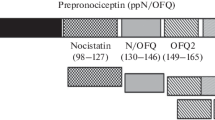
Abstract
Since the discovery of the NOP receptor and N/OFQ as the endogenous ligand, evidence has appeared demonstrating the involvement of this receptor system in pain. This was not surprising for members of the opioid receptor and peptide families, particularly since both the receptor and N/OFQ are highly expressed in brain regions involved in pain, spinal cord, and dorsal root ganglia. What has been surprising is the complicated picture that has emerged from 25 years of research. The original finding that N/OFQ decreased tail flick and hotplate latency, when administered i.c.v., led to the hypothesis that NOP receptor antagonists could have analgesic activity without abuse liability. However, as data accumulated, it became clear that not only the potency but the activity per se was different when N/OFQ or small molecule NOP agonists were administered in the brain versus the spinal cord and it also depended upon the pain assay used. When administered systemically, NOP receptor agonists are generally ineffective in attenuating heat pain but are antinociceptive in an acute inflammatory pain model. Most antagonists administered systemically have no antinociceptive activity of their own, even though selective peptide NOP antagonists have potent antinociceptive activity when administered i.c.v. Chronic pain models provide different results as well, as small molecule NOP receptor agonists have potent anti-allodynic and anti-hyperalgesic activity after systemic administration. A considerable number of electrophysiological and anatomical experiments, in particular with NOP-eGFP mice, have been conducted in an attempt to explain the complicated profile resulting from NOP receptor modulation, to examine receptor plasticity, and to elucidate mechanisms by which selective NOP agonists, bifunctional NOP/mu agonists, or NOP receptor antagonists modulate acute and chronic pain.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 NOP Receptors and N/OFQ, the Endogenous Ligand
The fourth member of the opioid receptor family, which was initially called ORL1, LC132, XOR1, kappa 3, ROR-C, C3 (Bunzow et al. 1994; Fukuda et al. 1994; Wang et al. 1994; Lachowicz et al. 1995; Meunier et al. 1995; Pan et al. 1995), was identified by homology with the other three opioid receptors, mu, delta, and kappa. Although this receptor had homology to the other receptors, basically as high as they had to each other, it did not bind either peptide or small molecule opiates with high affinity, nor was activity blocked by naloxone at normal opioid concentrations. Therefore, it was concluded that this fourth receptor, now called the NOP (Nociceptin/Orphanin FQ Peptide) receptor, is in the opioid receptor family, but is not an opioid receptor (Cox et al. 2015). Initial in situ hybridization studies demonstrated receptor mRNA in many brain regions, particularly those involved in emotion and cognition (Mollereau et al. 1994, 1996; Neal et al. 1999a). Nevertheless, because of the obvious opioid connection, when the endogenous agonist nociceptin/orphanin FQ (N/OFQ) was discovered to be the fourth member of the opioid peptide family, the first experiments conducted by Meunier et al. and Reinscheid et al. had to do with pain (Meunier et al. 1995; Reinscheid et al. 1995). The logical assumption was that N/OFQ would have antinociceptive activity, like the other members of the opioid peptide family. Both groups found the opposite, N/OFQ reduced hotplate (Meunier et al. 1995) and tail flick (Reinscheid et al. 1995) latency in mice, when administered i.c.v. Naturally, it was this apparently nociceptive property that led to the perhaps misleading name nociceptin given by Meunier and colleagues.
The idea that N/OFQ was actually nociceptive was somewhat short-lived. In two important publications, Grandy and colleagues demonstrated that N/OFQ could block antinociceptive activity induced by agonists to all three opioid receptors (Mogil et al. 1996b). In fact, N/OFQ didn’t turn out to be nociceptive per se, as much as it reduced the increase in tail flick latency induced by the i.c.v. injection itself (Mogil et al. 1996a). In other words, it blocked the injection-mediated stress-induced analgesia. Subsequent studies by a number of researchers demonstrated conclusively that N/OFQ, and small molecule NOP agonists, could block stress-induced analgesia, induced by a variety of stressors (Rizzi et al. 2001; Reiss et al. 2008; Xie et al. 2008).
All of the initial studies on N/OFQ were subsequent to i.c.v. administration of the peptide. Opiates also have actions in the spinal cord, and interestingly, when N/OFQ was administered intrathecally, it failed to block morphine antinociceptive activity but, in fact, potentiated morphine and had antinociceptive activity on its own (Tian et al. 1997). However, in other studies, N/OFQ had no activity when administered into the spinal cord (Vanderah et al. 1998). In a comprehensive study of several mouse strains, Mogil et al. demonstrated considerable mouse strain differences in both stress-induced analgesia and the anti-opioid actions of N/OFQ (Mogil et al. 1999). Additional early studies led to confusion about the actions of N/OFQ, as well as N/OFQ metabolites and other peptides derived from the prohormone. Not only do differences in route of administration lead to different actions of N/OFQ, this peptide was found to induce a pronociceptive response at very low doses (atto to femtomole) after intraplantar or intrathecal (i.t.) administration (Inoue et al. 1998). This could be blocked by NK1 receptor antagonists and therefore appeared to be due to stimulation of substance P release (Inoue et al. 1998; Sakurada et al. 1999). However, at higher doses (nanomole, i.t.), N/OFQ blocked substance P-induced pain response (Inoue et al. 1999). In addition, Pasternak and colleagues reported that i.c.v. administered N/OFQ was initially pronociceptive, but then over time, this developed into a naloxone-reversible antinociceptive action (Rossi et al. 1997), as if N/OFQ was being metabolized to a peptide that activates opioid receptors. In addition, this group also reported that two N/OFQ N-terminal fragments N/OFQ(1–7) and N/OFQ(1–11) had naloxone-reversible antinociceptive activity. It has never been made clear how this works since neither peptide has high affinity for the NOP receptor nor any of the classical opiate receptors (Rossi et al. 1997; Mathis et al. 1998). Studies in rats were also problematic, as Vanderah et al. could find no nociceptive or antinociceptive actions of N/OFQ in either the brain or spinal cord (Vanderah et al. 1998). These significant differences in reasonably straightforward experiments clearly indicate that NOP receptor-mediated analgesia is dependent upon strain, species, and the particular assay being conducted. Species differences in NOP receptor activity with respect to pain are probably most evident when comparing rodents and nonhuman primates (Ko et al. 2009; Ding et al. 2016).
Although initial in situ hybridization studies suggested NOP receptor activity being related to emotion and cognition, subsequent immunohistochemistry, in vitro autoradiography, and in situ hybridization studies by Watson and coworkers fully characterized the location of both NOP receptors and N/OFQ (Neal et al. 1999a, b). These experiments clearly demonstrated the presence of both NOP receptors and N/OFQ in brain regions involved in pain and analgesia. In particular, both peptide and receptor could be found in high concentrations in the ventral lateral periaqueductal gray (vlPAG) and the rostral ventromedial medulla (RVM), the brainstem descending analgesic pathway, as well as in the spinal cord and dorsal root ganglia. Electrophysiological studies in a variety of brain regions, including the vlPAG, demonstrated that NOP receptors acted like other members of the opioid receptor family and opened inwardly rectifying potassium (GIRK) channels, thereby hyperpolarizing and reducing the activity of neurons after receptor activation (Connor et al. 1996; Heinricher et al. 1997; Vaughan et al. 1997). In fact, NOP receptors appear to be more ubiquitous than the opioid receptors. In the vlPAG, the mu receptor is on about half of the neurons, while N/OFQ activated NOP receptors on virtually every neuron tested. The presence of NOP receptors in the vlPAG led Grandy to hypothesize that the anti-opiate actions of N/OFQ could be due to inactivation of this antinociceptive pathway. Mu opioid receptor activation on vlPAG neurons leads to antinociceptive activity, and this activity can be blocked by naloxone and also by N/OFQ if administered together with morphine directly into the vlPAG (Morgan et al. 1997). This was taken an important step further by Fields and colleagues (Pan et al. 2000).
