Abstract
Acute pancreatitis is an inflammatory disease that is mild and resolves without serious morbidity in 80% of patients. In the remaining 20%, it is complicated by substantial morbidity and mortality. The early distinction of these two groups of patients is desirable, as it is essential for their proper management. Even though positive diagnosis of acute pancreatitis is based on clinical and biological data, imaging, especially abdominal multidetector computed tomography (MDCT), has a key role in the initial phases of the disease. It allows the establishment of radiologic scoring systems with the aim of predicting which patients will have a severe debilitating hospital course and which patients will recover without major physiologic insult. The time of realization and the technique of MDCT are presently well defined. In the initial phase of the disease, MDCT allows firstly assessment of extension of initial lesions (pancreatic necrosis and fluid collections) and secondly depiction of early complications. Later, MDCT in association with MRI contributes to the etiological diagnosis and to the detection of late complications. Moreover, MDCT is helpful to plan and perform the treatment of the complications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Acute Pancreatitis
- Pancreatic Duct
- Fluid Collection
- Compute Tomography Examination
- Severe Acute Pancreatitis
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Acute pancreatitis is an inflammatory disease that is mild and resolves without serious morbidity in 80% of patients. In the remaining 20%, it is complicated by substantial morbidity and mortality (Frossard et al. 2008). The early distinction of these two groups of patients is desirable, as it is essential for their proper management. Even though positive diagnosis of acute pancreatitis is based on clinical and biological data, imaging, especially abdominal multidetector computed tomography (MDCT), has a key role in the initial phases of the disease. It allows the establishment of radiologic scoring systems with the aim of predicting which patients will have a severe debilitating hospital course and which patients will recover without major physiologic insult. The time of realization and the technique of MDCT are presently well defined. In the initial phase of the disease, MDCT allows firstly assessment of extension of initial lesions (pancreatic necrosis and fluid collections) and secondly depiction of early complications. Later, MDCT in association with MRI contributes to the etiological diagnosis and to the detection of late complications (Maher et al. 2004). Moreover, MDCT is helpful to plan and perform the treatment of the complications (Cannon et al. 2009; Kirby et al. 2008).
2 Epidemiology
The incidence of acute pancreatitis has been rising in the western world during the last 20 years (Whitcomb 2006), (5–80 per 1,000,000 inhabitants). But the incidence, varying widely depending on the country, is partly explained by the difference in alcohol consumption in the various countries (Yadav and Lowenfels 2006).
Complications are clearly related to severe pancreatitis (in opposition to mild pancreatitis), where in up to 20% of cases acute pancreatitis is complicated by substantial morbidity and mortality. However, the frequency of severe pancreatitis remains stable and the overall population mortality rate has remained unchanged in the 20 last years (Yadav and Lowenfels 2006).
The main causes of acute pancreatitis are chronic alcohol consumption, cholelithiasis, pancreatic tumors, and iatrogenic causes including following endoscopic retrograde cholangiopancreatography (ERCP), following gastric, pancreatic, or splenic surgery, and following blunt or penetrating trauma (Maher et al. 2004). Other causes are secondary to use of drugs, infection, hyperlipidemia, hypercalcemia, inflammatory bowel disease (Pitchumoni et al. 2010), and anatomic variations. In about 15–20% of cases, no cause of acute pancreatitis was found (Table 1) (Carroll et al. 2007)
The main cause, gallstones or alcohol consumption, depends on the country (Whitcomb 2006). Gallstone pancreatitis is more common in women, and alcoholic pancreatitis is more common in men. However, in the last 20 years, the incidence of gallstone pancreatitis has increased in all counties (Yadav and Lowenfels 2006; Nøjgaard et al. 2010). Alcohol-induced acute pancreatitis is associated with a greater incidence of severe acute pancreatitis (Lankisch et al. 1999).
3 Physiopathology
According to most authors, acute pancreatitis is caused by the unregulated activation of trypsinogen to trypsin within pancreatic acinar cells that leads to the autodigestion of the gland and local inflammation. The triggers of the activation are mainly gallstones, probably caused by an increase in intraductal pressure, and alcohol abuse.
The severity of pancreatic damage is not related to the cause that triggers the disease, but to the injury of acinar cells and to the activation of inflammatory and endothelial cells. Local complications (acinar cell necrosis, peripancreatic fat necrosis, acute fluid collection, then pseudocyst formation) might develop. Release of several mediators from the pancreas or from extrapancreatic organs such as the liver could lead to injury in remote organs (e.g., lungs, kidney) and could contribute to visceral failure (Pastor et al. 2003).
Classically, acute pancreatitis is classified as mild pancreatitis (called interstitial or edematous pancreatitis) and severe acute pancreatitis (called necrotizing pancreatitis). In mild pancreatitis, the inflammation is limited; there is no organ failure and there is spontaneous regression without complications. In 20% of cases, the acute pancreatitis is severe. This form will lead to high morbidity, and high mortality (death rates 30–50%) (McKay and Buter 2003; Papachristou et al. 2007).
