Abstract
As one of the candidates of the therapeutic strategy for asthma in addition to inhaled corticosteroids (ICS), leukotriene receptor antagonists (LTRAs) are known to be useful for long-term management of asthma patients complicated by allergic rhinitis (AR) or exercise-induced asthma (EIA). Currently available LTRAs are pranlukast hydrate, zafirlukast, and montelukast. These LTRAs have a bronchodilator action and inhibit airway inflammation, resulting in a significant improvement of asthma symptoms, respiratory function, inhalation frequency of as-needed inhaled β2-agonist, airway inflammation, airway hyperresponsiveness, dosage of ICSs, asthma exacerbations, and patients’ QOL. Although cys-LTs are deeply associated with the pathogenesis of asthma, LTRAs alone are less effective compared with ICS. However, the effects of LTRAs in combination with ICS are the same as those of LABAs in combination with ICS in steroid-naïve asthmatic patients. Concerning antiallergy drugs other than LTRAs, some mediator-release suppressants, H1 histamine receptor antagonists (H1RAs), thromboxane A2 (TXA2) inhibitors/antagonists, and Th2 cytokine inhibitor had been used mainly in Japan until the late 1990s. However, the use of these agents rapidly decreased after ICS/long acting beta agonist (LABA) combination was introduced and recommended for the management of asthma in the early 2000s. The effectiveness of other antiallergic agents on asthma management seems to be quite limited, and the safety of oral antiallergic agents has not been demonstrated in fetuses during pregnancy. Further effectiveness studies are needed to determine the true value of these orally administered agents in combination with ICS as an anti-asthma treatment.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Leukotriene Receptor Antagonists (LTRAs)
1.1 Pharmacology of Leukotrienes and Its Receptors
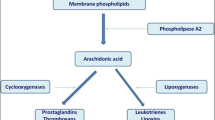
Cysteinyl leukotrienes (cys-LTs) are produced in eosinophils, basophils, mast cells, macrophages, and myeloid dendritic cells, involving several steps including the oxidation of arachidonic acid by 5-lipoxygenase (5-LO) (Laidlaw and Boyce 2012; Kanaoka and Boyce 2004, 2014; Clark et al. 1990). After the unstable epoxide LTA4 is synthesized, LTC4 synthase (LTC4S) conjugates LTA4 to reduced glutathione, forming LTC4, the parent of the cys-LTs (Reid et al. 1990; Lam et al. 1994). Once formed, LTC4 is transported to the extracellular space via the ATP-binding cassette (ABC) transporters 1 and 4 and then metabolized to LTD4 and LTE4 by γ-glutamyl transpeptidases and dipeptidases, respectively. The rapid extracellular metabolism of LTC4 and LTD4 results in short biologic half-lives relative to the stable mediator LTE4, which is abundant and readily detected in biologic fluids. Thus, three different ligands (LTC4, LTD4, and LTE4) arise from a single intracellular synthetic event by successive enzymatic conversions (Laidlaw and Boyce 2012; Kanaoka and Boyce 2014). In addition to this intracellular pathway, there is also a transcellular mechanism for cys-LTs generation that can be carried out by cells that express LTC4S but not the proximal enzyme 5-LO in the pathway (e.g., platelets, endothelial cells). In the latter mechanism, the LTC4S-expressing cells can convert extracellular LTA4 (released by neutrophils or other cells with an active 5-LO enzyme) (Maclouf et al. 1994) and may serve as an additional source of cys-LTs in certain inflammatory states (Laidlaw and Boyce 2012).
The bioactivities of the cys-LTs in the preclinical setting, particularly their potency as smooth muscle constrictors, spurred interest in these mediators as potential therapeutic targets in asthma (Laidlaw and Boyce 2012). The ability to monitor urinary levels of LTE4 as a reflection of systemic cys-LT generation in vivo provided the proof that cys-LTs are generated by subjects with acute asthma exacerbations (Drazen et al. 1992). Individuals with aspirin-induced asthma (AIA) have especially high baseline levels of urinary LTE4 and a marked further increment in these levels in response to oral challenge with aspirin (Christie et al. 1991). The role of cys-LTs in asthma has been well validated by clinical trials using the available drugs. The 5-LO inhibitor and selective antagonists of CysLT1 receptor both improve lung function, reduce the frequency of asthma exacerbations (Laidlaw and Boyce 2012; Liu et al. 1996; Israel et al. 1996), and reduce the severity of reactions to aspirin challenge in individuals with AIA (Berges-Gimeno et al. 2002).
