (2018b) Interaction of chemical pesticides and their formulation ingredients with microbes associated with plants and plant pests. J Agric Food Chem 66:7753–7561
Duke SO (2019) Enhanced metabolic degradation: the last evolved glyphosate resistance mechanism of weeds? Plant Physiol. https://doi.org/10.1104/pp.19.01245
Duke SO, Hoagland RE (1978) Effects of glyphosate on metabolism of phenolic compounds. I. Induction of phenylalanine ammonia-lyase activity in dark-grown maize roots. Plant Sci 11:185–190
Duke SO, Powles SB (2009) Glyphosate-resistant crops and weeds: now and in the future. AgBioforum 12:346–357
Duke SO, Reddy KN (2018) Is mineral nutrition of glyphosate-resistant crops altered by glyphosate treatment? Outlooks Pest Manag 29:206–209
Duke SO, Hoagland RE, Elmore CD (1980) Effects of glyphosate on metabolism of phenolic compounds. V. L-α-aminooxy-β-phenylpropionic acid and glyphosate effects on phenylalanine ammonia-lyase in soybean seedlings. Plant Physiol 65:17–21
Duke SO, Rimando AM, Duke MV, Paul RN, Ferreira JFS, Smeda RJ (1999) Sequestration of phytotoxins by plants: implications for biosynthetic production. In: Cutler HA, Cutler SJ (eds) Natural products: agrochemicals and pharmaceuticals. CRC Press, Boca Raton, pp 127–136
Duke SO, Baerson SR, Rimando AM (2003a) Herbicides: glyphosate. In: Plimmer JR, Gammon DW, Ragsdale NN (eds) Encyclopedia of agrochemicals. Wiley, New York. https://doi.org/10.1002/047126363X.agr119
Duke SO, Rimando AM, Pace PF, Reddy KN, Smeda RJ (2003b) Isoflavone, glyphosate, and aminomethylphosphonic acid levels in seeds of glyphosate-treated, glyphosate-resistant soybean. J Agric Food Chem 51:340–344
Duke SO, Wedge DE, Cerdeira AL, Mattalo MB (2007) Interactions of synthetic herbicides with plant disease and microbial herbicides. In: Vurro M, Gressel J (eds) Novel biotechnologies for biocontrol agent enhancement and management. Springer, Dordrecht, pp 277–296
Duke SO, Lydon J, Koskinen WC, Moorman TB, Chaney RL, Hammerschmidt R (2012) Glyphosate effects on plant mineral nutrition, crop rhizophere microbiota, and plant disease in glyphosate-resistant crops. J Agric Food Chem 60:10375–10397
Duke SO, Bajsa J, Pan Z (2013) Omics methods for probing the mode of action of natural and synthetic phytotoxins. J Chem Ecol 39:333–347
Duke SO, Pan Z, Bajsa-Hirschel J, Sánchez-Moreiras AM, Vaughn JN (2018a) Use of omics methods to determine the mode of action of natural phytotoxins. Am Chem Soc Symp Ser 1294:33–46
Duke SO, Rimando AM, Reddy KN, Cizdziel JV, Bellaloui N, Shaw DR, Williams MM, Maul JE (2018b) Lack of transgene and glyphosate effects on yield and mineral and amino acid content of glyphosate-resistant soybean. Pest Manag Sci 74:1166–1173
Dusky JA, Kang MS, Glaz B, Miller JD (1986) Response of eight sugarcane cultivars to glyphosine and glyphosate ripeners. J Plant Growth Regul 4:225–235
Eliel EL, Ramirez MB (1997) (-)-Quinic acid: configurational (stereochemical) descriptors. Tetrahedron Asymmetry 8:2551–3554
Enrich LB, Scheuermann ML, Mohadjer A, Matthias KR, Eller CF, Newman MS, Fujinaka M, Poon T (2008) Liquidamber styraciflua: a renewable source of shikimic acid. Tetrahedron Lett 49:2503–2505
Ermakova IT, Kiseleva NI, Shushkova T, Zharikov M, Zharikov GA, Leontievsky AA (2010) Bioremediation of glyphosate-contaminated soils. Appl Microbiol Biotechnol 88:585–594
Eschenburg S, Kabsch W, Healy ML, Schonbrunn E (2003) A new view of the mechanisms of UDP-N-acetylglucosamine enolpyruvyl transferase (MurA) and 5-enolpyruvylshikimate-3-phosphate synthase (AroA) derived from X-ray structures of their tetrahedral reaction intermediate states. J Biol Chem 278:49215–49222
Federspiel M, Fischer R, Hennig M, Mair H-J, Oberhouser T, Rimmler G, Ableiz T, Bruhin J, Estermann H, Gandert C, Göckel V, Götzö S, Hoffmann U, Huber G, Janatsch G, Lauper S, Rõckel-Stäbler O, Trussardi R, Zwahlen AG (1999) Industrial synthesis of the key precursor in the synthesis of the anti-influenza drug oseltamivir phosphate (Ro 64-0796/002, GS-4104-02): ethyl (3R,4S, 5S)-4,5-epoxy-3-(1-ethyl-propoxy)-cyclohex-1-ene-1-carboxylate. Org Proc Res Dev 3:266–274
Feng PCC, Sandbrink JJ, Sammons RD (2000) Retention, uptake, and translocation of 14C-glyphosate from track-spray applications and correlation to rainfastness in velvetleaf (Abutilon theophrasti). Weed Tech 14:127–132
Feng PCC, Baley GJ, Clinton WP, Bunkers GJ, Alibhai MF, Paulitz T, Kidwell KK (2005) Glyphosate inhibits rust diseases in glyphosate-resistant wheat and soybean. Proc Natl Acad Sci U S A 102:17290–17295
Feng PCC, Clark C, Andrade G, Balbi MC, Caldwell P (2008) The control of Asian rust by glyphosate in glyphosate-resistant soybeans. Pest Manag Sci 64:353–359
Fernando N, Manalil S, Florentine SK, Chauhan BS, Seneweera S (2016) Glyphosate resistance of C3 and C4 weeds under rising atmospheric CO2. Front Plant Sci 7:7. https://doi.org/10.3389/fpls.2016.00910
Firdous S, Iqbal S, Anwar S, Jabeen H (2018) Identification and analysis of 5-enolpyruvylshikimate-3-phosphate synthase EPSPS gene from glyphosate-resistant Ochrobacterium intermedium Sq20. Pest Manag Sci 74:1184–1196
Fischer RS, Berry A, Gaines CG, Jensen RA (1986) Comparative action of glyphosate as a trigger of energy drain in eubacteria. J Bacteriol 168:1147–1154
Franz JE, Mao MK, Sikorski JA (1997) Glyphosate: a unique global herbicide. Amer Chem Soc Monograph 189. American Chemical Society, Washington
Freedman B (1990) Controversy over the use of herbicides in forestry, with particular reference to glyphosate usage. J Environ Sci Health Part C 8:277–286
Fry DJ (1991) Centipedegrass response to plant growth regulators. HortSci 26:40–42
Fu S, Chen HYH, Bell FW, Sharma M, Delaney JR, Peterson G (2008) Effects of timing of glyphosate application on jack pine, black spruce, and white spruce plantations in northern Manitoba. For Chron 84:37–45
Funke T, Healy-Fried ML, Han H, Alberg DG, Bartlett PA, Schönbrunn E (2007) Differential inhibition of class I and class II 5-enolpyruvylshikimate-3-phosphate synthases by tetrahedral reaction intermediate analogues. Biochemistry 46:13344–13351
Gage KL, Krausz RF, Walters SA (2019) Emerging challenges for weed management in herbicide-resistant crops. Agriculture 9. https://doi.org/10.3390/agriculture9080180
Gaines TA (2018) The importance of glyphosate in non-GM settings. Outlooks Pest Manag 29:255–257
Gaines TA, Zhang W, Wang D, Bukun B, Chisholm ST, Shaner DL, Nissen SJ, Patzoldt WL, Tranel PJ, Culpepper AS, Grey TL, Webster TM, Vencill WK, Sammons RD, Jiang J, Preston C, Leach JE, Westra P (2010) Gene amplification confers glyphosate resistance in Amaranthus palmeri. Proc Natl Acad Sci U S A 107:1029–1034