In the literature, the terms “necrotizing pancreatitis” and “severe acute pancreatitis” are often used interchangeably. In many cases the clinical diagnosis of severe acute pancreatitis corresponds to the radiological diagnosis of necrotizing pancreatitis, but this is not necessarily true. Thus, patients with pancreatic necrosis may have minimal discomfort and no organ dysfunction in up to 50% of cases (Delrue et al. 2010).
Severe acute pancreatitis evolves in two phases. In the first 2 weeks there is an expansion of the inflammation, and the pancreatic and peripancreatic ischemia. Organ failure is the main determinant of the disease outcome. The second phase is marked by infection that occurs in 40–70% of cases (Takahashi et al. 2008).
4 Clinical Findings
There is constant acute abdominal pain. It begins in the epigastric area or in the right upper quadrant and becomes diffuse with irradiations to the back. Nausea and vomiting can be associated.
Physical findings depend on the severity of the disease. In mild disease, there is tenderness in the upper abdomen contrasting with the intensity of the abdominal pain. For 20% of patients, the disease is severe with some extrapancreatic complications which appear quickly. Physical examination can reveal ascites, ileus, hypovolemic shock, and hypoxemia. Increased capillary permeability, which conveys fluid accumulation within the interstitium, contributes to the decreased intravascular volume. Renal dysfunction is a severe complication that results from inadequate fluid resuscitation and septic complications. The incidence of pulmonary complications is high in severe pancreatitis (15–55% of cases), with a first peak upon admission (15% of patients); later, pulmonary injury might result from septic shock and complicate infection of the necrotic pancreas. Hepatic injury is usually mild during acute pancreatitis (Whitcomb 2006).
Biochemical findings supported the diagnosis of acute pancreatitis: a high serum lipase concentration of 3 times the normal upper limit indicates acute pancreatitis but it has no role in the assessment of disease severity. The serum trypsinogen level is not commonly available (Ueda et al. 2007).
Others markers are valuable to score the severity of the disease: serum creatinine level, white blood cell count, glucose level, lactate dehydrogenase (LDH) level, aspartate transaminase level, calcium serum level, and hematocrit.
Several clinical scoring systems are helpful for clinicians to assess the severity of the disease and to identify patients at risk of having adverse outcomes; they are based on physical and biochemical data. Many scores have been established: the SOFA scoring system, the Marshall scoring system, the Acute Physiology and Chronic Health Evaluation II (APACHE II), and the Ranson scoring system (Table 2). It is important to note that the Ranson score cannot be obtained before 48 h after admission and APACHE II can be calculated after 24 h.
The Atlanta classification, introduced in 1992, was a step toward a global consensus and is widely accepted. It divides acute pancreatitis into mild and severe. Severe acute pancreatitis is defined if a patient suffers at least one of the criteria detailed in Table 3. Moreover, the Atlanta classification includes the important role of computed tomography (CT) in describing disease severity.
5 CT Findings
CT has several goals:
-
To confirm the diagnosis of acute pancreatitis by excluding other diagnoses of acute abdominal pain
-
To assess the severity of the disease
-
To diagnose early an obstructive biliary cause
-
To assess complications and to manage their treatment
5.1 MDCT Technique
The best time for staging acute fluid collection is at 72 h and the appreciation of parenchymal necrosis is optimal at 96 h after the first symptoms (abdominal pain). Usually, CT is performed within 48–72 h following the clinical diagnosis of pancreatitis. The CT protocol must include two acquisitions:
-
The first acquisition without injection of iodine contrast medium is helpful to show biliary or pancreatic stones and to asses hemorrhage.
-
The second acquisition after injection of 2 mL of iodine contrast medium per kilogram (concentration 350 g/L) at 3 mL/s, performed in the portal phase (delay of 70 s).
Neither gastric nor bowel opacification is necessary.
5.2 Initial Lesions
Initial lesions contribute to scoring the acute pancreatitis; they can be isolated or associated with each other.
5.2.1 Pancreatic Necrosis
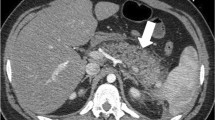
Pancreatic necrosis is defined as an area of nonviable pancreatic parenchyma; it consists of focal or a diffuse lack of parenchymal enhancement demonstrated after intravenous contrast medium administration (Fig. 1). This necrosis can involve a part or the entire pancreas, and can go over the pancreas in peripancreatic fat (Fig. 2). It is present in 5–20% of patients with acute pancreatitis. It can be hemorrhagic (Fig. 3), with hyperattenuation on the series before contrast medium injection. Its evolution to walled-off pancreatitis necrosis (WOPN) leads to an irregular fluid collection that develop within the area of pancreatic necrosis and can extend into the peripancreatic space. These WOPN have solid luminal content that develops as a late consequence of necrotizing pancreatitis (Fig. 4). They must be well differentiated from pseudocyst because the therapeutic options are different (Takahashi et al. 2008).
