Abstract
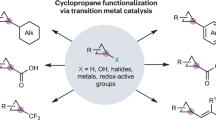
The present work describes a comprehensive review of the functionalization of cyclopropyl C–H bonds via transition-metal catalysis. Compared to the enormous number of publications related to direct sp2 and sp3 bond transformations in the last two decades, the first full account of direct cyclopropyl C(sp3)–H bond functionalization was only disclosed in 2011. Both intra- and intermolecular transformations are detailed in the review, including asymmetric reactions. In addition, mechanistic aspects of various Pd-catalyzed cyclopropane functionalizations are discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The cyclopropane ring is a versatile building block in organic synthesis [1]. Cyclopropanes possess unique physical, chemical, and electronic properties as a result of ring strain (27.5 kcal/mol) [2, 3]. Found in numerous natural products, the smallest carbocycle has been both a challenge and an inspiration for synthetic organic chemists [4, 5]. Cyclopropanes are also commonly encountered in current drug targets, where they act as conformational restrictors, thereby orienting a molecule into its bioactive conformation and potentially increasing its potency or providing an improvement in metabolic stability of certain compounds [6]. Recently, the cyclopropane ring was ranked 10th in a top 100 list of the most frequently used rings in the synthesis of small molecule drugs, thus highlighting its continual relevance in medicinally active compounds [7].
Significant efforts have been dedicated to the syntheses of cyclopropanes in both racemic or enantioenriched forms [8–10]. Cyclopropanes may also act as precursors en route toward accessing more chemical complexity, via involvement in various reactions such as cycloadditions, ring openings, or cross-couplings. In particular, there is ample literature precedence for the functionalization of cyclopropanes via cross-coupling reactions [11]. The cyclopropane can act as either the “nucleophile” (organometallic reagent) or “electrophile” (cyclopropyl halide). Both roles can require numerous synthetic steps to achieve the pre-functionalized partners, resulting in unnecessary waste. One solution to the problem of pre-functionalization would be to use only one pre-functionalized coupling partner and employ the cyclopropyl C–H bond as a functional group. The inherent ring strain of the three-membered ring and orbital rehybridization results in enhanced acidity of cyclopropane C–H bonds, thereby facilitating such transformations. A commonly encountered problem in the activation of alkanes with transition metals is their propensity for β-hydride elimination [12]. In cyclopropanes, β-hydride elimination would result in the formation of a cyclopropene species, which is unfavorable thermodynamically [11]. While C–H bond functionalization at sp2 and sp3 centers has been vastly explored in the last decades [13–16], full reports concerning cyclopropanes have only lately appeared in the literature. The current chapter presents a comprehensive review of the direct transformations of the cyclopropane C–H bond via transition-metal catalysis.
2 Early Contributions from the Yu and Sanford Groups
The functionalization of cyclopropanes such as 1 via amide-directed metalation followed by quenching with an electrophile (e.g., iodine) is well known in the literature (Scheme 1a) [17, 18]. However, it was not until 2005 that the first example of a transition-metal-catalyzed direct functionalization process of a cyclopropane C–H bond was disclosed [19]. The use of a chelating oxazoline-based auxiliary (derived from (S)-tert-leucinol) enabled iodination of primary C(sp3)–H bonds under Pd catalysis at room temperature. In one example, the secondary C–H group at the β-position of cyclopropyl substrate 4 was exclusively monoiodinated in 65% yield, in preference to the methyl group (Scheme 1b). However, long reaction times (4 days) were required. The oxazoline auxiliary could subsequently be removed under acidic conditions to provide the corresponding cyclopropane carboxylic acid in 99% ee.
Later, Yu and coworkers investigated the coupling of C(sp3)–H bonds with boronic acids, employing Pd(OAc)2 as the catalyst and benzoquinone (BQ)/Ag2O as oxidants [20] (Scheme 2). The strongly binding, but easily removed, O-methyl hydroxamic acid was employed as directing group. The use of 2,2,5,5-tetramethyltetrahydrofuran as solvent was necessary, as it was believed that the bulky solvent not only prevented homocoupling of the boronic acid but also β-hydride elimination. Phenethyl and iso-butyl boronic acids were employed as coupling partners to provide the corresponding cyclopropyl alkylated products 7 and 8 in 72% and 58% yields, respectively. To improve the practicality of the transformation, the Ag(I) salt could be replaced by air (20 atm) as oxidant.