Gaines TA, Shaner DL, Ward SM, Leach JE, Preston C, Westra P (2011) Mechanism of resistance of evolved glyphosate-resistant Palmer Amaranth (Amaranthus palmeri). J Agric Food Chem 59:5886–5889
Gattás F, Espinosa M, Babay P, Pizzaro H, Cataldo D (2020) Invasive species versus pollutants: potential of Limnoperna fortunei to degrade glyphosate-based commercial formulations. Ecotox Environ Saf 201. https://doi.org/10.1016/j.ecoenv.2020.110794
Ge X, d'Avignon A, Ackerman JJH, Ostrander E, Sammons RD (2013) Application of 31P-NMR spectroscopy to glyphosate studies in plants: insights into cellular uptake and vacuole sequestration correlated to herbicide resistance. In: Handbook on herbicides: biological activity, classification and health and environmental implications. Nova Science Publishers, pp 55–83
Ge X, d’Avignon A, Ackerman JJH, Sammons RD (2014) Glyphosate uptake, vacuolar sequestration, and tonoplast pump activity in glyphosate-resistant horseweed. Plant Physiol 166:1256–1268
Geiger DR, Bestman HD (1990) Self-limitation of herbicide mobility by phototoxic action. Weed Sci 38:324–329
Geiger DR, Kapitan SW, Tucci MA (1986) Glyphosate inhibits photosynthesis and allocation of carbon to starch in sugar beet leaves. Plant Physiol 82:468–472
Geisy JP, Dobson S, Solomon KR (2000) Ecotoxicological risk assessment for roundup® herbicide. Rev Environ Contam Toxicol 167:35–120
Gélinas P, Gagnon F, McKinnon C (2018) Wheat preharvest herbicide application, whole-grain flour properties, yeast activity and the degradation of glyphosate in bread. Int J Food Sci Technol 53:1597–1602
Ghanizadeh H, Harrington KC (2020) Perpective: root exudation of herbicides as a novel mode of herbicide resistance in weeds. Pest Manag Sci 76:2543–2547
Ghosh S, Chisti Y, Banerjee UC (2012) Production of shikimic acid. Biotech Adv (6):1425–1431
Gimsing AL, Borggaard OK (2001) Effect of KCl and CaCl2 as background electrolytes on the competitive adsorption of glyphosate and phosphate on goethite. Clays Clay Minerals 49:270–275
Givens WA, Shaw DR, Kruger GR, Johnson WG, Weller SC, Young BG, Wilson RG, Owen MDK, Jordan D (2009) Survey of tillage trends following the adoption of glyphosate-resistant crops. Weed Technol 23:15–155
Gomes MP, Smedbol E, Chalifour A, Hénault-Ethier L, Labrecque M, Lepage L, Lucotte M, Juneau P (2014) Alteration of plant physiology by glyphosate and its by-product aminomethylphosphonic acid: an overview. J Exp Bot 65:4691–4703
Gomes MP, Le Manac’h SG, Maccario S, Labreque M, Lucotte M, Juneau P (2016) Differential effects of glyphosate and aminomethylphosphonic acid (AMPA) on photosynthesis and chlorophyll metabolism in willow plants. Pestic Biochem Physiol 130:65–70
Gómez-Gallego C, Rainio M, Collado MC, Mantziari A, Salminen S, Saikkonen K, Helander M (2020) Glyphosate-based herbicide affects the composition of microbes associated with Colorado potato beetle (Leptinotarsa decemlineata). FEMS Microbiol Lett 367. https://doi.org/10.1093/femsle/fnaa050
Gougler JA, Geiger DR (1981) Uptake and distribution of N-phosphonomethylglycine in sugar beet plants. Plant Physiol 68:668–672
Green JM (2009) Evolution of glyphosate-resistant crop technology. Weed Sci 57:109–117
Green JM, Siehl DL (2020) History and outlook for glyphosate-resistant crops. Rev Environ Contam Toxicol. (this volume)
Green JM, Hazel CB, Forney DR, Pugh LM (2008) New multiple-herbicide crop resistance and formulation technology to augment the utility of glyphosate. Pest Manag Sci 64:332–339
Gressel J (2010) Herbicides as synergists for mycoherbicides, and vice versa. Weed Sci 58:324–328
Griffin JL, Boudreaux JM, Miller DK (2010) Herbicides as harvest aids. Weed Sci 58:355–358
Grossbard E (1985) Effects of glyphosate on the microflora: with reference to the decomposition of treated vegetation and interaction with some plant pathogens. In: Grossbard E, Atkinson D (eds) The herbicide glyphosate. Butterworths, London, pp 159–185
Grossbard E, Atkinson D (1985) The herbicide glyphosate. Butterworths, London, p 490
Haderlie LC, Widholm JM, Slife FW (1977) Effect of glyphosate on carrot and tobacco cells. Plant Physiol 60:40–43
Haidar R, Fermaud M, Calvo-Garrido C, Roudet J, Deshcamps A (2016) Modes of action for biocontrol of Botryis cinera by antagonistic bacteria. Phytopathol Mediterr 55:301–322
Hallas LE, Adams WJ, Heitkamp MA (1992) Glyphosate degradation by immobilized bacteria: field studies with industrial wastewater effluent. Appl Envir Microbiol 58:1215–1219
Hammer PE, Hinson TK, Duck NB, Koziel (2007) Methods to confer herbicide resistance. US patent application 20070107078 A1, pp 1-53
Hammerschmidt R (2018) How glyphosate affects plant disease development: it is more than enhanced susceptibility. Pest Manag Sci 74:1054–1063
Harikrishnan R, Yang XB (2002) Effects of herbicides on root rot and damping-off caused by Rhizoctonia solani in glyphosate-tolerant soybean. Plant Dis 86:1369–1373
Harring T, Streibig JC, Husted S (1998) Accumulation of shikimic acid: a technique for screening glyphosate efficacy. J Agric Food Chem 46:4406–4412
Harrington KC, Ghanizadeh H (2017) Herbicide application using wiper applicators – a review. Crop Prot 102:56–62
Harris CA, Gaston CP (2004) Effects of refining predicted dietary intakes of pesticide residues: a case study using glyphosate. Food Addit Contam 21:857–864
Harris D, Grossbard E (1979) Effects of the herbicides gramoxone W and roundup on Septoria nodorum. Trans Br Mycol Soc 73:27–34
Hart MM, Powell JR, Gulden RH, Dunfield KE, Pauls KP, Swanton CJ, Klironomos JN, Antune PM, Koch AM, Trevors JT (2009) Separating the effect of crop from herbicide on soil microbial communities in glyphosate-resistant corn. Pedobiologia 52:253–262
Hartzler RG (2010) Reduction in common milkweed (Asclepias syriaca) occurrence in Iowa cropland from 1999 to 2009. Crop Protect 29:1542–1544
Haughton AJ, Bell JR, Boatman ND, Wilcox A (2001) The effect of the herbicide glyphosate on non-target spiders: part II. Indirect effects on Lepthyphantes tenuis in field margins. Pest Manag Sci 57:1037–1042
Heap I, Duke SO (2018) Overview of glyphosate-resistant weeds worldwide. Pest Manag Sci 74:1040–1049
Hearon SE, Wang M, McDonald TJ, Phillips TD (2021) Decreased bioavailability of aminomethylphosphonic acid (AMPA) in genetically modified corn with activated carbon or calcium montmorillonite clay inclusion in soil. J Environ Sci 100:131–143
Helander M, Saloniemi I, Omacini M, Druille M, Salminen J-P, Saikkonen K (2018) Glyphosate decreases mycorrhizal colonization and affects plant-soil feedback. Sci Total Environ 642:285–291
Herbicide Resistance Action Committee (2020) HRAC mode of action classification 2020. https://hracglobal.com/files/HRAC_Revised_MOA_Classification_Herbicides_Poster.png. Accessed 8 Aug 2020