5.2.2 Acute Fluid Collections
Fluid collections contain enzymatic fluid secretions. They can be homogeneous or hetrogeneous. The presence of gas inside is a sign of gravity. A well-defined wall encloses fluid collections. They are more often located near the pancreatic gland and on the left and right anterior pararenal spaces (Fig. 5). This collection can have visceral atypical locations, such as hepatic, splenic, and even pleural. Present in 40% of patients with acute pancreatitis, they have a spontaneous resolution in 50% of patients. In the case of nonresolution, they evolve as pseudocyst (in a minimal time of 4 weeks) (Table 4).
Severe pancreatitis without pancreatic necrosis classified as Balthazar E or CT severity index (CTSI) 4. The pancreas has a smooth-contour appearance. The prepancreatic collection (circle) is heterogeneous and could correspond to fat necrosis or acute fluid collection. Perihepatic (star) and prerenal (arrow) collections are classified as acute fluid collections
5.2.3 Size and Morphology of the Pancreatic Gland
The size of the pancreas can be normal or increased owing to edema. This increase is diffuse or localized. Normal lobular contours can disappear with a smooth-contour appearance in a diffuse or focal way (Fig. 6).
5.2.4 Peripancreatic Fat Infiltration
A reticulation of the peripancreatic fat reveals fat stranding (Fig. 7). This infiltration is evident when it is associated with parenchyma necrosis, but even if there is no parenchyma necrosis on the CT scan, peripancreatic fat infiltration can be present.
5.2.5 Ascites
Ascites as a peritoneal reaction can be observed especially around the liver and the spleen, and in the pelvis. It is often associated with pleural effusion. It is important, but not easy, to differentiate ascites and acute fluid collection, to avoid an overestimation of the different CT scores that take into account this last sign but not the ascites.
5.3 CT Score
The establishment of a prognostic of the severity of the disease allows the best orientation and the best management of the patient. The CT scores mostly used are the Balthazar score and the CT severity index (CTSI). But new scores have been described, such as the extrapancreatic inflammation on CT (EPIC) score and the simple prognostic score, that try to take into account the visceral dysfunction, which remains the main factor determining outcome in the first period of severe acute pancreatitis. Other scores such as the pancreatic severity index (London et al. 1989) and the mesenteric edema and peritoneal score (King et al. 2003) have been proposed but are not often used in clinical practice.
5.3.1 Balthazar Score
The Balthazar score is based on a five-grade scale (Table 5). The parameters recorded are a reflection of the pancreatic and peripancreatic inflammation and the presence of fluid collection. The main advantages of this score are its simplicity and that contrast medium injection is not required. The main disadvantages are the lack of precision regarding the fluid collection (acute fluid collection, organized necrosis, walled-off pancreatic necrosis, postnecrotic fluid collection) and, above all, not taking into account the pancreatic necrosis. Morbidity and mortality are, respectively, 4 and 0% in patients with CT grade A, B, or C and 54 and 14% in patients with CT grade D or E. To obtain the best results, the CT must not be performed too early. One must wait at least 48–72 h after the beginning of the symptoms (Delrue et al. 2010; Casas et al. 2004; Balthazar et al. 1985) (Figs. 5, 6, 7, 8).
5.3.2 CT Severity Index
The CTSI is probably the most used CT score. It is based on the Balthazar score, but, in addition, it takes into account the percentage of pancreatic necrosis (Table 6). This score has a better correlation than the Balthazar score with the morbidity and the mortality, which are, respectively, 3 and 8% when the CTSI is 3 or less and 92 and 17% when it is 7 or more (Delrue et al. 2010; Balthazar et al. 1990; Vriens et al. 2005). The main advantage of this CTSI is the introduction of pancreatic necrosis, and thus the difference between edematous and necrotic pancreatitis (Fig. 9). As reported in “Pancreatic necrosis,” this is closely related to a high morbidity and mortality, in contrast to interstitial and edematous pancreatitis. The disadvantages of the CTSI are, firstly, the requirement of contrast medium injection, which has been reported by some authors to aggravate the course of the disease (Schmidt et al. 1995), and, secondly, the need for a delay of 72 h or better 96 h to define well the pancreatic necrosis. It is important to note that the potential aggravation by iodine contrast medium has been invalidated by more recent studies (Arvanitakis et al. 2004; Balthazar et al. 1990).
Severe pancreatitis with pancreatic necrosis, classified as Balthazar E or CTSI 8. CT examination shows multiple acute fluid collections. The enhancement of the pancreatic parenchyma is poor and heterogeneous (arrows). Almost all of the gland seems to be involved in the necrosis but not in a homogeneous way
A modified CTSI has been proposed (Mortelé et al. 2004) that associates with the signs of inflammation and necrosis of the classical CTSI the signs of extrapancreatic complications. This score seems to be more strongly correlated to the outcome of the disease than the traditional CTSI, but it has been poorly evaluated and the delay before CT is very long (within 1 week).
5.3.3 EPIC Score
The EPIC score is based not on pancreatic or peripancreatic lesions but only on extrapancreatic manifestations of the disease (Table 7). An EPIC score of 4 or more is predictive of a severe acute pancreatitis with a sensitivity of 100% and a specificity of 70.8%. The main advantages of the EPIC score are that injection of contrast medium is not required and that it can be performed in the first 24 h following the start of the symptoms (De Waele et al. 2007).