A unique example of direct olefination of a cyclopropane was also disclosed by the Yu lab [21]. An electron-deficient arylamide was employed as directing group, as the previously employed oxazoline or hydroxamic acid was unreactive in the alkenylation. The proposed mechanism for the reaction involves an amide-directed C–H insertion of the Pd(II) catalyst into the cyclopropane methylene C–H bond of 9, followed by olefin carbopalladation and β-hydride elimination to provide intermediate 10 (Scheme 3a). Pd(0) is re-oxidized back to Pd(II) by Ag(I)/Cu(II), and a tandem 1,4-addition between the amide moiety of 10 and the acrylate provides the corresponding γ-lactam 11 as the sole isolated product. In the presence of an α-methyl substituent, the cyclopropane C–H bond is olefinated exclusively in 84% yield to deliver a mixture of cis-/trans-isomers. However, if an α-aryl substituent is present, significant amounts (18% or 33% yield, respectively) of the competitive sp2 olefination products 14 and 17 are obtained. Direct cyclopropane carbonylation in the presence of an N-arylamide auxiliary is also possible under Pd catalysis [22]. After an amide-directed Pd activation of the cyclopropyl C–H bond of 18, a migratory insertion of CO takes place, and the subsequent Pd intermediate undergoes C–N reductive elimination to provide a succinimide product 19 in 86% yield (Scheme 3b). More recently, Chatani demonstrated cyclopropyl carbonylation in the presence of an N,N-bidentate group under Ru3(CO)12 catalysis [23].
Sanford and Kubota investigated the functionalization of cyclopropanes under oxidative conditions, employing directing groups such as oximes or pyridines [24]. Iodination of the cyclopropyl C–H bond was found to be dependent on the steric and electronic environment of the auxiliary; for example, in the absence of the methyl group at the α-position of oxazoline 16, there was no conversion to product (Scheme 4a). Various substituted pyridines (21, 23, and 25) could direct the iodination, albeit in low yields, even after long reaction times (Scheme 4a). Attempts at cyclopropane acetoxylation with PhI(OAc)2 using various directing groups, as shown for substrates 4 and 27, resulted in ring opening of the three-membered ring leading to allylic acetates 28 and 29 (Scheme 4b). In a subsequent communication, Sanford and coworkers disclosed one example of alkenylation of a cyclopropyl C–H bond, resulting in the formation of cyclized pyridinium product 30 in a modest 43% yield (Scheme 4c) [25]. The reaction employed a cationic Pd catalyst, as well as a vanadium-based heteropolyacid (H4[PMo11VO40]) and air as co-oxidants.
3 Intramolecular Direct Functionalization of Cyclopropanes
Similar to the intramolecular functionalization of aryl or alkyl substrates, use of a heteroatom containing-tether not only limits the degree of freedom in the system but also allows for coordination of a transition metal, thus facilitating the reaction.
Rousseaux, Liegault, and Fagnou reported the elegant formation of quinoline and tetrahydroquinoline derivatives via a Pd(0)-catalyzed C–H activation of cyclopropane methylene bond [26]. Both bromophenyl and chlorophenyl cyclopropyl carbamates 31 and 32 were found to be suitable substrates for the transformation into dihydroquinoline 33, albeit under slightly different conditions (Scheme 5). It was found that the resulting dihydroquinolines 33 were prone to decomposition; thus protocols for either oxidation or reduction of the unstable intermediates were developed. A variety of substituents (nitro, cyano, trifluoromethyl, methoxy, ester) were tolerated in the positions para and meta to the bromide or chloride. A methyl in the ortho position provided the corresponding tetrahydroquinoline in 99% yield, demonstrating that the reaction is not sensitive to steric effects.
The authors then investigated the reaction pathway, focusing on whether a concerted metalation–deprotonation (CMD) step occurred prior to the cyclopropane ring opening (recent examples of Pd-catalyzed ring opening of cyclopropanes: [27, 28], [29]). In the absence of a pivalate source, no dihydroquinoline 33 was observed in the cyclization, underlining the involvement of pivalate in the CMD step. Traces of cyclopropyl product 36 were isolated from the reaction of chlorophenyl cyclopropyl carbamate 32, suggesting the presence of 36 as a possible intermediate in the mechanism (Scheme 6). However, a subsequent control reaction demonstrated that 36 does not undergo ring opening when submitted to the reaction conditions. The result supports the mechanistic proposal that the C–H activation step occurs prior to ring opening and the ring opening of the cyclopropane precedes reductive elimination.
Based on the studies, the following mechanism was proposed: oxidative addition of Pd(0) into the aryl halide bond (step A, Scheme 7) is followed by ligand exchange to provide a Pd(II)-pivalate species (step B). Then, pivalate-assisted CMD of the cyclopropyl C–H bond results in the formation of a six-membered palladacycle (step C), which undergoes a cyclopropane ring opening/proton transfer to release ring strain (step D). Deprotonation and reductive elimination (step E) provides the dihydroquinoline 33.