Herrmann KM (1995) The shikimate pathway as an entry to aromatic secondary metabolism. Plant Physiol 107:7
Hoagland RE (1980) Effects of glyphosate on metabolism of phenolic compounds. VI. Effects of glyphosine and glyphosate metabolites on phenylalanine ammonia-lyase activity, growth, and protein, chlorophyll, and anthocyanin levels in soybean (Glycine max) seedlings. Weed Sci 28:393–400
Hoagland RE, Boyette CD, Jordan RH, Stetina KC (2018) Interaction of the bioherbicide Myrothecium verrucaria with technical-grade glyphosate on glyphosate-susceptible and –resistant palmer amaranth. Am J Plant Sci 9:2306–2319
Hobbie KA, Rooney N, Scott RP, Anderson KA (2017) An alternative method to produce shikimic acid chemical feedstock by applying glyphosate to forage crops. Crop Sci 57:945–950
Hove-Jensen B, Zechel DL, Jochimsen B (2014) Utilization of glyphosate as phosphate source: biochemistry and genetics of bacterial carbon-phosphorous lyase. Microbiol Mol Biol Rev 78:176–197
Itou T, Hayama K, Sakai A, Tanouchi H, Okuda S, Kushima H, Kajimoto T (2015) Developing an effective glyphosate application technique to control Bischofia javanica Blume, an invasive alien tree species in the Ogasawara Islands. J Forest Res 20:248–253
Jacob GS, Garbow JR, Hallas LE, Kimack NM, Kishore GM, Schaefer J (1988) Metabolism of glyphosate in Pseudomonas sp. strain LBr. Appl Environ Microbiol 54:2953–2958
Jang SJ, Mallory-Smith C, Kuk YI (2020) Inhibition of wheat growth planted after glyphosate application to weeds. Weed Sci 68:373–381
Jansons M, Pugajeva I, Bartkevičs V (2018) Occurrence of glyphosate in beer from the Latvian market. Food Additives Contamin 35A:1767–1775
Jaworski EG (1972) Mode of action of N-phosphonomethylglycine: inhibition of aromatic amino acid biosynthesis. J Agric Food Chem 20:1195–1198
Jeffery LS, English JR, Connell J (1981) The effects of fall application of glyphosate on corn (Zea mays), soybeans (Glycine max), and johnsongrass (Sorghum halepense). Weed Sci 29:190–195
Jenson RA (1985) The shikimate/arogenate pathway: link between carbohydrate metabolism and secondary metabolism. Physiol Plant 66:164–168
Johal GS, Huber DM (2009) Glyphosate effects on diseases of plants. Eur J Agron 31:144–152
Johnson BJ (1976) Glyphosate for weed control in dormant bermudagrass. Weed Sci 24:140–143
Johnson BJ (1990) Response of bahiagrass (Paspalium notatum) to plant growth regulators. Weed Technol 4:895–899
Kandel YR, Bradley CA, Wise KA, Chilvers MI, Tenuta AU, Davis VM, Esker PD, Smith DL, Licht MA, Mueller DS (2015) Effect of glyphosate application on sudden death syndrome of glyphosate-resistant soybean under field conditions. Plant Dis 99:347–354
Kawate MK, Kawate SC, Ogg AG, Kraft JM (1992) Response of Fusarium solani f. sp. pisi and Phythium ultimum to glyphosate. Weed Sci 40:497–502
Kay SH (1995) Efficacy of wipe-on applications of glyphosate and imazapyr on common reed in aquatic sites. J Aquat Plant Manag 33:25–26
Kepler RM, Epp-Schmidt DJ, Yarwood SA, Cavigelli MA, Reddy KN, Duke SO, Bradley CA, Williams MM, Buyer JS, Maul JE (2020) Soil microbial communities in diverse agroecosystems exposed to the herbicide glyphosate. Appl Environ Microbiol 86:e01744–e01719. https://doi.org/10.1128/AEM.01744-19
Khan S, Zhou JL, Ren L, Mojiri A (2020) Effects of glyphosate on germination, photosynthesis and chloroplast morphology in tomato. Chemosphere 258. https://doi.org/10.1016/j.chemosphere.2020.127350
King CA, Purcell LC, Vories ED (2001) Plant growth and nitrogenase activity of glyphosate-tolerant soybean in response to foliar glyphosate applications. Agron J 93:179–186
Kishore GM, Jacob GS (1987) Degradation of glyphosate by Pseudomonas sp. PG2982 via a sarcosine intermediate. J Biol Chem 262:12164–12168
Kishore GM, Shah DM (1988) Amino acid biosynthesis inhibitors as herbicides. Annu Rev Biochem 57:627–663
Kitchen LM, Witt WW, Rieck CE (1981) Inhibition of chlorophyll accumulation by glyphosate. Weed Sci 29:513–516
Knowles WS, Anderson KS, Andrew SS, Phillion DP, Ream JE, Johnson KA, Sikorski (1993) Synthesis and characterization of N-amino-glyphosate as a potent analog inhibitor of E.Coli EPSP synthase. Bioorgan Med Chem Lett 3:2863–2868
Kohn DC, South MS (2020) Use of glyphosate for disease suppression and yield enhancement in sobean. US10555527B2
Kolakowski BM, Miller L, Murray A, Leclair A, Bietlot H, van de Riet JM (2020) Analysis of glyphosate residues in foods from the Canadian retail markets between 2015 and 2017. J Agric Food Chem 68:5201–5211
Komoßa D, Gennity I, Sandermann H (1992) Plant metabolism of herbicides with C-P bonds: glyphosate. Pestic Biochem Physiol 43:85–94
Koskinen WC, Marek LJ, Hall KE (2016) Analysis of glyphosate and aminomethylphosphonic acid in water, plant materials and soil. Pest Manag Sci 72:423–432
Kremer RJ, Means NE (2009) Glyphosate and glyphosate-resistant crop interactions with rhizosphere microorganisms. Eur J Agron 31:153–161
Kremer RJ, Means NE, Kim KS (2005) Glyphosate affects soybean root exudation and rhizosphere microorganisms. Int J Environ Anal Chem 85:1165–1174
Krutz LJ, Locke MA, Steinrade RW Jr (2009) Interactions of tillage and cover crop on water, sediment, and pre-emergence loss in glyphosate-resistant cotton: implications for the control of glyphosate-resistant weed biotypes. J Environ Qual 38:1240–1247
Kryuchkova YV, Burygin GL, Gogoleva NE, Gogolev YV, Chernyshova MP, Makarov OE, Fedorov EE, Turkovskaya OV (2014) Isolation and characterization of glyphosate-degrading rhizosphere strain, Enterobacter cloacae K7. Microbiol Res 169:99–105
Krzyśko-Łupicka T, Orlik A (1997) Use of glyphosate as the sole source of phosphorus or carbon for the selection of soil-borne fungal strains capable to degrade this herbicide. Chemosphere 34:2601–2605
Kudsk P, Mathiasson SK (2020) Pesticide regulation in the European Union and the glyphosate controversy. Weed Res 68:214–222
Kuklinsky-Sobral J, Araújo WL, Mendes R, Pizzirani-Kleiner AA, Azevedo L (2005) Isolation and characterization of endophytic bacteria from soybean (Glycine max) grown in soil treated with glyphosate herbicide. Plant Soil 273:91–99
Laitenen P, Rämö S, Siimes K (2007) Glyphosate translocation from plants to soil – does it constitute a significant proportion of residues in soil? Plant Soil 300:51–60
Lanaro R, Costa JL, Cazenave SOS, Zanolli-Filho LA, Tavares MFM, Chasin AAM (2015) Determination of herbicides paraquat, glyphosate, and aminomethylphosphonic acid in marijuana samples by capillary electrophoresis. J Foren Sci 60:S241–S247