The descending pain modulatory pathway travels from the PAG to the brainstem RVM to the dorsal horn of the spinal cord, and morphine can block the pain signal at any locus. Fields had proposed a model whereby activation of primary (OFF) cells leads to analgesia, while activation of secondary (ON) cells leads to pain or hyperalgesia (Pan and Fields 1996). Morphine acts as an analgesic in this brain region by both inhibiting the ON cells and at the same time disinhibiting the OFF cells via inhibition of GABA interneurons. Kappa receptor activation inhibits the OFF cells, which leads to increased pain or hyperalgesia (Pan et al. 1997). In electrophysiological experiments, Fields and colleagues found that N/OFQ activated NOP receptors and therefore hyperpolarized both primary (OFF) and secondary (ON) cells. By this mechanism, N/OFQ blocked opioid analgesic activity by inactivating the analgesic OFF cells. Conversely, in the condition of opioid withdrawal, the pain-inducing ON cells are activated, leading to hyperalgesia, and these cells are also hyperpolarized by N/OFQ activation of NOP receptors. In this case N/OFQ would block this pain signal, leading to a net analgesic effect. These results clearly demonstrated that the actions of N/OFQ or other NOP agonists can be dependent upon the state of the animal (Pan et al. 2000).
2 Expression of NOP Receptors in Brain, Spinal Cord, and DRG
There have been several publications that have described the localization of NOP receptors and N/OFQ in the brain, spinal cord, and dorsal root ganglia (DRG) that give clues to the involvement of this receptor in pain and analgesia. The NOP receptor can be found throughout the brain, in large amounts in brain regions involved in anxiety, memory, reward, and pain. It can also be found in the dorsal horn of the spinal cord and in DRG, which obviously suggests a connection to pain. The location of NOP receptors was determined by using multiple histological approaches including in situ hybridization, immunohistochemistry, and in vitro autoradiography (Anton et al. 1996; Neal et al. 1999a; Florin et al. 2000; Chen and Sommer 2006). Each method has its own benefits and challenges. In situ hybridization is specific and sensitive but shows only the mRNA-containing cell bodies. In vitro autoradiography can be very specific, but is not particularly sensitive, nor does it provide cellular resolution. Immunohistochemistry can be sensitive, with excellent resolution, but immunostaining on accurate tissue regions is hugely affected by the selectivity of the antibodies. In fact, antibodies for the NOP receptor have been problematic. Although several papers have been published, one paper was retracted after the same antibodies identified virtually the same receptor localization in NOP receptor knockout mice (Anton et al. 1996; Corrigendum 1999). G protein-coupled receptor (GPCR) antibodies cannot be considered reliable until they are tested with knockout animals. At this point, there are no NOP receptor antibodies that have been validated in this manner.
2.1 NOP-eGFP Receptors in Brain
In order to develop a new tool for NOP receptor identification, Brigitte Kieffer developed a mouse with eGFP attached to the C-terminal tail of NOP receptors (Ozawa et al. 2015). These knock-in mice are similar to delta receptor-eGFP and mu receptor-mCherry mice also developed by Kieffer and colleagues (Scherrer et al. 2006). We have used the NOP-eGFP mice to explore the localization of the NOP receptor in brain, spinal cord, and DRG. Although these mice provide a sensitive method to identify the NOP receptor, the tagged receptor presents certain problems. First of all, receptor number is higher in the knock-in mouse than the wild type. Because this knock-in receptor maintains the normal NOP receptor promotor, the regional location should be identical to the wild type; however the large fusion protein may affect degradation and potentially trafficking. This has been a controversial issue for the delta-eGFP receptor (Wang et al. 2010), though ultimately many in situ hybridization studies in wild-type animals seem to confirm the delta-eGFP findings (Scherrer et al. 2009; Bardoni et al. 2014). With respect to the NOP-eGFP receptor, localization of this fusion protein is generally consistent with previous in situ hybridization and in vitro autoradiography studies (Neal et al. 1999a).
Location of the NOP-eGFP receptor in brain is similar to what was expected. Receptor level is very high in the PAG, locus coeruleus, and RVM, regions important to the ascending and descending pain pathways, as well as in the anterior cingulate cortex, a brain region important to the affective component of pain (Ozawa et al. 2015). NOP receptors are also on virtually every cell in the trigeminal ganglia and trigeminal nucleus caudalis, suggesting that the NOP system could be involved in migraine (Fig. 1). Interestingly, in the trigeminal ganglia, as with the dorsal root ganglia, there is some overlap with CGRP in the small diameter neurons. However, overall there are relatively few neurons that are double stained for NOP receptor and CGRP, a neuropeptide known to be involved in migraine (Edvinsson 2003). This is consistent with publications demonstrating that N/OFQ is dramatically decreased in the cerebral spinal fluid and blood during migraine (Ertsey et al. 2005), inhibits neurogenic dural vasodilatation (Bartsch et al. 2002; Capuano et al. 2007), and clearly suggests NOP receptors as potential target for treatment of migraine. As discussed previously, NOP-eGFP receptors are also highly expressed in the amygdala and hippocampus, regions involved in stress and learning, as well as nucleus accumbens, ventral tegmental area, and medial habenula, regions involved in reward and drug abuse.
2.2 NOP Receptors in Spinal Cord
In addition to the NOP-eGFP expression in the brain, NOP-eGFP receptors are highly expressed in the spinal cord (Ozawa et al. 2018). Consistent with an involvement in pain after intrathecal administration, NOP receptors are mostly distributed between laminae I through III in the spinal dorsal horn, regions important for the regulation of pain, itch, and touch. NOP-eGFP receptor immunoreactivity was very high in spinal laminae I, IIouter, and the dorsal border of lamina IIinner, where peptidergic (CGRP-positive) and non-peptidergic (isolectin B4 (IB4)-positive) nociceptive primary afferents project. The intense immunoreactivity extended into the ventral border of laminae IIinner and III, which are characterized by the presence of excitatory interneurons that express the gamma isoform of the protein kinase C – (PKCγ-positive interneurons). This region of the dorsal horn has been demonstrated to be important to injury-induced chronic mechanical allodynia (Malmberg et al. 1997). In addition to NOP receptor distribution in the dorsal horn, in general agreement with the location of the receptors by in vitro autoradiography in rats (Neal et al. 1999a), strong immunoreactivity was also detected in lamina X, and a moderate fluorescent signal was observed throughout the intermediate zone and ventral horn.
2.3 NOP Receptors in Dorsal Root Ganglia
NOP-eGFP receptors are also highly expressed in DRG (Ozawa et al. 2018). Interestingly, in situ hybridization studies indicated that NOP receptor mRNA was very abundant but NOP receptors were not detected in DRG in an initial in vitro autoradiographic study (Neal et al. 1999a). However, their presence of NOP receptors in DRG had been detected by electrophysiological and immunohistochemical studies (Chen and Sommer 2006; Murali et al. 2012; Anand et al. 2016). Furthermore, the type of DRG neurons that express NOP receptors is consistent with both the location of receptors in the spinal cord and the antinociceptive activity of NOP receptor agonists. DRG neurons are cell bodies for specialized cells that send axons both to the spinal cord and the periphery. Subtypes of DRG neurons are generally electrophysiologically distinguished by their conduction velocity, as well as their cell body size combined with histochemistry; fast-conducting, thickly myelinated, A-beta fibers (large diameter neurons); slower-conducting thinly myelinated A-delta (medium diameter neurons); and slowly conducting unmyelinated C-fiber (small diameter neurons) (Gebhart and Schmidt 2013).