5.3.4 Simple Prognosis Score
The simple prognosis score is a composite score based on the presence (1 point) or the absence (0 points) of the following biological and CT data: (1) serum LDH level (900 IU/L or higher), (2) blood urea nitrogen level (25 mg/dL or higher), and (3) the presence of pancreatic necrosis on the CT performed within the first 2 days of admission. A score higher than 2 or 3 is considered to reflect severe pancreatitis. The mortality rate, percentage of infection, and rate of organ failure are, respectively, 10, 7, and 37% when the score is 0 or 1 and 58, 51, and 91% when the score is 2 or 3. The main advantages of this score are that it reflects through the biological data the visceral dysfunction and its simplicity. The main disadvantage is its poor evaluation in the literature (Delrue et al. 2010).
5.3.5 Classification in Three Groups
A new classification in three categories has recently been proposed taking into account the CT score and multiple or persistent organ failure. Severe acute pancreatitis was defined as death, persistent organ failure (over 48 h), or multiple organ failure. Moderate acute pancreatitis was defined as the presence of acute collections and/or pancreatic necrosis. Mild acute pancreatitis was defined by exclusion. This classification seems to distinguish three homogeneous groups of severity but needs validation (De Madaria et al. 2010).
5.4 Etiological Orientations
5.4.1 In the Early Stage Some Cause Could Be Suspected
A common bile duct stone migration is the most important cause to diagnose initially because of its clinical impact (therapeutic ERCP). It could be suggested in the case of gallstones or a common bile duct dilatation visible on CT. Common bile duct stones are rarely obvious on CT. Ultrasonography has good sensibility performance for the diagnosis of gallstones (more than 90%) but poor sensibility for the diagnosis of common duct stones (40–60%). Nevertheless, ultrasonography in the first 24 h is mandatory to depict bile duct dilatation, small lithiasis in the gallbladder (large lithiasis do not migrate), or biliary duct lithiasis (Fig. 10).
Ultrasonography performed 24 h after the beginning of the symptoms of pancreatitis shows sludge (black arrow) and a small stone (white arrow) in the gallbladder. The extrahepatic bile duct is visible and ultrasonography demonstrates a small stone (head of arrow). These findings enable one to make the diagnosis of biliary pancreatitis and lead to the performance of an endoscopic retrograde cholangiography associated with a sphincterectomy
An alcoholic cause can be suggested in the case of liver abnormalities: steatosis (hepatic size increased with attenuation of the parenchyma) or cirrhosis (dysmorphism of the liver and portal hypertension signs) (Fig. 11).
Iatrogenic and traumatic causes are obvious causes because of the clinical context: ERCP, pancreatic surgery, car crash, for example.
5.4.2 Different Pancreatic Diseases
Different pancreatic diseases are to blame for acute pancreatitis. They are difficult to diagnose in the early stage of the disease because of the pancreatic parenchyma change. An exploration by CT or MRI is mandatory later.
5.4.2.1 Pancreatic Duct Abnormalities
-
Stenosis or intraductal stones in the case of chronic pancreatitis: CT features include pancreatic lithiasis, irregular ductal dilatation, and parenchyma atrophy.
-
Intraductal papillary mucinous tumors of the pancreas: the enlargement of the main pancreatic duct and/or branch ducts is well identified on MRI (Fig. 12) (Vullierme et al. 2005).
-
Congenital anomalies of the pancreatic duct (annular pancreas or pancreas divisum) explored by magnetic resonance cholangiopancreatography.
5.4.2.2 Tumors
-
Adenocarcinoma induces an enlargement of the main pancreatic duct but it is difficult to differentiate a hyperattenuating mass in the early phase due owing inflammation and necrosis from a pancreatic tumor. Although the diagnosis of adenocarcinoma is usually easy to perform by CT, it is important not to perform the examination too early after the acute episode (Fig. 13).
-
Lymphoma. Pancreas localization is more often secondary to a general disease; it can appear as a nodular or diffuse infiltrative lesion.
5.4.2.3 Autoimmune Pancreatitis
This disease is characterized by an autoimmune inflammatory process and can initially be revealed by an acute pancreatitis. It mimics a tumoral lesion with enlargement of the pancreas associated with a minimal peripancreatic stranding and a nondilated or diffusely narrowed pancreatic duct. The diagnosis is based on the association with extrapancreatic manifestations of autoimmune diseases, serologic markers (autoantibodies), and the response to corticosteroid treatment (Fig. 14) (Sahani et al. 2004).
5.4.2.4 General metabolic diseases
General metabolic diseases (hypercalcemia, hypertriglyceridemia) can be incriminated for acute pancreatitis, but they have no particular CT features.
5.5 Complications
CT allows the diagnosis of complications which can appear during the clinical course of the disease.
5.5.1 In the Acute Phase
In the acute phase complications are dominated by organ failures, but some local complications can occur.
Thrombosis usually concerns veins more than arteries, such as splenic, mesenteric, or portal vein (Fig. 15).
