Abstract
Sensory systems enable animals to generate a representation of the environment. One sensory system common to many aquatic vertebrates is the lateral line. This chapter reviews the functional organization of the peripheral lateral line system and describes the organization and distribution of the sensory structures termed neuromasts, their innervation patterns, and the relationship between their afferent innervation and the afferent termination sites in the brain. It details how neuronal encoding of information by lateral line afferents is partitioned in submodalities and summarizes their respective stimulus dimensions and response dynamics including frequency selectivity and threshold sensitivity. The authors further discuss the receptive fields, spatial selectivity, and the directional selectivity that enables the detection of local and global water disturbances across the fish body. Finally the current state of knowledge on peripheral encoding of various natural hydrodynamic stimuli such as large scale ambient water motions, dipole fields, translating objects, and surface waves are presented.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Aquatic vertebrates
- Canal and superficial neuromast
- Dipole
- Frequency tuning
- Hair cell
- Information coding and processing
- Natural and artificial hydrodynamic stimuli
- Octavolateral efferent system
- Peripheral organization
- Receptive field
1 Introduction
As a hydrodynamic sensory system, the lateral line enables fish and some aquatic amphibians to detect wide-scale, ambient water motions created by wind and gravity, as well as local water disturbances, such as those created by animal movements or by the interactions between ambient water motions and aquascape features. Like all sensory systems, the lateral line system must extract biologically significant information from a welter of stimuli that bombard it in order to increase the probability of an animal’s survival. Critically important stimulus features (e.g., those that permit determination of a predator’s approach speed and direction) must be extracted, while irrelevant background noises that interfere with the detection and processing of important information must often be suppressed. Thus, lateral line sense organs have evolved not only to transduce fluid motion into neuronal signals, but also to act as critical first-stage filters that shape the information that the brain receives and reduce the amount of central processing that is required.
Although it is impossible to know if fish actually form hydrodynamic images of the environment in the same way that, for example, humans might form visual images, it is possible to describe how hydrodynamic information is represented by the peripheral nervous system and how this representation changes over time and in different behavioral contexts. Technological advances in our ability both to measure and model the spatial characteristics of flow fields have led to significant advances in our understanding of how flow patterns can be used to obtain information about flow-generating sources. Examples include how Mexican blind cavefish (Astyanax mexicanus) can avoid obstacles based on obstacle-created distortions in their self-generated flow field (Windsor et al., 2010a,b), how piscivorous predators can obtain information about a prey’s whereabouts from the prey’s hydrodynamic trail (Hanke et al., 2000; Hanke & Bleckmann, 2004), and how the location and distance of a small vibrating body can be encoded in the spatial pattern of water motion along arrayed flow sensors in the lateral line (Ćurčić-Blake & van Netten, 2006; Goulet et al., 2008; Rapo et al., 2009).
To understand fully the nature of hydrodynamic information available to fish, one must first understand how incoming water motions are transformed and represented in the peripheral nervous system. Information available to the brain in the form of spike activity in primary afferent fibers can be shaped by many factors, including (1) boundary layers at the interface between the fish’s body and the surrounding water (see the chapter by McHenry & Liao); (2) the biophysical properties of the transduction elements (hair cells; Section 2) and the structures that surround them (see the chapter by van Netten & McHenry; and Section 5); (3) the spatial distribution, orientation and innervation of flow sensors (see chapter by Webb; and Section 3); and (4) descending influences of efferent innervation from the central nervous system (CNS; Section 6). This chapter examines how these factors influence stimulus encoding by primary afferent fibers, and in general, how these and other features of the peripheral nervous system shape hydrodynamic information received by the brain.
2 Sensory Transduction and Response Characteristics of Hair Cells
The transduction of hydrodynamic events into a neuronal code in the lateral line system is mediated by hair cells, the same receptor cells found in the vestibular and auditory systems of all vertebrates. Much of what is known today about the basic functional properties of lateral line hair cells comes from the classic studies of Åke Flock and his colleagues in the 1960s and early 1970s on the lateral line canal system of the freshwater burbot (Lota lota) (Flock, 1965a,b; Harris et al., 1970). These studies revealed two important features of hair cells that directly impact how stimulus information is encoded by the lateral line system. One is the directional sensitivity of the hair cell and the other is the nonlinearity of hair cell responses to opposing stimulus directions.
The directional selectivity of the hair cell arises from the morphological polarization of the transduction apparatus, which consists of an apical bundle of stereovillae with one elongated kinocilium at the periphery (Fig. 1a). The stereovillae increase in length in a systematic fashion in the direction of the eccentrically placed kinocilium. Displacement of the hair bundle toward the kinocilium results in a depolarization of the hair cell membrane and an increase in the firing rate of the afferent fiber, whereas displacement in the opposite direction causes a hyperpolarizing response and a decrease in the firing rate (Fig. 1a). Displacements in directions orthogonal to the axis of morphologic polarization result in no response, and off-axis directions result in response magnitudes that are a cosine function of the stimulus direction (Fig. 1b) (Flock, 1965b).
Opponent organization of lateral line neuromasts. (a) Hair cells on any given neuromast are oriented in one of two opposing (orange and green) directions, resulting in a single axis of best sensitivity. (Courtesy of H. Bleckmann.) (b) Hair cell responsiveness, modeled as a cosine function of the direction of hair bundle deflection for the two oppositely oriented populations of hair cells
A distinct, nonlinear feature of hair cells is that for equal displacements in opposite directions, the magnitude of the depolarizing response is greater than that of the hyperpolarizing response. Nevertheless, for a given direction of displacement, response magnitude increases linearly with displacement amplitude over several orders of magnitude (up to ~50 nm, RMS) (Kroese & van Netten, 1989).
Insofar as it has been investigated, lateral line hair cells do not appear to have any intrinsic electrical tuning (Kroese & van Netten, 1989), as has been reported for hair cells in other sensory systems (e.g., turtle cochlea). Rather, frequency tuning in the lateral line system appears to be dominated by biophysical factors associated with the number of hair cells per sense organ and structural features that are intrinsic (e.g., stereovillae length) or extrinsic (e.g., size and shape of surrounding structures) to the hair cell (see the chapter by McHenry & van Netten and Section 5). However, studies of hair cell physiology have involved only a few species, for example, the ruffe (Acerina cernua) and the burbot (Lota lota), with no direct comparisons between hair cells in different submodalities (superficial or canal neuromast; see Section 5) (Flock, 1965a,b; Kroese & van Netten, 1987). Thus, it is difficult to make sweeping generalizations about the intrinsic response properties of hair cells, especially because several features, such as stereovillae length, are known to vary between submodalities and species (Flock, 1971; Russell & Sellick, 1976; Faucher et al., 2005). For further details on hair cell function, readers are referred to Kroese and van Netten (1989) (lateral line hair cells) and Ashmore (1991) and Eatock et al. (2006) (hair cells in other systems).
3 Functional Organization of the Peripheral Lateral Line System
Although the number, type, and distribution pattern of lateral line flow sensors may vary greatly between species (Coombs et al., 1988; see also the chapter by Webb), the peripheral lateral line system of both fish and amphibians has at least four basic organizational features in common: (1) an opponent organization of hair cells that gives rise to a single axis of best sensitivity for each sense organ, the neuromast (Section 3.1); (2) the arrangement of multiple neuromasts into linear arrays on the head and trunk, typically in rostrocaudal and dorsoventral directions (Section 3.2); (3) the coalescence of afferent fibers from the head and trunk into separate cranial nerves, each of which projects to a different region of the hindbrain; and (4) the formation of an orderly topographical map of rostrocaudal body location within each trunk or head region of the hindbrain (Section 3.3). In addition, the lateral line system of fishes can be subdivided into two submodalities: superficial neuromasts (SNs) that reside on the skin surface and canal neuromasts (CNs) that are enclosed in fluid-filled canals just below the skin surface (see Section 5 for further details).
3.1 Opponent Organization of Hair Cells
With rare exceptions (e.g., Shardo, 1996), hair cells in lateral line neuromasts exhibit an organization of oppositely oriented hair cells (Flock & Wersäll, 1962; López-Schier et al., 2004) (Fig. 1). Oppositely oriented hair cells, which are spatially intermingled throughout the neuromast, are innervated by separate populations of afferent nerve fibers, each of which synapses with hair cells of the same orientation during development (Faucherre et al., 2009). As a result, each neuromast has a single, bidirectional axis of best sensitivity (or responsiveness). This means that stimulation in one direction along this axis will result in an increase in firing rate for one fiber population and a decrease in the other and vice versa for stimulation in the opposite direction (Görner, 1963) (Fig. 1a). The functional ramifications of this highly conserved, opponent organization has yet to be fully understood, but Wiese (1984, 1988) has suggested that it helps to preserve responsiveness to AC (oscillatory) flows in the presence of sustained DC (unidirectional) flows (e.g., those in a stream or those created by forward swimming motions). If parallels to the vertebrate vestibular system can be drawn, the opponent organization may also serve to amplify the signal to the brain. The hair cells of the semicircular canals are also arranged in an opponent fashion, with hair cells of, for example, the horizontal canal on the left side having orientations that are opposite to those on the right side. Thus, a horizontal head rotation in one direction excites one population while inhibiting the other. Such an arrangement means that the difference in neuronal activity between left and right sides can be interpreted by the brain as a head rotation in a given direction (Highstein et al., 2004) and that the amplitude of the difference signal will be greater than that of the signal from either side alone.
3.2 Neuromast Distribution, Orientation, and Innervation Patterns
The lateral line system most likely got its name from the highly visible line of scales running down the flanks of most fish. Within each scale is a short, open-ended tube (or canal) with a single canal neuromast inside. Although this “line” of CN scales represents only one of many not-so-visible CN and SN arrays in the lateral line system of bony fishes (Fig. 2) (see also the chapter by Webb), it nevertheless captures the hallmark features of the lateral line as a system of many flow sensors distributed over wide regions and organized (for the most part) into arrays. Although lateral line canals may exhibit some curvature, for example, to course above or below the eye, they generally follow a rostrocaudal (e.g., mandibular and trunk canals) or dorsoventral (e.g., pre-opercular canal) direction. In addition, there is a single (supratemporal) canal that runs across the top of the head in a lateromedial (left/right) direction. Thus, all three axes of a 3D-coordinate system are represented. SNs are also frequently organized into lines, some of which follow the course of canals as “accessory lines” (Coombs & Janssen, 1989; see also the chapter by Webb) and others that form rows that are independent of canals, for example, dorsoventrally oriented rows on trunk scales and rostrocaudally oriented rows along the caudal fin rays (Webb, 1989a; Schmitz et al., 2008) (Fig 2). Some species have large “fields” of SNs, but these typically consist of multiple, parallel lines of SNs. In addition, there is usually a line of SNs that form a ring around the nares.
General principles of neuromast distribution and orientation. The Lake Michigan mottled sculpin (Cottus bairdi, Scorpaeniformes) (top) and the Mexican tetra (Astyanax mexicanus, Characiformes) (bottom, adapted from Schemmel, 1967) differ dramatically in the number and spatial extent of SNs (small dots) on the body surface. Nevertheless, SNs in both species tend to form rows with axes of best sensitivity (double arrows) that are either parallel (e.g., SN rows on caudal fin rays, inset) or orthogonal (e.g., SN rows on trunk scales of the Mexican tetra, inset) to the row. CNs (large filled circles) are found in canals that run above (supraorbital) and below (infraorbital) the eye, down the cheek (preopercle), along the lower jaw (mandibular), across the top of the head (supratemporal), and down the trunk (trunk canal). The axis of best sensitivity of each CN is parallel to the long axis of the canal (top right insets)
Based on cases examined so far, the CN axis of best sensitivity is always parallel to the long axis of the canal (Flock & Wersäll, 1962; Janssen et al., 1987; Webb, 1989b) (Fig. 2). This no doubt follows from the fact that flow directions inside the canal are constrained to this axis. In contrast, SNs on fish are typically (but not always) free of surrounding structures that direct water motions. Experimental verification of SN orientations (axis of best sensitivity), based on SEM visualization of hair cell orientations, are limited to a few teleost species, including tilapia (Oreochromis aureus; Webb, 1989a), sea bass (Dicentrarchus labrax; Faucher et al., 2005), and goldfish (Carassius auratus; Schmitz et al., 2008). Although difficult to generalize to all 30,000+ species of fish, SNs in these few species have at least four features in common: (1) they maintain the same orientation within a given row, typically either parallel or orthogonal to the long axis of the row (Fig. 2); (2) they form rows that vary widely in their orientation on the head, but that are typically limited to rostrocaudal or dorsoventral directions on the trunk; (3) they form rows of rostrocaudally oriented SNs along the caudal fin rays (Fig. 2); and (4) they tend to have dorsoventral orientations when located near CNs on the trunk. In goldfish, which have thousands of SNs all over the body surface (a row on nearly every trunk scale), only a small minority of those on the trunk have dorsoventral orientations and these are found on scales with CNs (Schmitz et al., 2008).
The spatial distribution and orientation of neuromasts in aquatic amphibians are similar to those in fish, but direct comparisons are difficult because amphibians lack canal neuromasts. In the clawed frog (Xenopus laevis), short lines of 2–15 SNs, called stitches, form rows or lines that generally follow the same distribution patterns as canals in fishes (e.g., along the trunk, above and below the eye, etc.). Moreover, the orientations of stitches, like SN rows on fish, are varied on the head, but restricted to either rostrocaudal or dorsoventral directions on the trunk. In Xenopus, skin papillae on both sides of each SN form a channel that likely directs water motions along the SN axis of best sensitivity, which is always orthogonal to the long axis of the stitch (Görner, 1963).
The lateral line system of the amphibian axolotl (Ambystoma mexicanum) is innervated by five distinct cranial nerves, each with its own ganglion (Northcutt, 1992; Northcutt et al., 2000). In fishes, there are at least three and as many as seven cranial nerves supplying the lateral line (Northcutt, 1989). Typically, neuromasts on the head are innervated by afferent fibers in the anterior lateral line nerve (ALLN), those on the trunk by fibers in the posterior lateral line nerve (PLLN), and those in the middle (post-otic region on the head) by yet a third (middle) lateral line nerve (Northcutt, 1989; Song & Northcutt, 1991). For a phylogenetic/evolutionary perspective on the spatial distribution and innervation of lateral line neuromasts, the readers are referred to Northcutt (1989), Song and Northcutt (1991), and the chapter by Webb.