Immunohistochemical studies with NOP-eGFP mice indicated that the receptors are expressed in various subpopulations of DRG neurons and are co-expressed with many known cell markers (Fig. 2; Ozawa et al. 2018). These studies have demonstrated that a small percentage of small NOP-eGFP positive cells are IB4-positive (non-peptidergic), with a larger number co-expressing CGRP and mu receptors (and therefore are peptidergic). This is consistent with an electrophysiological study, which demonstrated that 85% of peptidergic C-nociceptors (small IB4-negative DRG neurons) are responsive to both N/OFQ and DAMGO, while a smaller percentage of small IB4-positive neurons were responsive to N/OFQ (Murali et al. 2012). These data suggest that the NOP-N/OFQ system can function by inhibition of peptidergic nociceptors, which are essential to acute heat pain and heat hyperalgesia. These neurons project to laminae I and IIouter of the spinal cord and are consistent with high expression of NOP receptors, as described above. In addition to heat stimuli-responsive NOP-eGFP-positive peptidergic C-nociceptors, the presence of NOP receptors on small IB4+ C-fibers suggests that NOP receptor activation may also modulate acute mechanical pain, similar to delta receptors, as described by Scherrer and colleagues (Scherrer et al. 2009; Bardoni et al. 2014). There are also a large number of myelinated DRG neurons expressing NOP-eGFP. Medium myelinated primary afferents express NOP-eGFP receptors in the absence of CGRP. These are not typical A-delta nociceptors but may represent myelinated low-threshold mechanoreceptors (LTMRs) that are important for touch (Luo et al. 2009; Abraira and Ginty 2013; Bardoni et al. 2014). These low-threshold primary afferents project to the ventral border of lamina IIinner and III, which also express NOP-eGFP immunoreactivity and are known to be involved in the development of injury-related allodynia (Malmberg et al. 1997; Neumann et al. 2008). Our recent studies have demonstrated that NOP receptors seem to be on large A-beta fibers that are also important for touch. All of these results are consistent with known actions of spinal and peripheral NOP receptor activation in both acute and chronic pain models (Khroyan et al. 2011b; Ozawa et al. 2018). Further molecular characterization using a variety of additional markers will be required to fully resolve the identity of these primary afferents. When considered together with the effects of N/OFQ discussed above when administered directly into the vlPAG or RVM, it becomes clear that the location of the NOP receptor and circuity explain the dichotomy of N/OFQ blocking opioid analgesia in the brain but being analgesic when administered into the spinal cord.
NOP-eGFP expressing DRG neurons. White arrows depict the cells co-expressing NOP-eGFP and cellular markers. Scale bar 100 μm. Reprinted with permission from Ozawa et al. (2015)
3 NOP Receptor Knockout Studies
NOP receptors were deleted by homologous recombination by Nishi et al. (1997), and Pintar and colleagues, who deleted both the receptor (Clarke et al. 2001) and the peptide (Kest et al. 2001). Pain mechanisms were studied extensively in these animals. Although these animals have no apparent difference in pain sensitivity itself, the homozygous KO animals developed morphine tolerance at a reduced rate compared with either wild-type or heterozygous animals (Ueda et al. 1997, 2000). In fact, physical signs of morphine dependence were also reduced in the NOP receptor KO mice. These results suggest that both tolerance and physical dependence develop due to an upregulation of the anti-opioid, NOP, system (Ueda et al. 2000). However, in another report, the effect of NOP receptor deletion on physical dependence, but not tolerance, could be reproduced (Mamiya et al. 2001). There was no change in the development of either thermal or mechanical pain due to chronic constriction injury (CCI) in NOP knockout versus wild-type mice (Bertorelli et al. 2002). A similar result was found when the gene for preproN/OFQ was deleted. In these animals, there was no difference in sensitivity to acute pain. However, there was increased nociceptive response during prolonged stimulation which occurs during the second phase of the formalin test (Depner et al. 2003). These results were confirmed both in NOP(−/−) mice (Rizzi et al. 2006) and rats (Rizzi et al. 2011). Overall, these results suggest that endogenous N/OFQ contributes the control of pain during prolonged nociceptive stimulation and that NOP receptor plasticity is likely involved in the development of tolerance and dependence to opiates.
4 NOP Receptor-Active Compounds as Analgesics
NOP receptor agonists and antagonists and both peptide and small molecules have been recently reviewed (Toll et al. 2016). However, a brief discussion here is necessary for a historical prospective on the investigations into and understanding of the actions of NOP receptors on pain itself.
4.1 Antagonists
After the discovery of N/OFQ, the initial theory was that antagonists could have antinociceptive or analgesic activity. Since N/OFQ blocked opioid analgesia, an antagonist could be analgesic per se by reducing the endogenous tone of N/OFQ. The first partial agonists and antagonists, developed by Guerrini, Calo, and colleagues, were peptides that were tested for antinociceptive activity in mice after i.c.v. administration. The first “antagonist” discovered, [Phe1Ψ(CH2-NH)Gly2]N/OFQ(1–13)-NH2, turned out to be a partial agonist, which had anti-opiate actions in vivo (Guerrini et al. 1998; Bertorelli et al. 1999). However, subsequent compounds, including [Nphe1]N/OFQ (1–13)-NH2 and [Nphe1,Arg14,Lys15]N/OFQ-NH2 (UFP-101), were discovered to be very selective competitive antagonists (Calo et al. 2000, 2002). Both of these compounds had potent and direct antinociceptive activity in the warm water tail withdrawal assay and potentiated morphine analgesia when administered i.c.v. These studies demonstrated that endogenous N/OFQ in the brain must be either activating pain pathways or blocking the action of endogenous opioids, probably enkephalin, from reducing pain signals.
Interestingly, the profiles of small molecule NOP receptor antagonists appear to be different than the peptides. The first selective NOP receptor antagonist discovered, J-113397, had no agonist activity in vitro, and when given systemically in vivo, it blocked the hyperalgesic activity of N/OFQ (Ozaki et al. 2000). This is consistent with data using SB-612111, a higher affinity and more selective NOP receptor antagonist. SB-612111 has no direct antinociceptive activity but reverses N/OFQ inhibition of morphine analgesia and morphine tolerance and other behavioral actions of N/OFQ (Zaratin et al. 2004; Rizzi et al. 2007). Similar results, i.e., no effect per se but blocking of N/OFQ actions, were obtained with the potent and selective NOP antagonist comp 24 (Goto et al. 2006; Fischetti et al. 2009). However, this is inconsistent with a different NOP receptor antagonist JTC-801, which appears to have potent analgesic activity in both acute and chronic pain models that was not reversed by naloxone (Yamada et al. 2002; Mabuchi et al. 2003; Suyama et al. 2003). This compound was tested in people and ultimately dropped after Phase II clinical trials. The reason why this antagonist but not other NOP receptor antagonists has antinociceptive activity in acute pain models is not clear. JTC-801 is less selective than other antagonists tested, which might account for the different behavioral actions. The difference between peptides and small molecule antagonists is also not clear. It is conceivable that the difference has to do with the site of administration, since the peptides are uniformly administered by an i.c.v. route, while the small molecules were administered systemically.
4.2 Small Molecule Agonists
4.2.1 Selective Agonists
Naturally, the first selective agonist to be tested in pain assays was N/OFQ itself. As a 17 amino acid peptide, it required direct injection into the brain or spinal cord to reach the CNS. The first selective small molecule NOP receptor agonist, Ro 64-6198, was developed by Roche originally as a potential anxiolytic (Jenck et al. 2000). In their original publication, when given systemically, Ro 64-6198, which has subnanomolar affinity for NOP receptors and full agonist activity in the [35S]GTPγS binding assay, had potent anxiolytic activity but had neither antinociceptive activity in the tail flick assay, nor did it induce allodynia (Jenck et al. 2000). This has often been the case, with selective small molecule agonists, such as SR16835 and SCH 225288, having anti-allodynic and antitussive activity, respectively, but no direct acute antinociception in rodents (McLeod et al. 2009; Khroyan et al. 2011b). However, as with many properties of NOP receptor activation, acute antinociceptive activity can be dependent upon the pain model. There are data demonstrating that selective NOP receptor agonists can have antinociceptive activity in rodents in the formalin and inflammatory pain test (Byford et al. 2007; Rizzi et al. 2016, 2017) and Ro 64-6198 has modest antinociceptive activity in hot plate, but not tail flick assay (Reiss et al. 2008). However, it should be taken into consideration that each of these pain assays is reflexive, in response to a painful stimulus. NOP agonists are often sedative, and it is possible that some of this “antinociceptive” activity could be a function of sedation rather than analgesia.
This appears to be quite different in nonhuman primates, where selective NOP agonists have very potent antinociceptive activity that is blocked by NOP receptor antagonists, but not by naloxone (Ko et al. 2009).
