Abstract
The spleen is located in the left upper quadrant of the abdomen and is typically covered by the 9th to 11th rib. It is an intraperitoneal organ with a ligamentous attachment to the greater curvature of the stomach (gastrosplenic ligament) and to the left kidney (splenorenal ligament). The spleen is typically wedge shaped, but often exhibits a wide variety of different shapes. Due to its often atypical shape, the most accurate way to compare the size of the spleen is to measure its volume (Harris et al. 2010). However, splenic volumetry is not routinely applied in clinical practice. Spleen size is typically determined by measuring the spleen length. In literature, a splenic length of 12 cm is defined as the upper limit of normal (Stiff et al. 2009; Sienz et al. 2011; Danila 2010). Nevertheless, it is important to know that the volume and length of the spleen are variable and might not show a normal distribution (Cruz-Romero et al. 2016). A study in 1230 healthy volunteers demonstrated that spleen length and volume are significantly influenced by sex, body height, and body weight (Chow et al. 2016). The median spleen length, anteroposterior dimension, width, and volume were 10.9 cm, 4.5 cm, 6.5 cm, and 166 cm3, respectively (Chow et al. 2016).
The original version of this chapter was revised. Water marks and line numbers have been removed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Imaging Anatomy and Function of the Spleen
The spleen is located in the left upper quadrant of the abdomen and is typically covered by the 9th to 11th rib. It is an intraperitoneal organ with a ligamentous attachment to the greater curvature of the stomach (gastrosplenic ligament) and to the left kidney (splenorenal ligament). The spleen is typically wedge shaped, but often exhibits a wide variety of different shapes. Due to its often atypical shape, the most accurate way to compare the size of the spleen is to measure its volume (Harris et al. 2010). However, splenic volumetry is not routinely applied in clinical practice. Spleen size is typically determined by measuring the spleen length. In literature, a splenic length of 12 cm is defined as the upper limit of normal (Stiff et al. 2009; Sienz et al. 2011; Danila 2010). Nevertheless, it is important to know that the volume and length of the spleen are variable and might not show a normal distribution (Cruz-Romero et al. 2016). A study in 1230 healthy volunteers demonstrated that spleen length and volume are significantly influenced by sex, body height, and body weight (Chow et al. 2016). The median spleen length, anteroposterior dimension, width, and volume were 10.9 cm, 4.5 cm, 6.5 cm, and 166 cm3, respectively (Chow et al. 2016). The authors observed that by applying 12 cm as the upper limit of normal for the spleen length, 6% of healthy women and 26% of healthy men would have been misdiagnosed with splenomegaly. Therefore, they suggested equations to estimate the upper limit of spleen length and volume for both genders based on body height (Table 1). In addition, a former study observed that splenic volume significantly correlates with age and decreases in older patients (Harris et al. 2010). In this study, a mean splenic volume of 171 cm3 was observed in patients in their 3rd decade, whereas the mean splenic volume was only 89.2 cm3 in patients in their 8th decade. Finally, the size of the spleen is also affected by physiologic changes. It can decrease with physiological stress situations such as hypovolemic shock, heavy exercise, or trauma (Cruz-Romero et al. 2016; Stewart and McKenzie 2002; Kiguchi et al. 2015).
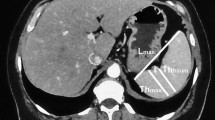
The parenchyma of the spleen is subdivided into the red and the white pulp, which cannot be differentiated in diagnostic imaging. The red pulp accounts mainly for the blood-filled sinuses and the white pulp accounts for lymphoid follicles. The spleen is arterially supplied by the splenic artery, which originates as one of three main branches from the celiac trunk. As a variant, the splenic artery can originate directly from the abdominal aorta. The venous drainage is performed by the splenic vein, which confluences with the superior and inferior mesenteric vein to the portal vein. The spleen has an attenuation of 40–50 Hounsfield units (HU) on unenhanced CT (Fig. 1a) (Tonolini and Bianco 2013). After administration of intravenous (i.v.) contrast medium, a heterogeneous, band-like enhancement is typically seen during the early arterial phase (Fig. 1b). Portal-venous and delayed phases show a homogeneous enhancement of the splenic parenchyma (Fig. 1c).
Congenital abnormalities of the spleen occur in heterotaxy syndrome, which is defined by an inaccurate left and right distribution of thoracic and abdominal organs. Heterotaxy syndrome is subdivided into left and right isomerism. Left isomerism is characterized by multiple spleens, also known as polysplenia syndrome (Fig. 2). In right isomerism, the spleen is absent (asplenia).
Ectopic spleen tissue is common and can be categorized in either accessory spleen tissue (splenunculus) or splenosis. Accessory spleen tissue is a congenital condition with a prevalence of up to 16% and presenting as round, well-marginated, and homogeneously enhancing masses (Mortele et al. 2004). Splenosis is an acquired autoimplantation of splenic tissue in other regions of the body, typically due to trauma or surgery (e.g., splenectomy) (Fig. 3).
Intrathoracic and abdominal splenosis. Patient with history of splenectomy (prior history of gunshot injury) developed multiple ovoid soft tissue masses in the left hypochondrium (a). Pleural-based soft tissue nodules were also seen in the left lung apex (b). 99m-Technetium-Sulfur-Colloid scan demonstrated increased uptake in the abovementioned soft tissue sites confirming the presence of splenosis (c)
In addition, there are two different variants of anatomy that represent potential pitfalls: the intrapancreatic accessory spleen and the wandering spleen. The intrapancreatic accessory spleen is most commonly located in the tail of the pancreas, where it can mimic a mass lesion like a neuroendocrine tumor (Fig. 4) (Kawamoto et al. 2012). It should be considered if a hypervascular mass is seen or if the attenuation pattern is similar to the spleen (Coquia et al. 2014). In questionable cases, a supermagnetic iron oxide-enhanced MRI can help to differentiate the two entities. The wandering spleen is rare and indicates a migration of the spleen to another anatomical region, usually the pelvis (Fig. 5). The migration is caused by a weakness of the suspensory ligaments of the spleen. Due to the migration, a vascular pedicle can form, which might twist and lead to a splenic torsion with a potential splenic infarction. The torsion leads to a whirl-sign in CT (Priyadarshi et al. 2013) and it is treated surgically.