Innervation patterns depend on submodality and hair cell polarity. In fish, afferent innervation of SNs and CNs differ in terms of the number of neuromasts that are contacted by a single fiber. SN fibers frequently innervate more than one SN (Münz, 1979; Nagiel et al., 2008; Faucherre et al., 2009). SNs innervated by the same fiber are typically part of the same row of SNs, as reported for tilapia (Sarotherodon niloticus) (Münz, 1985) and similarly, in the same stitch, as reported for the clawed frog (Mohr & Görner, 1996). Moreover, hair cells contacted by the same fiber appear to be of the same orientation (polarity) in all SNs, as indicated by both anatomical (Faucherre et al., 2009) and physiological (Montgomery & Coombs, 1992; Coombs et al., 1996) studies. In Tilapia, up to 10 superficial neuromasts on the same trunk scale may be innervated by a single fiber, but innervation of more than one CN by the same fiber is rare (<4%) (Münz, 1979, 1985). Most importantly, there is no evidence that single fibers innervate both SNs and CNs. In summary, SNs and CNs are innervated by separate nerve fibers, each of which integrates information from hair cells of the same orientation within a given SN or CN and in the case of SN fibers, also among SNs in the same row.
3.3 Relationship between Peripheral Innervation Patterns and Termination Sites in the Brain
Although it is beyond the scope of this chapter to discuss how information is processed by the CNS (see the chapter by Bleckmann & Mogdans), it is worth considering how or if organizational features of the peripheral nervous system (i.e., opponent organization of hair cells, spatial distribution of neuromasts, and subdivision of the system into two submodalities) are preserved centrally by the pattern of afferent termination sites in the brain. Tract-tracing neuroanatomical studies reveal that afferent fibers in bony fish project to four major regions of the brain (reviewed in McCormick, 1989; see also the chapter by Wullimann & Grothe): (1) the medial octavolateralis nucleus (MON) in the medulla, the only first-order nucleus that contributes to the ascending lemniscal pathway to the forebrain; (2) the caudal nucleus, also in the medulla; (3) the eminentia granularis in the cerebellum, which feeds back down to the MON as part of an adaptive filter network for suppressing repeated and expected stimuli (e.g., the ones caused by the animal’s own respiration) (Montgomery et al., 1995a); and (4) the Mauthner cell and reticulospinal network, which mediates rapid escape responses (Zottoli & Danielson, 1989) and other fast-start behaviors (e.g., Wöhl & Schuster, 2007). It is currently unknown whether individual afferent fibers terminate in all four areas of the brain, or whether different fibers project to different brain regions or some combination of both. Curiously, there is little anatomical evidence for a clear segregation of SN and CN inputs in the hindbrain (Puzdrowski, 1989), despite strong evidence for a functional dichotomy between the two submodalities (Section 5).
Nevertheless, response properties of central neurons in teleost fish indicate that information from the two submodalities, as well as that from oppositely oriented hair cells, are processed in separate, but parallel pathways—at least up to the level of the midbrain (Bleckmann, 2008; see also the chapter by Bleckman & Mogdans). A segregation of fibers from oppositely oriented hair cells is also likely in aquatic amphibians, based on the coalescence of primary afferent fibers into two distinct dorsomedial and ventrolateral fascicles as they enter the brain stem (Fritzsch, 1981; Altman & Dawes, 1983).
Recent anatomical and physiological studies on the development of trunk neuromast innervation in larval zebrafish are consistent with the idea that there may be two, functionally distinct, populations of developing afferent connections to the brain (Haehnel et al., 2012; Liao & Haehnel, 2012; Pujol-Martí et al., 2012). The first population consists of large, early-born and less excitable neurons that innervate multiple neuromasts and the second, of small, later-born and more sensitive neurons that innervate single neuromasts. The former projects to a more medial location in the hindbrain (consistent with the Mauthner cell location), while the latter projects to more lateral locations (consistent with the MON location). Based on these anatomical and physiological distinctions, the story that is beginning to emerge is that early-born cells mediate Mauthner-mediated escape responses to strong hydrodynamic stimuli that simultaneously stimulate wide regions of the body. In contrast, later-born cells are hypothesized to preserve the fine-grain resolution of spatial nonuniformities of hydrodynamic stimuli along the body surface—information that is presumably processed by the MON and the rest of the ascending lemniscal pathway.
Theories for how spatial patterns of flow activity along arrayed lateral line sensors contain information about, for example, the location of dipole sources or shed vortices in the wake of a swimming fish (Section 7) raise the question of whether or not information about instantaneous spatial patterns of activity is preserved and represented in the MON. Such a representation would seem to require that information from simultaneously activated neuromasts at different body locations (1) arrive at the MON at the same time and (2) form an orderly map of the body surface (i.e., a somatotopic map) in the MON. Evidence for the first requirement comes from a single study on PLLN fibers in the trout (Oncorhynchus mykiss) (Schellart & Kroese, 2002). Conduction velocities were found to be slowest (~13 m s–1) for fibers innervating neuromasts near the head and fastest (~33 m s–1) for those near the tail, suggesting that longer travel distances to the brain were compensated for (at least, somewhat) by faster conduction velocities. Recent studies on developing innervation patterns in larval zebrafish, however, suggest an alternative interpretation of these data—that fast conducting fibers from the tail region form part of a dedicated pathway for Mauthner-mediated escape responses (Haehnel et al., 2012; Liao & Haehnel, 2012; Pujol-Martí et al., 2012).
Evidence for somatotopic mapping comes from studies on the zebrafish (Danio rerio), in which ALLN and PLLN fiber terminals were found to project to two separate regions of the MON—a ventromedial region that maps relative rostrocaudal location of head neuromasts and a dorsolateral region that maps relative rostrocaudal location of trunk neuromasts (Alexandre & Ghysen, 1999). Whether or not there are orderly maps of other body axes (e.g., dorsoventral or lateromedial) remains to be seen, but lateral line maps of the body surface do not appear to be as precise and as comprehensive as electrosensory maps in the hindbrain of weakly electric fish (Carr, 1990).
Interestingly, the zebrafish data indicate that there may be two separate maps (one for the head and one for the trunk), rather than a single, continuous map of body surface from head to tail, as seems to be the case for electrosensory maps. This arrangement—segregation of PLLN and ALLN terminals into dorsolateral and ventromedial regions of the MON—has been reported for several different species of fish (e.g., McCormick, 1983; Puzdrowski, 1989; New & Singh, 1994), as well as the African clawed frog (Xenopus laevis; Will et al., 1985). Unfortunately, little if any information is available on the termination sites of additional lateral line nerve fibers (e.g., in the middle nerve) relative to those of the PLLN and ALLN.
4 Neuronal Encoding of Information by Lateral Line Afferents
Lateral line afferent fibers, like other sensory afferents, rely on spike trains to represent stimulus information. The ability of lateral line fibers to respond to a sinusoidal signal at a given phase angle (i.e., to phase-lock) is robust over the entire range of low frequencies to which the system responds (Coombs et al., 1988; Engelmann et al., 2002; Chagnaud et al., 2007b) (Fig. 3). Thus, temporal codes that rely on the precise timing of individual spikes with respect to the stimulus are clearly available for encoding temporal dimensions of the stimulus. Similarly, rate codes, which rely on the number of spikes that occur in a relatively short time period, increase linearly with stimulus amplitude over a 20-dB range (Coombs et al., 1988; Bleckman et al., 1989), and thus are available for encoding stimulus amplitude. However, because the lateral line system responds to a vector quantity (flow) using directionally sensitive hair cells (Flock, 1965a, b) (Section 2), the firing rate of afferent fibers depends not only on stimulus magnitude but also on stimulus direction. Thus, an increase or decrease in afferent firing rate could signify a change in stimulus magnitude, direction, or both and the nervous system must sort this all out. Consequently, population codes, which rely on the pattern of activity across different fibers, are likely to be very important for encoding spatial patterns of activity along arrayed sensors, as discussed in Section 7.
(a) Spike train response of a lateral line afferent fiber to a sustained sinusoidal signal (stimulus trace at very bottom of fig.). (b) Dot-raster representation of spikes from the same fiber to 10 presentations of the same stimulus and (c) peristimulus-time (left) and period (right) histograms of the same data shown in (b)
5 Partitioning of Information by Lateral Line Submodalities
Most, if not all sensory systems consist of submodalities (e.g., rods and cones in the visual system) that respond to different stimulus dimensions and that partition incoming stimuli into parallel streams of information. The lateral line system partitions information not only according to spatial location along the body surface (Section 3.3), but also according to different flow dimensions, as encoded by SN and CN submodalities. SN and CN submodalities differ in many ways other than their location relative to the skin surface (Table 1). Most importantly, SNs and CNs differ in their response properties and the kind of information that they encode, as summarized in this section.
5.1 Relevant Stimulus Dimensions and Response Dynamics
In the mechanosensory lateral line, the response dynamics of afferent fibers depend heavily on fluid viscosity, as well as the mass and elasticity of structural elements in and around the neuromast (see the chapters by McHenry & Liao and van Netten & McHenry). Because SNs are superficially situated, they are directly exposed to flow along the body surface and their response is normally driven by flow velocity and boundary layer shear stress (spatial velocity gradients) along the overlying hydrogelatinous structure (the cupula), which couples the motions of the surrounding water to the underlying hair cells (Denton & Gray, 1983; Kalmijn, 1989; see also the chapter by van Netten & McHenry). When considering fluid motion inside the canal, typical CNs, like SNs, are driven by flow velocity over the cupula and neuromast. However, flow inside the canal is impeded by the boundary layer developing on the internal walls of small-diameter canals. Thus, inertial forces must come into play before fluids can move inside the canal. As a result, inside fluid motion is proportional to the net acceleration between the fish and the surrounding water, which is also proportional to the pressure difference between the two adjacent canal pores (Denton & Gray, 1983, 1988; Kalmijn, 1988, 1989).
The clearest evidence for this functional dichotomy comes from a linear frequency-response analysis of afferent fibers in the African clawed frog (Kroese & van Netten, 1987) (Fig. 4a, c) and the trout (Oncorhynchus mykiss) (Kroese & Schellart, 1992) (Fig. 4b, d). In these studies, both the gain (% modulation of spontaneous activity) (Fig. 4a, b) and phase angle (preferred firing time during a sinusoidal cycle of stimulation) (Fig. 4c, d) of afferent fiber responses to a sinusoidally oscillating sphere (dipole stimulus) were measured. This analysis revealed that fibers fell into two response categories in which responses of SN fibers (open circles, Fig. 4) conformed to predictions for a velocity-driven response (dashed lines, Fig. 4) and those of CN fibers (filled circles) conformed to predictions for an acceleration-driven response (solid lines, Fig. 4). Although several other species show evidence of a similar dichotomy (Section 5.2), there may be exceptions to this rule in some species. Indeed, species-specific morphological variations in lateral line structures (Coombs et al., 1989; see also the chapter by Webb), coupled with theoretical predictions based on physics (Denton & Gray, 1989), indicate that some lateral line neuromasts may respond to displacement and in rare cases, even pressure (e.g., in clupeids) (Denton & Gray, 1979). Nevertheless, response dynamics that fall outside of the flow velocity/acceleration dichotomy are likely to be the exceptions rather than the general rule.
Gain (% modulation of spontaneous activity) (a, b) and phase angle (deg re: sphere displacement) (c, d) responses of SN (open circles) and CN (filled circles) fibers in response to a small sphere oscillating at different frequencies. Data for Xenopus (a, c) and trout (b, d) are replotted from Kroese et al. (1978) and Kroese and Schellart (1992). Dashed lines indicate the gain (20 dB/decade slopes) and phase angle (90° re: sphere displacement) predictions for a velocity driven response, whereas solid lines indicate the gain (40 dB/decade slope) and phase angle (180° re: sphere displacement) predictions for an acceleration-driven response
Conspicuously absent from the lateral line system are any obvious signs of amplitude and time (phase)-encoding submodalities, as is found in the closely related electrosensory system of fish and in mammalian auditory systems (reviewed in Carr, 1993). Both SN and CN fibers exhibit tonic to slowly adapting responses to maintained stimuli (Coombs & Janssen, 1989; Voigt et al., 2000) and peripheral features commonly associated with phase-sensitive pathways (e.g., minimal branching in the axonal arbor of primary afferents, large afferent terminals or calyceal synapses) have not yet been found. This is probably not too surprising, given that the lateral line system generally operates at frequencies below 200 Hz, where phase-locking abilities are robust (Fig. 3). Thus, both amplitude and phase can be adequately represented in the same fiber.
5.2 Frequency Selectivity and Threshold Sensitivity
The peripheral auditory system of mammals is well known for its partitioning of information in the frequency domain (e.g., Kiang et al., 1969). A bank of differently tuned auditory fibers, which span the entire frequency range of hearing, preserves the frequency selectivity found along the basilar membrane of the cochlea. This arrangement is well suited for a spectral analysis of complex sounds. In addition, the tuning of individual auditory fibers to different frequency regions improves signal detection in the presence of noise, based on spectral differences between signal and noise. Although there is little evidence for a continuous “bank” of differently tuned fibers in the lateral line system, velocity-sensitive SNs and acceleration-sensitive CNs nevertheless divide the frequency range of detection into low- and high-frequency regions.
The “view” of how the two submodality partition frequency information, however, is heavily influenced by whether displacement, velocity, or acceleration is chosen as the frame of reference (Kalmijn, 1989). In the displacement frame used by Kroese and colleagues, relative responsiveness (gain) is plotted for a fixed displacement amplitude at different oscillation frequencies (Fig. 4a, b). This generates a picture of SN and CN fibers as having band pass characteristics with a single best frequency that is typically lower for SNs than CNs for any given species (e.g., 30 and 80 Hz, respectively, for SN and CN fibers in the trout, as illustrated in Fig. 4b).