A further example of cyclopropyl C–H activation followed by ring opening and cyclization was reported by the Charette group in the synthesis of novel seven-membered benzo[c]azepine-1-one products [30]. Both bromo- and iodo-cyclopropyl benzamides 37 and 38 were effective substrates for the transformation, providing two isomeric benzazepine-type products 39 and 40 in excellent overall yield (Scheme 8a). When each isomer was separately resubmitted to the reaction conditions, no change was observed for 39, while 40 slightly isomerized to 39, suggesting that 40 is the kinetic product and 39 the thermodynamic one. When cyclopropyl benzamide 37 is submitted to Fagnou’s reaction conditions with cesium pivalate, a mixture of spirooxindoles 41 and 42, as well as benzazepine 39, is produced, suggesting the intermediacy of 41 in the reaction pathway. However, similar to the control reaction performed in the former Fagnou’s report (Scheme 6), 41 was fully recovered when submitted to the optimized reaction conditions. In contrast to other uses of Ag(I) salts as halide sequesters or oxidants, the authors highlight the employment of exactly 1 equiv. Ag+ as a halide abstractor to provide a reactive cationic Pd species.
The proposed mechanism involves an initial oxidative addition (step A, Scheme 9), followed by halide abstraction by the silver ion to provide a highly reactive cationic Pd species (step B). Acetate-mediated CMD (step C) provides a rare seven-membered ring palladacycle which undergoes ring opening (step D), followed by deprotonation/reductive elimination (step E) to provide the desired products.
Additionally, Charette and coworkers disclosed the synthesis of biologically active 3,3′-cyclopropyl oxindole 44 via a Pd-catalyzed, Ag-promoted C–H functionalization of cyclopropanecarboxamide 43 derived from 2-bromoaniline (Scheme 10) [31]. Substitution on the aryl ring or on the cyclopropane, including heterocycles such as furan or thiophene, was well tolerated. A mixture of diastereomers was obtained when aryl substitution was present on the cyclopropane. X-ray crystallography confirmed the structure of both diastereomers.
To investigate whether an enolate arylation was occurring, the authors prepared enantioenriched cyclopropane substrate 45 and submitted it to the reaction conditions (Scheme 11). After 3 h, all starting material was consumed, and spirooxindole product 46 showed little erosion of enantioselectivity. Furthermore, the kinetic isotope effect was determined via parallel reactions to be 3.9, identifying C–H cleavage as a rate-determining step. This observation is not consistent with an enolate-like pathway. Furthermore, the use of a weak base (K2CO3) makes the enolate pathway quite unlikely.
Based on all the observations, the mechanism shown in Scheme 12 was proposed. An initial oxidative addition step A is followed by bromide abstraction by Ag+ to give a cationic Pd species (step B), which can undergo a concerted metalation–deprotonation step mediated by carbonate (or phosphate, C and D). The six-membered palladacycle then undergoes reductive elimination (step E) to give the product and regenerate the Pd0 catalyst.
A related synthesis of 3,3′-cyclopropyl oxindoles was reported by Takemoto et al. [32]. Carbamoyl chloride 47, containing a cyclopropyl ring at the ortho position, undergoes a Pd-catalyzed cyclization onto the benzylic C–H bond of the cyclopropane to provide the corresponding spirooxindole 44 in 60% yield (Scheme 13). Intramolecular competition reactions investigated the chemoselectivity of the cyclization on the cyclopropane C–H bond versus ethyl (48), methyl (49), phenyl (50), and alkene (51). The order of reactivity under the optimized conditions was determined to be the Heck reaction > cyclopropyl C(sp3)–H activation > C(sp2)–H activation > methyl C(sp3)–H activation. In addition, the stereochemistry of the starting material is transmitted to the substrate, as trans-substituted 52 provided the corresponding trans-spirooxindole in 85% yield.
The Cramer group further reported a synthesis of cyclopropyl spiroindolines [33]. Cyclopropyl substrate 53, containing a triflyl-protected aniline, undergoes a Pd(0)-catalyzed, pivalic acid-mediated methine C–H activation via a CMD process (Scheme 14). Remarkably, only the five-membered spiroindoline 54 is isolated which can be explained through the easier formation of a six- versus seven-membered palladacycle intermediate. Various functional groups are tolerated in the transformation, including substitution on the cyclopropane with an aryl or alkyl group. In the latter cases, the stereochemistry of the starting material (either cis or trans) is transferred to the products. A malonate-containing substrate can successfully replace the N-triflyl group, but the oxygen analog failed to cyclize to the desired product. In case of 2,6-dibromoaniline 56, the intramolecular cyclopropane arylation could be followed by a Suzuki coupling to provide 57 in 77% yield or intermolecular C–H arylation to provide 58 in 78% yield in a domino-type reaction (Scheme 15). The triflyl protecting group can be removed by reaction with Red-Al.