Pseudoaneurysms are rare during the initial phase of acute pancreatitis and are more often discovered on CT in the follow-up of the disease. They are due to direct erosion of the artery wall by the pancreatic enzymes of the fluid collection. They appear as an image of addition, enhanced with the same timing as the arterial vessels. CT should be considered as the first investigation in diagnosis and for planning intervention. Multiplanar reformation and maximum intensity projection reconstructions allow one to identify the artery from which the pseudoaneurysm rises and the pseudoaneurysm morphology. Their treatment must be endovascular first (Fig. 16) (Kirby et al. 2008).
Severe pancreatitis with pancreatic necrosis, complicated by a pseudoaneurysm. The enhanced CT examination performed 16 days after the beginning of the disease shows a pseudoaneurysm (arrow) of the gastroduodenal artery close to a voluminous collected necrosis. Embolization by the sandwich technique (heads of arrow) allows complete exclusion of the pseudoaneurysm
Acute fluid collection can injure abdominal organs such as liver, spleen, gallbladder, and gut and may be responsible for necrosis (Fig. 17), infarction, hematoma, and even spleen rupture (Habib et al. 2000).
Severe pancreatitis with digestive necrosis and spleen infarction. Enhanced CT examination shows a voluminous acute fluid collection in the omental bursa (star). Presence of gas in the gastric wall (white arrow) and in the intrahepatic portal vessel (black arrow) demonstrates the digestive necrosis. Well-delineated segmental hypoattenuation of the spleen corresponds to an infarction
Intestinal perforation is rare but can occur owing to spread of pancreatic enzymes to the intestinal wall (Van Minnen et al. 2004). According to the literature, this complication seems to involve almost exclusively the colon and not the small bowel. Diagnosis is often difficult because the signs of colon perforation (pericolitis, bubble of gas) are frequent in acute pancreatitis.
Infection of necrosis is a frequent and severe complication (Whitcomb 2006). The diffuse or local area of nonviable parenchyma is initially sterile and can become infected by bacteria of gut origin. Mortality in sterile and infected necrosis is, respectively, 10 and 25%. This complication develops during the second or third week in 40–70% of patients with severe pancreatitis. Suspicion of infection of the necrosis is made on the basis of clinical and biological criteria. The only CT sign is the presence of a bubble of gas within the necrosis area. But this sign is not very sensitive (Fig. 18). Thus, to discriminate between sterile and infected pancreatic necrosis, CT-guided needle aspirations of pancreatic or peripancreatic tissues are done repeatedly.
A positive diagnosis of infection can lead to surgical debridement. Endoscopic or percutaneous drainage is limited in the case of infected necrosis because these lesions are mainly made up of solid residues with a poor liquid component (Freeny et al. 1998). But percutaneous drainage can be performed when surgery is contraindicated or for residual collections after surgical debridement (Fig. 19).
Disconnection of the pancreatic duct (Sandrasegaran et al. 2007) is underestimated. However, patients with a disconnected pancreatic duct are at a increased risk of persistent pancreatic fistula. Therefore, surgical treatment should be discussed in this situation.
Diagnosis of disconnected pancreatic duct could be advocated if all of the following signs are present: necrosis of at least 2 cm of pancreas; viable pancreatic tissue upstream of the site of necrosis, and extravasation of contrast medium injected into the main pancreatic duct on pancreatography or on MRI with secretin injection.
5.5.2 In a Later Phase (After 4 Weeks)
Local complications are mainly due to a pseudocyst. Pseudocysts are the evolution of the acute fluid collection that has not been resolved. On CT, pseudocysts appear at least after 4 weeks of evolution of the acute pancreatitis (Fig. 20). Pancreatic pseudocysts are defined as localized amylase-rich fluid collections located within the pancreatic tissue or adjacent to the pancreas (Yeo et al. 1990). They are more frequently unilocular than multilocular and are surrounded by a fibrous wall without epithelial lining. The CT findings of a pseudocyst include a round or oval fluid collection, well delineated, with an homogeneous content and a thin or thick, but regular wall enhanced after contrast medium injection (Kim et al. 2005). They occur in 5–40% of patients with acute pancreatitis.
Classic complications of pseudocyst are compression on digestive, biliary, or vascular structures, rupture, infection, and hemorrhage. In the literature, an etiological or morphological factor is predictive of the evolution (regression or complication of the pseudocyst) (Maringhini et al. 1999).
Infection of the pseudocyst does not dramatically change the feature of the pseudocyst on CT, but a light heterogeneity of the content could appear. Treatment consists in drainage that could be endoscopic or per cutaneous (Loveday et al. 2008).
Compression can involve the gut, the biliary system, or vessels (Fig. 21). CT is used to determine the relation between the pseudocyst and its adjacent structures, and thus to guide the choice of the therapeutic management, which can be endoscopic or surgical.
Intracystic hemorrhage is due to the erosion of an artery by the pancreatic enzymes of the pseudocyst fluid. Diagnosis could follow a hypovolemic shock, but it is more often made following a systematic CT examination in the follow-up of the acute pancreatitis. The density of the pseudocyst is spontaneously high and after contrast medium injection leakage of contrast medium within the pseudocyst is observed (Fig. 22). The first treatment should be endovascular.