2 Trauma
2.1 Introduction
Splenic injury can result from blunt or penetrating trauma. In blunt abdominal trauma, the spleen is one of the most frequently injured organs (Haan et al. 2005; Yao et al. 2002). After splenic injury, the following pathologies may occur: hematoma (subcapsular or intraparenchymal), laceration, active extravasation, pseudoaneurysma, and arteriovenous (AV) fistula. Splenic injury is associated with injuries to other nearby structures like the left hemidiaphragm, left lung, ribs, left lobe of the liver, left kidney, or pancreatic tail. Imaging is particularly important to stratify patient management and determine the need for a splenectomy. In the past decades, a trend towards nonoperative management occurred because it has been shown that splenectomy increases the risk of postoperative infectious complications. Overwhelming post-splenectomy infections (OPSIs) are a lifelong threat after splenectomy and can occur with a lifetime risk of 1–4 per 1000 patients (Schwartz et al. 1982). OPSI may result in multiorgan failure and death in more than half of these patients. Nonoperative management is favored, especially in the hemodynamically stable patient, and includes surveillance and splenic artery embolization. In a nationwide study in Taiwan, it has been demonstrated that the trend towards nonoperative management did not result in increased mortality (Soo et al. 2015).
2.2 Classification
Splenic trauma can be graded according to the American Association for the Surgery of Trauma (AAST) classification which includes five grades (Table 2). Grades I-II are defined as low-grade while grades III-V are defined as high-grade injuries.
However, it has been shown in former studies that the AAST classification is a poor predictor of patient’s benefit from surgical or nonsurgical treatment (Becker et al. 1994; Sutyak et al. 1995; Cohn et al. 2009). Marmery et al. proposed another grading system which showed to better predict the need for splenic intervention (Table 3) (Marmery et al. 2007). Their grading system takes into account the presence of active bleeding on CT, because vascular injuries are a predictor of failure of nonoperative management. Patients with a grading of four are candidates for splenic artery embolization or surgery.
2.3 Imaging Characteristics
CT is the modality of choice to evaluate splenic trauma. It is recommended to perform imaging always during the portal-venous phase to avoid misinterpretation of parenchymal lesions due to the heterogeneous enhancement of the spleen in earlier phases. An additional arterial phase can be useful to diagnose vascular injuries like pseudoaneurysms or AV fistulas. However, the literature about an additional arterial phase is controversial. A retrospective study showed only minimal improvement in patient’s outcome when applying a routine arterial phase CT in blunt trauma patients (Corwin et al. 2016). Another, more recent reader study demonstrated improved diagnostic confidence for all types of blunt splenic injuries by applying a dual-phase CT protocol consisting of an arterial and portal-venous phase (Boscak et al. 2013). In this study, a dual-phase CT protocol provided an “excellent confidence” in 85% of cases for vascular injuries and in 93% of cases for nonvascular injuries like parenchymal injuries or hematoma. In CT, lacerations are demarcated as linear hypodensities. Subcapsular hematoma presents as low-density blood collection between the capsule and the parenchyma of the spleen. In contrast to para- or perisplenic fluid collections, the subcapsular hematoma leads to an indentation of the spleen’s margin. Active bleeding shows high-density and increases in size in delayed phases, while pseudoaneurysms or AV fistulas are stable in delayed phases. Delayed rupture (5–6% of nonsurgically managed cases), splenic abscess, or pseudoaneurysms are potential complications after splenic trauma.
2.4 Image Examples (Figs. 6–10)
2.5 Treatment Options
In clinical routine, the decision between surgery and nonoperative management is in general based on the initial clinical presentation of the patient. A patient in hemorrhagic shock or with other intraabdominal injuries qualifies for surgical treatment (Stassen et al. 2012). Nonoperative management is preferred in the hemodynamically stable patient. However, there is a higher failure rate with increasing grade of injury. The preferred treatment for hemodynamically stable patients with AAST grade IV or V injury is still controversial. Nonoperative management contains splenic artery embolization (SAE) and surveillance. SAE is indicated if active extravasation or a pseudoaneurysm/AV fistula is evident on CT (Olthof et al. 2013). SAE is performed either as coil embolization of the proximal splenic artery or as superselective embolization of the bleeding arterial branch. Complications of SAE are persistent hemorrhage, coil migration, and splenic infarction or abscess. The rate of severe complications is less than 4% (Skattum et al. 2013; Frandon et al. 2014). Surveillance is a treatment option in hemodynamically stable patients without active contrast extravasation or blush in CT and AAST grade I-III. A study by Saksobhavivat et al. showed that the CT-based classification scale proposed by Marmery et al. was the best individual predictor for decision making between surveillance and splenic intervention in hemodynamically stable patients (Saksobhavivat et al. 2015). A close monitoring is required due to the risk of secondary splenic rupture. The risk of secondary rupture declines over time, and 92% of secondary ruptures take place in the first 6 days after injury (Peitzman et al. 2000). Follow-up CT imaging should only be performed selectively based on the patient’s clinical status. The duration of observation should be based on the clinical presentation and severity of the initial injury.
3 Benign Lesions
3.1 General Principles
Benign splenic lesions expose certain specific imaging characteristics. They are usually well circumscribed, and the majority is hypodense to the splenic parenchyma in unenhanced and early contrast medium phases (Cave: hamartoma).
3.2 Cysts
Splenic cysts are the most common focal lesions of the spleen. They can be subdivided into primary/congenital cysts and secondary cysts. Furthermore, from an etiological standpoint, they can be divided into parasitic and nonparasitic cysts. About 20% of splenic cystic lesions are primary cysts, which are typically incidental findings at imaging. They are true cysts lined with epithelial cells. Secondary splenic cysts or pseudocysts account for about 80% of splenic cystic lesions and are typically posttraumatic after hematoma or splenic infarction. Secondary cysts present more often calcifications compared to primary cysts. In CT imaging, both types present as well-defined, rounded masses with water-equivalent attenuation (Fig. 11). Cysts do not exhibit an enhancement in contrast-enhanced phases. Differential diagnoses of nonparasitic cysts are splenic abscess and splenic hydatid cyst. Here, the patient’s history can help to differentiate among these entities.
3.3 Epidermoid Cyst
Splenic epidermoid cyst belongs to the family of primary, true splenic cysts. They are congenital and show a variation of clinical symptoms from asymptomatic to nausea, abdominal pain, and splenomegaly, especially at young ages (Rana et al. 2014). In CT, they present as hypodense, well-demarcated lesions with a thin wall and without uptake of contrast medium. Calcifications of the wall may occur. Partial splenectomy is the treatment of choice in patients with symptomatic epidermoid cysts.
3.4 Hemangioma
Hemangiomas are the second most frequent focal splenic lesions (Luna et al. 2006). They are usually asymptomatic and found incidentally. Hemangiomas are slow-flow venous malformations, and the majority show a cavernous type. Typically, a hypo- to isodense mass is seen on unenhanced CT (Fig. 12a). Hemangiomas are usually smaller than 2 cm and may show calcifications and a central, stellate scar (Fotiadis et al. 2009). After administration of intravenous contrast medium, they show peripheral enhancement, followed by centripetal filling and persistent contrast enhancement in late phase images (Fig. 12b, c). Secondary hemangiomas can be present in the course of a systemic angiomatosis like Klippel-Trénaunay-Weber syndrome or Beckwith-Wiedemann syndrome. The presence of multiple splenic hemangiomas which replace the entire parenchyma of the spleen is known as splenic hemangiomatosis. Splenic hemangiomatosis is also associated with the Klippel-Trénaunay-Weber syndrome and the Kasabach-Meritt syndrome. Complications of hemangiomas include hemorrhage, infarction, thrombosis, and very rarely rupture.