Baudoin et al. showed that both methine and methylene cyclopropane C–H bonds can be activated under Pd catalysis to provide the corresponding indanes 60 and 62 (Scheme 16) [34]. The methine functionalization is more efficient (68% vs. 39% yield), due to the formation of a less strained five-membered ring.
4 Intermolecular Direct Functionalization of Cyclopropanes
The concept of bidentate coordination being able to promote sp2 or sp3 C–H activation has been well known in the literature [35]. In comparison to monodentate coordination, bidentate groups maintain a higher degree of stereochemical control around the metal center, which enables the formation of more rigid cyclometalated species. Daugulis et al. introduced the picolinamide and aminoquinolinamide auxiliaries for Pd-catalyzed direct arylation of C(sp2)–H and C(sp3)–H centers with aryl iodides [36, 37]. The initial report by Daugulis inspired many other groups to utilize the abovementioned auxiliaries in a wide range of transformations, including C(sp3)–H arylation or C–heteroatom bond formation [38].
In 2013, Babu et al. [39] and Charette et al. [40] disclosed the direct arylation of cyclopropanes employing the aminoquinolamide and picolinamide auxiliaries, respectively. Thus, the Babu group showed that the methylene C–H bond of cyclopropyl substrate 63 can be functionalized with excess aryl iodide in the presence of catalytic Pd(OAc)2 and stoichiometric AgOAc (Scheme 17) [39]. 2-Methylthioanilide 67 could also be employed as auxiliary; however the arylated cyclopropanes 68 and 69 were obtained in lower yields. Monoarylated cyclopropanes 64–66 and 68–69 were obtained as the cis-diastereomer; moreover, cis-diarylated cyclopropanes can be obtained when excess (8 equiv.) aryl iodide is employed. It is also possible to access mixed triarylated cyclopropylcarboxamides such as 71 starting from trans-disubstituted cyclopropane 70 (Scheme 18). Later, Zeng et al. disclosed one example of the arylation of aminoquinolamide cyclopropane 63 with 4-bromoanisole; however, the yield was only 18% even after 36 h at 140°C [41].
The latter report from the Charette lab disclosed two distinct reaction conditions for the arylation of cyclopropylmethyl picolinamide 72, employing either excess Ag+ in the form of Ag3PO4 (condition A) or catalytic PivOH (condition B), along with catalytic Pd(OAc)2 (Scheme 19) [40]. The cis-diastereomer 73–77a is obtained exclusively, but in many cases traces of diarylated cyclopropanes 73–77b (cis and trans) were observed. The picolinamide group could be transformed into the corresponding tert-butylcarbamate by reaction with Boc2O, followed by oxidative cleavage in the presence of LiOH and H2O2. Only aryl iodides were tolerated as coupling partners in the reports from Babu and Charette.
Mechanistic evidence from the Daugulis lab [36, 37] and others [42] supports a Pd(II)/(IV) pathway for bidentate-directed C–H functionalization. Sustac and Charette also investigated the mechanism of their cyclopropane arylation [40]. It was determined that an acetate source was necessary for the reaction to proceed, because it is presumably involved in the CMD step. Both Pd(0) and Pd(II) sources could be employed in the Ag-mediated reaction. The silver source is proposed to aid with catalyst regeneration by removing iodide or, in the case of Pd(0) sources, to oxidize them to Pd(II). The proposed mechanism is shown in Scheme 20. Coordination of Pd(OAc)2 to the picolinamide 72 to give complex A occurs with loss of one acetate molecule. Then, acetate-mediated concerted metalation–deprotonation provides complex B. Oxidative addition of the aryl iodide gives rise to a highly unstable PdIV complex C, which undergoes reductive elimination to provide complex D. Loss of iodide mediated by Ag3PO4 or Na2CO3 and product dissociation and coordination of PdII to another picolinamide molecule 72 close the catalytic cycle.
The aminoquinolinamide auxiliary was also shown to mediate the construction of 1,1,2-trisubstituted arylcyclopropanes [43]. Substrate 78, bearing a cis-substituted cyclopropyl moiety, reacted smoothly with a variety of (hetero)aryl iodides in a Pd-catalyzed, Ag-mediated transformation (Scheme 21). Notably, substituents such as an aldehyde, a hydroxyl, or an unprotected indole were tolerated under the reaction conditions. Additionally, the reactivity of trans-cyclopropyl substrate 79 was investigated (Scheme 21). The challenge here was overcoming the steric effect of the bulky TBDPS group. It was discovered that adding the base K3PO4 improved the yield of the reaction (41% without vs. 75% with 1 equiv. K3PO4). Various aryl iodides acted as coupling partners in modest to good yields; in general, the yields were lower than in the case of the cis-substrate, presumably due to steric effects. Nonetheless, the reaction represents one of the few examples of intermolecular formation of quaternary centers via tertiary C(sp3)–H functionalization of cyclopropanes.