Pseudocyst close to the head of the pancreas, responsible for an erosion of the gastroduodenal artery with intracystic hemorrhage. On enhanced CT examination, the pseudocyst is filled by contrast medium (star). On angiography, the gastroduodenal artery is pushed into a horizontal position by the pseudocyst. Leakage of contrast medium in the pseudocyst is clearly demonstrated (arrow)
6 CT Pitfalls
Considering that the diagnosis of acute pancreatitis is based on clinical and biological data, any pancreatic or peripancreatic abnormality noticed on CT is used to classify the severity of the disease. Thus, the only pitfalls are the etiological diagnosis that could be hidden by the signs of pancreatitis.
7 Impact of CT on the Management
7.1 Positive Diagnosis
The only rule of CT on positive diagnosis is to exclude other causes of acute abdominal pain such as bowel perforation, mesenteric ischemia, or aortic aneurysm rupture.
7.2 CT Scoring
The main rule of CT is to establish the severity of the disease (mild or severe) to determine the gravity of the disease, leading to a specific management.
The choice of the score to use is not so easy because, on one hand, it is important to have an early risk stratification of patients with acute pancreatitis. On the other hand, scoring systems based on necrosis are limited because they need an evolution of the disease of at least 48 h or better 72 h to be efficient. In this situation, more recent radiologic scoring systems based on signs of organ dysfunction could be useful in the prediction of severity.
Thus, we suggest two options. For a patient without any clinical sign of severity, i.e., with no problems other than low to moderate abdominal pain, we can wait 72 h to perform the CT and use the CTSI. (Of course, if the patient is referred to the clinician more than 72 h after the beginning of the symptoms, the situation is the same.) For a patient with serious symptoms, i.e., serious pain, abdominal tenderness, hypovolemic or respiratory symptoms, CT is performed without contrast medium in the first 24 h and the EPIC score is used; the scoring is then completed after 72 h with a CT scan with injection of contrast medium.
7.3 Looking for Acute Complications
Complications of acute pancreatitis generally do not appear in the first few days of the evolution of the disease but appear in the first few weeks, or later in the case of pseudocyst complications. But even then uncommon early complications can occur. Thus, first CT, even it is mainly performed to establish a predictive score, should be used to look carefully for vascular, digestive, or visceral associated lesions.
7.4 First Etiological Orientations
During the first scoring CT scan, it is essential to look for signs that could be oriented to specific causes: hepatic abnormality (steatosis, cirrhosis); biliary or pancreatic lithiasis.
Other causes are, in most of cases, impossible to affirm in the early stage of the disease, especially when acute pancreatitis is severe, owing to the pancreatic parenchymal change. Therefore, when the etiological investigations (including clinical, biological, and first CT examinations) are not successful, new morphological examinations, which could be CT or MRI depending on the clinical suspicions, must be carried out after the clinical resolution of the acute pancreatitis. In this situation, it is important not to perform the examination too early after the acute episode, to prevent an inconclusive examination.
7.5 Following Acute Pancreatitis by CT
-
There is no well-established rhythm for following acute pancreatitis by CT. It is not so easy to determine a good delay between the beginning of the symptoms and the first CT examination regarding the opposing needs of an early risk stratification and establishment of an accurate score (48–72 h).
-
After this first CT examination has been performed to stage and score the disease, there is no need for a new CT examination until at least 4 weeks later, except in the case of a clinical or biological change. In this specific situation, a new CT examination could lead to the discovering of complications, worsening of score graduation or a change from edematous to necrotic pancreatitis (in 13 and 8%, respectively) (Lankisch et al. 2001).
-
At 4 weeks CT could be performed in the following cases: (1) when there have been no etiological findings; (2) in the case of resolute pancreatitis if it was necrotic or with a Balthazar grade of D or higher, with the goal of detecting complications; (3) and when the disease is not yet resolved. In this latest case, a CT scan is performed during the course of the disease in accordance with the clinical evolution.
7.6 Radiological Treatments
CT has a major role in the therapeutic management, to guide bacteriologic necrosis or fluid collection samples, to diagnose and plan treatment of abscess or vascular complications.
8 Diagnostic Strategy
-
1.
Positive diagnosis of acute pancreatitis is based on clinical symptoms and blood pancreatic enzyme level.
-
2.
CT is the unique morphological examination to perform at the initial stage of the disease. The ideal delay between the beginning of the symptom and this CT is 48–72 h. This CT allows the first staging of the lesions and predicts the evolution risk of the pancreatitis through the use of CT scores.
-
3.
In the first 24 h it could be useful to perform biliary ultrasonography. The goal of this examination is definitively not to detect any pancreatic signs but to look after signs of lithiasis. If a biliary pancreatitis is diagnosed or suspected, an early ERCP with endoscopic sphincterotomy is recommended (Wada et al. 2010; Johnson and Lévy 2010).
-
4.