3.5 Lymphangioma
Lymphangioma is a rare, benign neoplasm, which is primarily seen in children. They are congenital malformations of the lymphatic system, which show cystic dilatation of the lymphatic vessels (Kim et al. 2015). The cystic appearance develops slowly, due to an abnormal or absence communication between the lymphatic vessels. Lymphangiomas typically occur in the neck or axillary region, but they are rarely found in the spleen. Histologically they are divided into three subtypes: capillary, cavernous, or cystic (Rodriguez-Montes et al. 2016). Clinical presentation ranges from asymptomatic to left upper abdominal pain due to a mass effect and compression of adjacent structures. Multiple lymphangiomas of the spleen can occur in systemic lymphangiomatosis. In CT, they present as thin-walled, hypodense masses without enhancement. They are typically located subcapsular. While septations can be seen in ultrasound and MRI, they are usually too thin to be demarcated in CT. Cystic lymphangiomas may express peripheral calcifications. Complications of very large lymphangiomas are bleeding, splenomegaly, and secondary portal hypertension. Very rarely a transformation into a malignant lymphangiosarcoma has been described (Feigenberg et al. 1983).
3.6 Hamartoma
Hamartomas are benign, solid lesions that can have a size of up to 19 cm (Lam et al. 1999). They consist of an anomalous mixture of tumor tissue and normal splenic tissue with red and white pulp. They are usually incidental findings, but larger lesions may lead to splenomegaly and rupture. Clinical symptoms are therefore linked to the mass effect of larger lesions. Hamartomas of the spleen are associated with solid (thymoma) and hematological malignancies, with tuberous sclerosis and Wiskott-Aldrich-like syndrome (Lee and Maeda 2009). In CT, hamartomas are often isodense to the spleen in unenhanced imaging as well as after administration of contrast medium, which makes them difficult to detect. A change or distortion of the splenic contour may be the only finding in these cases. However, some hamartomas show a heterogenous enhancement after i.v. contrast ingestion (Fig. 13). Imaging is important to differentiate hamartomas from other malignant solid masses of the spleen like lymphomas or metastases. However, splenectomy is often needed to exclude malignancy (Carlomagno et al. 2015).
3.7 Sclerosing Angiomatoid Nodular Transformation
Sclerosing angiomatoid nodular transformation (SANT) is a solitary, non-neoplastic vascular tumor, which develops secondary to a vascular injury or an inflammation. Histopathologically, it is composed out of multiple angiomatoid nodules surrounded by fibrous tissue (Martel et al. 2004). SANT is usually asymptomatic. However, like most benign lesions of the spleen, large lesions can lead to abdominal pain and splenomegaly due to mass effect. After administration of contrast medium, the lesion is hypodense compared to the splenic parenchyma in arterial and portal-venous phase and becomes isodense in late phase due to centripetal filling. The first differential diagnosis is hemangioma. Diagnosis is usually established by splenectomy, rather than percutaneous biopsy because of the increased rate of complications in a vascular lesion (Imamura et al. 2016).
3.8 Angiomyolipoma
Angiomyolipoma of the spleen is extremely rare and usually associated with tuberous sclerosis. In tuberous sclerosis, additional angiomyolipomas can usually be found in the kidneys. In CT, angiomyolipomas show areas with negative, fat-equivalent attenuation, and they may show areas of contrast medium uptake due to increased vascularity (Thipphavong et al. 2014).
4 Semi-malignant Lesions
4.1 Littoral Cell Angioma
Littoral cell angioma is a rare vascular tumor, which is characterized by multiple spongelike vascular spaces. It can occur at any age and is usually benign. However, malignant transformation has been reported (Ben-Izhak et al. 2001). Littoral cell angioma is typically symptomatic leading to anemia, thrombocytopenia, and splenomegaly. Characteristic in CT are multiple, hypodense nodules in unenhanced, arterial, and portal-venous phases (Fig. 14a). However, littoral cell angioma is isodense to the splenic parenchyma in late phase (Fig. 14b). The differential diagnosis is broad and includes benign and malignant lesions. Therefore, splenectomy is usually performed to establish the diagnosis.
4.2 Hemangioendothelioma
Hemangioendothelioma is a very rare, borderline-malignant, vascular tumor. It occurs in pediatric patients or more commonly in young adults. Clinical presentation is nonspecific. In CT, hemangioendothelioma is hypodense in unenhanced images and a radiative peripheral enhancement in arterial phase followed by centripetal filling in late phase has been described (Wang et al. 2015). However, imaging alone will usually not allow to establish the diagnosis. Partial or total splenectomy has been described as treatment options.
5 Malignant Lesions
5.1 Lymphoma
Lymphoma is the most common malignancy in the spleen. It can occur primary or secondary to a systemic lymphatic disease. The spleen is involved in about one third of Hodgkin and non-Hodgkin lymphomas (Saboo et al. 2012), while primary lymphoma is extremely rare (<1%). Diagnosis is established by a combination of findings in the peripheral blood, the bone marrow, and imaging. CT appearance is variable and includes solitary or multiple lesions, diffuse infiltration, or non-mass homogeneous enhancement (Bowerson et al. 2015) (Fig. 15). Lesions show a mild enhancement, but are typically hypoenhancing compared to the surrounding splenic parenchyma on portal-venous phase (Li et al. 2013). Areas of necrosis can occur in primary lymphomas. Treatment of primary lymphoma may include splenectomy and chemotherapy.
Axial CT image of a patient with a diffuse large B-cell non-Hodgkin lymphoma demonstrates multiple, round, hypodense lesions in the spleen and liver in portal-venous phase (a). PET scan exhibits increased uptake of the abovementioned lesions in the liver and the spleen and in additional lesions in the right axillary region (b)
5.2 Splenic Metastases
The spleen is an infrequent site for metastatic disease (only 3.4% of metastatic carcinoma) (Giovagnoni et al. 2005). Splenic metastases are found in the late stage of widespread metastatic disease and are therefore a sign of poor prognosis. The most common primary sources are melanoma, breast, lung, ovarian, or colorectal cancer (Giovagnoni et al. 2005; Comperat et al. 2007). Splenic metastases are typically hypodense on unenhanced CT. Their contrast enhancement and attenuation characteristics follow the pattern of the primary cancer (Fig. 16). Biopsy is possible to determine the primary cancer.