However, SN and CN responses are not proportional to displacement, but rather to velocity and acceleration, respectively. A different approach in which the frequency response properties of lateral line fibers are measured with stimuli of equal velocity or acceleration amplitudes reveals that both CN (Fig. 5a, c) and SN (Fig. 5b, c) fibers have low-pass characteristics when plotted in terms of their relevant stimulus dimension (Coombs & Montgomery, 1992, 1994; Montgomery & Coombs, 1992; Montgomery et al., 1994). That is, they respond maximally and equally well over a limited range of low frequencies, reinforcing the idea of SNs as flow velocity detectors and CNs as flow acceleration detectors.
The upper limit to this low-frequency range depends on a number of factors that govern the mechanical properties of the neuromast and surrounding structures (see the chapter by van Netten & McHenry). In the Antarctic fish Trematomus bernachii, the high-frequency cutoff (frequency at which responsiveness drops to 50% of maximum) varies according to neuromast size for SNs, but not CNs. That is, the largest SNs on the ventral trunk line (TrV in Fig. 5b) have the lowest high-frequency cutoff (~30 Hz) and the smallest SNs on the head (Md3, PO2 in Fig. 5b) have the highest high-frequency cutoffs (~50 Hz). Although nonadaptive explanations for these small differences have been proposed (Coombs & Montgomery, 1994), it remains to be seen whether they are functionally significant or not. In contrast to SNs, the high-frequency cutoffs of CNs at different body locations are all very similar (~30 Hz) (Fig. 5a), despite a wide range of neuromast and canal diameters (100–600 μm) (Coombs & Montgomery, 1992). The absence of response variability across CN fibers provides evidence for the extent to which canal dimensions can vary without sacrificing essential canal functions (defined later in this section) and serves as a cautionary reminder that morphological variation does not always signify functional variation.
Normalized response amplitude (% of maximum spike rate) of CN (a) and SN (b) fibers innervating neuromasts in different canals or body locations in Trematomus bernachii. Iso-acceleration stimuli were used to measure CN responsiveness in (a), whereas iso-velocity stimuli were used to measure SN responsiveness in (b). (c) Direct comparisons of a SN and CN fiber responding to iso-velocity stimuli. Md, mandible; PO-h, horizontal arm of preopercle; PO-v, vertical arm of preopercle; Tr, trunk. (Data replotted from Coombs and Montgomery, 1992, 1994)
It is now generally accepted that canals function not only to protect neuromasts from damage, but also as mechanical filters to reduce low-frequency noise (Montgomery et al., 1994, 2009; see also the chapter by Montgomery et al.). When plotted in the same velocity frame of reference as SNs, it can be seen that the responsiveness of CN fibers to low frequency stimuli (< ~20 Hz) are reduced relative to that of SN fibers and may even be enhanced somewhat at higher frequencies (> ~30 Hz) (Fig. 5c). The reduction is due to the high-pass filtering action of canals, which reduce the amplitude of water velocity inside the canal relative to that outside the canal for low compared to high frequencies (Denton & Gray, 1983; Coombs & van Netten, 2005; see also the chapter by van Netten & McHenry). Enhancement, on the other hand, can happen if canal diameter decreases in the vicinity of the neuromast, resulting in increased flow speed in the narrow region relative to wide regions of the canal. From an information-processing point of view, canals can thus be seen to improve signal-to-noise ratios for the detection of high-frequency signals (e.g., from prey) in the presence of low-frequency noises, such as those present in ambient currents or currents produced by the fish’s own swimming motions (see also the chapter by Montgomery et al.; and Section 7.1).
Tuning curve data from PLLN fibers in the Lake Michigan mottled sculpin (Cottus bairdi) confirm that afferent fibers can be divided into two populations of velocity- and acceleration-sensitive fibers, with the latter having greater sensitivity at higher frequencies than the former (Fig. 6a) (Coombs & Janssen, 1989). Further, compared to velocity-sensitive tuning curves, acceleration-sensitive tuning curves are a good match, both in terms of sensitivity and bandwidth, for behaviorally measured thresholds obtained under nearly identical stimulus conditions (same source, same source location). A similar story can be told for ALLN fibers innervating supraorbital CNs in the surface-feeding topminnow (Aplocheilus lineatus; Fig. 6b) (Bleckmann & Topp, 1981). In both cases, the broad tuning characteristics of a single population of acceleration-sensitive (CN) fibers are sufficient to account for the entire frequency range of behavioral detection. Thus, there is at present little evidence that the lateral line system uses differently tuned fibers for performing any kind of spectral analysis, as is done by the mammalian auditory system. In fact, a filter-bank representation of spectral characteristics is unnecessary, given that frequency can be encoded temporally by the phase-locking abilities of afferent fibers.
Neuronal (thin dashed and solid lines) threshold curves for PLLN fibers in the mottled sculpin (a) and ALLN fibers in the surface-dwelling topminnow (b) compared to behavioral thresholds (thick solid lines) in each species. Neuronal tuning curves from four different species of cartilaginous and bony fish from very different environments and water temperatures are compared in (c): three benthic species, one from a temperate marine environment (thornback ray) (Görner & Kalmijn, 1989), one from a frigid (Antarctic) marine environment (Trematomus bernachii) (Montgomery & Coombs, 1992), and the third from a cool freshwater environment (Lake Michigan mottled sculpin) (Coombs & Janssen, 1990). The fourth species is the surface-dwelling, tropical water killifish (topminnow) (Topp, 1983)
From a comparative look at available tuning curve data, it can be seen that high-frequency cutoffs (frequency at which acceleration-sensitivity starts to decline) vary from ~30 Hz (thornback ray; Platyrhinoidis triseriata) to ~100 Hz (mottled sculpin and topminnow) (Fig. 6c). In this regard, it is worth noting that temperature also impacts the frequency responsiveness of lateral line fibers, as has been experimentally determined in the cold-water ruffe (Acerina cernua) and the warm-water African clown knifefish (Notopterus chitala; Wiersinga-Post & van Netten, 2000). An increase in temperature produces an upward shift in the frequency of maximum hair cell response, as measured by the amplitude of the summed extracellular potentials. In the cold-water ruffe, the frequency of maximum hair cell response (in a displacement frame of reference) for supraorbital CNs is 116 Hz at the normal habitat temperature of this species (4°C), but 290 Hz at 20°C. By comparison, the frequency of maximum hair cell response in the warm-water clown knifefish is 460 Hz at its normal habitat temperature (28°C). This is the highest “best” frequency ever reported for the lateral line system in any species. Remarkably, the best frequency of the temperature-independent cupula response matches that of the hair cell response at the normal habitat temperature of each species. This suggests that structural features have coevolved to take advantage of the upper physiological limit dictated by the animals normal temperature (Wiersinga-Post & van Netten, 2000; van Netten, 2006).
Absolute sensitivity also depends on a number of factors, including structural features that produce mechanical resonances and the number of hair cells innervated by a single fiber. Tuning curve estimates of best acceleration sensitivity range from ~10–5 (ALLN fibers in topminnow) to ~10-2 m s–2 (PLLN fibers in thornback ray) for CNs responding to sinusoidal (AC) stimuli in the region of best sensitivity (Fig. 6c). In mottled sculpin, an acceleration sensitivity of 0.001 ms−2 corresponds to a pressure-gradient sensitivity of 0.002 Pa/2 mm, where 2 mm is the interpore spacing on the trunk canal of a 10-cm long fish. Estimates of velocity sensitivity in SNs depend on whether a DC (unidirectional flow) or AC (oscillating flow) is used. Velocity thresholds estimated from peak or peak–peak velocity levels in AC signals fall within 10–5–10–4 m/s for both fish (Coombs & Janssen, 1989; Liao, 2010) and amphibians (Görner, 1963), and as a group are generally lower than estimates obtained with DC stimuli, which fall between 10–3 and 10–2 m/s (Engelmann et al., 2000, Voigt et al., 2000).
However, given that DC flow over the skin surface of a fish may result in microturbulence with AC components (Chagnaud et al., 2008a) (Section 7.1), it is difficult to interpret these measures. As such, experimental evidence for SN responses to “pure” DC stimuli is absent and difficult to obtain, but there is nevertheless good evidence that SNs respond extremely well to very low frequencies (i.e., 0.1 Hz) (Kroese et al., 1989) (Fig. 4a,c). One way to think about SNs is that they respond to low-frequency AC signals, but also to low-frequency modulations of DC stimuli. Finally, it should be pointed out that estimates of best displacement sensitivity (in the 10–9–10–10 m range, as mathematically derived from velocity and acceleration thresholds) are on par with those from hair cells in other sensory systems, including the mammalian auditory system (Hudspeth & Markin, 1994).
5.3 Receptive Fields and Spatial Selectivity
Receptive field data obtained with unidirectional flow sources (e.g., water jet) are lacking and those obtained with bidirectional (dipole) sources are difficult to interpret owing to the spatial complexity of dipole flow fields, which are bilaterally but not radially symmetrical (Kalmijn, 1988, 1989). Receptive fields to dipole sources are complicated further by the fact that hair cells are directionally sensitive, meaning that the receptive field size and shape for any given neuromast/afferent fiber will depend not only on source amplitude, but also on flow direction, which depends on both source location and orientation (Fig. 7). Nevertheless, two points about spatial selectivity and receptive fields can be made. First, the physics of lateral line stimulation by dipoles dictate that for any given source amplitude and orientation, responsiveness will decline steeply with distance from the neuromast (Fig. 10b). This is due to the fact that flow amplitude (whether measured in displacement, velocity or acceleration units) declines steeply with 1/distance3 from a dipole source (Kalmijn, 1988). Given that the lateral line responds to the net motion (velocity or acceleration) between the fish and the surrounding water, the effective amplitude will decline even more steeply (1/distance4). Second, based on response properties and peripheral innervation patterns, CNs are likely to preserve information about the spatial heterogeneities of a flow field, whereas SNs are more likely to smooth (average) them out. This is because SN fibers respond to flow velocity and integrate hair cell inputs from multiple neuromasts, whereas CN fibers respond to spatial differences in flow velocity (acceleration) and typically integrate hair cell inputs from within a single neuromast. How the receptive field size of a group of mutually innervated SNs compares to that of a single CN for stimuli of equal effectiveness (e.g., at the most effective frequency, orientation, etc.) remains to be seen. However, groups of mutually innervated SNs can extend over large areas, for example, along the entire length of the caudal fin (Münz, 1979, 1985). The idea that each CN fiber is driven by local flow anomalies in a restricted region of space is further supported by the absence of any evidence for mechanical coupling between adjacent CNs (Denton & Gray, 1983; Coombs et al., 1996). Neuronal responses of CN fibers can be predicted solely by the pressure difference across the surrounding two canal pores (see Fig. 10e, f), indicating little if any influence from adjacent canal segments.
Illustration of how flow direction over a given (e.g., supraorbital) neuromast (gray oval) varies with dipole source location when source orientation is fixed (a, b) and with source orientation when location is fixed (a, c). For this particular type of source, it is doubtful that flow direction over a single neuromast carries useful information about either source location or orientation
5.4 Directional Selectivity
The directional selectivity (or sensitivity) of the lateral line system can be described in terms of (1) the direction of flow over a given neuromast with respect to the axis of hair cell polarization or (2) the location or orientation of a flow source with respect to some reference point on the fish (Fig. 7). Source orientation may be described in terms of the direction of translational movement, or, in the case of oscillating sources, as the axis of bidirectional oscillation. Because flow fields around discrete sources (e.g., moving animals) are spatially complex, flow direction in the vicinity of a single SN will vary in complex ways with source location and orientation (Fig. 7). Further, even relatively uniform, unidirectional flow fields (e.g., a stream) can result in local perturbations of flow direction and amplitude along the body and lateral line system (Voigt et al., 2000; Chagnaud et al., 2008a; and Section 7.1). Therefore, the spatial distribution of local flow amplitudes and directions along arrayed neuromasts is likely to be an important source of information about either large-scale currents or discrete flow sources.
Given that hair cell responsiveness is a cosine function of the direction of hair bundle displacement (Flock, 1965a, b) (see Fig. 1b), it is tempting to assume that the firing rate of an afferent fiber varies in a similar fashion with the direction of flow over the neuromast. However, direct measures of this kind have rarely been made, and thus it is difficult to know how or if surrounding structures such as the cupula may further sharpen the inherent directional tuning of the neuromast hair cells. In any event, it is reasonable to assume that neuromasts respond best (if not exclusively) to flow directions along the axis of hair cell polarization (see Fig. 2), as has been confirmed experimentally in Xenopus laevis (Görner, 1963; Görner & Mohr, 1989).
For CNs, flow inside the canal is constrained to one of two directions along the canal axis, and thus flow direction inside the canal bears no simple relationship to flow direction outside the canal. For this and other reasons, CNs are not likely to be well suited for encoding information about flow direction. In contrast, flow around SNs is normally (but not always; see Schwarz et al., 2011) free from physical constraint. Thus, by comparing the response magnitude of dorsoventrally and rostrocaudally oriented neuromasts, it should theoretically be possible to compute the resulting flow direction along the skin surface. Moreover, the fact that individual fibers innervate multiple SNs in a row means that local deviations about the mean overall flow direction will likely be averaged out. In support of these theoretical distinctions, SNs, but not CNs, appear to underlie rheotactic abilities of fish in slow currents (Montgomery et al., 1997).
The directional sensitivity of single neuromasts to different source angles has been measured for surface wave sources, which produce radially symmetrical, slowly propagating waves with dominant motions in the vertical plane (Bleckmann, 1985). In the surface-feeding African clawed frog, SN afferents innervating stitches on the head were found to be omnidirectional, responding to surface wave sources from all directions around the head (Zittlau et al., 1986). Indices of directional specificity (length of the mean vector) were quite low (0.26) compared to those obtained from optic tectum cells in the midbrain (>0.8) under identical stimulus conditions. In contrast, hair cell potentials from large CNs on the head of two species of surface-feeding fish (Aplocheilus lineatus and Pantodon buchholzi) exhibited strong directional preferences, most likely shaped by the orientation of canals and grooves (developmentally stunted, half-formed canals) on the head (Bleckmann et al., 1989a; Schwarz et al., 2011). Further, the unconditioned, prey-orienting responses of these surface-feeding fishes have been used to demonstrate high levels of orientation accuracy for a wide range (360°) of source angles. Nevertheless, sensory deprivation experiments point to time-of-arrival cues at different neuromasts, rather than directional tuning of individual neuromasts, as the likely mechanism of source localization abilities in these fish (reviewed in Bleckmann et al., 1985; Görner & Mohr, 1989; see also the chapter by Montgomery et al.).