Lange AH, Fischer BB, Elmore CL, Kempen HM, Schlesselman J (1975) Roundup – the end of perennial weeds in tree and vine crops? Calif Agric:6–7
Lazzaro L, Feretti G, Bianchi E, Benesperi R (2019) Treatment by glyphosate-based herbicide allowed recovering native species after Oxalis pes-caprae L. invasion: indications from a Mediterranean island. Plant Biosyst 153:651–659
Lee LJ, Ngim J (2000) A first report of glyphosate-resisant goosegrass (Eleusine indica (L) Gaertn) in Malaysia. Pest Manag Sci 56:336–339
Lee CD, Penner D, Hammerschmidt R (2000) Influence of formulated glyphosate and activator adjuvants on Sclerotinia sclerotiorumin glyphosate-resistant and -susceptible Glycine max. Weed Sci 48:710–715
Lee CD, Penner D, Hammerschmidt R (2003) Glyphosate and shade effects on glyphosate-resistant soybean defense response to Sclerotinia sclerotiorum. Weed Sci 51:294–298
Leegood RC (2002) C4 photosynthesis: principles of CO2 concentration and prospects for introduction into C3 plants. J Exp Bot 53:581–590
Legendre BL, Finger CK (1987) Response of sugarcane varietis to the chemical ripener glyphosate. Proc. Plant Growth Reg Soc Am 14:479–484
Lerbs W, Stock M, Parthier B (1990) Physiological aspects of glyphosate degradation in Alcaligenes spec. strain GL. Arch Microbiol 153:146–150
Lescano MR, Masin CE, Rodriguez AR, Godoy JL, Zalazar CS (2020) Earthworms to improve glyphosate degradation in biobeds. Environ Sci Pollut Res 27:27023–27031
Lévesque CA, Rahe JE (1992) Herbicide interactions with fungal root pathogens, with special reference to glyphosate. Annu Rev Phytopathol 30:579–602
Linz GM, Homan HJ (2011) Use of glyphosate for managing invasive cattail (Typha spp.) to disperse blackbird (Icteridae) roosts. Crop Protect 30:98–104
Locke MA, Bryson CT (1997) Herbicide-soil interaction in reduced tillage and plant residue management systems. Weed Sci 45:307–320
Lockhart WL, Billeck BN, Baron CL (1989) Bioassays with a floating aquatic plant (Lemna minor) for effects of sprayed and dissolved glyphosate. Hyrobiologia 188:353–359
Lopez EG (1993) Effect of glyphosate on different densities of water hyacinth. J Aquat Plant Manag 31:255–257
Lundgren JG, Gassmann AJ, Bernal J, Duan JJ, Ruberson J (2009) Ecological compatibility of GM crops and biological control. Crop Prot 28:1017–1030
Lydon J, Duke SO (1988) Glyphosate induction of elevated levels of hydroxybenzoic acids in higher plants. J Agric Food Chem 36:813–818
Madsen KH, Heitholt JJ, Duke SO, Smeda RJ, Streibig JC (1986) Photosynthetic parameters in glyphosate-treated sugarbeet (Beta vulgaris L.). Weed Res 35:81–88
Maeda H, Dudareva N (2012) The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu Rev Plant Biol 63:73–105
Malalgoda M, Ohm J-B, Howatt KA, Green A, Simsek S (2020) Effects of pre-harvest glyphosate use on protein composition and shikimic acid accumulation in wheat. Food Chem 332:127422
Manea A, Leishman MR, Downey PO (2011) Exotic C4 grasses have increased tolerance to glyphosate under elevated carbon dioxide. Weed Sci 59:28–36
Marble SC, Prior SA, Runion GB, Torbert HA (2015) Control of yellow and purple nutsedge in elevated CO2 environment with glyphosate and halosulfuoron. Front Plant Sci 6:Article 1. https://doi.org/10.3389/fpls.2015.00001
Maroli AS, Nandula VK, Duke SO, Tharayil N (2016) Stable isotope resolved metabolomics reveals the role of anabolic and catabolic processes in glyphosate-induced amino acid accumulation in Amaranthus palmeri biotypes. J Agric Food Chem 64:7040–7048
Maroli AS, Gaines TA, Foley ME, Duke SO, Doğramici M, Anderson JV, Horvath DP, Chao WS, Tharayil N (2018a) Omics in weed science: a perspective from genomics, transcriptomics, and metabolomics approaches. Weed Sci 66:681–695
Maroli AS, Nandula VK, Duke SO, Gerard P, Tharayil N (2018b) Comparative metabolomic analysis of two Ipomoea lacunosa biotypes with contrasting glyphosate tolerance captures glyphosate-induced differential perturbations in cellular physiology. J Agric Food Chem 66:2027–2039
Marrs RH, Frost AJ, Plant RA, Lunnis P (1993) Determination of buffer zones to protect seedlings of non-target plants from the effects of glyphosate spray drift. Agric Ecosyst Environ 45:283–293
Marshall EJP, Solomon KR, Carrasquilla G (2009) Coca (Erythroxylum coca) control is affected by glyphosate formulations and adjuvants. J Toxicol Environ Heath 72A:930–936
Martinez DA, Loening UE, Graham MC (2018) Impacts of glyphosate-based herbicides on disease resistance and health of crops: a review. Environ Sci Eur 30:2. https://doi.org/10.1186/s12302-018-0131-7
Marzabadi MR, Gruys KJ, Pansegrau PD, Walker MC, Yuen HK, Sikorski JA (1996) An EPSP synthase inhibitor joining shikimate 3-phosphate with glyphosate: synthesis and ligand bonding studies. Biochemistry 35:4199–4210
Matallo MB, Almeida SDB, Franco DAS, Cerdeira AL, Gazziero DLP (2014) Glyphosate as a tool to produce shikimic acid in plants. Planta Daninha 32:601–608
Matarczyk JA, Willis AJ, Vranjic JA, Ash JE (2002) Herbicides, weeds and endangered species: management of Bitou bush (Chrysanthemoides monilifera spp. rotunda) with glyphosate and impacts on the endangered shrub, Pimelea spicata. Biol Conserv 108:133–141
Mateos-Naranjo E, Redondo-Gómez S, Cox L, Cornejo J, Figueroa ME (2009) Effectiveness of glyphosate and imazamox on the control of the invasive cordgrass Spartina densiflora. Ecotoxicol Environ Saf 72:1694–1700
Matzrafi M, Brunharo C, Tehranchian P, Hanson BD, Jasieniuk M (2019) Increased temperatures and elevated CO2 levels reduce the sensitivity of Conyza canadensis and Chenopodium album to glyphosate. Sci Rep 9:2228. https://doi.org/10.1038/s41598-019-38729-x
McAllister RS, Haderlie LC (1985) Translocation of 14C-glyphosate and 14CO2-labeled photoassimilates in Canada thistle (Cirsium arvense). Weed Sci 33:153–159
McConkey GA, Pinney JW, Westhead DR, Plueckhahn K, Fitzpatrick TB, Mcheroux P, Kappes B (2004) Annotating the Plasmodium genome and the enigma of the shikimate pathway. Trends Parasitol 20:60–65
McElroy JS, Hall ND (2020) Echinochloa colona with reported resistance to glyphosate conferred by aldo-keto reductase also contains a Pro-106-Thr EPSPS target site mutation. Plant Physiol. https://doi.org/10.1104/pp.20.00064
McWhorter DB, Derting CW (1985) Methods of application of glyphosate. In: Grossbard E, Atkinson D (eds) The herbicide glyphosate. Butterworths, London, pp 241–259
Melander B, Munier-Jolain N, Charles R, Wirth J, Schwarz J, van der Weide R, Bonin L, Jensen PK, Kudsk P (2013) European perspectives on the adoption of nonchemical weed management in reduced-tillage systems for arable crops. Weed Technol 27:231–240
Mendonca CM, Reed ML, Kukurugya MA, Aristilde L (2019) Adverse metabolic outcomes in soil Pseudomonas species exposed to polyethoxylated tallow amine and glyphosate. Envion Sci Technol Lett 6:448–455