Splenic metastasis of a patient with sarcoma. Initial axial contrast-enhanced CT of the upper abdomen demonstrates a round, hypodense, partially cystic, and slightly enhancing lesion in the spleen with a diameter of 2.5 cm (a). In a 1-month follow-up CT, the lesion increased in size to a diameter of 4.5 cm (b), indicating malignancy
5.3 Angiosarcoma
Angiosarcoma is the most common primary, nonhematolymphoid malignancy of the spleen. Its origin is the epithelial-type cell of blood vessels. Angiosarcoma is very aggressive and shows a high rate of metastasis. Metastases are usually found in the liver, but also in the lungs, bone, or lymphatic system. Angiosarcoma effects more frequently older patients between the 5th and 7th decade (Hamid et al. 2010). Patients suffer from left upper abdominal pain, anemia, or thrombocytopenia. The symptoms typically initiate the imaging work-up. Hemoperitoneum is possible due to rupture of the tumor (Vrachliotis et al. 2000). Splenectomy is the treatment of choice. However, angiosarcoma is often diagnosed in an advanced stage, and prognosis is poor with a 6-month survival rate of 20% (Vrachliotis et al. 2000). A few reports indicate that angiosarcoma might develop from hemangioma or hemangioendothelioma (Alt et al. 1985; Falk et al. 1993).
CT appearance is variable depending on the amount of central necrosis, hemorrhage, or calcifications. Unenhanced CT usually shows splenomegaly due to a solitary or multiple enlarged, ill-defined, heterogeneous masses with hypo- and hyperdense components. The lesions are hypoenhancing, but some lesions show peripheral enhancement (Fig. 17) (Bowerson et al. 2015). Metastases of the liver can be hypo- or more commonly hypervascular (Thompson et al. 2005).
5.4 Hemangiopericytoma
Hemangiopericytoma is a rare vascular tumor that can be considered as a soft tissue sarcoma with low-grade malignant potential. It usually arises in the retroperitoneum of the pelvis or the lower extremities and is very rarely seen as a primary tumor in the spleen (Illuminati et al. 2015). Clinical presentation ranges from asymptomatic to abdominal discomfort and splenomegaly. Splenectomy is treatment of choice, but recurrence can occur up to 20 years after treatment. The overall survival rate is about 71% after 5 years (Spitz et al. 1998). Metastases have been shown in the lung or bone (Enzinger and Smith 1976). In case of recurrence, resection is combined with adjuvant adriamycin-based chemotherapy. In CT, the tumor is well delineated, shows a polylobulated contour, and is hypodense to the splenic parenchyma in unenhanced imaging. After administration of contrast medium, slight hyperattenuation of the solid parts can be observed.
6 Infection
6.1 Abscess
Splenic abscesses are overall a very rare condition. Potential causes are infection, trauma, splenic infarction, and immunodeficiency. Infections are caused either per continuitatem by a renal or pancreatic infection or they are most commonly caused by hematogenous spread of another infectious source like endocarditis (Fig. 18). Clinical presentation includes fever, leukocytosis, and left upper quadrant pain. CT is the imaging modality of choice, especially to detect small abscesses. Abscesses are typically multiple, ill-defined, hypodense lesions of 5–10 mm (Thipphavong et al. 2014). CT attenuation ranges from 20 to 40 HU, and a rim enhancement can be observed in some cases (Tonolini and Bianco 2013). As a rule of thumb, bacterial abscesses are usually solitary and large, while fungal or mycobacterial abscesses are usually multiple, small, and associated with conditions of immunodeficiency. Candida, Aspergillus, and Cryptococcus are the most common fungal species. Treatment includes antibacterial and antimycotic therapy, percutaneous drainage in selected patients, or splenectomy in severe cases. Treatment of the primary source is essential in secondary abscesses caused by hematogenous spread.
Splenic abscess. Coronal image of the upper abdomen shows a round, hypodense lesion in the spleen with CT attenuation values of about 30 HU (white arrows), representing an abscess. An additional abscess in the renal parenchyma (dark arrow) increases the likelihood of a hematogenous spread due to another primary infectious source
6.2 Hydatid Infection
Hydatid infection or echinococcosis is most commonly caused by the tapeworm Echinococcus granulosus (95%). The disease is endemic in the Middle East, Asia, Mediterranean, South America, India, and Australia (Rasheed et al. 2013). Carnivores, usually dogs, are the primary host of the parasite. Humans become an intermediate host through contact with a primary host or by ingestion of contaminated vegetables or water. In humans, the parasite most commonly affects the liver (70%) or lung (15–20%) causing cystic lesions filled with larval tapeworms (Pedrosa et al. 2000). The spleen is rarely involved (0.9–8%). Hydatid infection of the spleen can be subdivided into either primary (only in the spleen) or, more commonly, secondary infection due to hematogenous dissemination or intraperitoneal spread from a ruptured cyst in the liver (Franquet et al. 1990). The cysts have a primary size of about 1 cm and show only a slow growth of 2–3 cm per year. Based on the slow growth rate, patients with splenic hydatid cyst are usually asymptomatic, and the cyst is discovered as an incidental finding during imaging or if it causes symptoms due to compression of adjacent structures. The cysts are composed of three different layers: (1) an outer layer with a fibrous zone called the “pericyst,” (2) an acellular middle laminated membrane, and (3) an inner germinal layer. Layers 2 and 3 form the so-called endocyst (Pedrosa et al. 2000).
Imaging findings change during the life cycle of the parasite. Unenhanced CT is sufficient in uncomplicated cysts, because the cysts do not enhance after administration of i.v. contrast medium. In early disease, a simple, hypodense cyst with water-equivalent attenuation surrounded by a thin wall can be observed. The wall is noncalcified, but is markedly hyperdense in unenhanced CT. However, the attenuation of the cyst depends on the intracystic content. Intracystic debris, inflammatory cells, or so-called hydatid sand can cause higher attenuation values. The next stage is characterized by the development of “daughter cysts.” Daughter cysts are multiple additional cysts which develop along the wall of the central “mother cyst.” Typically, the mother cyst shows higher attenuation values of 30–40 HU compared to the daughter cysts (0–10 HU) (Kalovidouris et al. 1986). In later stages, a detachment of the endocyst from the pericyst can be seen due to degeneration or therapy. Complete detachment is seen as floating membranes within the cyst and has been described primary in ultrasound as “water lily sign” (Beggs 1985). In addition, peripheral calcifications are seen and indicate the healing process (Fig. 19). However, calcification does not necessarily prove the death of the parasite (Beggs 1985). Only complete calcification of the cyst is a sign for the full elimination.