6 Efferent Modulation of Peripheral Activity
Insofar as it is understood, the octavolateralis efferent system functions as a gain control system to modulate hair cell sensitivity in the vestibular and auditory systems of all vertebrates (Köppl, 2011), as well as in the lateral line system of fishes and aquatic amphibians (Roberts & Meredith, 1989). In each case, efferent neurons make synaptic contacts with both hair cells and the afferent fibers innervating them (Hama, 1978; Köppl, 2011). Lateral line efferent fibers are anatomically easy to identify, as they typically possess smaller diameters than afferent fibers (e.g., Münz, 1985; Northcutt, 1992), indicating that conduction velocities are relatively slow and temporal fidelity unimportant. This is in strong contrast to afferent fibers for which fiber diameters and thus conduction velocities are believed to increase with increasing distances from the brain (see Section 3.3). Efferent cell bodies reside in the bilaterally paired octavolateralis efferent nucleus (OEN), which straddles the midline and is in close association with the branchiomotor columns in the hindbrain medulla in both fish and amphibians (Roberts and Meredith, 1989; see also the chapter by Wullimann & Grothe). The OEN receives inputs from a variety of sources, including locomotor and sensory nuclei (Roberts & Meredith, 1989; see also the chapter by Wullimann & Grothe). In fishes and amphibians there is no clear segregation of the OEN into distinct cell populations, according to whether their targets are auditory, vestibular, or lateral line (Roberts & Meredith, 1989). However, there is a tendency for a crude topographic segregation, with efferents innervating lateral line organs on the head region being more rostrally located than those supplying the trunk (reviewed in Wagner & Schwartz, 1996; Bricaud et al., 2001). This tendency appears to be species specific, with some species showing evidence of distinct rostral and caudal subdivisions whereas others do not (Wagner & Schwartz, 1996; Bricaud et al., 2001). However, evidence for a strict topographic map (an orderly representation of relative rostrocaudal position) is lacking. Moreover, rostral and caudal subdivisions seem to differ in terms of their topographic organization. In Aplocheilus, for example, approximately 66% of efferents from the rostral subdivision innervate lateral line organs on the head, whereas those in the caudal subdivision are evenly divided between those that supply the head and trunk (Wagner & Schwartz, 1996).
The total number of OEN neurons in most, if not all species studied so far, is far less than the total number of neuromasts, indicating a high degree of divergence and low spatial selectivity of OEN innervation (Bricaud et al., 2001). Thus, it is assumed that a single efferent neuron innervates more than one neuromast (Köppl, 2011). Further, in fish, a given efferent fiber can innervate all hair cells within a given neuromast, including those of opposite polarities (Faucherre et al., 2009), as well as hair cells in different lateral line submodalities (i.e., both SNs and CNs; Münz, 1985), on different sides of the body (Roberts & Meredith, 1989) and in other octavolateralis modalities (e.g., vestibular and auditory systems; Münz, 1985). Nevertheless, efferent fibers do show some degree of differential innervation of head and trunk regions of the lateral line, which likely relates to the rostrocaudal segregation of cell bodies in the OEN (Wagner & Schwartz, 1996) and separate rostral and caudal nerve rami (Song & Northcutt, 1991).
OEN neurons are predominantly cholinergic (Flock & Lam, 1974), reflecting their motoneuronal origin. However, other neuroactive peptides known to modulate hair cell sensitivity, including γ-aminobutyric acid (GABA), may be present in lateral line efferent synapses as well (Roberts & Meredith, 1989). The action of the efferent system on lateral line hair cells has been shown to reduce both spontaneous and evoked activity in afferent neurons (Russell, 1971; Tricas & Highstein, 1991). Despite the predominant inhibitory activity of OEN efferents, several reports have shown excitatory effects exemplified by an increase in discharge rate of afferents innervating vestibular (Highstein & Baker, 1985) and lateral line afferents (Flock & Russell, 1973).
The efferent system can be activated in one of two major ways: (1) by stimulation of different sensory modalities, including visual (Tricas & Highstein, 1990) and somatosensory (Roberts & Russell, 1972) systems or (2) by motor acts (e.g., vocalization, swimming motions) that self-stimulate the octavolateralis sense organs (Tricas & Highstein, 1991; Weeg et al., 2005). Clear evidence of self-stimulation of the lateral line comes from physiological recordings of afferent activity during respiratory gill (Montgomery et al., 1996) and swimming (Russell & Roberts, 1974; Ayali et al., 2009) movements of restrained fish, as well as from freely swimming fish (Palmer et al., 2005). Although flows generated by self-movement can serve a useful function (e.g., in active flow-sensing of stationary objects by Mexican blind cavefish) (Teyke, 1985; Windsor et al., 2008, 2010a,b), they can also create noise interference and saturation problems for the detection of other flow sources (e.g., prey). Thus, one of the proposed functions of the efferent system is that it inhibits hair cell activity under circumstances when self-motion is expected to over swamp the system (Roberts & Meredith, 1989).
All in all, the function of the efferent system in the processing of lateral line information is somewhat complicated because OEN efferents exhibit different modes of activation (sensory or motor), different effects on hair cells (excitatory or inhibitory) and diffuse influences on hair cells of different polarities, different submodalities of the lateral line, and different octavolateralis modalities all together. The most parsimonious view of the efferent system is thus as a context-dependent, gain-control system that operates in both feedback and feed forward modes (Roberts & Meredith, 1989). This duality permits control over hair cell sensitivity in context-appropriate ways. In the case of continued strong stimulation (self-generated or otherwise), both modes of the efferent system can be used to turn down the gain so as to prevent overstimulation of hair cells and improve overall signal-to-noise ratios. In the feedback mode, the system responds to sensory inputs about overstimulation and in the feed forward mode, it responds to premotor inputs that inform the OEN about motor instructions for behavioral actions in anticipation of overstimulation. In the case of sensory-evoked states of arousal (Highstein & Baker, 1985; Tricas & Highstein, 1991), the efferent system could operate in a feed forward mode to turn up the gain in anticipation of either predator or prey. This might be particularly true in cases in which sensory systems such as vision, with long-range sensing abilities, can inform the OEN about anticipated stimulation of shorter-range systems, such as the lateral line.
It is conceivable that the rostral and caudal subdivisions of the OEN subserve feedback and feed forward circuits, respectively (Bricaud et al., 2001). This idea stems from the observation that sensorimotor areas of the brain for instructing C-start escape behaviors (i.e., the Mauthner cell and reticulospinal system) are in close proximity to caudal OEN cells in at least two species of fish (Metcalfe et al., 1985; Wagner & Schwartz, 1996). Not only does the Mauthner-mediated escape behavior produce strong hydrodynamic stimuli (Tytell & Lauder, 2008), but activation of the Mauthner cell also leads to activation of the efferent system. Thus, it is easy to see how feed forward inhibition of the lateral line to block overstimulation during this fast-start behavior could be advantageous in this circumstance (Zottoli & Danielson, 1989).
In addition to the efferent innervation by the OEN, the peripheral lateral line system also receives diencephalic efferent input in zebrafish (Metcalfe et al., 1985; Bricaud et al., 2001), goldfish (Zottoli & van Horne, 1983; Puzdrowski, 1989), and catfish (New & Singh, 1994). Contrary to the OEN neurons, diencephalic neurons appear to be catecholaminergic and excitatory (Bricaud et al., 2001), but their function remains elusive.
7 Peripheral Encoding of Hydrodynamic Stimuli
The hydrodynamic stimuli that can be detected and encoded by the lateral line system can be grouped into three broad categories: (1) large-scale ambient water motions (e.g., streams, tidal currents, ocean waves) created by wind, temperature gradients or gravity, and local flow perturbations created by (2) stationary or (3) moving sources. Whereas animate sources create local flows via whole-body or body part movements, inanimate (or immobile) sources act as bluff bodies to create nearby flow disturbances in the presence of large-scale water motions. Each type of hydrodynamic stimulus provides important information to fish. For example, the direction of ambient water motion can provide stream-dwelling fish with the information they need to conserve energy and prevent downstream displacement, to travel to an upstream natal spawning site or odorous prey, or to maximize their interception of downstream planktonic drift (see the chapter by Montgomery et al.). Likewise, the hydrodynamic signatures of animate sources theoretically contribute information about the identity of the source, how big it is, where it is with respect to self, how fast it is moving, and what direction it moves. Finally, local flow disturbances caused by inanimate sources are thought to provide fish with information about habitat structure, including the location of flow refuges or prime foraging sites. All of this information is likely extracted from the spatiotemporal pattern of flow along the arrayed sensors of the lateral line system.
Flow patterns around different animate and inanimate bodies can vary dramatically, as Fig. 8 illustrates. Flow patterns depend not only on body size and the relative speed of motion between the body and the surrounding water (as predicted by the Reynolds number) (see the chapter by McHenry & Liao), but also on location with respect to the leading or trailing edge of a moving body (Fig. 8d, e) or a stationary body in flow (Fig. 8e). The forward translational motion of a fish, for example, can arguably be modeled as a mechanical dipole, defined by the difference that exists between a positive (above ambient) pressure at the leading edge and negative (below ambient) pressure at the trailing edge (e.g., compare leading edge flow patterns in Fig. 8c, d). Movement along more than one axis, such as that produced by undulatory movements of the caudal body and fin during fish swimming, can result in more complex, multipole fields. Small, slow-moving bodies (or small bodies in slow flows) (Reynolds number <10) will produce laminar flows with more or less parallel streamlines, whereas large, fast-moving bodies (or large stationary bodies in fast flows) will in addition produce trailing vortices (intermediate Reynolds numbers) or fully turbulent wakes (high Reynolds numbers). All things considered, it is easy to see how flow patterns can provide fish with rich information about their natural environment, including habitat structure and animate sources of interest.
Examples of water flow patterns around different flow-generating or flow-disrupting sources. (a) Pulsating bubble (monopole source). (b) Vibrating sphere (dipole source), frequently used as lateral line stimulus in experimental studies. (c) Gliding motions of a fish during the coast phase of burst and coast swimming, as modeled for Mexican blind cavefish by Hassan (1985). (d) Swimming motions (caudal body and fin undulations) of a giant Danio. (From Wolfgang et al., 1999.) (e) Bluff body in a stream generating a von Kármán vortex wake. (f) Surface waves produced by a whirlygig beetle. (g) Water jets produced by salp (Madin, 1990). (h) Exhalant siphon of a bivalve mollusc. (From Montgomery et al., 1995.) Boxes in (d) and (e) emphasize differences in flow patterns between leading and trailing edges of a moving (d) or stationary (e) body
7.1 Encoding of Large-Scale Ambient Water Motions
Large-scale unidirectional flows (currents) are prevalent in many aquatic environments, including streams, rivers, and oceans. Aquatic animals show a strong orienting response (rheotaxis) to these large-scale flows in which they typically orient upstream (positive rheotaxis) to minimize drag, but sometimes also downstream (negative rheotaxis) (see also the chapter by Montgomery et al.). Because rheotaxis is a robust behavior that utilizes visual, vestibular, tactile, and lateral line cues (Arnold, 1974; Montgomery et al., 1997), the exact nature of lateral line contributions to this multisensory behavior is difficult to discern and likely to be context dependent. Nevertheless, behavioral experiments show that SNs but not CNs are necessary for rheotactic behaviors at low flow speeds (<~8 cm s–1)-a finding that has been replicated in several different fish species (Montgomery et al., 1997; Baker & Montgomery, 1999).
As might be expected from the different response properties of SN and CN fibers to AC (dipole) stimuli (Section 5), afferent fiber responses to unidirectional (DC) stimuli also fall into two categories (Engelmann et al., 2000, 2002; Voigt et al., 2000) (Fig. 9a). This dichotomy appears to hold for both rheophilic (e.g., stream-dwelling trout) and limnophilic (e.g., pond-dwelling goldfish) species. Fibers responding to unidirectional flows increased their firing rate in a linear fashion to increasing flow speed (Voigt et al., 2000; Carton & Montgomery, 2002; Chagnaud et al., 2008a). The most sensitive of these fibers (type I fibers presumably innervating velocity-sensitive SNs) are characterized by steep rate/level slopes and nonadapting (tonic) responses to a maintained stimulus, whereas insensitive or weakly sensitive fibers (type II fibers presumably innervating acceleration-sensitive CNs) have flat or shallow rate/level slopes and adapting responses (Voigt et al., 2000).
To determine if stream-like flows created noise-interference problems for fish, the responsiveness of type I and II fibers to a suprathreshold AC stimulus (50 Hz vibrating sphere) was investigated in the presence and absence of a stream-like flow (Engelmann et al., 2002; Chagnaud et al., 2007b) (Fig. 9b). In the absence of flow, the responses of type I and II fibers to the AC stimulus were similar. However, in the presence of a 10 cm s–1 flow, the responses of type I fibers to the AC stimulus were masked, whereas those of type II fibers were not. This finding underscores the functional utility of CNs for detecting AC signals in the presence of flow noise. Moreover, the physiological results are consistent with behavioral results from mottled sculpin showing that the CN-mediated ability of these fish to detect AC signals is little affected by DC flow noise (Coombs et al., 2001).