4.2.2 Nonselective Agonists
Many studies from several investigators had demonstrated the N/OFQ and NOP receptor agonists could modulate opiate activity. As described above, NOP receptor agonists, when given i.c.v. or systemically, had the ability to block opioid analgesia, diminish opioid reward, and block opioid tolerance and dependence. This led to the hypothesis that a compound with appropriate efficacy at NOP and mu opioid receptors might retain antinociceptive activity but with other side effects, such as abuse liability, reduced. Zaveri, Toll, and colleagues explored this hypothesis extensively, publishing results of several nonselective compounds with differing ratios of NOP to mu affinity and efficacy, attempting to titrate these parameters to design an analgesic with reduced side effects. They proved the initial hypothesis to be correct, as a compound such as SR16435, a high-affinity partial agonist at NOP and mu receptors, had naloxone-reversible antinociception and was rewarding (induced a conditioned place preference (CPP)) but with reduced tolerance development (Khroyan et al. 2007) and SR16507, a potent full agonist at both NOP and mu receptor, had potent antinociceptive activity but was rewarding. Presumably in these compounds, the mu agonist activity was too high for the rewarding aspect to be blocked by the NOP partial or even full agonist activity. However, SR14150, a weak partial agonist at mu receptors and a potent partial agonist at NOP receptors, had naloxone-reversible antinociceptive activity but did not induce a CPP (Toll et al. 2009). This compound clearly demonstrated that with the correct parameters of affinity and efficacy in a single compound, the NOP agonist activity could reduce the reward but still leave antinociceptive actions of the mu component. Interestingly, buprenorphine itself has high affinity at opioid receptors and moderate affinity at NOP receptor (Wnendt et al. 1999; Lester and Traynor 2006). Both the antinociceptive and rewarding aspects of buprenorphine appear to be reduced by its inherent NOP agonist activity (Ciccocioppo et al. 2007; Khroyan et al. 2009; Lutfy et al. 2003). These results led to additional compounds by Husbands and colleagues in which the NOP receptor activity was increased in buprenorphine-type compounds, once again leading to compounds with antinociceptive activity but reduced reinforcing effects (Khroyan et al. 2011a; Ding et al. 2016). Recently, two additional nonselective NOP/mu agonists have proven interesting in nonhuman primate and human studies. Cebranopadol, a full mu/full NOP agonist from Grunenthal, is a very potent analgesic, which is particularly potent in chronic pain assays and is in Phase III clinical trials (Linz et al. 2014; Scholz et al. 2018). AT-121, from Zaveri, is a mixed partial agonist with potent antinociceptive activity in nonhuman primates and appears devoid of unwanted opioid side effects (Ding et al. 2018).
5 Effect of N/OFQ on Opioid Tolerance
Because N/OFQ has anti-opiate activity, it was hypothesized that the NOP receptor system might be involved in the development of tolerance to opiates. One could imagine that if the NOP system is upregulated with chronic opiate treatment, this could functionally inhibit further actions of the opiate, which would result as tolerance to the drug. This was initially tested in NOP receptor knockout mice, for which morphine tolerance was reduced (Ueda et al. 1997). Conversely, chronic morphine treatment led to an upregulation of NOP receptor mRNA in the spinal cord, and morphine tolerance was reduced by a subcutaneous or intrathecal administration of the NOP receptor antagonist J-113397 or the more selective antagonist SB-612111 suggesting that the reduction in antinociceptive activity (tolerance) after chronic morphine can, at least partially, be attributed to an upregulation of the NOP system. (Ueda et al. 2000; Zaratin et al. 2004) In fact, administration of J-113397 directly into the vlPAG alone was sufficient to block the expression of tolerance after chronic morphine administration (Scoto et al. 2010). In the knockout mice for the N/OFQ precursor protein (preproN/OFQ), there is no consensus, as one group found no development of morphine tolerance with up to 3 weeks of morphine treatment (Chung et al. 2006), while another group using a similar genotype found the development of tolerance to morphine equivalent to wild type (Kest et al. 2001). However, naloxone-induced withdrawal jumping was significantly reduced, indicating that in the absence of the peptide, morphine dependence is reduced (Kest et al. 2001). Interestingly, although NOP receptor antagonists block the expression of tolerance, N/OFQ itself, when administered into the brain daily after systemic morphine treatment, blocks the development of tolerance (Lutfy et al. 2001). The explanation for this phenomenon is not perfectly clear, but might suggest that the presence of N/OFQ concurrently with morphine blocks the ability of morphine to upregulate the NOP system, thereby attenuating the development of tolerance. Overall, perhaps it is not surprising that a receptor that opposes the actions of morphine in the brain can modulate tolerance development as well.
6 Chronic Pain
It turns out the effects of NOP receptor agonist activation appear to be considerably clearer with respect to chronic than acute pain (Schroder et al. 2014). Early studies examining the effects of N/OFQ on pain induced by inflammation or sciatic nerve injury suggested potential neuroplasticity, as the peptide was very effective in inducing anti-allodynic and anti-hyperalgesic activity in these chronic pain models (Hao et al. 1998; Bertorelli et al. 1999). In the rat chronic constriction injury (CCI) model, spinally administered N/OFQ inhibited thermal hyperalgesia as well as mechanical allodynia, while it had no effect on mechanical pain thresholds in naïve rats (Courteix et al. 2004). Similarly, the selective NOP receptor agonist Ro 64-6198 inhibited mechanical and cold allodynia after peripheral and spinal administration in CCI rats, while it had no effect on naïve animals (Obara et al. 2005). Similar results were obtained with less selective NOP/mu agonists. SR14150, a partial agonist at both receptors, has mu-mediated (naloxone-reversible) antinociceptive activity in the tail flick test but anti-allodynic activity in SNL mice that was blocked by the NOP antagonist SB612111. As with Ro 64-6198, the more selective NOP full agonist SR16835 had no activity in the tail flick in naïve mice but potent anti-allodynic activity, which was reversed by SB612111, in SNL mice (Khroyan et al. 2011b).
One explanation for changes in antinociceptive activities of NOP receptor agonists in chronic pain animals could be changes in gene expression and subsequent changes in NOP receptor or N/OFQ levels. The level of NOP receptors, NOP receptor mRNA, and N/OFQ have all been examined subsequent to both peripheral or spinal nerve injury and to inflammatory pain models. Initial studies using semiquantitative rtPCR indicated a pain-induced increase in mRNA of both NOP receptors and N/OFQ (Andoh et al. 1997; Briscini et al. 2002; Ma et al. 2005). This appears to be consistent with an increase in efficacy of NOP agonists for chronic as opposed to acute pain. More recent studies using NOP-eGFP and wild-type mice produced different results. In these animals, spinal nerve ligation (SNL) to induce chronic pain caused a dramatic decrease in NOP receptors in specific spinal cord laminae and in DRG (Figs. 3 and 4; Ozawa et al. 2018). As seen in Fig. 3, SNL greatly decreased NOP-eGFP receptors in the ipsilateral but not contralateral spinal dorsal horn laminae I and IIouter, with no apparent change in PKCγ-stained region of lamina IIinner and lamina III. As discussed above, these regions containing PKCγ-positive neurons are thought to be important for peripheral nerve injury-induced mechanical pain (Neumann et al. 2008). NOP receptor changes in the dorsal horn are consistent with a corresponding decrease in NOP receptor mRNA levels in spinal cord of wild-type SNL mice (Ozawa et al. 2018). Similar results were found in DRG, a large decrease in both NOP-eGFP receptors and NOP receptor mRNA levels in SNL mice (Fig. 4). Furthermore, the DRG neurons that were most greatly diminished were the small diameter CGRP and mu receptor-containing cells that correspond to C-nociceptors with axon terminals in laminae I and IIouter.