Advanced stage hydatid infection of the spleen and liver. Axial image in portal-venous phase shows multiple, ill-defined, heterogeneous, hypoattenuating, partially calcified lesions in the spleen and liver. Peripheral calcifications indicate the healing process, but they do not prove the death of the parasite
Treatment options are splenectomy, spleen-preserving surgery like partial splenectomy or cyst enucleation, and minimally invasive approaches like “puncture, aspiration, injection, and reaspiration” (PAIR) (Rasheed et al. 2013). Surgery and PAIR are increasingly combined with chemotherapy (Albendazole or Mebendazole) (Teggi et al. 1993). A rare, but life-threatening, complication of hydatid disease is the rupture of a cyst into the peritoneal cavity (Arikanoglu et al. 2012). This rupture can be spontaneous, posttraumatic, or after surgery and lead to severe anaphylactic reactions.
6.3 Tuberculosis
Splenic tuberculosis is extremely rare and typically associated with immunodeficiency (Lee et al. 2012). It is caused by the bacterium Mycobacterium tuberculosis. Splenic tuberculosis is seen either as a solitary form or, more commonly, as part of disseminated tuberculosis. Other, more frequent, sites of extrapulmonary, abdominal tuberculosis are lymph nodes, the peritoneum, the genitourinary tract, and the gastrointestinal tract (Lee et al. 2012). The clinical presentation of splenic tuberculosis is unspecific. Fever, fatigue, and weight loss are the most common symptoms. In addition, splenomegaly can occur. In solitary splenic tuberculosis, CT typically shows a mass lesion which is hypoenhancing compared to the surrounding splenic parenchyma. The enhancement is heterogenous, and areas of central necrosis can be observed (Lin et al. 2016). In the disseminated form, the most common appearance is splenomegaly combined with multiple hypoenhancing lesions (Fig. 20). However, the diagnosis is impossible to establish without histopathological examination. Needle biopsy and splenectomy are potential options to obtain a tissue sample. Treatment includes antitubercular medication and/or splenectomy.
Disseminated form of splenic tuberculosis. Typical image appearance with splenomegaly and multiple hypodense lesions distributed in a miliary pattern. Reprinted from “CT appearances of abdominal tuberculosis” by Lee et al. (2012) published on p. 601, with permission from Elsevier
6.4 Malaria
Malaria is an endemic infectious disease in tropical or subtropical regions, resulting in almost half a million deaths in 2015 (WHO n.d.). It is caused by Plasmodium species (P. falciparum, P. vivax, P. ovale, P. malariae, P. knowlesi), which are transmitted by the bite of female Anopheles mosquitoes. Malaria is subdivided based on the clinical presentation and course of the disease in Malaria tropica, Malaria tertiana, and Malaria quartana. Clinical symptoms are episodic fever, malaise, anemia, and abdominal pain. The spleen plays a major role in the immunological defense against the disease. Infected red blood cells are entrapped in the spleen followed by an immunological response. As a consequence, splenic congestion occurs which leads to an increase in splenic size. This pathophysiological process explains the typical imaging finding of malaria. Hepatosplenomegaly is the most common finding in CT. Another reported finding is a poor contrast enhancement in arterial phase, due to the congestion of the spleen (Bae and Jeon 2006). Splenic infarction and splenic rupture are important complications. Splenic infarction was reported in cases of P. falciparum and P. vivax malaria as heterogeneous hypodense areas (Bonnard et al. 2005; Kim et al. 2007). A subcapsular hematoma is believed to be the precursor of splenic rupture (Waweru et al. 2014). Splenic rupture was observed most frequently in P. vivax malaria (Jimenez et al. 2007; Kim et al. 2010).
7 Miscellaneous
7.1 Splenic Infarction
Splenic infarctions are most commonly caused by septic embolization of infectious endocarditis (Tonolini and Bianco 2013). In addition, there are multiple other causes including vascular diseases, lymphoproliferative disorders, hemoglobinopathies, and infectious diseases that are related to splenomegaly, for example, mononucleosis or malaria. Infarction of the entire spleen is typically caused by occlusion of the whole splenic artery. In CT, splenic infarcts present as wedge-shaped, well-demarcated, hypodense regions with subcapsular distribution (Fig. 21). In follow-up imaging, these regions decrease in size and become atrophic (Tonolini and Bianco 2013).
7.2 Extramedullary Hematopoiesis
Extramedullary hematopoiesis (EMH) is a consequence of insufficient erythropoiesis in the bone marrow. Possible causes are myeloproliferative disorders (e.g., polycythemia vera or myelofibrosis), hemoglobinopathies (e.g., sickle cell disease), bone marrow infiltration (e.g., by diffuse osseous metastatic disease or leukemia), and thalassemia. The liver and the spleen are the organs most frequently involved. However, EMH can also occur in various different organs, for example, lymph nodes, pleura, lungs, or even in the skin. A typical finding is an enlargement of the affected organ or a circumscribed mass, as, for example, a fat-containing paraspinal mass in the mediastinum. Hepatosplenomegaly is most frequently observed in CT and it can occur with or, more commonly, without focal lesions. Multiple mildly enlarged retroperitoneal lymph nodes can be observed (Sohawon et al. 2012). Focal lesions of EMH appear as homogeneous, well-circumscribed, hypodense lesions in CT (Orphanidou-Vlachou et al. 2014). Their size ranges from 2.5 to 7 cm (Singer et al. 2004). These lesions often require a biopsy, because lymphoma, metastases, and sarcoma are potential differential diagnoses (Roberts et al. 2016). Clinical history can help to distinguish EMH from other entities. Care has to be taken while planning and performing a biopsy, because lesions are vascular, and hemorrhagic complications are possible. Treatment is only necessary in symptomatic patients and includes blood transfusion, radiotherapy, or resection. Interestingly, it has been shown that spleen volume, measured by CT, is an independent predictor for survival in splenomegaly caused by EMH due to primary myelofibrosis (Song et al. 2016).
7.3 Splenic Sarcoidosis
Sarcoidosis is a systemic inflammatory disease that can affect any organ system. Its etiology is unknown. Characteristic of sarcoidosis is the formation of noncaseating granulomas. Extrapulmonary involvement is observed in about 30% of patients with sarcoidosis, and the abdomen is the most frequent extrapulmonary site (Gezer et al. 2015). Patients are most commonly between 20 and 50 years old, and women are more likely affected. In the abdomen, sarcoidosis most frequently involves the liver and the spleen. Most often, a mild hepato- and/or splenomegaly can be observed. Multiple, hypoattenuating, mass-like nodules of the liver and spleen with a size of 5–20 mm are found in 10–15% of cases (Fig. 22) (Warshauer and Lee 2004). The nodules may also lead to contour irregularity of the liver and spleen. However, abdominal involvement in sarcoidosis usually does not cause clinical symptoms. Clinical symptoms are confined to patients with massive splenomegaly, which is a rather rare condition. Potential differential diagnosis of hypoattenuating nodules in both the liver and the spleen are lymphoma, metastasis, tuberculosis, and abscess (Gezer et al. 2015). Glucocorticoids are typically the first-line treatment.