Mertins M, Hoss S, Neumann G, Afzal J, Reichenbecher W (2018) Glyphosate, a chelating agent-relevant for eccological risk assessment? Environ Sci Pollut Res Internat 25:5298–5317
Mesnage R, Antoniou MN (2017) Facts and fallacies in the debate on glyphosate toxicity. Front Public Health. https://doi.org/10.3389/fpubh.2017.00316
Mills PJ, Kania-Korwel I, Fagan J, McEvoy LK, Laughlin GA, Barrett-Conner E (2017) Excretion of the herbicide glyphosate in older adults between 1993 and 2016. JAMA 318:1610–1611
Mir R, Jallu S, Singh TP (2015) The shikimate pathway: review of amino acid sequence, function and three-dimensional structures of the enzymes. Crit Rev Microbiol 41:172–189
Miyazaki J, Bauer-Panskus A, Bøhn T, Reichenbecher W, Then C (2019) Insufficient risk assessment of herbicide-tolerant genetically engineered soybeans intended for import into the EU. Environ Sci Eur 31:92. https://doi.org/10.1186/s12302-19-0274-1
Moerschbacher BM, Noll U, Gorrichon L, Reisener H-J (1990) Specific inhibition of lignification breaks hypersensitive resistance of wheat to stem rust. Plant Physiol 93:465–470
Mohseni-Moghadam M, Wolfe S, Dami I, Doohan D (2016) Response of wine grape cultivars to simulated drift rates of 2,4-D, dicamba, and glyphosate, and 2,4-D or dicamba plus glyphosate. Weed Technol 30:807–814
Mollaee M, Matloob A, Mobli A, Thompson M, Chauhan BS (2020) Response of glyphosate-resistant and susceptible biotypes of Echinochloa colona to low doses of glyphosate in different soil moisture conditions. PLoS One 15:e0233428. https://doi.org/10.1371/journal.pone.0233428
Monsanto (2020) Sprayers and water volumes. https://www.monsanto-ag.co.uk/roundup/roundup-amenity/application-information/sprayers-and-water-volumes/#:~:text=Knapsack%20Spraying,and%20250%20litres%20per%20hectare
Moorman TB, Becerril JM, Lydon J, Duke SO (1992) Production of hydroxybenzoic acids by Bradyrhizobium japonicum strains after treatment with glyphosate. J Agric Food Chem 40:289–293
Moretti ML, van Horn CR, Robertson R, Segobye K, Weller SC, Young BG, Johnson WG, Sammons RD, Wang D, Ge X, d’Avignon A, Gaines TA, Westra P, Green AC, Jeffery T, Lespérance MA, Tardif FJ, Sikkema PH, Hall JC, McLean MD, Lawton MB, Schulz B (2018) Glyphosate resistance in Ambrosia trifida: part 2. Rapid response physiology and non-target-site resistance. Pest Manag Sci 74:1079–1088
Morillo E, Undabeytia T, Maqueda C, Ramos A (2000) Glyphosate adsorption on soils of different characteristics. Influence of copper addition. Chemosphere 40:103–107
Morin F, Vera V, Nurit F, Tissut M, Marigo G (1997) Glyphosate uptake in Catharanthus roseus cells: role of a phosphate transporter. Pestic Biochem Physiol 58:13–22
Morishita DW (2018) Impact of glyphosate-resistant sugar beet. Pest Manag Sci 74:1050–1053
Nandula VK, Reddy KN, Rimando AM, Duke SO, Poston DH (2007) Glyphosate-resistant and -susceptible soybean (Glycine max) and canola (Brassica napus) dose response and metabolism relationships with glyphosate. J Agric Food Chem 55:3540–3545
Nandula VK, Riechers DE, Ferhatoglu Y, Marrett M, Duke SO, Dayan FE, Goldberg-Cavellari A, Tétard-Jones C, Wortley DU, Onkokesung N, Brazier-Hicks M, Edwards R, Gaines T, Iwakami S, Jugulam M, Ma R (2019) Herbicide metabolism: crop selectivity, bioactivation, resistance mechanisms, and regulation. Weed Sci 67:149–175
Nascente AS, Crusciol CAC, Stone LF, Cobucci T (2013) Upland rice yield as affected by previous summer crop rotation (soybean or upland rice) and glyphosate management of cover crops. Planta Daninha 31:147–155
Nelson KA, Renner KA, Hammerschmidt R (2002) Cultivar and herbicide selection affects soybean development and the incidence of Sclerotinia stem rot. Agron J 94:1270–1281
Nguyen CT, Dang LH, Nguyen DT, Tran KP, Giang BL, Tran NQ (2019) Effect of GA3 and gly plant growth regulators on productivity and sugar content of sugarcane. Agriculture 9:136. https://doi.org/10.3390/agriculture9070136
Nicolia A, Ferradini N, Molla G, Biagetti E, Pollegioni L, Veronesi F, Rosellini D (2014) Expression of an evolved engineered variant of a bacterial glycine oxidase leads to glyphosate resistance in alfalfa. J Biotechnol 184:201–208
Njiti VN, Myers O Jr, Schroeder D, Lightfoot D (2003) Roundup ready soybean: glyphosate effects on Fusarium solani root colonization and sudden death syndrome. Agron J 95:1140–1145
Nolan BL (2016) The effects of tillage, glyphosate, and genetic modification on bacterial root endophyte composition in Zea mays. Honors Thesis 138. https://egrove.olemiss.edu/hon_thesis/138
Norsworthy JK, Burgos NR, Oliver LR (2001) Differences in weed tolerance to glyphosate involve different mechanisms. Weed Technol 15:725–731
Obojska A, Ternan NG, Lejczak B, Kafarski P, McMullan G (2002) Organophosphonate utilizatjon by the thermophile Geobacillus caldoxyylosilyticus T20. Appl Environ Microbiol 68:2081–2084
Onyon LJ, Volans GN (1987) The epidemiology and prevention of paraquat poisoning. Human Toxicol 6:19–29
Orcaray L, Igal M, Marino D, Royuela M (2010) The possible role of quinate in the mode of action of glyphosate and acetolactate synthase inhbitors. Pest Manag Sci 66:262–269
Pan L, Yu Q, Han H, Mao L, Nyporko A, Fan L, Bai L, Powles S (2019) Aldo-keto reductase metabolizes glyphosate and confers glyphosate resistance in Echinocloa colona. Plant Physiol. https://doi.org/10.1104/pp.19.00979
Pankey JH, Griffin JL, Colyer PD, Schneider RW, Miller DK (2005) Preemergence herbicide and glyphosate effect on seedling disease in glyphosate-resistant cotton. Weed Technol 19:312–318
Patterson EL, Saski C, Küpper A, Beffa R, Gaines TA (2020) Omics potential in herbicide-resistant weed management. Plan Theory 8:607. https://doi.org/10.3390/plants8120607
Pazdiora PC, Piasecki C, Dorneles KR, Agostinetto D, Vargas L, Dallagnol LJ (2019) Glyphosate-resistant Conyza bonariensis is susceptible to powdery mildew caused by Podosphaera erigerontis-canadensis. Plant Dis 103:365
Pehl CE, Shelnutt HE (1990) Hexazinone influences on Pinus taeda seedlings. Forest Ecol Manage 35:271–276
Pereira FCM, Tayengwa R, Alves PLDCA, Peer WA (2019) Phosphate status affects phosphate transporter expression and glyphosate uptake and transport in grand eucalyptus (Eucalyptus grandis). Weed Sci 67:29–40
Pincelli-Souza RP, Bortolheiro FPAP, Carbonari CA, Velini ED, Silva MA (2020) Hormetic effect of glyphosate persists during the entire growth period and increase sugarcane yield. Pest Manag Sci 76:2388–2394
Pleasants JM, Oberhauser KS (2013) Milkweed loss in agricultural fields because of herbicide use: effect on the monarch butterfly population. Insect Conserv Diver 6:135–144