NOP-eGFP receptor distribution in the spinal cord in sham and SNL mice. Ip ipsilateral, C contralateral. Scale bar 100 μm. Reprinted with permission from Ozawa et al. (2018)
NOP-eGFP expression in DRG under a chronic pain condition. (a) Representative images of L4 DRG neurons derived from sham-operated and SNL mice. (b) NOP-eGFP expression level in DRG neurons. (c) Population of NOP-expressing DRG neurons in sham and SNL mice. Scale bar 100 μm. Reprinted with permission from Ozawa et al. (2018)
Based upon these changes in NOP receptors, it was hypothesized that N/OFQ, when administered i.t., would attenuate mechanical allodynia induced by SNL, measured by using von Frey filaments poked into the injured paw, but be less effective on heat pain in the hotplate test, since many of these NOP receptor-containing cells are missing in DRG and spinal cord of SNL mice. This would be similar to analgesic actions demonstrated for delta opioid receptors in mice treated with complete Freund’s adjuvant (CFA) (Scherrer et al. 2009). Surprisingly, N/OFQ was actually more effective in blocking heat pain than it was attenuating cold pain or mechanical allodynia. This suggests two possibilities. One possibility is that there are C-nociceptors that modulate heat and cold pain that contain NOP receptors but do not co-express CGRP, which are not affected by SNL, allowing N/OFQ to maintain effectiveness; or more likely, NOP receptors can be found on spinal projection neurons that transmit the heat pain signal to the brain. This along with our hypothesis as to how NOP receptors reduce pain signals in the spinal cord of naïve and SNL mice is shown in Fig. 5. In this way, intrathecally administered N/OFQ would still block hotplate-induced heat pain despite the fact that NOP receptors are missing on a significant portion of potential nociceptors. In naïve animals, NOP receptor agonists presynaptically inhibit C-fiber-evoked responses in the spinal dorsal horn in order to mediate an antinociceptive response (Wang et al. 1996; Lai et al. 1997; Liebel et al. 1997; Carpenter et al. 2000). In SNL mice, spinal NOP receptor activation apparently directly inhibits heat- and cold-specific projection neurons in the dorsal horn to induce an analgesic response. Electrophysiological experiments should directly address this issue.
7 Conclusions
The involvement of NOP receptors and N/OFQ in pain transmission has been definitively proven, with a mixed NOP/mu agonist now in clinical trials. Furthermore, selective NOP agonists seem poised for development as analgesics as well. Nevertheless, there are many significant issues and hurdles remaining. NOP agonists have demonstrated the potential for considerable side effects. NOP receptor agonists are often very sedative (Higgins et al. 2001; Byford et al. 2007), although this seems to be diminished in cebranopadol and AT-121, for reasons that are not clear (Linz et al. 2014; Ding et al. 2018). One possibility for reduced sedation is ligand bias, as cebranopadol seems to internalize NOP receptors to a lesser extent than other agonists and is less efficacious at β-arrestin activation than G protein coupling (Rizzi et al. 2016). NOP agonists also block long-term potentiation, as well as spatial memory (Sandin et al. 1997; Bongsebandhu-Phubhakdi and Manabe 2007; Kuzmin et al. 2009). This will have to be examined carefully in people. It may be of great value that NOP agonists are more effective in blocking chronic than acute pain since that is certainly a bigger problem, particularly since long-term administration of opiates is greatly discouraged. The mechanism by which NOP receptor agonists have increased efficacy in chronic pain is still unknown and upon further investigation could not only uncover actions of the NOP system but could lead to a better understanding of the transition to chronic pain.
References
Abraira VE, Ginty DD (2013) The sensory neurons of touch. Neuron 79:618–639
Anand P, Yiangou Y, Anand U, Mukerji G, Sinisi M, Fox M, McQuillan A, Quick T, Korchev YE, Hein P (2016) Nociceptin/orphanin FQ receptor expression in clinical pain disorders and functional effects in cultured neurons. Pain 157:1960–1969
Andoh T, Itoh M, Kuraishi Y (1997) Nociceptin gene expression in rat dorsal root ganglia induced by peripheral inflammation. Neuroreport 8:2793–2796
Anton B, Fein J, To T, Li X, Silberstein L, Evans CJ (1996) Immunohistochemical localization of ORL-1 in the central nervous system of the rat. J Comp Neurol 368:229–251
Bardoni R, Tawfik VL, Wang D, Francois A, Solorzano C, Shuster SA, Choudhury P, Betelli C, Cassidy C, Smith K, de Nooij JC, Mennicken F, O'Donnell D, Kieffer BL, Woodbury CJ, Basbaum AI, MacDermott AB, Scherrer G (2014) Delta opioid receptors presynaptically regulate cutaneous mechanosensory neuron input to the spinal cord dorsal horn. Neuron 81:1312–1327
Bartsch T, Akerman S, Goadsby PJ (2002) The ORL-1 (NOP1) receptor ligand nociceptin/orphanin FQ (N/OFQ) inhibits neurogenic dural vasodilatation in the rat. Neuropharmacology 43:991–998
Bertorelli R, Corradini L, Rafiq K, Tupper J, Calo G, Ongini E (1999) Nociceptin and the ORL-1 ligand [Phe1psi (CH2-NH)Gly2]nociceptin(1-13)NH2 exert anti-opioid effects in the Freund’s adjuvant-induced arthritic rat model of chronic pain. Br J Pharmacol 128:1252–1258
Bertorelli R, Bastia E, Citterio F, Corradini L, Forlani A, Ongini E (2002) Lack of the nociceptin receptor does not affect acute or chronic nociception in mice. Peptides 23:1589–1596
Bongsebandhu-phubhakdi S, Manabe T (2007) The neuropeptide nociceptin is a synaptically released endogenous inhibitor of hippocampal long-term potentiation. J Neurosci 27:4850–4858
Briscini L, Corradini L, Ongini E, Bertorelli R (2002) Up-regulation of ORL-1 receptors in spinal tissue of allodynic rats after sciatic nerve injury. Eur J Pharmacol 447:59–65
Bunzow JR, Saez C, Mortrud M, Bouvier C, Williams JT, Low M, Grandy DK (1994) Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a mu, delta or kappa opioid receptor type. FEBS Lett 347:284–288
Byford AJ, Anderson A, Jones PS, Palin R, Houghton AK (2007) The hypnotic, electroencephalographic, and antinociceptive properties of nonpeptide ORL1 receptor agonists after intravenous injection in rodents. Anesth Analg 104:174–179
Calo G, Guerrini R, Bigoni R, Rizzi A, Marzola G, Okawa H, Bianchi C, Lambert DG, Salvadori S, Regoli D (2000) Characterization of [Nphe(1)]nociceptin(1-13)NH(2), a new selective nociceptin receptor antagonist. Br J Pharmacol 129:1183–1193
Calo G, Rizzi A, Rizzi D, Bigoni R, Guerrini R, Marzola G, Marti M, McDonald J, Morari M, Lambert DG, Salvadori S, Regoli D (2002) [Nphe1,Arg14,Lys15]nociceptin-NH2, a novel potent and selective antagonist of the nociceptin/orphanin FQ receptor. Br J Pharmacol 136:303–311
Capuano A, Curro D, Dello Russo C, Tringali G, Pozzoli G, Di Trapani G, Navarra P (2007) Nociceptin (1-13)NH2 inhibits stimulated calcitonin-gene-related-peptide release from primary cultures of rat trigeminal ganglia neurones. Cephalalgia 27:868–876