7.4 Splenic Amyloidosis
Amyloidosis is characterized by an accumulation of fibrillar proteins in the extracellular space of tissues resulting in organ dysfunction. Splenic amyloidosis can be primary or secondary as part of systemic amyloidosis. Primary splenic amyloidosis is extremely rare. In systemic amyloidosis, however, the spleen is involved in 5–10% of cases (Renzulli et al. 2009). Clinical presentation is usually asymptomatic. Complications of amyloidosis are splenic rupture, splenic infarction, and hyposplenism. Imaging findings are splenomegaly and heterogeneous or diffuse hypoperfusion with focal hypoenhancing areas in postcontrast CT (Fig. 23) (Monzawa et al. 2002; Kim et al. 2003; Mainenti et al. 2005). However, splenomegaly is rather uncommon (4–13% of cases) (Mainenti et al. 2005).
Splenic amyloidosis. Axial image of the upper abdomen in delayed phase. Hypoenhancing of the spleen compared to the liver is seen, which represents hypoperfusion due to amyloidosis (© With permission of Springer: Mainenti et al. 2005)
8 Differential Diagnosis Based on Imaging Finding
8.1 Splenomegaly
Group of disease | Subgroup | Entity |
|---|---|---|
Hematological disease | Anemia | Sickle cell disease |
Thalassemia | ||
Thrombotic thrombocytopenic purpura (TTP) | ||
Neoplastic/proliferative | Leukemia | |
Extramedullary hematopoiesis | ||
Polycythemia rubra vera | ||
Osteopetrosis | ||
Infectious | Viral | EBV, CMV, HSV |
Bacterial | Abscess | |
Tuberculosis | ||
Fungal | Histoplasmosis | |
Candidiasis | ||
Parasitic | Malaria | |
Hydatid disease | ||
Neoplastic (nonhematologic) | Benign | Hemangioma |
Malignant | Lymphoma | |
Metastases | ||
Angiosarcoma | ||
Trauma | Hematoma | |
Pseudocyst | ||
Storage and metabolic disease | Hemochromatosis | |
Amyloidosis | ||
Glycogen storage disease | ||
Gaucher disease | ||
Connective tissue disorders | Rheumatoid arthritis | Felty syndrome |
Juvenile rheumatoid arthritis | ||
Systemic lupus erythematosus |
8.2 Splenic Infarction
Etiology |
|---|
Hematological disease Sickle cell disease β-Thalassemia |
Embolic Infective endocarditis |
Other Malaria (microthrombosis) Splenic arterial aneurysm Splenic artery compression by tumor Splenic vascular disease |
8.3 Splenic Rupture
Etiology |
|---|
Hematological malignancy Leukemia Lymphoma |
Primary splenic neoplasia Hemangioma Hamartoma Angiosarcoma |
Infectious EBV, CMV, malaria |
Primary and secondary amyloidosis |
Trauma |
Iatrogenic Colonoscopy Abdominal surgery |
References
Alt B, Hafez GR, Trigg M, Shahidi NT, Gilbert EF (1985) Angiosarcoma of the liver and spleen in an infant. Pediatr Pathol 4(3–4):331–339
Arikanoglu Z, Taskesen F, Aliosmanoglu I, Gul M, Cetincakmak MG, Onder A et al (2012) Spontaneous intraperitoneal rupture of a hepatic hydatid cyst. Int Surg 97(3):245–248
Bae K, Jeon KN (2006) CT findings of malarial spleen. Br J Radiol 79(946):e145–e147
Becker CD, Spring P, Glättli A, Schweizer W (1994) Blunt splenic trauma in adults: can CT findings be used to determine the need for surgery? AJR Am J Roentgenol 162(2):343–347
Beggs I (1985) The radiology of hydatid disease. AJR Am J Roentgenol 145(3):639–648
Ben-Izhak O, Bejar J, Ben-Eliezer S, Vlodavsky E (2001) Splenic littoral cell haemangioendothelioma: a new low-grade variant of malignant littoral cell tumour. Histopathology 39(5):469–475
Bonnard P, Guiard-Schmid JB, Develoux M, Rozenbaum W, Pialoux G (2005) Splenic infarction during acute malaria. Trans R Soc Trop Med Hyg 99(1):82–86
Boscak AR, Shanmuganathan K, Mirvis SE, Fleiter TR, Miller LA, Sliker CW et al (2013) Optimizing trauma multidetector CT protocol for blunt splenic injury: need for arterial and portal venous phase scans. Radiology 268(1):79–88
Bowerson M, Menias CO, Lee K, Fowler KJ, Luna A, Yano M et al (2015) Hot spleen: hypervascular lesions of the spleen. Abdom Imaging 40(7):2796–2813
Carlomagno N, Duraturo F, Candida M, De Rosa M, Varone V, Ciancia G et al (2015) Multiple splenic hamartomas and familial adenomatous polyposis: a case report and review of the literature. J Med Case Reports 9:154
Chow KU, Luxembourg B, Seifried E, Bonig H (2016) Spleen size is significantly influenced by body height and sex: establishment of normal values for spleen size at US with a cohort of 1200 healthy individuals. Radiology 279(1):306–313
Cohn SM, Arango JI, Myers JG, Lopez PP, Jonas RB, Waite LL et al (2009) Computed tomography grading systems poorly predict the need for intervention after spleen and liver injuries. Am Surg 75(2):133–139
Comperat E, Bardier-Dupas A, Camparo P, Capron F, Charlotte F (2007) Splenic metastases: clinicopathologic presentation, differential diagnosis, and pathogenesis. Arch Pathol Lab Med 131(6):965–969
Coquia SF, Kawamoto S, Zaheer A, Bleich KB, Blackford AL, Hruban RH et al (2014) Intrapancreatic accessory spleen: possibilities of computed tomography in differentiation from nonfunctioning pancreatic neuroendocrine tumor. J Comput Assist Tomogr 38(6):874–878
Corwin MT, Fananapazir G, Lamba R, Salcedo ES, Holmes JF (2016) Arterial phase CT for the detection of splenic injuries in blunt trauma: would it improve clinical outcomes? Clin Imaging 40(2):212–216
Cruz-Romero C, Agarwal S, Abujudeh HH, Thrall J, Hahn PF (2016) Spleen volume on CT and the effect of abdominal trauma. Emerg Radiol 23(4):315–323
Danila M (2010) The ultrasound examination of the spleen. Med Ultrason 12(3):253–254
Enzinger FM, Smith BH (1976) Hemangiopericytoma. An analysis of 106 cases. Hum Pathol 7(1):61–82
Falk S, Krishnan J, Meis JM (1993) Primary angiosarcoma of the spleen. A clinicopathologic study of 40 cases. Am J Surg Pathol 17(10):959–970