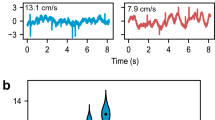
Effect of unidirectional flow on primary lateral line afferent responses. (a) Relationship between flow velocity and mean discharge rate of primary afferents in the New Zealand long-fin eel Anguilla dieffenbachii. Note that the majority of fibers increased their discharge rate with increasing flow velocity. (Data adapted from Voigt et al., 2000.) (b) Raster plots of evoked spikes from a type I (likely innervating a SN) and type II (likely innervating a CN) afferent to a vibrating sphere (respective sphere displacement indicated at the left) under no-flow and unidirectional flow conditions. Note that both afferents respond similarly under still water conditions. Under flow conditions, however, the type I afferent (likely innervating a SN) response to the vibrating sphere is reduced. (Data adapted from Engelmann et al., 2000)
One surprising result of neurophysiological studies with stream-like stimuli is that reversals in flow direction do not produce the expected results, given that fibers innervate hair cells of one polarity only (Section 3.1). That is, a switch from, for example, rostrocaudal to caudorostral flow directions does not cause an increase in firing rate followed by a decrease in firing rate (or vice versa, depending on the orientation of the innervated hair cells with respect to the current direction). Rather, the vast majority of primary afferents were found to respond with increases in firing rate (Voigt et al., 2000), regardless of the flow direction (Chagnaud et al., 2008a). One explanation may be that under the conditions of these experiments there are micro and macroscopic turbulences along the fish’s skin surface, as evidenced by the burst-like firing patterns of afferents to unidirectional flow (e.g., Engelmann et al., 2002). In essence, the turbulence contributes an AC component to the otherwise DC flow, preventing complete saturation and adaptation of the afferent fiber response to maintained stimulation in one direction. An alternative explanation is that sampling biases (i.e., recording from a large population of CN fibers in addition to some SN fibers) could lead to the false conclusion that SN fibers respond to AC rather than DC components of the flow (Montgomery et al., 2009). Unfortunately, such a sampling bias is difficult to rule out when recording blindly from cranial nerves with mixed SN and CN fibers without using time-consuming procedures to distinguish between them (e.g., linear frequency-response analysis of Kroese et al., 1989) (Section 5.1).
The common turbulences in natural water flows may also provide usable cues for determining water flow direction and speed. As an individual packet of turbulence moves along the fish surface in the prevailing direction of bulk flow, it should lead to sequential activation of neighboring neuromasts (Chagnaud et al., 2008b). A comparison of activity in afferent fibers that innervate adjacent neuromasts could consequently be used to determine both flow velocity (from the time interval between sequentially activated neuromasts) and direction (from the sequence of activated neuromasts). Thus, the neuronal activity of closely spaced neuromasts can be correlated over time, demonstrating the potential to extract both flow velocity and flow direction via a cross correlation mechanism of lateral line afferent responses (Chagnaud et al., 2008b). Alternatively, given the directional tuning of neuromasts (Section 3.1) and their varying orientations on the body surface (Section 3.2), it is possible that flow direction and speed could be computed by vector addition of inputs from multiple neuromasts, a task that seems best suited to SNs (Section 5.4).
7.2 Dipole Fields and Spatial Activity Patterns Along Lateral Line Arrays
Dipole fields have played an important, but incomplete role in our understanding of how spatial patterns of activity along a linear array of sensors might encode information about flow sources, such as their location, distance, and orientation (Coombs et al., 1996, 2001) (Fig. 10). Dipole-like fields can be generated in different ways: by the translational or oscillatory movements of a solid body or by the presence of a stationary body in moving water. Under ideal (unbounded) conditions and with certain simplifying assumptions, the field around these bodies can be mathematically modelled with potential flow theory (PFT), an approach that has been used to model flow patterns around Mexican blind cavefish (Astyanax mexicanus) as they glide past stationary obstacles (Hassan, 1992; Windsor et al., 2010a), as well as those emanating from artificial prey vibrations in the context of prey-orienting behaviors of mottled sculpin (Coombs et al., 1996). One important assumption of PFT models, that is, freedom from vorticity, is violated in the boundary layer (solid–water interface) and other regions (e.g., in the trailing wake of a swimming fish or an object in a stream) where shear stresses and vortices predominate. Thus, PFT is not adequate for modeling vortex structures and cannot adequately describe the stimulus to SNs responding to shear stresses in the boundary layer. Nevertheless, it is very useful for describing the pressure-gradient stimulus to CNs, which are impervious to boundary layer effects (Rapo et al., 2009; see also the chapter by van Netten & McHenry).
Illustration of the sequential pattern of activation of a single CN (x-axis title = source position re: CN) when the source changes its location along a linear transect with respect to CN location (x = 0). The same figure also illustrates the instantaneous spatial pattern of activation across an array of CNs when the location of the source is fixed (at x = 0) (x-axis title = CN location re: source). Both sequential and instantaneous activation patterns vary with different source characteristics, including (a) source amplitude, (b) source distance, (c) source orientation, that is, the axis of source vibration is parallel or perpendicular to the long axis of the sensory array and (d) source direction, that is, whether the source is moving forward or backward along its axis of vibration. In (a–c), the pressure gradient across the surrounding two canal pores at each source position is plotted for the same phase of the sinusoidal cycle of source oscillation. In (d), the pressure gradient is plotted for two opposite phases of the sinusoidal cycle to show how the pressure-gradient direction (fore or aft along the canal axis as indicated by positive and negative values on the y-axis) oscillates over time at any given position. In (e) and (f), the magnitude (e) and phase angle (f) response of a PLLN fiber (red closed symbols) are plotted relative to spontaneous response rates (black open symbols) and modeled predictions for the pressure-gradient stimulus in blue. (Data from Coombs et al., 1996.) The slight mismatch between neuronal and modeled predictions are due to the fact that the fish’s body was tilted slightly downward with respect to the axis of source translocation in these neurophysiological studies
PFT models can be used to predict how various source parameters (e.g., amplitude, distance, and orientation) affect the instantaneous spatial pattern of stimulus amplitudes and directions (modeled as pressure gradients) along an array of CNs for a fixed source location (Fig. 10a–d, x-axis labeled CN position re: source). By the same token, they can also predict how the stimulus to a single sensor changes over time to the changing positions of a translocating source (Fig. 10a–f, x-axis labeled Source position re: CN). For an oscillating source at a fixed location and orientation, the pressure-gradient direction at any given CN location reverses its polarity over time (Fig. 10d) and the response magnitude and phase angle of each CN fiber faithfully represent the amplitude and direction (polarity) of the local pressure difference between surrounding canal pores (Fig. 10e, f). From modeling efforts like these, it can be seen that several prominent features in the spatial activation carry information about the source (Table 2). For example, the location of peak excitatory regions provides information about the somatotopic location of the source and the shape of the activation pattern carries information about source orientation (Fig. 10c). Because the peak amplitude in the pattern varies with several source parameters, including source distance, size, and amplitude, this simple feature is not a very useful piece of information in and of itself. However, when the bandwidth of the peak excitatory region and/or its spatial slope is normalized to peak amplitude, unambiguous information about source distance is provided (von der Emde et al., 1998; Coombs et al., 2002). The sign or the polarity of pressure gradients also carries information. The sign corresponds to the polarity of flow direction inside the canal (e.g., fore or aft), and in combination with the shape of the pattern, carries information about the relative direction of movement between source and fish. Precisely how this information is extracted and used by the CNS to guide behavior is unknown, but models such as these provide testable hypotheses of behavioral performance (e.g., Coombs & Patton, 2009). These will no doubt improve as the field progresses to more realistic and sophisticated models that use computational fluid dynamics to determine viscous effects and that also determine activation patterns over the entire lateral line system (e.g., Rapo et al., 2009), rather than just a single line of neuromasts along the trunk (e.g., Coombs et al., 1996).
Several signal-processing approaches have been used to extract information from dipole activation patterns, including the use of prominent features (e.g., location of minima, maxima and zero (polarity reversal) points (Franosch et al., 2005; Goulet et al., 2008), template matching using preobtained “banks” of patterns for known source parameters (Pandya et al., 2006), wavelet transform (Ćurčić-Blake & van Netten, 2006), beamforming (Yang et al., 2010), model-based estimation (Abdulsadda & Xiaobo, 2011), and even artificial neuronal networks (Abdulsadda & Xiaobo, 2011). Some of these processing strategies are unlikely to be implemented by the CNS. Template-matching would seem to require a huge bank of templates to cover every scenario and thus unlikely to be very efficient. Feature-based approaches also have serious drawbacks since various source parameters interact in complex ways to affect prominent features in the activation pattern. For example, peak excitatory regions to signify source location (Fig. 10a) are confounded by changes in source orientation (Fig. 10c) (Coombs & Patton, 2009). In short, it is unknown at present how or if any of these algorithms are implemented by the CNS of fish and amphibians.
7.3 Flow Disturbances: Vortex Shedding
Vortex rings are highly sustained patterns of spiral motions that can occur in many different biological situations (e.g., Vogel, 1996; see also the chapter by Liao & McHenry) (Fig. 8d, e, g, h). Vortices are typically shed in the wake of swimming fish (Blickhan et al., 1992; Drucker & Lauder, 1999; Nauen & Lauder, 2002) and the wake contains information about fish size, swimming speed, and swimming direction (Blickhan et al., 1992; Hanke et al., 2000; Hanke & Bleckmann, 2004). In addition, objects placed in a unidirectional flow may generate an alternating (left, right) series of vortices behind it termed Kármán vortex streets (see the chapter by McHenry & Liao) (Fig. 8e). Behavioral experiments show that fish can detect vortex rings (Tou, 1991) and that piscivorous catfish can follow the vortex trails of their prey in the dark (Pohlmann et al., 2001, 2004). Further, fish exploit vortex streets behind bluff bodies for station holding (Sutterlin & Waddy, 1975; Przybilla et al., 2010) and to reduce the costs of locomotion (Liao et al., 2003a,b). Thus, vortices provide useful hydrodynamic information to fish about vortex-generating sources, whether animate or inanimate.
In response to a single vortex ring travelling along the fish’s body, primary lateral line afferents of goldfish display highly reproducible changes in discharge rate as the vortex ring passes over the innervated neuromast (Chagnaud et al., 2006; Franosch et al., 2009) (Fig. 11). The pattern of response depends primarily on the direction of water motions in the vortex, re: the orientation of the innervated hair cells (e.g., compare Fig. 11a, b). Mathematical models of vortex encoding by the lateral line indicate that a single neuromast fiber could encode the orientation (spin direction) of the vortex ring, re: the neuromast’s axis of best sensitivity, but not its radius, distance, or velocity (Franosch et al., 2009). Nevertheless, the spatiotemporal pattern of activity along fibers from differently oriented neuromasts could theoretically provide information about many if not all of these remaining parameters.
Responses from a goldfish lateral line afferent to a single vortex travelling from head to tail (a) and from tail to head (b) along the fish’s body. (Top) 2D plot of water particle motion. (Middle) Dot-raster plot of evoked spike activity during repeated stimulus presentations. (Bottom) Peri-stimulus time histogram based on dot-raster data shown in middle panel. (Adapted from Chagnaud et al., 2006)
For stationary bluff bodies in a stream, the frequency of vortex shedding depends on the flow velocity and the diameter of the bluff body (Vogel, 1996; see also the chapter by McHenry & Liao). A Fourier analysis of the temporal firing pattern of lateral line afferents to a vortex street reveals that the vortex shedding frequency is indeed represented by the peak in the amplitude spectrum (Chagnaud et al., 2007a). Moreover, the “goodness” of the information about shedding frequency (i.e., the amplitude of the vortex-shedding peak) depends on the position of the fish relative to the bluff body (in this case, a cylinder). Lateral positions (behind, but to the left or right of the cylinder) resulted in stronger representations of the shedding frequency than positions centered on the cylinder, as would be expected by the lateral positions of shed vortices (Fig. 8e). That fish position themselves to maximize vortex interception is evidenced by the lateral entrainment of stream-dwelling fish to bluff bodies (Sutterlin & Waddy, 1975; Przybilla et al., 2010) and by the lateral positions that piscivorous catfish hold with respect to the hydrodynamic trails that they follow (Pohlmann et al., 2001, 2004).
7.4 Models of Unidirectional Body Movement: Translating Objects
Within their environment fish are constantly exposed to moving bodies, such as prey or predators. Fish can readily detect the speed and direction of moving objects in behavioral experiments (Vogel & Bleckmann, 2001). The responses of peripheral lateral line fibers to an object moving at constant speed along the length of a fish show several distinct features, including highly reproducible patterns of response that are direction dependent (Bleckmann & Zelick, 1993; Mogdans & Bleckmann, 1998; Mogdans & Geisen, 2009) (Fig. 12a). The reproducible responses to the approaching and passing object are followed by less reproducible and more variable responses in the wake of the object (Mogdans & Bleckmann, 1998; Montgomery & Coombs, 1998). Nevertheless, the direction, speed, and translocation of the object are represented in the temporal pattern of firing activity in a single neuromast fiber (Fig. 12). Response magnitude (peak firing rate), on the other hand, is likely to vary with multiple parameters, such as object speed, size, and distance (Fig. 12b) and thus, by itself, is not a very useful code for any of these parameters. In principle, the pressure-gradient pattern around a translating body (minus its wake features) can be modeled with PFT (Section 7.2). In this respect, the reproducible components of the response patterns to the approaching and passing object are a good match to modeled and measured pressure-gradient patterns (Montgomery & Coombs, 1998; Mogdans & Geisen, 2009). Finally, it should be noted that type II fibers presumed to innervate CNs exhibit robust responses to the passage of a translating object, even in the presence of a high (>10 cm s–1) background flow (Engelmann et al., 2003).