Carpenter KJ, Vithlani M, Dickenson AH (2000) Unaltered peripheral excitatory actions of nociceptin contrast with enhanced spinal inhibitory effects after carrageenan inflammation: an electrophysiological study in the rat. Pain 85:433–441
Chen Y, Sommer C (2006) Nociceptin and its receptor in rat dorsal root ganglion neurons in neuropathic and inflammatory pain models: implications on pain processing. J Peripher Nerv Syst 11:232–240
Chung S, Pohl S, Zeng J, Civelli O, Reinscheid RK (2006) Endogenous orphanin FQ/nociceptin is involved in the development of morphine tolerance. J Pharmacol Exp Ther 318:262–267
Ciccocioppo R, Economidou D, Rimondini R, Sommer W, Massi M, Heilig M (2007) Buprenorphine reduces alcohol drinking through activation of the nociceptin/orphanin FQ-NOP receptor system. Biol Psychiatry 61:4–12
Clarke S, Chen Z, Hsu MS, Pintar J, Hill R, Kitchen I (2001) Quantitative autoradiographic mapping of the ORL1, mu-, delta- and kappa-receptors in the brains of knockout mice lacking the ORL1 receptor gene. Brain Res 906:13–24
Connor M, Vaughan CW, Chieng B, Christie MJ (1996) Nociceptin receptor coupling to a potassium conductance in rat locus coeruleus neurones in vitro. Br J Pharmacol 119:1614–1618
Corrigendum (1999) Corrigendum. J Comp Neurol 412:708
Courteix C, Coudore-Civiale MA, Privat AM, Pelissier T, Eschalier A, Fialip J (2004) Evidence for an exclusive antinociceptive effect of nociceptin/orphanin FQ, an endogenous ligand for the ORL1 receptor, in two animal models of neuropathic pain. Pain 110:236–245
Cox BM, Christie MJ, Devi L, Toll L, Traynor JR (2015) Challenges for opioid receptor nomenclature: IUPHAR review 9. Br J Pharmacol 172:317–323
Depner UB, Reinscheid RK, Takeshima H, Brune K, Zeilhofer HU (2003) Normal sensitivity to acute pain, but increased inflammatory hyperalgesia in mice lacking the nociceptin precursor polypeptide or the nociceptin receptor. Eur J Neurosci 17:2381–2387
Ding H, Czoty PW, Kiguchi N, Cami-Kobeci G, Sukhtankar DD, Nader MA, Husbands SM, Ko MC (2016) A novel orvinol analog, BU08028, as a safe opioid analgesic without abuse liability in primates. Proc Natl Acad Sci U S A 113:E5511–E5518
Ding H, Kiguchi N, Yasuda D, Daga PR, Polgar WE, Lu JJ, Czoty PW, Kishioka S, Zaveri NT, Ko MC (2018) A bifunctional nociceptin and mu opioid receptor agonist is analgesic without opioid side effects in nonhuman primates. Sci Transl Med 10:eaar3483
Edvinsson L (2003) New therapeutic target in primary headaches – blocking the CGRP receptor. Expert Opin Ther Targets 7:377–383
Ertsey C, Hantos M, Bozsik G, Tekes K (2005) Plasma nociceptin levels are reduced in migraine without aura. Cephalalgia 25:261–266
Fischetti C, Camarda V, Rizzi A, Pela M, Trapella C, Guerrini R, McDonald J, Lambert DG, Salvadori S, Regoli D, Calo G (2009) Pharmacological characterization of the nociceptin/orphanin FQ receptor non peptide antagonist compound 24. Eur J Pharmacol 614:50–57
Florin S, Meunier J, Costentin J (2000) Autoradiographic localization of [3H]nociceptin binding sites in the rat brain. Brain Res 880:11–16
Fukuda K, Kato S, Mori K, Nishi M, Takeshima H, Iwabe N, Miyata T, Houtani T, Sugimoto T (1994) cDNA cloning and regional distribution of a novel member of the opioid receptor family. FEBS Lett 343:42–46
Gebhart GF, Schmidt RF (2013) Primary afferents/neurons. In: Encyclopedia of pain. Springer, Berlin
Goto Y, Arai-Otsuki S, Tachibana Y, Ichikawa D, Ozaki S, Takahashi H, Iwasawa Y, Okamoto O, Okuda S, Ohta H, Sagara T (2006) Identification of a novel spiropiperidine opioid receptor-like 1 antagonist class by a focused library approach featuring 3D-pharmacophore similarity. J Med Chem 49:847–849
Guerrini R, Calo G, Rizzi A, Bigoni R, Bianchi C, Salvadori S, Regoli D (1998) A new selective antagonist of the nociceptin receptor. Br J Pharmacol 123:163–165
Hao JX, Xu IS, Wiesenfeld-Hallin Z, Xu XJ (1998) Anti-hyperalgesic and anti-allodynic effects of intrathecal nociceptin/orphanin FQ in rats after spinal cord injury, peripheral nerve injury and inflammation. Pain 76:385–393
Heinricher MM, McGaraughty S, Grandy DK (1997) Circuitry underlying antiopioid actions of orphanin FQ in the rostral ventromedial medulla. J Neurophysiol 78:3351–3358
Higgins GA, Grottick AJ, Ballard TM, Richards JG, Messer J, Takeshima H, Pauly-Evers M, Jenck F, Adam G, Wichmann J (2001) Influence of the selective ORL1 receptor agonist, Ro64-6198, on rodent neurological function. Neuropharmacology 41:97–107
Inoue M, Kobayashi M, Kozaki S, Zimmer A, Ueda H (1998) Nociceptin/orphanin FQ-induced nociceptive responses through substance P release from peripheral nerve endings in mice. Proc Natl Acad Sci U S A 95:10949–10953
Inoue M, Shimohira I, Yoshida A, Zimmer A, Takeshima H, Sakurada T, Ueda H (1999) Dose-related opposite modulation by nociceptin/orphanin FQ of substance P nociception in the nociceptors and spinal cord. J Pharmacol Exp Ther 291:308–313
Jenck F, Wichmann J, Dautzenberg FM, Moreau JL, Ouagazzal AM, Martin JR, Lundstrom K, Cesura AM, Poli SM, Roever S, Kolczewski S, Adam G, Kilpatrick G (2000) A synthetic agonist at the orphanin FQ/nociceptin receptor ORL1: anxiolytic profile in the rat. Proc Natl Acad Sci U S A 97:4938–4943
Kest B, Hopkins E, Palmese CA, Chen ZP, Mogil JS, Pintar JE (2001) Morphine tolerance and dependence in nociceptin/orphanin FQ transgenic knock-out mice. Neuroscience 104:217–222
Khroyan TV, Zaveri NT, Polgar WE, Orduna J, Olsen C, Jiang F, Toll L (2007) SR 16435 [1-(1-(bicyclo[3.3.1]nonan-9-yl)piperidin-4-yl)indolin-2-one], a novel mixed nociceptin/orphanin FQ/mu-opioid receptor partial agonist: analgesic and rewarding properties in mice. J Pharmacol Exp Ther 320:934–943
Khroyan TV, Polgar WE, Jiang F, Zaveri NT, Toll L (2009) Nociceptin/orphanin FQ receptor activation attenuates antinociception induced by mixed nociceptin/orphanin FQ/mu-opioid receptor agonists. J Pharmacol Exp Ther 331:946–953
Khroyan TV, Polgar WE, Cami-Kobeci G, Husbands SM, Zaveri NT, Toll L (2011a) The first universal opioid ligand, (2S)-2-[(5R,6R,7R,14S)-N-cyclopropylmethyl-4,5-epoxy-6,14-ethano-3-hydroxy-6-methoxymorphinan-7-yl]-3,3-dimethylpentan-2-ol (BU08028): characterization of the in vitro profile and in vivo behavioral effects in mouse models of acute pain and cocaine-induced reward. J Pharmacol Exp Ther 336:952–961
Khroyan TV, Polgar WE, Orduna J, Montenegro J, Jiang F, Zaveri NT, Toll L (2011b) Differential effects of nociceptin/orphanin FQ (NOP) receptor agonists in acute versus chronic pain: studies with bifunctional NOP/mu receptor agonists in the sciatic nerve ligation chronic pain model in mice. J Pharmacol Exp Ther 339:687–693
Ko MC, Woods JH, Fantegrossi WE, Galuska CM, Wichmann J, Prinssen EP (2009) Behavioral effects of a synthetic agonist selective for nociceptin/orphanin FQ peptide receptors in monkeys. Neuropsychopharmacology 34:2088–2096
Kuzmin A, Madjid N, Johansson B, Terenius L, Ogren SO (2009) The nociceptin system and hippocampal cognition in mice: a pharmacological and genetic analysis. Brain Res 1305(Suppl):S7–S19