The Yu group employed a weakly coordinating N-arylamide group for the monoarylation of a cyclopropane 80 with p-iodotoluene in the presence of 2-isobutoxyquinoline (82) as the ligand (Scheme 22) [44]. The ligand had the property of being “mutually repulsive”: it allowed for single coordination of Pd to its pyridine portion but also coordination of Pd to the arylamide group of 80. Such a coordination mode results in an overall lowering the transition state energy of the C(sp3)–H activation.
Furthermore, selective monoarylation of 1-aminocyclopropane-1-carboxylic acid derivative 83 was achieved in the presence of alkoxy-substituted quinoline 85 in good yield (Scheme 23) [45]. Only one diastereomer of the unnatural amino acid derivative 84 was produced.
5 Enantioselective Direct Functionalization of Cyclopropanes
The asymmetric synthesis of cyclopropanes has attracted continual efforts in organic synthesis, due to their relevance in natural products and biologically active compounds. The prevalent methods employed include halomethylmetal mediated processes in the presence of chiral auxiliaries/catalysts (Simmons–Smith-type reactions), transition-metal-catalyzed decomposition of diazoalkanes, Michael-induced ring closures, or asymmetric metalations [8–10, 46]. However, the asymmetric preparation of unfunctionalized cyclopropanes remains relatively undisclosed. The enantioselective activation of unactivated C–H bonds via transition-metal catalysis is an area of active research in organic chemistry [47–49]. Recently, a few groups investigated the enantioselective synthesis of cyclopropanes by direct functionalization reactions.
An intramolecular process for the asymmetric Pd-catalyzed C–H arylation of cyclopropanes was published by the Cramer group [50]. An initial screening of different classes of ligands found TADDOL-type phosphoramidites promising in the synthesis of tetrahydroquinoline 87. Fine-tuning of the TADDOL structure revealed that ligand 86, containing 3,5-xylyl substituents, along with the addition of catalytic amounts of pivalic acid to the reaction, provided the best yields and enantiomeric excesses (Scheme 24). The transformation also represented a rare example of the formation of a seven-membered palladacycle as a reaction intermediate. Notably, the cyclization can be performed with catalyst loadings as low as 1 mol%, without affecting the yield or enantioselectivity. Various α-substituted cyclopropanes were tolerated in the reaction to provide the corresponding tetrahydroquinolines 87–90. Of note, the presence of a phenyl or benzyl group in compounds 88 and 89, respectively, did not result in a competing C(sp2)–H functionalization. In contrast, the absence of α-substitution led to the synthesis of spiroindolines (Scheme 14, vide supra). The triflyl group of 88 was cleaved by reaction with Red-Al in an excellent 99% yield.
A system consisting of a chiral NHC ligand and a Pd(0) catalyst was shown to activate racemic cyclopropyl substrate 91 in a publication by Kündig et al. [51]. The reaction was not selective as a 1:1 mixture of products arising from the reaction of the cyclopropyl methine C–H bond (compound 92) and methyl C–H bond (compound 93) was obtained (Scheme 25).
In 2011, the Yu group disclosed the first example of intermolecular enantioselective C–H functionalization of cyclopropanes [52]. Initially, the cross-coupling of amide cyclopropane 95 with phenylboronic acid pinacol ester (Ph-BPin) was investigated in the absence of a chiral ligand. It was established that the transformation takes place at 100°C, in the presence of Pd(OAc)2 as the catalyst, giving rise to a 2:1 mixture of mono- and diarylated cis-cyclopropanes 96 and 97, respectively. Alkyl potassium trifluoroborates were also compatible coupling partners, when the base was changed to Li2CO3 (Scheme 26).
A thorough screen of amino acids and their derivatives led to the application of chiral ligand 98 derived from phenylalanine in the intermolecular arylation of cyclopropane derivatives (Scheme 27) [52]. Albeit not practical, the addition of the catalyst and ligand in two batches at 40°C was optimal for the yield and enantioselectivity. Traces of water were also required in the transformation, and it was speculated that water aided in the transmetalation step. Ph-BPin was the best nucleophile, while the employment of alkyl boronic esters required increased temperatures (70°C), leading to slightly lower yields and enantioselectivities. It was also necessary to block the α-position of the cyclopropane in all examples, leading in some cases to lengthy syntheses of the starting materials. Nonetheless, groups such as methyl, isopropyl, cyclopentyl, β-benzyl ethers, γ-protected amines, or even aryls were tolerated in the α-position.