Feigenberg Z, Wysenbeek A, Avidor E, Dintsman M (1983) Malignant lymphangioma of the spleen. Isr J Med Sci 19(2):202–204
Fotiadis C, Georgopoulos I, Stoidis C, Patapis P (2009) Primary tumors of the spleen. Int J Biomed Sci 5(2):85–91
Frandon J, Rodiere M, Arvieux C, Michoud M, Vendrell A, Broux C et al (2014) Blunt splenic injury: outcomes of proximal versus distal and combined splenic artery embolization. Diagn Interv Imaging 95(9):825–831
Franquet T, Montes M, Lecumberri FJ, Esparza J, Bescos JM (1990) Hydatid disease of the spleen: imaging findings in nine patients. AJR Am J Roentgenol 154(3):525–528
Gezer NS, Basara I, Altay C, Harman M, Rocher L, Karabulut N et al (2015) Abdominal sarcoidosis: cross-sectional imaging findings. Diagn Interv Radiol 21(2):111–117
Giovagnoni A, Giorgi C, Goteri G (2005) Tumours of the spleen. Cancer Imaging 5:73–77
Haan JM, Bochicchio GV, Kramer N, Scalea TM (2005) Nonoperative management of blunt splenic injury: a 5-year experience. J Trauma 58(3):492–498
Hamid KS, Rodriguez JA, Lairmore TC (2010) Primary splenic angiosarcoma. JSLS 14(3):431–435
Harris A, Kamishima T, Hao HY, Kato F, Omatsu T, Onodera Y et al (2010) Splenic volume measurements on computed tomography utilizing automatically contouring software and its relationship with age, gender, and anthropometric parameters. Eur J Radiol 75(1):e97–101
Illuminati G, Pizzardi G, Calio F, Pacile MA, Carboni F, Palumbo P et al (2015) Hemangiopericytoma of the spleen. Int J Surg 15:6–10
Imamura Y, Nakajima R, Hatta K, Seshimo A, Sawada T, Abe K et al (2016) Sclerosing angiomatoid nodular transformation (SANT) of the spleen: a case report with FDG-PET findings and literature review. Acta Radiol Open 5(8):2058460116649799
Jimenez BC, Navarro M, Huerga H, Lopez-Velez R (2007) Spontaneous splenic rupture due to Plasmodium vivax in a traveler: case report and review. J Travel Med 14(3):188–191
Kalovidouris A, Pissiotis C, Pontifex G, Gouliamos A, Pentea S, Papavassiliou C (1986) CT characterization of multivesicular hydatid cysts. J Comput Assist Tomogr 10(3):428–431
Kawamoto S, Johnson PT, Hall H, Cameron JL, Hruban RH, Fishman EK (2012) Intrapancreatic accessory spleen: CT appearance and differential diagnosis. Abdom Imaging 37(5):812–827
Kiguchi T, Higuchi T, Takahashi N, Shimokoshi T, Yamazaki M, Yoshimura N et al (2015) CT measurement of splenic volume changes as a result of hypovolemic shock. Jpn J Radiol 33(10):645–649
Kim SH, Han JK, Lee KH, Won HJ, Kim KW, Kim JS et al (2003) Abdominal amyloidosis: spectrum of radiological findings. Clin Radiol 58(8):610–620
Kim A, Park YK, Lee JS, Chung MH, Kim ES (2007) A case of symptomatic splenic infarction in vivax malaria. Korean J Parasitol 45(1):55–58
Kim EM, Cho HJ, Cho CR, Kwak YG, Kim MY, Cho YK (2010) Abdominal computed tomography findings of malaria infection with Plasmodium vivax. Am J Trop Med Hyg 83(6):1202–1205
Kim SY, Kwon HJ, Park HW, Lee SY, Son BH, Kim MS (2015) Multiple cystic lymphangiomas of the spleen: radiologic and histopathologic findings. J Med Ultrason (2001) 42(3):409–412
Lam KY, Yip KH, Peh WC (1999) Splenic vascular lesions: unusual features and a review of the literature. Aust N Z J Surg 69(6):422–425
Lee H, Maeda K (2009) Hamartoma of the spleen. Arch Pathol Lab Med 133(1):147–151
Lee WK, Van Tonder F, Tartaglia CJ, Dagia C, Cazzato RL, Duddalwar VA et al (2012) CT appearances of abdominal tuberculosis. Clin Radiol 67(6):596–604
Li M, Zhang L, Wu N, Huang W, Lv N (2013) Imaging findings of primary splenic lymphoma: a review of 17 cases in which diagnosis was made at splenectomy. PLoS One 8(11):e80264
Lin SF, Zheng L, Zhou L (2016) Solitary splenic tuberculosis: a case report and review of the literature. World J Surg Oncol 14(1):154
Luna A, Ribes R, Caro P, Luna L, Aumente E, Ros PR (2006) MRI of focal splenic lesions without and with dynamic gadolinium enhancement. AJR Am J Roentgenol 186(6):1533–1547
Mainenti PP, Camera L, Nicotra S, Cantalupo T, Soscia E, Di Vizio D et al (2005) Splenic hypoperfusion as a sign of systemic amyloidosis. Abdom Imaging 30(6):768–772
Marmery H, Shanmuganathan K, Alexander MT, Mirvis SE (2007) Optimization of selection for nonoperative management of blunt splenic injury: comparison of MDCT grading systems. AJR Am J Roentgenol 189(6):1421–1427
Martel M, Cheuk W, Lombardi L, Lifschitz-Mercer B, Chan JK, Rosai J (2004) Sclerosing angiomatoid nodular transformation (SANT): report of 25 cases of a distinctive benign splenic lesion. Am J Surg Pathol 28(10):1268–1279
Monzawa S, Tsukamoto T, Omata K, Hosoda K, Araki T, Sugimura K (2002) A case with primary amyloidosis of the liver and spleen: radiologic findings. Eur J Radiol 41(3):237–241
Moore EE, Cogbill TH, Jurkovich GJ, Shackford SR, Malangoni MA, Champion HR (1995) Organ injury scaling: spleen and liver (1994 revision). J Trauma 38(3):323–324
Mortele KJ, Mortele B, Silverman SG (2004) CT features of the accessory spleen. AJR Am J Roentgenol 183(6):1653–1657
Olthof DC, Joosse P, van der Vlies CH, de Haan RJ, Goslings JC (2013) Prognostic factors for failure of nonoperative management in adults with blunt splenic injury: a systematic review. J Trauma Acute Care Surg 74(2):546–557
Orphanidou-Vlachou E, Tziakouri-Shiakalli C, Georgiades CS (2014) Extramedullary hemopoiesis. Semin Ultrasound CT MR 35(3):255–262
Pedrosa I, Saiz A, Arrazola J, Ferreiros J, Pedrosa CS (2000) Hydatid disease: radiologic and pathologic features and complications. Radiographics 20(3):795–817