Lachowicz JE, Shen Y, Monsma FJ Jr, Sibley DR (1995) Molecular cloning of a novel G protein-coupled receptor related to the opiate receptor family. J Neurochem 64:34–40
Lai CC, Wu SY, Dun SL, Dun NJ (1997) Nociceptin-like immunoreactivity in the rat dorsal horn and inhibition of substantia gelatinosa neurons. Neuroscience 81:887–891
Lester PA, Traynor JR (2006) Comparison of the in vitro efficacy of mu, delta, kappa and ORL1 receptor agonists and non-selective opioid agonists in dog brain membranes. Brain Res 1073-1074:290–296
Liebel JT, Swandulla D, Zeilhofer HU (1997) Modulation of excitatory synaptic transmission by nociceptin in superficial dorsal horn neurones of the neonatal rat spinal cord. Br J Pharmacol 121:425–432
Linz K, Christoph T, Tzschentke TM, Koch T, Schiene K, Gautrois M, Schroder W, Kogel BY, Beier H, Englberger W, Schunk S, De Vry J, Jahnel U, Frosch S (2014) Cebranopadol: a novel potent analgesic nociceptin/orphanin FQ peptide and opioid receptor agonist. J Pharmacol Exp Ther 349:535–548
Luo W, Enomoto H, Rice FL, Milbrandt J, Ginty DD (2009) Molecular identification of rapidly adapting mechanoreceptors and their developmental dependence on ret signaling. Neuron 64:841–856
Lutfy K, Hossain SM, Khaliq I, Maidment NT (2001) Orphanin FQ/nociceptin attenuates the development of morphine tolerance in rats. Br J Pharmacol 134:529–534
Lutfy K, Eitan S, Bryant CD, Yang YC, Saliminejad N, Walwyn W, Kieffer BL, Takeshima H, Carroll FI, Maidment NT, Evans CJ (2003) Buprenorphine-induced antinociception is mediated by mu-opioid receptors and compromised by concomitant activation of opioid receptor-like receptors. J Neurosci 23:10331–10337
Ma F, Xie H, Dong ZQ, Wang YQ, Wu GC (2005) Expression of ORL1 mRNA in some brain nuclei in neuropathic pain rats. Brain Res 1043:214–217
Mabuchi T, Matsumura S, Okuda-Ashitaka E, Kitano T, Kojima H, Nagano T, Minami T, Ito S (2003) Attenuation of neuropathic pain by the nociceptin/orphanin FQ antagonist JTC-801 is mediated by inhibition of nitric oxide production. Eur J Neurosci 17:1384–1392
Malmberg AB, Chen C, Tonegawa S, Basbaum AI (1997) Preserved acute pain and reduced neuropathic pain in mice lacking PKCgamma. Science 278:279–283
Mamiya T, Noda Y, Ren X, Nagai T, Takeshima H, Ukai M, Nabeshima T (2001) Morphine tolerance and dependence in the nociceptin receptor knockout mice. J Neural Transm 108:1349–1361
Mathis JP, Goldberg IE, Rossi GC, Leventhal L, Pasternak GW (1998) Antinociceptive analogs of orphanin FQ/nociceptin(1-11). Life Sci 63:PL 161–PL 166
McLeod RL, Tulshian DB, Ho GD, Fernandez X, Bolser DC, Parra LE, Zimmer JC, Erickson CH, Fawzi AB, Jayappa H, Lehr C, Erskine J, Smith-Torhan A, Zhang H, Hey JA (2009) Effect of a novel NOP receptor agonist (SCH 225288) on Guinea pig irritant-evoked, feline mechanically induced and canine infectious tracheobronchitis cough. Pharmacology 84:153–161
Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B (1995) Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 377:532–535
Mogil JS, Grisel JE, Reinscheid RK, Civelli O, Belknap JK, Grandy DK (1996a) Orphanin FQ is a functional anti-opioid peptide. Neuroscience 75:333–337
Mogil JS, Grisel JE, Zhangs G, Belknap JK, Grandy DK (1996b) Functional antagonism of mu-, delta- and kappa-opioid antinociception by orphanin FQ. Neurosci Lett 214:131–134
Mogil JS, Nessim LA, Wilson SG (1999) Strain-dependent effects of supraspinal orphanin FQ/nociceptin on thermal nociceptive sensitivity in mice. Neurosci Lett 261:147–150
Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC (1994) ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett 341:33–38
Mollereau C, Simons M-J, Soularue P, Liners F, Vassart G, Meunier J-C, Parmentier M (1996) Structure, tissue distribution, and chromosomal localization of the prepronociceptin gene. Proc Natl Acad Sci U S A 93:8666–8670
Morgan MM, Grisel JE, Robbins CS, Grandy DK (1997) Antinociception mediated by the periaqueductal gray is attenuated by orphanin FQ. Neuroreport 8:3431–3434
Murali SS, Napier IA, Rycroft BK, Christie MJ (2012) Opioid-related (ORL1) receptors are enriched in a subpopulation of sensory neurons and prolonged activation produces no functional loss of surface N-type calcium channels. J Physiol 590:1655–1667
Neal CR Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Akil H, Watson SJ Jr (1999a) Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with (125)I-[(14)Tyr]-orphanin FQ binding. J Comp Neurol 412:563–605
Neal CR Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ Jr (1999b) Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol 406:503–547
Neumann S, Braz JM, Skinner K, Llewellyn-Smith IJ, Basbaum AI (2008) Innocuous, not noxious, input activates PKCgamma interneurons of the spinal dorsal horn via myelinated afferent fibers. J Neurosci 28:7936–7944
Nishi M, Houtani T, Noda Y, Mamiya T, Sato K, Doi T, Kuno J, Takeshima H, Nukada T, Nabeshima T, Yamashita T, Noda T, Sugimoto T (1997) Unrestrained nociceptive response and disregulation of hearing ability in mice lacking the nociceptin/orphaninFQ receptor. EMBO J 16:1858–1864
Obara I, Przewlocki R, Przewlocka B (2005) Spinal and local peripheral antiallodynic activity of Ro64-6198 in neuropathic pain in the rat. Pain 116:17–25
Ozaki S, Kawamoto H, Itoh Y, Miyaji M, Azuma T, Ichikawa D, Nambu H, Iguchi T, Iwasawa Y, Ohta H (2000) In vitro and in vivo pharmacological characterization of J-113397, a potent and selective non-peptidyl ORL1 receptor antagonist. Eur J Pharmacol 402:45–53
Ozawa A, Brunori G, Mercatelli D, Wu J, Cippitelli A, Zou B, Xie XS, Williams M, Zaveri NT, Low S, Scherrer G, Kieffer BL, Toll L (2015) Knock-in mice with NOP-eGFP receptors identify receptor cellular and regional localization. J Neurosci 35:11682–11693
Ozawa A, Brunori G, Cippitelli A, Toll N, Schoch J, Kieffer BL, Toll L (2018) Dissecting the spinal NOP receptor distribution under a chronic pain model using NOP-eGFP knock-in mice. Br J Pharmacol 175:2662
Pan ZZ, Fields HL (1996) Endogenous opioid-mediated inhibition of putative pain-modulating neurons in rat rostral ventromedial medulla. Neuroscience 74:855–862
Pan YX, Cheng J, Xu J, Rossi G, Jacobson E, Ryan-Moro J, Brooks AI, Dean GE, Standifer KM, Pasternak GW (1995) Cloning and functional characterization through antisense mapping of a k3-related opioid receptor. Mol Pharmacol 47:1180–1188
Pan ZZ, Tershner SA, Fields HL (1997) Cellular mechanism for anti-analgesic action of agonists of the kappa-opioid receptor. Nature 389:382–385
Pan Z, Hirakawa N, Fields HL (2000) A cellular mechanism for the bidirectional pain-modulating actions of orphanin FQ/nociceptin. Neuron 26:515–522
Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ Jr, Civelli O (1995) Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science 270:792–794
Reiss D, Wichmann J, Tekeshima H, Kieffer BL, Ouagazzal AM (2008) Effects of nociceptin/orphanin FQ receptor (NOP) agonist, Ro64-6198, on reactivity to acute pain in mice: comparison to morphine. Eur J Pharmacol 579:141–148