Pollegioni L, Schonbrunn E, Siehl D (2011) Molecular basis of glyphosate resistance – different approaches through protein engineering. FEBS J 278:2753–2766
Pombo G, Orzolek MD, Tukey LD, Pyzik TP (1985) The effect of paclobutrazol, diaminozide, glyphosate and 2,4-D in gel on the emergence and growth of germinated tomato seeds. J Hort Sci 60:353–367
Pradhan S, Flores FJ, Melouk H, Walker NR, Molineros JE, Garzon CD (2017) Chemical hormesis on plant pathogenic fungi and oomycetes. Amer Chem Soc Symp Ser 1249:121–133
Qiao C, Wang C, Pang R, Tian F, Han L, Guo L, Luo J, Li J, Pang T, Xie H, Fang J (2020) Environmental behavior and influencing factors in peach orchard ecosystem. Ecotoxicol Environ Saf 206:111209. https://doi.org/10.1016/j.ecoenv.2020.111209
Rajaa R, Nguyen TT, Slaughter DC, Fennimore SA (2020) Real-time weed-crop classification and localization technique for robotic weed control in lettuce. Biosyst Eng 192:257–274
Ramirez-Villacis DX, Finkel OM, Salas-González I, Fitzpatrick CR, Dangl JL, Jones CD, Leon-Reyes A (2020) Root microbiome modulates plant growth promotion induced by low doses of glyphosate. mSphere 5(4):e00484–e00420
Ratcliff AW, Busse MD, Shestak CJ (2006) Changes in microbial community structure following herbicide (glyphosate) additions to forest soils. App Soil Ecol 34:114–124
Reddy KN, Koger CH (2004) Live and killed hairy vetch cover crop effects on weeds and yield in glyphosate-resistant corn. Weed Technol 18:835–840
Reddy KN, Zablotowicz RM (2003) Glyphosate-resistant soybean response to various salts of glyphosate and glyphosate accumulation in soybean nodules. Weed Sci 51:496–502
Reddy KN, Hoagland RE, Zablotowicz RM (2001) Effect of glyphosate on growth, chlorophyll, and nodulation in glyphosate-resistant and susceptible soybean (Glycine max) varieties. J New Seeds 2:37–52
Reddy KN, Duke SO, Rimando AM (2004) Aminomethlphosphonic acid, a metabolie of glyphosate, causes injury in glyphosate-treated, glyphosate-resistant soybean. J Agric Food Chem 52:5139–5143
Reddy KN, Rimando AM, Duke SO, Nandula VK (2008) Aminomethylphosphonic acid accumulation in plant species treated with glyphosate. J Agric Food Chem 56:2125–2130
Reddy KN, Ding W, Zablotowicz RM, Thomson SJ, Huang Y, Krutz LJ (2010) Biological responses to glyphosate drift from aerial application in non-glyphosate-resistant corn. Pest Manag Sci 66:1148–1154
Reddy KN, Cizdziel JV, Williams MM, Maul JE, Rimando A, Duke SO (2018) Glyphosate resistance technology has minimal effect on maize mineral nutrition and yield. J Agric Food Chem 66:10139–10146
Renné P, Dressen U, Hebbeker U, Hille D, Flügge UI, Westhoff P, Weber APM (2003) The Arabidopsis mutant dct is deficient in the plastidic glutamate/malate translocator DiT2. Plant J 35:316–331
Roberts F, Roberts CW, Johnson JJ, Kyle DE, Krell T, Coggins JR, Coombs GH, Milhous WK, Tzipori S, Ferguson DJ, Chakrabarti D, McLeod R (1998) Evidence for the shikimate pathway in apicomplexan parasites. Nature 393:801–805
Roberts CW, Roberts F, Lyons RE, Kirisits MJ, Mui EJ, Finnerty J, Johnson JJ, Ferguson DJP, Coggins JR, Krell T et al (2002) The shikimate pathway and its branches in apicomplexan parasites. J Infect Dis 185(Suppl):S25–S36
Rodrigues JJ, Worsham AD, Corbin FT (1982) Exudation of glyphosate from wheat (Triticum aestivum) plants and its effects on interplanted corn (Zea mays) and soybeans (Glycine max). Weed Sci 30:316–320
Rodríguez-Gil JL, Prosser RS, Duke SO, Solomon KR (2020) Ecotoxicology of glyphosate, its formulants, and environmental degradation products. Rev Environ Contam Toxicol. (this volume)
Roisch U, Lingens F (1980) The mode of action of the herbicide N-(phosphonomethyl)glycine: its effect on the growth and enzymes of aromatic amino biosynthesis of Escherichia coli. Hoppe Seylers Z Physiol Chem 361:1049–1058
Rose-Fricker C (2002) Glyphosate tolerant fescue grass variety. US Patent 6,423,887 B1, July, 23, 2002
Samac D, Foster-Hartnett D (2012) Effect of glyphosate application on foliar diseases of glyphosate-tolerant alfalfa. Plant Dis 96:1104–1110
Sammons RD, Gaines TA (2014) Glyphosate resistance: state of knowledge. Pest Manag Sci 70:1367–1377
Sammons RD, Gruys KJ, Anderson KS, Johnson KA, Sikorski JA (1995) Reevaluating glyphosate as a transition-state inhibitor of EPSP synthase: identification of an EPSP synthase•EPSP•glyphosate ternary complex. Biochemistry 34:6433–6440
Sammons RD, You J, Qi Y, Flasinski S, Kavanaugh C, Washam J, Ostrander E, Wang D, Heck G (2018) Evaluation of glyphosate resistance in Arabidopsis thaliana expressing an altered target site EPSPS. Pest Manag Sci 74:1174–1183
Samsel A, Seneff S (2013) Glyphosate’s suppression of cytochrome P450 enzymes and amino acid biosynthesis by the gut microbiome: pathways to modern diseases. Entropy 15:1416–1463
Sanogo S, Yang XB, Lundeen P (2001) Field response of glyphosate-tolerant soybean to herbicides and sudden death syndrome. Plant Dis 85:773–779
Saunders LE, Pezeshki R (2015) Glyphosate in runoff waters and in the root-zone: a review. Toxics 3:462–480
Schafer JR, Hallett SG, Johnson WG (2012) Response of giant ragweed (Ambrosia trifida), horseweed (Conyza canadensis), and common lambsquaters (Chenopodium album) biotypes to glyphosate in the presence of absence of soil microorganisms. Weed Sci 60:641–649
Schafer JR, Hallett SG, Johnson WG (2013) Soil microbial root colonization of glyphosate-treated giant ragweed (Ambrosia trifida), horseweed (Conyza canadensis), and common lambsquarters (Chenopodium album) biotypes. Weed Sci 61:289–295
Schlatter DC, Yin C, Hulbert S, Burke I, Paulitz T (2017) Impact of repeated use on wheat-associated bacterial are small and depend on glyphosate use history. Appl Environ Microbiol 83:e0134–e0117. https://doi.org/10.1128/AEM.0134-17
Schönherr J (2002) A mechanistic analysis of penetration of glyphosate salts across astomatous cuticular membranes. Pest Manag Sci 58:342–351
Schrübbers LC, Valverde BE, Sørensen JC, Cedergreen N (2014) Glyphosate spray drift in Coffea arabica – sensitivity of coffee plants and possible use of shikimic acid as a biomarker for glyphosate exposure. Pest Biochem Physiol 115:15–22
Sebastian DJ, Nissen SJ, Sebastian JR, Beck KG (2017) Seed bank depletion: the key to long-term downy brome (Bromus tectorum L.) management. Rangeland Ecol Manag 70:477–483
Selvapanidiyan A, Bhatnagar RK (1994) Isolation of a glyphosate-metabolizing Pseudomonas: detection, partial purification and localization of carbon-phosphorous lyase. Appl Microbiol Biotechnol 40:876–882
Sengupta D, Naik D, Reddy AR (2015) Plant aldo-keto reductases (AKR) as multi-tasking soldiers involved in diverse plant metabolic processes and stress defense: a structure-function update. J Plant Physiol 179:40–55