Peitzman AB, Heil B, Rivera L, Federle MB, Harbrecht BG, Clancy KD et al (2000) Blunt splenic injury in adults: Multi-institutional Study of the Eastern Association for the Surgery of Trauma. J Trauma 49(2):177–187 discussion 87-9
Priyadarshi RN, Anand U, Kumar B, Prakash V (2013) Torsion in wandering spleen: CT demonstration of whirl sign. Abdom Imaging 38(4):835–838
Rana AP, Kaur M, Singh P, Malhotra S, Kuka AS (2014) Splenic epidermoid cyst – a rare entity. J Clin Diagn Res 8(2):175–176
Rasheed K, Zargar SA, Telwani AA (2013) Hydatid cyst of spleen: a diagnostic challenge. N Am J Med Sci 5(1):10–20
Renzulli P, Schoepfer A, Mueller E, Candinas D (2009) Atraumatic splenic rupture in amyloidosis. Amyloid 16(1):47–53
Roberts AS, Shetty AS, Mellnick VM, Pickhardt PJ, Bhalla S, Menias CO (2016) Extramedullary haematopoiesis: radiological imaging features. Clin Radiol 71(9):807–814
Rodriguez-Montes JA, Collantes-Bellido E, Marin-Serrano E, Prieto-Nieto I, Perez-Robledo JP (2016) Splenic lymphangioma. A rare tumour. Presentation of 3 cases and a literature review. Cir Cir 84(2):154–159
Saboo SS, Krajewski KM, O'Regan KN, Giardino A, Brown JR, Ramaiya N et al (2012) Spleen in haematological malignancies: spectrum of imaging findings. Br J Radiol 85(1009):81–92
Saksobhavivat N, Shanmuganathan K, Chen HH, DuBose JJ, Richard H, Khan MA et al (2015) Blunt splenic injury: use of a multidetector CT-based splenic injury grading system and clinical parameters for triage of patients at admission. Radiology 274(3):702–711
Schwartz PE, Sterioff S, Mucha P, Melton LJ, Offord KP (1982) Postsplenectomy sepsis and mortality in adults. JAMA 248(18):2279–2283
Sienz M, Ignee A, Dietrich CF (2011) Reference values in abdominal ultrasound – biliopancreatic system and spleen. Z Gastroenterol 49(7):845–870
Singer A, Maldjian P, Simmons MZ (2004) Extramedullary hematopoiesis presenting as a focal splenic mass: a case report. Abdom Imaging 29(6):710–712
Skattum J, Naess PA, Eken T, Gaarder C (2013) Refining the role of splenic angiographic embolization in high-grade splenic injuries. J Trauma Acute Care Surg 74(1):100–103 discussion 3-4
Sohawon D, Lau KK, Lau T, Bowden DK (2012) Extra-medullary haematopoiesis: a pictorial review of its typical and atypical locations. J Med Imaging Radiat Oncol 56(5):538–544
Song MK, Chung JS, Lim SN, Lee GW, Lee SM, Lee NK et al (2016) Usefulness of spleen volume measured by computed tomography for predicting clinical outcome in primary myelofibrosis. Int J Hematol 104(4):476–484
Soo KM, Lin TY, Chen CW, Lin YK, Kuo LC, Wang JY et al (2015) More becomes less: management strategy has definitely changed over the past decade of splenic injury--a nationwide population-based study. Biomed Res Int 2015:124969
Spitz FR, Bouvet M, Pisters PW, Pollock RE, Feig BW (1998) Hemangiopericytoma: a 20-year single-institution experience. Ann Surg Oncol 5(4):350–355
Stassen NA, Bhullar I, Cheng JD, Crandall ML, Friese RS, Guillamondegui OD et al (2012) Selective nonoperative management of blunt splenic injury: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg 73(5 Suppl 4):S294–S300
Stewart IB, McKenzie DC (2002) The human spleen during physiological stress. Sports Med 32(6):361–369
Stiff PJ, Bensinger W, Abidi MH, Gingrich R, Artz AS, Nademanee A et al (2009) Clinical and ultrasonic evaluation of spleen size during peripheral blood progenitor cell mobilization by filgrastim: results of an open-label trial in normal donors. Biol Blood Marrow Transplant 15(7):827–834
Sutyak JP, Chiu WC, D'Amelio LF, Amorosa JK, Hammond JS (1995) Computed tomography is inaccurate in estimating the severity of adult splenic injury. J Trauma 39(3):514–518
Teggi A, Lastilla MG, De Rosa F (1993) Therapy of human hydatid disease with mebendazole and albendazole. Antimicrob Agents Chemother 37(8):1679–1684
Thipphavong S, Duigenan S, Schindera ST, Gee MS, Philips S (2014) Nonneoplastic, benign, and malignant splenic diseases: cross-sectional imaging findings and rare disease entities. AJR Am J Roentgenol 203(2):315–322
Thompson WM, Levy AD, Aguilera NS, Gorospe L, Abbott RM (2005) Angiosarcoma of the spleen: imaging characteristics in 12 patients. Radiology 235(1):106–115
Tonolini M, Bianco R (2013) Nontraumatic splenic emergencies: cross-sectional imaging findings and triage. Emerg Radiol 20(4):323–332
Vrachliotis TG, Bennett WF, Vaswani KK, Niemann TH, Bova JG (2000) Primary angiosarcoma of the spleen – CT, MR, and sonographic characteristics: report of two cases. Abdom Imaging 25(3):283–285
Wang Z, Zhang L, Zhang B, Mu D, Cui K, Li S (2015) Hemangioendothelioma arising from the spleen: a case report and literature review. Oncol Lett 9(1):209–212
Warshauer DM, Lee JK (2004) Imaging manifestations of abdominal sarcoidosis. AJR Am J Roentgenol 182(1):15–28
Waweru P, Macleod J, Gikonyo A (2014) Complicated malaria and a covert ruptured spleen: a case report. J Surg Case Rep 2014(11):1–2
WHO Malaria Fact sheet http://www.who.int/mediacentre/factsheets/fs094/en/
Yao DC, Jeffrey RB, Mirvis SE, Weekes A, Federle MP, Kim C et al (2002) Using contrast-enhanced helical CT to visualize arterial extravasation after blunt abdominal trauma: incidence and organ distribution. AJR Am J Roentgenol 178(1):17–20
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Euler, A., Schindera, S.T. (2017). Spleen. In: Nikolaou, K., Bamberg, F., Laghi, A., Rubin, G.D. (eds) Multislice CT. Medical Radiology(). Springer, Cham. https://doi.org/10.1007/174_2016_101
Download citation
DOI: https://doi.org/10.1007/174_2016_101
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-42585-6
Online ISBN: 978-3-319-42586-3
eBook Packages: MedicineMedicine (R0)