Rizzi A, Marzola G, Bigoni R, Guerrini R, Salvadori S, Mogil JS, Regoli D, Calo G (2001) Endogenous nociceptin signaling and stress-induced analgesia. Neuroreport 12:3009–3013
Rizzi A, Nazzaro C, Marzola GG, Zucchini S, Trapella C, Guerrini R, Zeilhofer HU, Regoli D, Calo G (2006) Endogenous nociceptin/orphanin FQ signalling produces opposite spinal antinociceptive and supraspinal pronociceptive effects in the mouse formalin test: pharmacological and genetic evidences. Pain 124:100–108
Rizzi A, Gavioli EC, Marzola G, Spagnolo B, Zucchini S, Ciccocioppo R, Trapella C, Regoli D, Calo G (2007) Pharmacological characterization of the nociceptin/orphanin FQ receptor antagonist SB-612111 [(-)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrah ydro-5H-benzocyclohepten-5-ol]: in vivo studies. J Pharmacol Exp Ther 321:968–974
Rizzi A, Molinari S, Marti M, Marzola G, Calo G (2011) Nociceptin/orphanin FQ receptor knockout rats: in vitro and in vivo studies. Neuropharmacology 60:572–579
Rizzi A, Cerlesi MC, Ruzza C, Malfacini D, Ferrari F, Bianco S, Costa T, Guerrini R, Trapella C, Calo G (2016) Pharmacological characterization of cebranopadol a novel analgesic acting as mixed nociceptin/orphanin FQ and opioid receptor agonist. Pharmacol Res Perspect 4:e00247
Rizzi A, Ruzza C, Bianco S, Trapella C, Calo G (2017) Antinociceptive action of NOP and opioid receptor agonists in the mouse orofacial formalin test. Peptides 94:71–77
Rossi GC, Leventhal L, Bolan E, Pasternak GW (1997) Pharmacological characterization of orphanin FQ/nociceptin and its fragments. J Pharmacol Exp Ther 282:858–865
Sakurada T, Katsuyama S, Sakurada S, Inoue M, Tan-No K, Kisara K, Sakurada C, Ueda H, Sasaki J (1999) Nociceptin-induced scratching, biting and licking in mice: involvement of spinal NK1 receptors. Br J Pharmacol 127:1712–1718
Sandin J, Georgieva J, Schott PA, Ogren SO, Terenius L (1997) Nociceptin/orphanin FQ microinjected into hippocampus impairs spatial learning in rats. Eur J Neurosci 9:194–197
Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Laustriat D, Cao YQ, Basbaum AI, Dierich A, Vonesh JL, Gaveriaux-Ruff C, Kieffer BL (2006) Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc Natl Acad Sci U S A 103:9691–9696
Scherrer G, Imamachi N, Cao Y-Q, Contet C, Mennicken F, O’Donnell D, Kieffer BL, Basbaum AI (2009) Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell 137:1148–1159
Scholz A, Bothmer J, Kok M, Hoschen K, Daniels S (2018) Cebranopadol: a novel, first-in-class, strong analgesic: results from a randomized phase IIa clinical trial in postoperative acute pain. Pain Physician 21:E193–E206
Schroder W, Lambert DG, Ko MC, Koch T (2014) Functional plasticity of the N/OFQ-NOP receptor system determines analgesic properties of NOP receptor agonists. Br J Pharmacol 171:3777–3800
Scoto GM, Arico G, Iemolo A, Ronsisvalle G, Parenti C (2010) Selective inhibition of the NOP receptor in the ventrolateral periaqueductal gray attenuates the development and the expression of tolerance to morphine-induced antinociception in rats. Peptides 31:696–700
Suyama H, Kawamoto M, Gaus S, Yuge O (2003) Effect of JTC-801 (nociceptin antagonist) on neuropathic pain in a rat model. Neurosci Lett 351:133–136
Tian JH, Xu W, Fang Y, Mogil JS, Grisel JE, Grandy DK, Han JS (1997) Bidirectional modulatory effect of orphanin FQ on morphine-induced analgesia: antagonism in brain and potentiation in spinal cord of the rat. Br J Pharmacol 120:676–680
Toll L, Khroyan TV, Polgar WE, Jiang F, Olsen C, Zaveri NT (2009) Comparison of the antinociceptive and antirewarding profiles of novel bifunctional nociceptin receptor/mu-opioid receptor ligands: implications for therapeutic applications. J Pharmacol Exp Ther 331:954–964
Toll L, Bruchas MR, Calo G, Cox BM, Zaveri NT (2016) Nociceptin/orphanin FQ receptor structure, signaling, ligands, functions, and interactions with opioid systems. Pharmacol Rev 68:419–457
Ueda H, Yamaguchi T, Tokuyama S, Inoue M, Nishi M, Takeshima H (1997) Partial loss of tolerance liability to morphine analgesia in mice lacking the nociceptin receptor gene. Neurosci Lett 237:136–138
Ueda H, Inoue M, Takeshima H, Iwasawa Y (2000) Enhanced spinal nociceptin receptor expression develops morphine tolerance and dependence. J Neurosci 20:7640–7647
Vanderah TW, Raffa RB, Lashbrook J, Burritt A, Hruby V, Porreca F (1998) Orphanin-FQ/nociceptin: lack of anti nociceptive, hyperalgesic or allodynic effects in acute thermal or mechanical tests following intracerebroventricular or intrathecal administration to mice or rats. Eur J Pain 2:267–278
Vaughan CW, Ingram SL, Christie MJ (1997) Actions of the ORL1 receptor ligand nociceptin on membrane properties of rat periaqueductal gray neurons in vitro. J Neurosci 17:996–1003
Wang JB, Johnson PS, Imai Y, Persico AM, Ozenberger BA, Eppler CM, Uhl GR (1994) cDNA cloning of an orphan opiate receptor gene family member and its splice variant. FEBS Lett 348:75–79
Wang XM, Zhang KM, Mokha SS (1996) Nociceptin (orphanin FQ), an endogenous ligand for the QRL1 (opioid-receptor-like1) receptor; modulates responses of trigeminal neurons evoked by excitatory amino acids and somatosensory stimuli. J Neurophysiol 76:3568–3572
Wang HB, Zhao B, Zhong YQ, Li KC, Li ZY, Wang Q, Lu YJ, Zhang ZN, He SQ, Zheng HC, Wu SX, Hokfelt TG, Bao L, Zhang X (2010) Coexpression of delta- and mu-opioid receptors in nociceptive sensory neurons. Proc Natl Acad Sci U S A 107:13117–13122
Wnendt S, Kruger T, Janocha E, Hildebrandt D, Englberger W (1999) Agonistic effect of buprenorphine in a nociceptin/OFQ receptor-triggered reporter gene assay. Mol Pharmacol 56:334–338
Xie X, Wisor JP, Hara J, Crowder TL, LeWinter R, Khroyan TV, Yamanaka A, Diano S, Horvath TL, Sakurai T, Toll L, Kilduff TS (2008) Hypocretin/orexin and nociceptin/orphanin FQ coordinately regulate analgesia in a mouse model of stress-induced analgesia. J Clin Invest 118:2471–2481
Yamada H, Nakamoto H, Suzuki Y, Ito T, Aisaka K (2002) Pharmacological profiles of a novel opioid receptor-like1 (ORL(1)) receptor antagonist, JTC-801. Br J Pharmacol 135:323–332
Zaratin PF, Petrone G, Sbacchi M, Garnier M, Fossati C, Petrillo P, Ronzoni S, Giardina GA Scheideler MA (2004) Modification of nociception and morphine tolerance by the selective opiate receptor-like orphan receptor antagonist (-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol (SB-612111). J Pharmacol Exp Ther 308:454–461
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Toll, L., Ozawa, A., Cippitelli, A. (2019). NOP-Related Mechanisms in Pain and Analgesia. In: Ko, MC., Caló, G. (eds) The Nociceptin/Orphanin FQ Peptide Receptor. Handbook of Experimental Pharmacology, vol 254. Springer, Cham. https://doi.org/10.1007/164_2019_214
Download citation
DOI: https://doi.org/10.1007/164_2019_214
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-20185-2
Online ISBN: 978-3-030-20186-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)