The place of MRI in acute pancreatitis has not been established. Regarding detection of necrosis and acute fluid collection, as well as predicting scoring, the performance of MRI is reported to be equivalent to that of CT (Arvanitakis et al. 2007; Xiao et al. 2010). Potential advantages of MRI are the lack of radiation, which might be suitable for patients with multiple follow-up reviews, and providing in the same examination information regarding the pancreatic parenchyma and biliary tree (Fig. 23), as well as the visualization of the pancreatic duct to evaluate integrity or rupture (Lau et al. 2001). The main disadvantages are the lower availability of MRI, the difficulty to achieve breathhold in patients with great pain, which could alter image quality, and the difficulty to manage the intensive care devices, in the case of severe patient conditions, in a magnetic environment.
In practice, MRI is rarely used in the initial staging of acute pancreatitis, but it could have an important place in the etiological assessment.
References
Arvanitakis M, Delhaye M, De Maertelaere V, Bali M, Winant C, Coppens E, Jeanmart J, Zalcman M, Van Gansbeke D, Devière J, Matos C (2004) Computed tomography and magnetic resonance imaging in the assessment of acute pancreatitis. Gastroenterology 126:715–723
Arvanitakis M, Koustiani G, Gantzarou A, Grollios G, Tsitouridis I, Haritandi-Kouridou A, Dimitriadis A, Arvanitakis C (2007) Staging of severity and prognosis of acute pancreatitis by computed tomography and magnetic resonance imaging—a comparative study. Dig Liver Dis 39:473–482
Balthazar EJ, Ranson JHC, Naidich DP et al (1985) Acute-pancreatitis—prognostic value of CT. Radiology 3:767–772
Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH (1990) Acute pancreatitis: value of CT in establishing prognosis. Radiology 2:331–336
Cannon JW, Callery MP, Vollmer CM Jr (2009) Diagnosis and management of pancreatic pseudocysts: what is the evidence? J Am Coll Surg 209(3):385–393
Carroll JK, Herrick B, Gipson T, Lee SP (2007) Acute pancreatitis: diagnosis, prognosis, and treatment. Am Fam Physician 10:1513–1520
Casas JD, Diaz R, Valderas G, Mariscal A, Cuadras P (2004) Prognostic value of CT in the early assessment of patients with acute pancreatitis. AJR Am J Roentgenol 3:569–574
De Madaria E, Soler-Sala G, Lopez-Font I, Zapater P, Martínez J, Gómez-Escolar L, Sánchez-Fortún C, Sempere L, Pérez-López J, Lluís F, Pérez-Mateo M (2010) Update of the Atlanta classification of severity of acute pancreatitis: should a moderate category be included. Pancreatology 10(5):613–619
De Waele JJ, Delrue L, Hoste EA et al (2007) Extrapancreatic inflammation on abdominal computed tomography as an early predictor of disease severity in acute pancreatitis—evaluation of a new scoring system. Pancreas 2:185–190
Delrue LJ, De Waele JJ, Duyck PO (2010) Acute pancreatitis: radiologic scores in predicting severity and outcome. Abdom Imaging 35(3):349–361
Freeny PC, Hauptmann E, Althaus SJ, Traverso LW, Sinanan M (1998) Percutaneous CT-guided catheter drainage of infected acute necrotizing pancreatitis: techniques and results. AJR Am J Roentgenol 170(4):969–975
Frossard JL, Steer ML, Pastor CM (2008) Acute pancreatitis. Lancet 371(9607):143–152
Habib E, Elhadad A, Slama JL (2000) Diagnosis and treatment of spleen rupture during pancreatitis. Gastroenterol Clin Biol 24(12):1229–1232
Johnson C, Lévy P (2010) Detection of gallstones in acute pancreatitis: when and how? Pancreatology 10(1):27–32
Kim YH, Saini S, Sahani D, Hahn PF, Mueller PR, Auh YH (2005) Imaging diagnosis of cystic pancreatic lesions: pseudocyst versus nonpseudocyst. Radiographics 25(3):671–685
King NK, Powell JJ, Redhead D, Siriwardena AK (2003) A simplified method for computed tomographic estimation of prognosis in acute pancreatitis. Scand J Gastroenterol 4:433–436
Kirby JM, Vora P, Midia M, Rawlinson J (2008) Vascular complications of pancreatitis: imaging and intervention. Cardiovasc Intervent Radiol 31(5):957–970
Lankisch PG, Assmus C, Pflichthofer D, Struckman K, Lehnick D (1999) Which etiology causes the most severe pancreatitis? Int J Pancreat 26:55–57
Lankisch PG, Struckmann K, Assmus C, Lehnick D, Maisonneuve P, Lowenfels AB (2001) Do we need a computed tomography examination in all patients with acute pancreatitis within 72 h after admission to hospital for the detection of pancreatic necrosis? Scand J Gastroenterol 36(4):432–436