(a) Responses from a single anterior lateral line nerve fiber in the goldfish to a translating object as a function of travel direction (tail to head in top set of graphs; head to tail in bottom set). Top trace in each set shows dot-raster plot of spikes over repeated stimulus presentations; bottom panel: Peri-stimulus time histograms of spike activity shown in dot-raster. (b) Single-point measurement of water velocity to a translating object of small (0.1 cm square, top), medium (1.0 cm square, middle), and large (4 cm square, bottom) size and at three distances from the sensor (1, 2, and 3 cm). [Data from a and b adapted from Mogdans & Geisen, 2009)
7.5 Surface Waves
Surface waves, such as those created by terrestrial insects that have fallen into the water or aquatic insects that skim the water’s surface (e.g., whirligig beetles) (see Fig. 8f) are potent lateral line stimuli. Not surprisingly, surface-feeding fish such as the topminnow (Aplocheilus lineatus) have several behavioral (see the chapter by Montgomery et al. ) and anatomical adaptations for intercepting and detecting surface waves, including specializations of the lateral line (e.g., enlarged canal neuromasts in topless canals) on the flattened, dorsal surface of their heads (Bleckmann et al., 1989a). Unfortunately, there are very few data on the response properties of afferent fibers to surface waves, and the few data that do exist fail to characterize the spatiotemporal pattern of response to, for example, a spreading surface wave. Nevertheless, two important points can be made. One, surface waves and subsurface water disturbances (Sections 7.1–7.4) differ from one another in many significant ways, including propagation speed and how water disturbances change with distance from the source. Thus, peripheral codes for various source characteristics may be very different in each case. For example, hydrodynamic disturbances created by dipole sources propagate at the speed of sound (Kalmijn, 1989), whereas those in surface waves propagate very slowly (cm’s/s) (Bleckmann et al., 1989a). Thus, instantaneous spatial patterns of activity may be more useful for representing various dipole source parameters, such as distance and location, than for surface wave sources. Indeed, behavioral experiments on prey-orienting responses of mottled sculpin to subsurface dipole sources and of topminnow to surface-wave sources indicate that for source location, spatial patterns are used in the former case (Conley & Coombs, 1998) and time of arrival cues are used in the latter (reviewed in Bleckmann, 1985; Görner & Mohr, 1989). As a second example, the amplitude of particle motion in a surface wave decreases in a frequency-dependent fashion, with high-frequency components attenuating more rapidly with distance than low-frequency components. In contrast, a frequency-dependent attenuation of water motion does not occur for subsurface sources in similar unbounded (free field) conditions. As a result, temporal (frequency-following) codes, as reported for afferent fibers in the surface-feeding clawed frog (Zittlau et al., 1986; Elepfandt & Wiedemer, 1987), provide information about source distance, an idea that is supported by behavioral experiments.
As a second important point, lateral line afferents from neuromasts on the dorsal surface of some surface-feeding fishes respond to surface wave sources in a directionally selective manner with each neuromast being “tuned” to different directions, depending on its location and orientation on the head (Bleckmann et al., 1989a; Schwarz et al., 2011). Thus, the pattern of activity across different spatially tuned neuromasts could provide information about source location. Nevertheless, behavioral experiments indicate that time of arrival cues (for this slowly propagating wave) across spatially distributed neuromasts are used to determine source location, not directional tuning of neuromasts (Bleckmann et al., 1989).
8 Summary and Conclusions
The peripheral lateral line system is characterized by several hallmark features: (1) it consists of many (from <50 to >1000) individual flow sensors distributed all over the head and body; (2) each sensor has an opponent organization of hair cells that results in a single axis of bidirectional sensitivity; (3) flow sensors are subdivided into functionally distinct submodalities (CNs and SNs); and (4) sensors are arranged into discrete arrays. Despite many speculations about the functional ramifications of these features, even some of the most basic features have yet to be fully understood and appreciated—largely because so little is known about how information is processed in the brain, but also because well-designed behavioral and physiological tests of specific functions are lacking. A good example of a poorly understood feature that could benefit from this approach is the opponent organization of receptor cells (Section 3.1).
Another example involves the widely held belief that the spatially distributed nature of this system is ideally suited for encoding spatiotemporal features of complex flows and for forming hydrodynamic images of the environment. Recent technological advances in our ability to measure and model flow patterns have indeed led to a far richer appreciation of the kinds of information that these complex patterns hold (Sections 7.2–7.4). Nevertheless, we still know very little about how spatiotemporal information is processed and used by the CNS to direct actual behavior. In the case of spatiotemporal information about the location of discrete flow-generating sources (e.g., a stationary dipole; Section 7.2), the processing strategies for orienting behaviors must ultimately transform a 2-D, somatotopic representation of source parameters along the body surface into a 3-D egocentric representation with respect to a single, head-centered frame of reference (Coombs & Patton, 2009; see also the chapter by Montgomery et al.). This transformation undoubtedly involves the optic tectum of the midbrain, a highly conserved site of multisensory and motorsensory integration that subserves orienting behaviors in all vertebrates (Stein & Meredith, 1993). Thus, the many processing strategies and algorithms put forward by engineering and computational modeling groups must ultimately be tested with respect to how or if these algorithms are implemented in the brain to direct behavior.
The functional dichotomy between SNs and CNs has come into much sharper focus in recent years—largely because of well-motivated behavioral and physiological experiments to test behaviorally and ecologically relevant hypotheses (see the chapter by Montgomery et al.). In particular, CNs have been identified as playing a key role in detecting and localizing (AC) signals from discrete sources and for being able to do so, even in the presence of flow noise (Sections 5.2 and 7.1). In contrast, SNs have been identified as playing a key role in detecting wide-scale, ambient currents and in mediating rheotaxis to slow currents (see the chapter by Montgomery et al.). Both behavioral and physiological lines of evidence suggest that superficial neuromasts (SNs) function in the spatiotemporal integration of information across multiple receptors (e.g., to compute the general direction of a uniform or large-scale current) and conversely, canal neuromasts (CNs) function in the spatiotemporal segregation of information for encoding the spatial heterogeneities associated with small, current-generating or current-distorting sources) (reviewed in Kanter & Coombs, 2003). Nonetheless, multiple studies have revealed that information from the different submodalities may be combined in different ways under different behavioral contexts. Whereas a single submodality (either SNs or CNs) may be used for relatively simple tasks, for example, rheotaxis at low current speeds (SN) or prey orientation in still water (CN), both submodalities are likely to work together in ways not yet understood for more demanding tasks (e.g., active flow sensing by Mexican blind cavefish).
Recent work on the developing lateral line system in zebrafish has also shed new light on the organization of the peripheral lateral line system, raising several interesting questions regarding the function of CNs and SNs and how they are wired up to the brain. This work indicates that there may be fast-conducting pathways from widely distributed, mutually innervated neuromasts to the Mauthner cell/reticulospinal escape network (Liao & Haehnel, 2012; Pujol-Martí et al., 2012; and Section 3.3). Clearly, this finding must be reconciled with the prevailing view that in adult fish a single CN fiber innervates a single neuromast and mutually innervated SNs are restricted to a small locale (e.g., to a single scale). Unfortunately, all neuromasts are superficial at larval stages, so the submodal destination of the mutually innervated, widely distributed neuromasts is in question—that is, it is not known whether they remain superficial or become enclosed in canals at later stages of development (see the chapter by Webb).
Further complicating the issue is the fact that the majority of neuromasts on the trunk remain superficial in adult zebrafish because canal formation has been developmentally arrested. In other words, this small fish has retained some of its larval characteristics through a process known as paedomorphosis. Thus, it is difficult to know if the mutually innervated neuromasts belong to the SN or presumptive CN categories (see the chapter by Webb). If SNs, then the larval zebrafish finding may be consistent with what is known about mutually innervated SNs in other adult fish, albeit at a somewhat larger scale of spatial integration than currently believed. If CNs, then this may be evidence of a separate “pool” of CN fibers never before recognized. Such a pool would suggest that in addition to fiber populations being segregated according to hair cell orientation and submodality, they are also segregated into slow and fast conducting pathways according to fiber diameter and termination site (i.e., Mauthner cell vs. MON) in the brain. Indeed, there is evidence that thin and thick saccular (S1 and S2) afferents to goldfish auditory organs are segregated along these lines (Furukawa & Ishii, 1967). Further research is needed to address the question of whether the putative fast-conducting pathway in larval zebrafish persists in adult fish and if so, whether or not fast and slow afferent pathways represent a common organizational plan in other species.
Despite our advanced understanding of the physics that shape the SN/CN response dichotomy (see the chapter by van Netten & McHenry), there is still a prominent lack of data on the basic electrophysiological response properties of “naked” lateral line hair cells. Do lateral line hair cells, like vertebrate auditory hair cells (Eatock, 2000), adapt to a constant (DC) deflection? If they adapt, what is the time course of adaptation? Data on this question are needed to understand fully how neuromasts respond under conditions of constant exposure to unidirectional, ambient flow. Also lacking is detailed information on the intrinsic response properties of SN and CN hair cells. For example, given that SNs are presumably “designed” for responding to DC and low-frequency stimuli, do their hair cells show less adaptation than CN hair cells? Do hair cells in the two submodalities have other electrical or micromechanical properties that further differentiate their response properties? There is some anatomical (Song & Northcutt, 1991) and pharmacological (Song et al., 1995) evidence to suggest that hair cells in the two submodalities are fundamentally different. Comparative electrophysiological studies of hair cell responses coupled with pharmacological characterizations of hair cell ionic channels are needed to address potential differences in the intrinsic response properties of lateral line hair cells.
As a final note, our understanding of peripheral processing by the lateral line will undoubtedly improve as we gain more realistic, 3D “pictures” of complex flow fields using techniques like particle imaging velocimetry. Techniques like these, coupled with computational fluid dynamics to construct 3D models of spatiotemporal activation patterns along the lateral line system of a given species (e.g., Rapo et al., 2009), will be particularly instructive, especially if they incorporate both viscous and potential flow effects to model the effective stimulus to both SNs and CNs.
Abbreviations
- ALLN:
-
anterior lateral line nerve
- CN:
-
canal neuromast
- CNS:
-
central nervous system
- MON:
-
medial octavolateral nucleus
- OEN:
-
octavolateral efferent nucleus
- PFT:
-
potential flow theory
- PIV:
-
particle image velocimetry
- PLLN:
-
posterior lateral line nerve
- SN:
-
superficial neuromast
References
Abdulsadda, A. T., & Xiaobo, T. (2011). Underwater source localization using an IPMC-based artificial lateral line Paper presented at the IEEE International Conference on Robotics and Automation (ICRA), May 9–13, 2011, Shanghai.
Alexandre, D., & Ghysen, A. (1999). Somatotopy of the lateral line projection in larval zebrafish. Proceedings of the National Academy of Sciences of the USA, 96(13), 7558–7562.
Altman, J. S., & Dawes, E. A. (1983). A cobalt study of medullary sensory projections from lateral line nerves, associated cutaneous nerves, and the VIIIth nerve in adult Xenopus. The Journal of Comparative Neurology, 213(3), 310–326.
Arnold, G. (1974). Rheotropism in fishes. Biological Reviews, 49, 515–576.
Ashmore, J. F. (1991). The electrophysiology of hair cells. Annual Review of Physiology, 53(1), 465–476.
Ayali, A., Gelman, S., Tytell, E. D., & Cohen, A. H. (2009). Lateral-line activity during undulatory body motions suggests a feedback link in closed-loop control of sea lamprey swimming. Canadian Journal of Zoology, 87(8), 671–683.
Baker, C. F., & Montgomery, J. C. (1999). Lateral line mediated rheotaxis in the Antarctic fish Pagothenia borchgrevinki. Polar Biology, 21(5), 305–309.
Bleckmann, H. (1985). Perception of water surface waves: how surface waves are used for prey identification, prey localization, and intraspecific communication. In D. Ottoson (Ed.), Progress in sensory physiology (pp. 147–166). Heidelberg: Springer-Verlag.
Bleckmann, H. (2008). Peripheral and central processing of lateral line information. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 194(2), 145–158.
Bleckmann, H., & Topp, G. (1981). Surface wave sensitivity of the lateral line organs of the topminnow Aplocheilus lineatus. Naturwissenschaften, 68(12), 624–625.
Bleckmann, H., & Zelick, R. (1993). The responses of peripheral and central mechanosensory lateral line units of weakly electric fish to moving objects. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 172(1), 115–128.
Bleckmann, H., Tittel, G., & Blübaum-Gronau, E. (1989a). The lateral line system of surface-feeding fish: Anatomy, physiology, and behavior. In S. Coombs, P. Görner, & H. Münz (Eds.), The mechanosensory lateral line: Neurobiology and evolution (pp. 501–526). New York: Springer-Verlag.
Bleckmann, H., Weiss, O., & Bullock, T. H. (1989b). Physiology of lateral line mechanoreceptive regions in the elasmobranch brain. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 164(4), 459–474.
Blickhan, R., Krick, C., Breithaupt, T., Zehren, D., & Nachtigall, W. (1992). Generation of a vortex-chain in the wake of a subundulatory swimmer. Naturwissenschaften, 79, 220–221.
Bricaud, O., Chaar, V., Dambly-Chaudière, C., & Ghysen, A. (2001). Early efferent innervation of the zebrafish lateral line. The Journal of Comparative Neurology, 434(3), 253–261.
Carr, C. E. (1990). Neuroethology of electric fish. Bioscience, 40(4), 259–267.
Carr, C. E. (1993). Processing of temporal information in the brain. Annual Review of Neuroscience, 16(1), 223–243.
Carton, A., & Montgomery, J. (2002). Responses of lateral line receptors to water flow in the Antarctic notothenioid, Trematomus bernacchii. Polar Biology, 25(10), 789–793.
Chagnaud, B. P., Bleckmann, H., & Engelmann, J. (2006). Neural responses of goldfish lateral line afferents to vortex motions. Journal of Experimental Biology, 209(2), 327–342.
Chagnaud, B. P., Bleckmann, H., & Hofmann, M. H. (2007a). Kármán vortex street detection by the lateral line. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 193(7), 753–763.
Chagnaud, B. P., Hofmann, M. H., & Mogdans, J. (2007b). Responses to dipole stimuli of anterior lateral line nerve fibres in goldfish, Carassius auratus, under still and running water conditions. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 193(2), 249–263.
Chagnaud, B. P., Bleckmann, H., & Hofmann, M. H. (2008a). Lateral line nerve fibers do not code bulk water flow direction in turbulent flow. Zoology (Jena), 111(3), 204–217.
Chagnaud, B. P., Brücker, C., Hofmann, M. H., & Bleckmann, H. (2008b). Measuring flow velocity and flow direction by spatial and temporal analysis of flow fluctuations. Journal of Neuroscience, 28(17), 4479–4487.
Conley, R. A., & Coombs, S. (1998). Dipole source localization by mottled sculpin. III. Orientation after site-specific, unilateral denervation of the lateral line system. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 183(3), 335–344.
Coombs, S., & Janssen, J. (1989). Peripheral processing by the lateral line system of the mottled sculpin (Cottus bairdi). In S. Coombs, P. Görner & H. Münz (Eds.), The mechanosensory lateral line: Neurobiology and evolution (pp. 299–319). New York: Springer-Verlag.
Coombs, S., & Janssen, J. (1990). Behavioral and neurophysiological assessment of lateral line sensitivity in the mottled sculpin, Cottus bairdi. Journal of Comparative Physiology A: Sensory Neural and Behavioral Physiology, 167(4), 557–567.