Most recently, the Yu group employed a triflyl-protected amine as a directing group in the asymmetric, intermolecular, and Pd-catalyzed functionalization of cyclopropylmethylamine 99 with aryl iodides (Scheme 28) [53]. Judicious screening of chiral mono-N-protected amino acids revealed Boc-L-Val-OH as the ideal ligand. In general, amino acid side chains that were branched (vs. linear) provided better enantioselectivities, while carbamates (e.g., Boc, Fmoc, Cbz) were desirable as N-protecting groups. Remarkably, the reaction provided exclusively the monoarylated product 100, as in all cases the remaining mass balance consisted of unreacted starting material. Furthermore, in contrast to the previous report [52], the presence of a substituent in the α-position of the cyclopropane was not required for reactivity.
The cyclopropyl methylene C–H bond was exclusively functionalized in the presence of competing aryl, benzyl, or methyl C–H bonds (Scheme 29). Additionally, a wide array of substituted aryl iodides could be employed as coupling partners in excellent yields and enantioselectivities; notably, an ester substituent on the cyclopropane was tolerated, affording the corresponding product in 42% yield. However, heteroaryl iodides were unreactive which represents a limitation to the methodology.
In contrast with the initial report employing boronic esters that follow a Pd(II)/(0) pathway [52], the current methodology is proposed to occur via a Pd(II)/(IV) mechanism. No reaction was observed in the presence of a Pd(0) source or in the absence of the silver salt. A plausible catalytic cycle involves an initial C–H activation step of palladium complex A to provide palladacycle B, followed by oxidative addition of the aryl iodide to give a highly reactive Pd(IV) complex C, which undergoes rapid reductive elimination to provide the desired product and a Pd(II) complex D (Scheme 30). The last step is loss of iodide, mediated by the silver salt. The use of the weakly coordinating –NHTf group by the Yu lab has allowed for the development of the first intermolecular and enantioselective C(sp3)–H activation via a Pd(II)/(IV) catalytic cycle.
6 Conclusion and Outlook
Over the last decade, continued interest in transition-metal-catalyzed C(sp3)–H bond functionalization reactions has allowed for the development of several methodologies for direct transformations of cyclopropanes. Intramolecular reactions resulted in the synthesis of biologically relevant cyclopropyloxindoles, as well as access to complex quinoline- or benzazepine-type products. Intermolecular transformations employed strongly binding auxiliaries or weakly coordinating directing groups to achieve arylation of cyclopropanes. A significant breakthrough represented the asymmetric direct arylation of cyclopropanes; in particular, contributions from the Yu group have put forward the use of mono-protected amino acids as chiral ligands in Pd(0)/(II) or Pd(II)/(IV) catalysis. Although at the moment the use of Pd salts as catalysts for the activation of cyclopropanes is predominant, it is desirable that less expensive metals such as Cu, Fe, Ni, or Co are also explored for the transformation.
References
Wong HNC, Hon MY, Tse CW, Yip YC, Tanko J, Hudlicky T (1989) Use of cyclopropanes and their derivatives in organic synthesis. Chem Rev 89(1):165–198
de Meijere A (1979) Bonding properties of cyclopropane and their chemical consequences. Angew Chem Int Ed 18(11):809–826
Exner K, Schleyer PR (2001) Theoretical bond energies: a critical evaluation. J Phys Chem A 105(13):3407–3416
Chen DYK, Pouwer RH, Richard J-A (2012) Recent advances in the total synthesis of cyclopropane-containing natural products. Chem Soc Rev 41(13):4631–4642
Pietruszka J (2003) Synthesis and properties of oligocyclopropyl-containing natural products and model compounds. Chem Rev 103(4):1051–1070
Reichelt A, Martin SF (2006) Synthesis and properties of cyclopropane-derived peptidomimetics. Acc Chem Res 39(7):433–442
Taylor RD, MacCoss M, Lawson ADG (2014) Rings in drugs. J Med Chem 57(14):5845–5859
Lebel H, Marcoux J-F, Molinaro C, Charette AB (2003) Stereoselective cyclopropanation reactions. Chem Rev 103(4):977–1050