Lau ST, Simcuck EJ, Kozarek RA, Traverso W (2001) A pancreatic ductal leak should be sought to direct treatment in patients with acute pancreatitis. Am J Surg 181:411–417
London NJ, Neoptolemos JP, Lavelle J, Bailey I, James D (1989) Contrast-enhanced abdominal computed tomography scanning and prediction of severity of acute pancreatitis: a prospective study. Br J Surg 3:268–272
Loveday BP, Mittal A, Phillips A, Windsor JA (2008) Minimally invasive management of pancreatic abscess, pseudocyst, and necrosis: a systematic review of current guidelines. World J Surg 32(11):2383–2394
Maher MM, Lucey BC, Gervais DA, Mueller PR (2004) Acute pancreatitis: the role of imaging and interventional radiology. Cardiovasc Intervent Radiol 27(3):208–225
Maringhini A, Uomo G, Patti R et al (1999) Pseudocysts in acute nonalcoholic pancreatitis: incidence and natural history. Dig Dis Sci 44:1669–1673
McKay CJ, Buter A (2003) Natural history of organ failure in acute pancreatitis. Pancreatology 2:111–114
Mortelé KJ, Wiesner W, Intriere L et al (2004) A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. AJR Am J Roentgenol 5:1261–1265
Nøjgaard C, Bendtsen F, Matzen P, Becker U (2010) The aetiology of acute and chronic pancreatitis over time in a hospital in Copenhagen. Dan Med Bull 57(1):A4103
Papachristou GI, Clermont G, Sharma A, Yadav D, Whitcomb DC (2007) Risk and markers of severe acute pancreatitis. Gastroenterol Clin N Am 2:277–296
Pastor CM, Matthay M, Frossard JL (2003) Pancreatitis-associated lung injury: new insights. Chest 124:2341–2351
Pitchumoni CS, Rubin A, Das K (2010) Pancreatitis in inflammatory bowel diseases. J Clin Gastroenterol 44(4):246–253
Sahani DV, Kalva SP, Farrell J, Maher MM, Saini S, Mueller PR, Lauwers GY, Fernandez CD, Warshaw AL, Simeone JF (2004) Autoimmune pancreatitis: imaging features. Radiology 233(2):345–352
Sandrasegaran K, Tann M, Jennings SG, Maglinte DD, Peter SD, Sherman S, Howard TJ (2007) Disconnection of the pancreatic duct: an important but overlooked complication of severe acute pancreatitis. Radiographics 27(5):1389–1400
Schmidt J, Hotz HG, Foitzik T et al (1995) Intravenous contrastmedium aggravates the impairment of pancreatic microcirculation in necrotizing pancreatitis in the rat. Ann Surg 3:257–264
Takahashi N, Papachristou GI, Schmit GD, Chahal P, LeRoy AJ, Sarr MG, Vege SS, Mandrekar JN, Baron TH (2008) CT findings of walled-off pancreatic necrosis (WOPN): differentiation from pseudocyst and prediction of outcome after endoscopic therapy. Eur Radiol 18(11):2522–2529
Ueda T, Takeyama Y, Yasuda T et al (2007) Simple scoring system for the prediction of the prognosis of severe acute pancreatitis. Surgery 1:51–58
Van Minnen LP, Besselink MG, Bosscha K, Van Leeuwen MS, Schipper ME, Gooszen HG (2004) Colonic involvement in acute pancreatitis. A retrospective study of 16 patients. Dig Surg 21(1):33–38
Vriens PW, van de Linde P, Slotema ET, Warmerdam PE, Breslau PJ (2005) Computed tomography severity index is an early prognostic tool for acute pancreatitis. J Am Coll Surg 4:497–502
Vullierme MP, Giraud M, Hammel P, Couvelard A, Sauvanet A, Belghiti J, Ruszniewski P, Vilgrain V (2005) Intraductal papillary mucinous tumours of the pancreas: imaging features. J Radiol 86(6 Pt 2):781–794
Wada K, Takada T, Hirata K, Mayumi T, Yoshida M, Yokoe M, Kiriyama S, Hirota M, Kimura Y, Takeda K, Arata S, Hirota M, Sekimoto M, Isaji S, Takeyama Y, Gabata T, Kitamura N, Amano H (2010) Treatment strategy for acute pancreatitis. J Hepatobiliary Pancreat Sci 17(1):79–86
Whitcomb DC (2006) Acute pancreatitis. N Engl J Med 20:2142–2150
Xiao B, Zhang XM, Tang W, Zeng NL, Zhai ZH (2010) Magnetic resonance imaging for local complications of acute pancreatitis: a pictorial review. World J Gastroenterol 16(22):2735–2742
Yadav D, Lowenfels AB (2006) Trends in the epidemiology of the first attack of acute pancreatitis: a systematic review. Pancreas 33:323–330
Yeo CJ, Bastidas JA, Lynch-Nyhan A et al (1990) The natural history of pancreatic pseudocysts documented by computed tomography. Surg Gynecol Obstet 170:411–417
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Ridereau-Zins, C., Aubé, C. (2011). Acute Pancreatitis. In: Taourel, P. (eds) CT of the Acute Abdomen. Medical Radiology(). Springer, Berlin, Heidelberg. https://doi.org/10.1007/174_2010_138
Download citation
DOI: https://doi.org/10.1007/174_2010_138
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-89231-1
Online ISBN: 978-3-540-89232-8
eBook Packages: MedicineMedicine (R0)