Coombs, S., & Montgomery, J. (1992). Fibers innervating different parts of the lateral line system of an Antarctic notothenioid, Trematomus bernacchii, have similar frequency responses, despite large variation in the peripheral morphology. Brain Behavior and Evolution, 40(5), 217–233.
Coombs, S., & Montgomery, J. (1994). Function and evolution of superficial neuromasts in an Antarctic notothenioid fish. Brain Behavior and Evolution, 44(6), 287–298.
Coombs, S., & van Netten, S. (2005). The hydrodynamics and structural mechanics of the lateral line system. In E. S. Robert & V. L. George (Eds.), Fish physiology, Vol. 23 (pp. 103–139). San Diego: Academic Press.
Coombs, S., & Patton, P. (2009). Lateral line stimulation patterns and prey orienting behavior in the Lake Michigan mottled sculpin Cottus bairdi. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 195(3), 279–297.
Coombs, S., Janssen, J., & Webb, J. F. (1988). Diversity of lateral line systems: Evolutionary and functional considerations. In J. Atema, R. R. Fay, A. N. Popper, & W. N. Tavolga (Eds.), Sensory biology of aquatic animals (pp. 553–593). New York: Springer-Verlag.
Coombs, S., Fay, R. R., & Janssen, J. (1989). Hot-film anemometry for measuring lateral line stimuli. Journal of the Acoustical Society of America, 85(5), 2185–2193.
Coombs, S., Hastings, M., & Finneran, J. (1996). Modeling and measuring lateral line excitation patterns to changing dipole source locations. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 178(3), 359–371.
Coombs, S., Braun, C. B., & Donovan, B. (2001). The orienting response of Lake Michigan mottled sculpin is mediated by canal neuromasts. Journal of Experimental Biology, 204(2), 337–348.
Coombs, S., New, J. G., & Nelson, M. (2002). Information-processing demands in electrosensory and mechanosensory lateral line systems. Journal of Physiology (Paris), 96(5–6), 341–354.
Ćurčić-Blake, B., & van Netten, S. M. (2006). Source location encoding in the fish lateral line canal. Journal of Experimental Biology, 209(8), 1548–1559.
Denton, E. J., & Gray, J. A. B. (1979). The analysis of sound by the sprat ear. Nature, 282, 406–407.
Denton, E. J., & Gray, J. (1983). Mechanical factors in the excitation of clupeid lateral lines. Proceedings of the Royal Society of London B: Biological Sciences, 218(1210), 1–26.
Denton, E. J., & Gray, J. A. B. (1988). Mechanical factors in the excitation of the lateral lines of fishes (pp. 595–617). In J. Atema, R. R. Fay, A. N. Popper & W. N. Tavolga (Eds.), Sensory biology of aquatic animals. New York: Springer-Verlag.
Denton, E. J., & Gray, J. A. B. (1989). Some observations on the forces acting on neuromasts in fish lateral line canals. In S. Coombs, P. Görner, & H. Münz (Eds.), The mechanosensory lateral line: Neurobiology and evolution (pp. 229–246). New York: Springer-Verlag.
Drucker, E. G., & Lauder, G. V. (1999). Locomotor forces on a swimming fish: Three-dimensional vortex wake dynamics quantified using digital particle image velocimetry. Journal of Experimental Biology, 202(18), 2393–2412.
Eatock, R. A. (2000). Adaptation in hair cells. Annual Review of Neuroscience, 23(1), 285–314.
Eatock, R. A., Fay, R. R., & Popper, A. N., Eds. (2006). Vertebrate hair cells. New York: Springer.
Elepfandt, A., & Wiedemer, L. (1987). Lateral-line responses to water surface waves in the clawed frog, Xenopus laevis. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 160(5), 667–682.
Engelmann, J., Hanke, W., Mogdans, J., & Bleckmann, H. (2000). Hydrodynamic stimuli and the fish lateral line. Nature, 408(6808), 51–52.
Engelmann, J., Hanke, W., & Bleckmann, H. (2002). Lateral line reception in still- and running water. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 188(7), 513–526.
Engelmann, J., Kröther, S., Bleckmann, H., & Mogdans, J. (2003). Effects of running water on lateral line responses to moving objects. Brain Behavior and Evolution, 61(4), 195–212.
Faucher, K., Lagardère, J.-P., & Aubert, A. (2005). Quantitative aspects of the spatial distribution and morphological characteristics of the sea bass (Dicentrarchus labrax L.; Teleostei, Serranidae) trunk lateral line neuromasts. Brain Behavior and Evolution, 65, 231–243.
Faucherre, A., Pujol-Martí, J., Kawakami, K., & López-Schier, H. (2009). Afferent neurons of the zebrafish lateral line are strict selectors of hair-cell orientation. PLoS ONE, 4(2), e4477.
Flock, Å. (1965a). Transducing mechanisms in the lateral line canal organ receptors. Cold Spring Harbor Symposia on Quantitative Biology, 30, 133–145.
Flock, Å. (1965b). Electron microscopic and electrophysiological studies on the lateral line canal organ. Acta Oto-laryngologica Supplementum, S199, 7–90.
Flock, Å. (1971). Sensory transduction in hair cells. In W. Loewenstein (Ed.), Handbook of sensory physiology, Vol. 1 (pp. 396–441). New York: Springer-Verlag.
Flock, Å., & Wersäll, J. (1962). A study of the orientation of the sensory hairs of the receptor cells in the lateral line organ of fish, with special reference to the function of the receptors. The Journal of Cell Biology, 15(1), 19–27.
Flock, Å., & Russell, I. J. (1973). The post-synaptic action of efferent fibres in the lateral line organ of the burbot Lota lota. Journal of Physiology, 235(3), 591–605.
Flock, Å. K. E., & Lam, D. M. K. (1974). Neurotransmitter synthesis in inner ear and lateral line sense organs. Nature, 249(5453), 142–144.
Franosch, J.-M., Sichert, A., Suttner, M., & Hemmen, J. (2005). Estimating position and velocity of a submerged moving object by the clawed frog Xenopus and by fish—A cybernetic approach. Biological Cybernetics, 93(4), 231–238.
Franosch, J.-M. P., Hagedorn, H. J. A., Goulet, J., Engelmann, J., & van Hemmen, J. L. (2009). Wake tracking and the detection of vortex rings by the canal lateral line of fish. Physical Review Letters, 103(7), 078102.
Fritzsch, B. (1981). The pattern of lateral-line afferents in urodeles: A horseradish-peroxidase study. Cell & Tissue Research, 218(3), 581–594.
Furukawa, T., & Ishii, Y. (1967). Neurophysiological studies on hearing in goldfish. Journal of Neurophysiology, 30, 1377–1403.
Görner, P. (1963). Untersuchungen zur Morphologie und Elektrophysiologie des Seitenlinienorgans vom Krallenfrosch (Xenopus laevis Daudin). Zeitschrift für vergleichende Physiologie, 47, 316–338.
Görner, P., & Kalmijn, A. J. (1989). Frequency response of lateral line neuromasts in the thornback ray (Platyrhinoidis triseriata). In J. Erber, R. Menzel, H.-J. Pflüger, & D. Todt (Eds.), Proceedings of the 2nd international congress of neuroethology, 1st ed. Stuttgart: Thieme.
Görner, P., & Mohr, C. (1989). Stimulus localization in Xenopus: Role of directional sensitivity of lateral line stitches. In S. Coombs, P. Görner & H. Münz (Eds.), The mechanosensory lateral line. Neurobiology and evolution (pp. 543–560).New York: Springer-Verlag.
Goulet, J., Engelmann, J., Chagnaud, B., Franosch, J.-M., Suttner, M., & van Hemmen, J. (2008). Object localization through the lateral line system of fish: Theory and experiment. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 194(1), 1–17.
Haehnel, M., Taguchi, M., & Liao, J. C. (2012). Heterogeneity and dynamics of lateral line afferent innervation during development in zebrafish (Danio rerio). The Journal of Comparative Neurology, 520(7), 1376–1386.
Hama, K. (1978). A study of the fine structure of the pit organ of the common Japanese sea eel Conger myriaster. Cell and Tissue Research, 189(3), 375–388.
Hanke, W., & Bleckmann, H. (2004). The hydrodynamic trails of Lepomis gibbosus (Centrarchidae), Colomesus psittacus (Tetraodontidae) and Thysochromis ansorgii (Cichlidae) investigated with scanning particle image velocimetry. Journal of Experimental Biology, 207(9), 1585–1596.
Hanke, W., Brücker, C., & Bleckmann, H. (2000). The ageing of the low-frequency water disturbances caused by swimming goldfish and its possible relevance to prey detection. Journal of Experimental Biology, 203(7), 1193–1200.
Harris, G. G., Frishkopf, L. S., & Flock, Å. (1970). Receptor potentials from hair cells of the lateral line. Science, 167(3914), 76–79.
Hassan, E. S. (1985). Mathematical analysis of the stimulus for the lateral line organ. Biological Cybernetics, 52(1), 23–36.
Hassan, E.-S. (1992). Mathematical description of the stimuli to the lateral line system of fish derived from a three-dimensional flow field analysis. Biological Cybernetics, 66(5), 443–452.
Highstein, S. M., & Baker, R. (1985). Action of the efferent vestibular system on primary afferents in the toadfish, Opsanus tau. Journal of Neurophysiology, 54(2), 370–384.
Highstein, S. M., Fay, R. R., & Popper, A. N. (2004). The vestibular system Springer Verlag.
Hudspeth, A. J., & Markin, V. S. (1994). The ear’s gears: Mechanoelectrical transduction by hair cells. Physics Today, 47(2), 22–28.
Janssen, J., Coombs, S., Hoekstra, D., & Platt , C. (1987). Anatomy and differential growth of the lateral line system of the mottled sculpin, Cottus bairdi (Scorpaeniformes: Cottidae). Brain, Behavior and Evolution, 30(3–4), 220–229.
Kalmijn, A. J. (1988 ). Hydrodynamic and acoustic field detection. In J. Atema, R. R. Fay, A. N. Popper, & W. N. Tavolga (Eds.), Sensory biology of aquatic animals. New York: Springer-Verlag,.
Kalmijn, A. J. (1989). Functional evolution of lateral line and inner ear sensory systems. In S. Coombs, P. Görner, & H. Münz (Eds.), The mechanosensory lateral line: Neurobiology and evolution (pp. 187–215). New York: Springer-Verlag.
Kanter, M. J., & Coombs, S. (2003). Rheotaxis and prey detection in uniform currents by Lake Michigan mottled sculpin (Cottus bairdi). Journal of Experimental Biology, 206(1), 59–70.
Kiang, N. Y. S., Baer, T., Marr, E. M., & Demont, D. (1969). Discharge rates of single auditory-nerve fibers as functions of tone level. The Journal of the Acoustical Society of America, 46(1A), 106.
Köppl, C. (2011). Evolution of the octavolateral efferent system. In D. K. Ryugo, R. R. Fay, & A. N. Popper (Eds.), Auditory and vestibular efferents (pp. 217–259). New York: Springer-Verlag.
Kroese, A. B., & van Netten, S. M. (1987). The application of incident light polarization microscopy for the visualization of vertebrate sensory hair cells in vivo. Journal of Microscopy, 145( 3), 309–317.
Kroese, A., & van Netten, S. (1989). Sensory transduction in lateral line hair cells. In S. Coombs, P. Görner, & H. Münz (Eds.), The mechanosensory lateral line: Neurobiology and evolution. New York: Springer-Verlag.
Kroese, A. B., & Schellart, N. A. (1992). Velocity- and acceleration-sensitive units in the trunk lateral line of the trout. Journal of Neurophysiology, 68(6), 2212–2221.
Kroese, A. B., Van der Zalm, J. M., & Van den Bercken, J. (1978). Frequency response of the lateral-line organ of Xenopus laevis. Pflügers Archive, 375(2), 167–175.
Kroese, A. B. A., Prins, M., & Schellart, N. A. M. (1989). Regional differences in conduction velocity and fibre diameter in posterior lateral line afferent axons in the trout. Journal of Physiology, 418, 136.
Liao, J. C., & Haehnel, M. (2012). Physiology of afferent neurons in larval zebrafish provides a functional framework for lateral line somatotopy. Journal of Neurophysiology, 107(10), 2615–2623.
Liao, J. C., Beal, D. N., Lauder, G. V., & Triantafyllou, M. S. (2003a). Fish exploiting vortices decrease muscle activity. Science, 302(5650), 1566–1569.
Liao, J. C., Beal, D. N., Lauder, G. V., & Triantafyllou, M. S. (2003b). The Kármán gait: Novel body kinematics of rainbow trout swimming in a vortex street. Journal of Experimental Biology, 206(6), 1059–1073.
López-Schier, H., Starr, C. J., Kappler, J. A., Kollmar, R., & Hudspeth, A. J. (2004). Directional cell migration establishes the axes of planar polarity in the posterior lateral-line organ of the zebrafish. Developmental Cell, 7(3), 401–412.
Madin, L. P. (1990). Aspects of jet propulsion in salps. Canadian Journal of Zoology, 68(4), 765–777.
McCormick, C. A. (1983). Central connection of the octavolateralis nerves in the pike cichlid, Crenicichla lepidota. Brain Research, 265(2), 177–185.
McCormick, C. A. (1989). Central lateral line mechanosensory pathways in bony fish. In S. Coombs, P. Görner, & H. Münz (Eds.), The mechanosensory lateral line: Neurobiology and volution (pp. 79–97). New York: Springer-Verlag.
Metcalfe, W. K., Kimmel, C. B., & Schabtach, E. (1985). Anatomy of the posterior lateral line system in young larvae of the zebrafish. The Journal of Comparative Neurology, 233(3), 377–389.
Mogdans, J., & Bleckmann, H. (1998). Responses of the goldfish trunk lateral line to moving objects. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 182, 659–676.
Mogdans, J., & Geisen, S. (2009). Responses of the goldfish head lateral line to moving objects. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 195(2), 151–165.
Mohr, C., & Görner, P. (1996). Innervation patterns of the lateral line stitches of the clawed frog, Xenopus laevis, and their reorganization during metamorphosis. Brain, Behavior and Evolution, 48(2), 55–69.