Servaites JC, Tucc MA, Geiger DR (1987) Glyphosate effects on carbon assimilation, ribulose bisphosphate carboxylase activity, and metabolite levels in sugar beet leaves. Plant Physiol 85:370–374
Shaner DL, Nadler-Hassar T, Henry WB, Koger CH (2005) A rapid in vivo shikimate accumulation assay with excised leaf discs. Weed Sci 53:769–774
Sharma U, Adee EA, Pfender WF (1989) Effect of glyphosate herbicide on pseudothecia formation by Pyrenophora triticirepentis in infested wheat straw. Plant Dis 73:647–650
Sharon A, Amsellem Z, Gressel J (1992) Glyphosate suppression of an elicited response. Increased susceptibility of Cassia obtusifolia to a mycoherbicide. Plant Physiol 98:654–659
Shieh W-J, Geiger DR, Buczynski SR (1993) Distribution of imported glyphosate in quackgrass (Elytrigia repens) rhizomes in relation to assimilate accumulation. Weed Sci 41:7–11
Simonsen L, Fomsgaard IS, Svensmark B, Spliid NH (2008) Fate and availability of glyphosate and AMPA in agricultural soil. J Environ Sci Health 43B:365–375
Singh BK, Shaner DL (1998) Rapid determination of glyphosate injury to plants and identification of glyphosate-resistant plants. Weed Technol 12:527–530
Singh S, Kumar V, Datta S, Wani AB, Dhanjal D, Romero R, Singh J (2020) Glyphosate uptake, translocation, resistance emergence in crops, analytical monitoring, toxicity and degradation: a review. Environ Chem Lett 18:663–702
Solomon KR (2020) Estimated exposure to glyphosate in humans via environmental, occupational, and dietary pathways: an updated review of the scientific literature. Pest Manag Sci 76:2878–2885
Solomon KR, Anadón A, Carrasquilla G, Cerdeira AL, Marshall J, Sanin L-H (2007) Coca and poppy eradication in Colombia: environmental and human health assessment of aerially applied glyphosate. Rev Environ Contam Toxicol 190:43–125
Sørensen FW, Gregersen M (1999) Rapid lethal intoxication caused by the herbicide glyphosate-trimesium (touchdown). Human Exp Toxicol 18:735–737
Steinrücken HC, Amrhein N (1980) The herbicide glyphosate is a potent inhibitor of 5-enolpyruvyl-shikimic acid-3-phosphate synthase. Biochem Biophys Res Commun 94:1207–1212
Steinrücken HC, Amrhein N (1984) 5-Enolpyruvylshikimate-3-phosphate synthase of Klebsiella pneumoniae. 2. Inhibition by glyphosate [N-(phosphonomethyl)glycine]. Eur J Biochem 143:351–357
Sturtz AV, Christie BR, Nowak J (2000) Bacterial endophytes: potential role in developing sustainable systems of crop production. Crit Rev Plant Sci 19:1–30
Suzuki N, Koussevitsky S, Mittler R, Miller G (2012) ROS and redox signaling in the response of plants to abiotic stress. Plant Cell Environ 35:259–270
Takano HK, Dayan FE (2020) Glufosinate-ammonium: a review of the current state of knowledge. Pest Manag Sci 76:3911–3925
Takano HK, Patterson EL, Nissen SJ, Dayan FE, Gaines TA (2019) Predicting herbicide movement across semi-permeable membranes using three phase partitioning. Pestic Biocchem Physiol 159:22–26
Tétard-Jones C, Edwards R (2016) Potential roles for microbial endophytes in herbicide tolerance in plants. Pest Manag Sci 72:202–209
Thogmartin WE, Wiederholt R, Oberhauser K, Drum RG, Diffendorfer JE, Altizer S, Taylor OR, Pleasants J, Semmens D, Semmens B, Erickson R, Libby K, Lopez-Hoffman L (2017) Monarch butterfly population decline in North America: identifying the threatening processes. R Soc Open Sci 4:170760. https://doi.org/10.1098/rsos.170760
Tohge T, Watanabe M, Hoefgen R, Fernie AR (2013) Shikimate and phenylalanine biosynthesis in green linage. Front Plant Sci 4:64
Torstensson NTL, Aamisepp A (1977) Detoxification of glyphosate in soil. Weed Res 17:209–212
Tuffi-Santos LD, Graça RN, Alfenas AC, Ferreira FA, Melo CA, Machado MS (2011) Glyphosate reduces urediniospore development and Puccinia psidii disease severity on Eucalyptus grandis. Pest Manag Sci 67:876–880
United States Electronic Code of Federal Regulations, §180.364 Glyphosate; tolerances for residues. https://www.ecfr.gov/cgi-bin/retrieveECFR?gp=1&SID=725587cc6c286ff9ca686aca984ab681&ty=HTML&h=L&mc=true&r=SECTION&n=se40.26.180_1364. Accessed 23 Jul 2020
United States Geological Survey (2020) Estimated annual agricultural pesticide use, Pesticide use maps – glyphosate. https://water.usgs.gov/nawqa/pnsp/usage/maps/show_map.php?year=2016&map=GLYPHOSATE&hilo=L&disp=Glyphosate. Accessed 28 Jul 2019
USDA AMS (2020) United States Department of Agriculture Agricultural Marketing Service, Pesticide Data Program. https://www.ams.usda.gov/datasets/pdp. Accessed 26 Jan 2020
Van Bruggen AHC, He MM, Shin K, Mai V, Jeong KC, Finckh MR, Morris JG (2018) Environmental and health effects of the herbicide glyphosate. Sci Total Environ 616–617:255–268
van Horn CR, Moretti ML, Robertson RR, Segobye K, Weller SC, Young BG, Johnson WG, Schulz B, Green AC, Jeffery T, Lespérance MA, Tardiff FJ, Sikkema PH, Hall JC, McLean MD, Lawton MB, Sammons RD, Wang D, Westra P, Gaines TA (2018) Glyphosate resistance in Ambrosia trifida: part 1. Novel rapid cell death response to glyphosate. Pest Manag Sci 74:107–1078
Velini ED, Alves E, Godoy MC, Meschede DK, Souza RT, Duke SO (2008) Glyphosate applied at low doses can stimulate plant growth. Pest Manag Sci 64:489–496
Velini ED, Trindade MLB, Barberis LRM, Duke SO (2010) Growth regulation and other secondary effects of herbicides. Weed Sci 58:351–354
Velini ED, Carbonari CA, Trindade MLB, Gomes GLGC, Antuniassi UR (2017) Variations in pesticide doses under field conditions. Am Chem Soc Symp Ser 1249:47–60
Vemanna RS, Vennapusa AR, Easwaran M, Chandrashekar BK, Rao H, Ghanti K, Sudhakar C, Mysore KS, Makarla U (2017) Aldo-keto reductase enzymes detoxify glyphosate and improve herbicide resistance in plants. Plant Biotechnol J 15:794–804
Viana RS, Lisboa LAM, Figueredo PAM, Ramos SB, Ferrari S, May A, Prado EP, Miasaki CT, Ferreira IS, Brenha JAM (2019) Productivity and biochemical characteristics of sugarcane when submitted to the action of chemical ripeners. Aust J Basic Appl Sci 13:64–71
Wagner R, Kogan M, Parada AM (2003) Phytotoxic activity of root absorbed glyphosate in corn seedlings (Zea mays L.). Weed Biol Manag 3:228–232
Wang Z-Y, Brummer EC (2012) Is genetic engineering ever going to take off in forage, turf and bioenergy crop breeding? Ann Bot 110:1317–1325
Wang WZ, Yang BP, Feng XY, Cao ZY, Feng CL, Wang JG, Xiong GR, Shen LB, Zeng J, Zhao TT, Zhang SZ (2017) Development and characterization of transgenic sugarcane with insect resistance and herbicide tolerance. Front Plant Sci 8:1535. https://doi.org/10.3389/fpls.2017.01535