Pellissier H (2008) Recent developments in asymmetric cyclopropanation. Tetrahedron 64(30–31):7041–7095
Gagnon A, Duplessis M, Fader L (2010) Arylcyclopropanes: properties, synthesis and use in medicinal chemistry. Org Prep Proc Int 42:1–73
Rubin M, Rubina M, Gevorgyan V (2007) Transition metal chemistry of cyclopropenes and cyclopropanes. Chem Rev 107(7):3117–3179
Ackermann L (2010) Metal-catalyzed direct alkylations of (hetero)arenes via C-H bond cleavages with unactivated alkyl halides. Chem Commun 46(27):4866–4877
Engle KM, Mei T-S, Wasa M, Yu J-Q (2012) Weak coordination as a powerful means for developing broadly useful C–H functionalization reactions. Acc Chem Res 45(6):788–802
Neufeldt SR, Sanford MS (2012) Controlling site selectivity in palladium-catalyzed C–H bond functionalization. Acc Chem Res 45(6):936–946
Lyons TW, Sanford MS (2010) Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem Rev 110(2):1147–1169
Alberico D, Scott ME, Lautens M (2007) Aryl–aryl bond formation by transition-metal-catalyzed direct arylation. Chem Rev 107(1):174–238
Eaton PE, Daniels RG, Casucci D, Cunkle GT, Engel P (1987) Amide activation for cyclopropane ortho-lithiation. J Org Chem 52(10):2100–2102
Zhang M-X, Eaton PE (2002) BuMgNiPr2: a new base for stoichiometric, position-selective deprotonation of cyclopropane carboxamides and other weak CH acids. Angew Chem Int Ed 41(12):2169–2171
Giri R, Chen X, Yu J-Q (2005) Palladium-catalyzed asymmetric iodination of unactivated C–H bonds under mild conditions. Angew Chem Int Ed 44(14):2112–2115
Wang D-H, Wasa M, Giri R, Yu J-Q (2008) Pd(II)-catalyzed cross-coupling of sp3 C–H bonds with sp2 and sp3 boronic acids using air as the oxidant. J Am Chem Soc 130(23):7190–7191
Wasa M, Engle KM, Yu J-Q (2010) Pd(II)-catalyzed olefination of sp3 C–H bonds. J Am Chem Soc 132(11):3680–3681
Yoo EJ, Wasa M, Yu J-Q (2010) Pd(II)-catalyzed carbonylation of C(sp3)–H bonds: a new entry to 1,4-dicarbonyl compounds. J Am Chem Soc 132(49):17378–17380
Hasegawa N, Shibata K, Charra V, Inoue S, Fukumoto Y, Chatani N (2013) Ruthenium-catalyzed cyclocarbonylation of aliphatic amides through the regioselective activation of unactivated C(sp3)–H bonds. Tetrahedron 69(22):4466–4472
Kubota A, Sanford MS (2011) Palladium-catalyzed ligand-directed oxidative functionalization of cyclopropanes. Synthesis 2011(16):2579–2589
Stowers KJ, Fortner KC, Sanford MS (2011) Aerobic Pd-catalyzed sp3 C–H olefination: a route to both N-heterocyclic scaffolds and alkenes. J Am Chem Soc 133(17):6541–6544
Rousseaux S, Liegault B, Fagnou K (2012) Palladium(0)-catalyzed cyclopropane C-H bond functionalization: synthesis of quinoline and tetrahydroquinoline derivatives. Chem Sci 3(1):244–248
Dos Santos A, El Kaïm L, Grimaud L, Ramozzi R (2012) Palladium-catalyzed ring opening of aminocyclopropyl Ugi adducts. Synlett 2012(3):438–442
Rosa D, Orellana A (2011) Palladium-catalyzed cross-coupling of cyclopropanols with aryl halides under mild conditions. Org Lett 13(1):110–113
He Z, Yudin AK (2006) Palladium-catalyzed oxidative activation of arylcyclopropanes. Org Lett 8(25):5829–5832
Ladd CL, Roman DS, Charette AB (2013) Palladium-catalyzed ring-opening of cyclopropyl benzamides: synthesis of benzo[c]azepine-1-ones via C(sp3)–H functionalization. Tetrahedron 69(22):4479–4487
Ladd CL, Sustac Roman D, Charette AB (2013) Silver-promoted, palladium-catalyzed direct arylation of cyclopropanes: facile access to spiro 3,3′-cyclopropyl oxindoles. Org Lett 15(6):1350–1353
Tsukano C, Okuno M, Takemoto Y (2013) Synthesis of spirooxindoles from carbamoyl chlorides via cyclopropyl methine C(sp3)–H activation using palladium catalyst. Chem Lett 42(7):753–755
Saget T, Perez D, Cramer N (2013) Synthesis of functionalized spiroindolines via palladium-catalyzed methine C–H arylation. Org Lett 15(6):1354–1357
Janody S, Jazzar R, Comte A, Holstein PM, Vors J-P, Ford MJ, Baudoin O (2014) Synthesis of 1-indanols and 1-indanamines by intramolecular palladium(0)-catalyzed C(sp3)–H arylation: impact of conformational effects. Chem Eur J 20(35):11084–11090