Montgomery, J., & Coombs, S. (1992). Physiological characterization of lateral line function in the Antarctic fish Trematomus bernacchii. Brain, Behavior and Evolution, 40(5), 209–216.
Montgomery, J., & Coombs, S. L. (1998). Peripheral encoding of moving sources by the lateral line system of a sit-and-wait predator. Journal of Experimental Biology, 201(1), 91–102.
Montgomery, J., Coombs, S., & Janssen, J. (1994). Form and function relationships in lateral line systems: Comparative data from six species of Antarctic notothenioid fish. Brain, Behavior and Evolution, 44(6), 299–306.
Montgomery, J. C., Coombs, S., Conley, R. A., & Bodznick, D. (1995a). Hindbrain sensory processing in lateral line, electrosensory and auditory systems: A comparative overview of anatomical and functional similarities. Auditory Neuroscience, 1, 207–231.
Montgomery, J., Coombs, S., & Halstead, M. (1995b). Biology of the mechanosensory lateral line in fishes. Reviews in Fish Biology and Fisheries, 5(4), 399–416.
Montgomery, J., Bodznick, D., & Halstead, M. (1996). Hindbrain signal processing in the lateral line system of the dwarf scorpionfish Scopeana papillosus. Journal of Experimental Biology, 199(4), 893–899.
Montgomery, J., Baker, C. F., & Carton, A. G. (1997). The lateral line can mediate rheotaxis in fish. Nature, 389, 960–963.
Montgomery, J. C., Windsor, S., & Bassett, D. (2009). Behavior and physiology of mechanoreception: Separating signal and noise. Integrative Zoology, 4(1), 3–12.
Münz, H. (1979). Morphology and innervation of the lateral line system of Sarotherodon niloticus L. (Cichlidae, Teleostei). Zoomorphology, 93, 73–86.
Münz, H. (1985). Single unit activity in the peripheral lateral line system of the cichlid fish, Sarotherodon niloticus L. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 157, 555–568.
Nagiel, A., Andor-Ardó, D., & Hudspeth, A. J. (2008). Specificity of afferent synapses onto plane-polarized hair cells in the posterior lateral line of the zebrafish. The Journal of Neuroscience, 28(34), 8442–8453.
Nauen, J. C., & Lauder, G. V. (2002). Hydrodynamics of caudal fin locomotion by chub mackerel, Scomber japonicus (Scombridae). Journal of Experimental Biology, 205(Pt 12), 1709–1724.
New, J. G., & Singh, S. (1994). Central topography of anterior lateral line nerve projections in the channel catfish, Ictalurus punctatus. Brain, Behavior and Evolution, 43(1), 34–50.
Northcutt, G. (1989). The phylogenetic distribution and innervation of craniate mechanoreceptive lateral lines. In S. Coombs, P. Görner, & H. Münz (Eds.), The mechanosensory lateral line: Neurobiology and evolution (pp. 17–78). New York: Springer-Verlag.
Northcutt, R. G. (1992). Distribution and innervation of lateral line organs in the axolotl. Journal of Comparative Neurology, 325(1), 95–123.
Northcutt, R. G., Holmes, P. H., & Albert, J. S. (2000). Distribution and innervation of lateral line organs in the channel catfish. Journal of Comparative Neurology, 421(4), 570–592.
Palmer, L. M., Deffenbaugh, M., & Mensinger, A. F. (2005). Sensitivity of the anterior lateral line to natural stimuli in the oyster toadfish, Opsanus tau (Linnaeus). Journal of Experimental Biology, 208(18), 3441–3450.
Pandya, S., Yang, Y., Jones, D. L., Engel, J., & Liu, C. (2006). Multisensor processing algorithms for underwater dipole localization and tracking using MEMS artificial lateral-line sensors. EURASIP Journal on Applied Signal Processing, 2006, 1–9.
Pohlmann, K., Grasso, F. W., & Breithaupt, T. (2001). Tracking wakes: The nocturnal predatory strategy of piscivorous catfish. Proceedings of the National Academy of Sciences of the USA, 98(13), 7371–7374.
Pohlmann, K., Atema, J., & Breithaupt, T. (2004). The importance of the lateral line in nocturnal predation of piscivorous catfish. Journal of Experimental Biology, 207(17), 2971–2978.
Przybilla, A., Kunze, S., Rudert, A., Bleckmann, H., & Brücker, C. (2010). Entraining in trout: A behavioural and hydrodynamic analysis. Journal of Experimental Biology, 213(17), 2976–2986.
Pujol-Martí, J., Zecca, A., Baudoin, J.-P., Faucherre, A., Asakawa, K., Kawakami, K., & López-Schier, H. (2012). Neuronal birth order identifies a dimorphic sensorineural map. The Journal of Neuroscience, 32(9), 2976–2987.
Puzdrowski, R. L. (1989). Peripheral distribution and central projections of the lateral-line nerves in goldfish, Carassius auratus. Brain Behavior and Evolution, 34(2), 110–131.
Rapo, M. A., Jiang, H., Grosenbaugh, M. A., & Coombs, S. (2009). Using computational fluid dynamics to calculate the stimulus to the lateral line of a fish in still water. Journal of Experimental Biology, 212(10), 1494–1505.
Roberts, B. L., & Russell, I. J. (1972). The activity of lateral-line efferent neurones in stationary and swimming dogfish. Journal of Experimental Biology, 57(2), 435–448.
Roberts, B. L., & Meredith, G. E. (1989). The efferent system. In S. Coombs, P. Görner, & H. Münz (Eds.), The mechanosensory lateral line: Neurobiology and evolution (pp. 445–459). New York: Springer-Verlag.
Russell, I. J. (1971). The pharmacology of efferent synapses in the lateral-line system of Xenopus laevis. Journal of Experimental Biology, 54(3), 643–658.
Russell, I. J., & Roberts, B. L. (1974). Active reduction of lateral-line sensitivity in swimming dogfish. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 94(1), 7–15.
Russell, I. J., & Sellick, P. M. (1976). Measurement of potassium and chloride ion concentrations in the cupulae of the lateral lines of Xenopus laevis. The Journal of Physiology, 257(1), 245–255.
Schellart, N. S., & Kroese, A. K. (2002). Conduction velocity compensation for afferent fiber length in the trunk lateral line of the trout. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 188(7), 561–576.
Schemmel, C. (1967). Vergleichende Untersuchungen an den Hautsinnesorganen ober- und unterirdisch lebender Astyanax-Formen. Zoomorphology, 61(2), 255–316.
Schmitz, A., Bleckmann, H., & Mogdans, J. (2008). Organization of the superficial neuromast system in goldfish, Carassius auratus. Journal of Morphology, 269(6), 751–761.
Schwarz, J. S., Reichenbach, T., & Hudspeth, A. J. (2011). A hydrodynamic sensory antenna used by killifish for nocturnal hunting. Journal of Experimental Biology, 214(11), 1857–1866.
Shardo, J. (1996 ). Radial polarity of the first neuromast in embryonic American shad, Alosa sapidissima (Teleostei: Clupeomorpha). Copeia, 226–228.
Song, J., & Northcutt, R. G. (1991). Morphology, distribution and innervation of the lateral-line receptors of the florida gar, Lepisosteus platyrhincus. Brain Behavior and Evolution, 37, 10–37.
Song, J., Yan, H. Y., & Popper, A. N. (1995). Damage and recovery of hair cells in fish canal (but not superficial) neuromasts after gentamicin exposure. Hearing Research, 91(1–2), 63–71.
Stein, B. E., & Meredith, M. A. (1993). The merging of the senses. Cambridge, MA: The MIT Press.
Sutterlin, A. M., & Waddy, S. (1975). Possible role of the posterior lateral line in obstacle entrainment by brook trout (Salvelinus fontinalis). Journal of the Fisheries Research Board of Canada, 32, 2441–2446.
Teyke, T. (1985). Collision with and avoidance of obstacles by blind cave fish Anoptichthys jordani; (Characidae). Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 157(6), 837–843.
Topp, G. (1983). Primary lateral line response to water surface waves in the topminnow Aplocheilus lineatus (Pisces, Cyrinodontidae). Pflügers Archive, 397, 62–67.
Tou, S. K. W. (1991). A statistical and experimental study on fish response subject to vortex ring motion. Journal of Environmental Science and Health A, 26(5), 755–775.
Tricas, T. C., & Highstein, S. M. (1990). Visually mediated inhibition of lateral line primary afferent activity by the octavolateralis efferent system during predation in the free-swimming toadfish, Opsanus tau. Experimental Brain Research, 83(1), 233–236.
Tricas, T. C., & Highstein, S. M. (1991). Action of the octavolateralis efferent system upon the lateral line of free-swimming toadfish, Opsanus tau. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 169(1), 25–37.
Tytell, E. D., & Lauder, G. V. (2008). Hydrodynamics of the escape response in bluegill sunfish, Lepomis macrochirus. Journal of Experimental Biology, 211(21), 3359–3369.
van Netten, S. (2006). Hydrodynamic detection by cupulae in a lateral line canal: Functional relations between physics and physiology. Biological Cybernetics, 94(1), 67–85.
Vogel, D., & Bleckmann, H. (2001). Behavioral discrimination of water motions caused by moving objects. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 186(12), 1107–1117.
Vogel, S. (1996). Life in moving fluids. Princeton, NJ: Princeton University Press.
Voigt, R., Carton, A. G., & Montgomery, J. C. (2000). Responses of anterior lateral line afferent neurones to water flow. Journal of Experimental Biology, 203(16), 2495–2502.
von der Emde, G., Schwarz, S., Gomez, L., Budelli, R., & Grant, K. (1998). Electric fish measure distance in the dark. Nature, 395(6705), 890–894.
Wagner, T., & Schwartz, E. (1996). Efferent neurons of the lateral line system and their innervation of lateral line branches in a euteleost and an osteoglossomorph. Anatomy and Embryology, 194(3), 271–278.
Webb, J. F. (1989a). Neuromast morphology and lateral line trunk canal ontogeny in two species of cichlids: An SEM study. Journal of Morphology, 202(1), 53–68.
Webb, J. F. (1989b). Developmental constraints and evolution of the lateral line system in teleost fishes In S. Coombs, P. Görner, & H. Münz (Eds.), The mechanosensory lateral line: Neurobiology and evolution (pp. 79–97). New York: Springer-Verlag.
Weeg, M. S., Land, B. R., & Bass, A. H. (2005). Vocal pathways modulate efferent neurons to the inner ear and lateral line. Journal of Neuroscience, 25(25), 5967–5974.
Wiersinga-Post, J. E. C., & van Netten, S. M. (2000). Temperature dependency of cupular mechanics and hair cell frequency selectivity in the fish canal lateral line organ. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 186(10), 949–956.
Wiese, K. (1984). Neural enhancement of directionality in sensory pathways of two arthropods, the cricket and the crayfish. In D. Varju & H. U. Schnitzler (Eds.), Localization and orientation in biology and engineering (pp. 186–191). New York: Springer-Verlag.
Wiese, K. (1988). The representation of hydrodynamic parameters in the CNS of the crayfish, Procambarus. In J. Atema, R. R. Fay, A. N. Popper, & W. N. Tavolga (Eds.), Sensory biology of aquatic animals (pp. 665–686). New York: Springer-Verlag.
Will, U., Luhede, G., & Görner, P. (1985). The area octavo-lateralis in Xenopus laevis. Cell and Tissue Research, 239(1), 163–175.
Windsor, S. P., Tan, D., & Montgomery, J. C. (2008). Swimming kinematics and hydrodynamic imaging in the blind Mexican cave fish (Astyanax fasciatus). Journal of Experimental Biology, 211(18), 2950–2959.
Windsor, S. P., Norris, S. E., Cameron, S. M., Mallinson, G. D., & Montgomery, J. C. (2010a). The flow fields involved in hydrodynamic imaging by blind Mexican cave fish (Astyanax fasciatus). Part I: Open water and heading towards a wall. Journal of Experimental Biology, 213(22), 3819–3831.
Windsor, S. P., Norris, S. E., Cameron, S. M., Mallinson, G. D., & Montgomery, J. C. (2010b). The flow fields involved in hydrodynamic imaging by blind Mexican cave fish (Astyanax fasciatus). Part II: Gliding parallel to a wall. Journal of Experimental Biology, 213(22), 3832–3842.
Wöhl, S., & Schuster, S. (2007). The predictive start of hunting archer fish: A flexible and precise motor pattern performed with the kinematics of an escape C-start. Journal of Experimental Biology, 210(2), 311–324.
Wolfgang, M. J., Anderson, J. M., Grosenbaugh, M. A., Yue, D. K., & Triantafyllou, M. S. (1999). Near-body flow dynamics in swimming fish. Journal of Experimental Biology, 202(17), 2303–2327.
Yang, Y., Nguyen, N., Chen, N., Lockwood, M., Tucker, C., Hu, H., Bleckmann, H., Liu, C., & Jones, D. L. (2010). Artificial lateral line with biomimetic neuromasts to emulate fish sensing. Bioinspiration & Biomimetics, 5, 016001.
Zittlau, K. E., Claas, B., & Münz, H. (1986). Directional sensitivity of lateral line units in the clawed toad Xenopus laevis; Daudin. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 158(4), 469–477.
Zottoli, S. J., & Van Horne, C. (1983). Posterior lateral line afferent and efferent pathways within the central nervous system of the goldfish with special reference to the Mauthner cell. The Journal of Comparative Neurology, 219(1), 100–111.
Zottoli, S. J., & Danielson, P. D. (1989). Lateral line afferent and efferent systems of the goldfish with special reference to the Mauthner cell. In S. Coombs, P. Görner, & H. Münz (Eds.), The mechanosensory lateral line: Neurobiology and evolution (pp. 461–478). New York: Springer-Verlag.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Chagnaud, B.P., Coombs, S. (2013). Information Encoding and Processing by the Peripheral Lateral Line System. In: Coombs, S., Bleckmann, H., Fay, R., Popper, A. (eds) The Lateral Line System. Springer Handbook of Auditory Research, vol 48. Springer, New York, NY. https://doi.org/10.1007/2506_2013_15
Download citation
DOI: https://doi.org/10.1007/2506_2013_15
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-8850-7
Online ISBN: 978-1-4614-8851-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)