Wauchope RD (1976) Acid dissociation constants or arsenic acid, methylarsonic acid (MAA), dimethylarsinic acid (cacodylic acid), and N-(phosphonomethyl) glycine. J Agric Food Chem 24:717–721
Weaver MA, Krutz LJ, Zablotowicz RM, Reddy KN (2007) Effects of glyphosate on soil microbial communities and its mineralization in a Mississippi soil. Pest Manag Sci 63:388–393
Weise A, Schulte M, Theuvsen L, Steinmann H-H (2018) Interactions of glyphosate use with farm characteristics and cropping systems in Central Europe. Pest Manag Sci 74:1155–1165
Westerman RB, Murray DS (1994) Silverleaf nightshade (Solanum elaeagnifolium) control in cotton (Gossipium hirsutum) with glyphosate. Weed Technol 8:720–727
Weston LA (1990) Cover crop and herbicide influence on row crop seedling establishment. Weed Sci 38:166–171
Whigham DK, Stoller EW (1979) Soybean desiccation by paraquat, glyphosate, and ametryn to accelerate harvest. Agron J 71:630–633
Wicke D, Schulz LM, Lentes S, Scholz P, Poehlein A, Gibhardt J, Daniel R, Ischebeck T, Commichau FM (2019) Identification of the first glyphosate transporter by genomic adaptation. Envion Microbiol 21:1287–1305
Wiese A, Schulte M, Theuvsen L, Steinmann H-H (2018) Interactions of glyphosate use with farm characteristics and cropping patterns in Central Europe. Pest Manag Sci 74:1155–1165
Williams MM, Bradley CA, Duke SO, Maul JE, Reddy KM (2015) Goss’s wilt incidence in sweet corn is independent of transgenic traits and glyphosate. HortScience 50:1791–1794
Wong PTW, Dowling PM, Tesoriero LA, Nicol HI (1993) Influence of preseason weed management and in-crop treatments on two successive wheat crops. 2. Take-all severity and incidence of Rhizoctonia root rot. Aust J Exp Agric 33:173–177
Wood LJ (2019) The presence of glyphosate in forest plants with different life strategies one year after application. Can J For Res 49:586–594
Xiang W-S, Wang X-J, Ren T-R, Ju X-L (2005) Expression of a wheat cytochrome P450 monooxygenase in yeast and its inhibition by glyphosate. Pest Manag Sci 61:402–406
Xu J, Smith S, Smith G, Wang W, Li Y (2019) Glyphosate contamination in grains and foods: an overview. Food Control 106:106710
Yael JA, Fuhr JD, Bocan GA, Millone AD, Tognalli N, dos Santos Afonso M, Martiarena ML (2014) Abiotic degradation of glyphosate into aminomethylphosphonic in the presence of metals. J Agric Food Chem 62:9651–9656
Yamada T, Kremer RJ, Castro PRC, Wood BW (2009) Glyphosate interactions with physiology, nutrition, and disease of plants: threat to agricultural sustainability. Eur J Agron 31:111–113
Yu Q, Jalaludin A, Han H, Chen M, Sammons RD, Powles SB (2015) Evolution of a double amino acid substitution in the 5-enolpyruvylshikimate-3-phosphate synthase in Eleusine indica conferring high-level glyphosate resistance. Plant Physiol 167:1440–1447
Zabaloy MC, Carné I, Viassolo R, Gómez MA, Gornez E (2016) Soil ecotoxicity assessment of glyphosate use under field conditions: microbial activity and community structure of eubacteria and ammonia-oxidizing bacteria. Pest Manag Sci 72:684–691
Zabalza A, Orcaray L, Fernández-Escalada M, Zulet-González A, Royuela M (2017) The pattern of shikimate pathway and phenylpropanoids after inhibition by glyphosate or quinate feeding in pea roots. Pestic Biochem Physiol 141:96–102
Zabalza A, Zulet-González A, Barco-Antoñanzas EMV, Gil-Monreal M, Royuela M (2020) Physiological approach to the use of the natural compound quinate in the control of sensitive and resistant Papaver rhoeas. Plan Theory 9:1215. https://doi.org/10.3390/plants9091215
Zablotowicz RM, Reddy KN (2007) Nitrogenase activity, nitrogen content, and yield responses to glyphosate in glyphosate-resistant soybean. Crop Prot 26:370–376
Zapiola ML, Mallory-Smith CA (2012) Crossing the divide: gene flow produces intergenic hybrids in feral transgenic creeping bentgrass population. Mol Ecol 21:4672–4680
Zboińska E, Maliszewska I, Lejczak B, Kafarski P (1992) Degradation of organophosphonates by Penicillium citrinum. Lett App Micobiol 15:269–272
Zhan H, Feng Y, Fan X, Chen S (2018) Recent advances in glyphosate biodegradation. Appl Microbiol Biotechnol 102:5033–5043
Ziska LH (2014) Climate, CO2 and invasive weed management. In: Dukes JS (ed) Ziska LH. CAB International, Invasive species and global climate change, pp 293–304
Ziska LH, Goins EW (2006) Elevated atmospheric carbon dioxide and weed populations in glyphosate treated soybean. Crop Sci 46:1354–1359
Ziska LH, Teasdale BJA (1999) Future atmospheric carbon dioxide may increase tolerance to glyphosate. Weed Sci 47:608–615
Ziska LH, Teasdale JR (2000) Sustained growth and increased tolerance to glyphosate observed in a C3 perrenial weed, quackgrass (Elytrigia repens), grown at elevated carbon dioxide. Funct Plant Biol 27:159–166
Ziska LH, Faulkner S, Lydon J (2004) Changes in biomass and root:shoot ration of field-grown Canada thistle (Cirsium arvense), as noxious, invasive weed, with elevated CO2: implications for control with glyphosate. Weed Sci 52:584–588
Zobiole LHS, Oliveira RS, Huber DM, Constantin J, Castro C, Oliviera FA, Oliveira A (2010) Glyphosate reduces shoot concentrations of mineral nutrients in glyphosate-resistant soybeans. Plant Soil 328:57–79
Zoller O, Rhyn P, Rupp H, Zarn JA, Geiser C (2018) Glyphosate residues in Swiss market foods: monitoring and risk evaluation. Food Additives Contam 11B:83–91
Zulet A, Zabalza A, Royuela M (2013) Phytotoxic and metabolic effects of exogenous quinate on Pisum sativum L. J Plant Growth Regul 32:779–788
Zulet-González A, Fernández-Escalada M, Zabalza A, Royuela M (2019) Enhancement of glyphosate efficiency on Amaranthus palmeri by exogenous quinate application. Pestic Biochem Physiol 158:1–11
Zulet-González A, Barco-Antoñanzas M, Gil-Monreal M, Royuela M, Zabalza A (2020) Increased glyphosate-induced gene expression in the shikimate pathway is abolished in the presence of aromatic amino acids and mimicked by shikimate. Front Plant Sci. https://doi.org/10.3389/fpls.2020.00459
Acknowledgments
I thank Drs. Ana Zabalza and Douglas Sammons for their advice about certain aspects of the review.
The author has no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Duke, S.O. (2020). Glyphosate: Uses Other Than in Glyphosate-Resistant Crops, Mode of Action, Degradation in Plants, and Effects on Non-target Plants and Agricultural Microbes. In: Knaak, J.B. (eds) Reviews of Environmental Contamination and Toxicology Volume 255. Reviews of Environmental Contamination and Toxicology, vol 255. Springer, Cham. https://doi.org/10.1007/398_2020_53
Download citation
DOI: https://doi.org/10.1007/398_2020_53
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-68482-2
Online ISBN: 978-3-030-68483-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)