Alsters PL, Engel PF, Hogerheide MP, Copijn M, Spek AL, van Koten G (1993) Rigid five- and six-membered C, N, N′-bound aryl-, benzyl-, and alkylorganopalladium complexes: sp2 vs. sp3 carbon-hydrogen activation during cyclopalladation and palladium(IV) intermediates in oxidative addition reactions with dihalogens and alkyl halides. Organometallics 12(5):1831–1844
Shabashov D, Daugulis O (2010) Auxiliary-assisted palladium-catalyzed arylation and alkylation of sp2 and sp3 carbon − hydrogen bonds. J Am Chem Soc 132(11):3965–3972
Zaitsev VG, Shabashov D, Daugulis O (2005) Highly regioselective arylation of sp3 C–H bonds catalyzed by palladium acetate. J Am Chem Soc 127(38):13154–13155
Rouquet G, Chatani N (2013) Catalytic functionalization of C(sp2)–H and C(sp3)–H bonds by using bidentate directing groups. Angew Chem Int Ed 52(45):11726–11743
Parella R, Gopalakrishnan B, Babu SA (2013) Auxiliary-enabled Pd-catalyzed direct arylation of methylene C(sp3)–H bond of cyclopropanes: highly diastereoselective assembling of di- and trisubstituted cyclopropanecarboxamides. Org Lett 15(13):3238–3241
Sustac Roman D, Charette AB (2013) C–H functionalization of cyclopropanes: a practical approach employing a picolinamide auxiliary. Org Lett 15(17):4394–4397
Wei Y, Tang H, Cong X, Rao B, Wu C, Zeng X (2014) Pd(II)-catalyzed intermolecular arylation of unactivated C(sp3)–H bonds with aryl bromides enabled by 8-aminoquinoline auxiliary. Org Lett 16(8):2248–2251
He G, Chen G (2011) A practical strategy for the structural diversification of aliphatic scaffolds through the palladium-catalyzed picolinamide-directed remote functionalization of unactivated C(sp3)–H bonds. Angew Chem Int Ed 50(22):5192–5196
Hoshiya N, Kobayashi T, Arisawa M, Shuto S (2013) Palladium-catalyzed arylation of cyclopropanes via directing group-mediated C(sp3)–H bond activation to construct quaternary carbon centers: synthesis of cis- and trans-1,1,2-trisubstituted chiral cyclopropanes. Org Lett 15(24):6202–6205
Wasa M, Chan KSL, Zhang X-G, He J, Miura M, Yu J-Q (2012) Ligand-enabled methylene C(sp3)–H bond activation with a Pd(II) catalyst. J Am Chem Soc 134(45):18570–18572
He J, Li S, Deng Y, Fu H, Laforteza BN, Spangler JE, Homs A, Yu J-Q (2014) Ligand-controlled C(sp3)–H arylation and olefination in synthesis of unnatural chiral α–amino acids. Science 343(6176):1216–1220
Lauru S, Simpkins NS, Gethin D, Wilson C (2008) Enantioselective synthesis of cyclopropylcarboxamides using s-BuLi-sparteine-mediated metallation. Chem Commun 42:5390–5392
Giri R, Shi B-F, Engle KM, Maugel N, Yu J-Q (2009) Transition metal-catalyzed C-H activation reactions: diastereoselectivity and enantioselectivity. Chem Soc Rev 38(11):3242–3272
Nakanishi M, Katayev D, Besnard C, Kündig EP (2011) Fused indolines by palladium-catalyzed asymmetric C–C coupling involving an unactivated methylene group. Angew Chem Int Ed 50(32):7438–7441
Martin N, Pierre C, Davi M, Jazzar R, Baudoin O (2012) Diastereo- and enantioselective intramolecular C(sp3)–H arylation for the synthesis of fused cyclopentanes. Chem Eur J 18(15):4480–4484
Saget T, Cramer N (2012) Palladium(0)-catalyzed enantioselective C–H arylation of cyclopropanes: efficient access to functionalized tetrahydroquinolines. Angew Chem Int Ed 51(51):12842–12845
Katayev D, Larionov E, Nakanishi M, Besnard C, Kündig EP (2014) Palladium–N-heterocyclic carbene (NHC)-catalyzed asymmetric synthesis of indolines through regiodivergent C(sp3)–H activation: scope and DFT study. Chem Eur J 20(46):15021–15030
Wasa M, Engle KM, Lin DW, Yoo EJ, Yu J-Q (2011) Pd(II)-catalyzed enantioselective C–H activation of cyclopropanes. J Am Chem Soc 133(49):19598–19601
Chan KSL, Fu H-Y, Yu J-Q (2015) Palladium(II)-catalyzed highly enantioselective C–H arylation of cyclopropylmethylamines. J Am Chem Soc 137(5):2042–2046
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Sustac Roman, D., Charette, A.B. (2015). Catalytic C–H Bond Functionalization of Cyclopropane Derivatives. In: Dixneuf, P., Doucet, H. (eds) C-H Bond Activation and Catalytic Functionalization II. Topics in Organometallic Chemistry, vol 56. Springer, Cham. https://doi.org/10.1007/3418_2015_118
Download citation
DOI: https://doi.org/10.1007/3418_2015_118
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-24802-8
Online ISBN: 978-3-319-29319-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)