While the three cys-LTs (C4, D4, E4) share certain functions in vivo, including smooth muscle contraction and vascular leak (Weiss et al. 1982a; Griffin et al. 1983), important differences were identified in early studies that suggested additional and distinct functions for each. Pharmacological profiling of guinea pig lung demonstrated that LTC4 and LTD4 were equipotent as constrictors, whereas LTE4 was inactive (Laidlaw and Boyce 2012). Remarkably, however, LTE4 was ten times more potent for inducing guinea pig tracheal ring contractions in vitro than LTC4 or LTD4 (Lee et al. 1984; Drazen et al. 1982). Together, these in vitro and in vivo functional findings predicted the existence of at least three receptors for cys-LTs: a high-affinity receptor for LTD4, a lower-affinity receptor for LTC4, and a separate receptor for LTE4, with the latter potentially capable of eliciting the secondary production of a prostanoid (Laidlaw and Boyce 2012).
Studies on human subjects also provided compelling evidence for the existence of at least three receptors for cys-LTs (Weiss et al. 1982a, b, 1983). Unlike non-asthmatic subjects, asthmatic subjects were much more sensitive to LTE4 in terms of bronchoconstriction (Davidson et al. 1987). However, asthmatic and non-asthmatic subjects had equivalent dose responses to LTC4 and LTD4 (Griffin et al. 1983). Moreover, subjects with AIA demonstrated even greater sensitivity to LTE4-induced bronchoconstriction than did aspirin-tolerant asthmatic controls (Christie et al. 1993). In another study, inhalation of LTE4, but not of LTD4, provoked the accumulation of eosinophils and basophils into the bronchial mucosa and sputum of asthmatic subjects when the two cys-LTs were administered at doses titrated to produce an equivalent degree of bronchoconstriction (Gauvreau et al. 2001). Inhalation of LTE4 also enhanced the sensitivity of asthmatic subjects to histamine-induced bronchoconstriction, an effect that was blocked by pretreatment with oral indomethacin (Christie et al. 1992). These studies support the concept that LTE4 acts through a receptor(s) that is distinct from those responsible for the actions of LTC4 and LTD4 and suggest that the expression and/or function of the LTE4 receptor may be selectively upregulated in asthma, specifically enhancing the pulmonary responsiveness to it (Laidlaw and Boyce 2012).
The CysLT1 and CysLT2 receptors are both G-protein-coupled receptors (GPCRs) and were cloned and characterized several years after the original descriptive pharmacology predicted their properties (Laidlaw and Boyce 2012). Human and mouse CysLT1 receptors are 87 % identical (Mollerup et al. 2001), and the CysLT2 receptors are 74 % identical (Hui et al. 2001), suggesting a high level of functional conservation through evolution. Both receptors are structural homologues of the purinergic (P2Y) receptors, which are specialized to recognize extracellular nucleotides, with 25–34 % identity at the amino acid level (Mellor et al. 2001). The CysLT1 receptor binds LTD4 with high affinity (10−9 M) and LTC4 with lesser affinity (10−8 M), whereas the CysLT2 receptor binds both LTC4 and LTD4 with equal affinity (10−8 M) (Laidlaw and Boyce 2012). Neither receptor exhibits substantial affinity for LTE4 in radioligand binding assays, nor does LTE4 elicit strong signaling responses in cells expressing CysLT1 receptor or CysLT2 receptor in isolation (Lynch et al. 1999; Heise et al. 2000). It is therefore unlikely that the pharmacology of LTE4 in vivo is attributable to the CysLT1 receptor and CysLT2 receptor alone (Laidlaw and Boyce 2012). The CysLT1 and CysLT2 receptors are broadly expressed by structural and hematopoietic cells. Some cell types (vascular smooth muscle) express mostly CysLT1 receptors (Heise et al. 2000), whereas others (endothelial cells) dominantly express CysLT2 receptors (Hui et al. 2001). Both receptors are expressed by cells of the innate (macrophages, monocytes, eosinophils, basophils, mast cells, dendritic cells) and adaptive (T cells, B cells) immune system, implying potentially cooperative functions in immunity and inflammation (Kanaoka and Boyce 2004, 2014). Although the CysLT1 and CysLT2 receptors both mediate calcium flux and activate signaling cascades, the blockade or knockdown of CysLT1 receptors in mast cells eliminated most LTD4-mediated signaling despite the presence of CysLT2 receptors on these cells (Laidlaw and Boyce 2012; Mellor et al. 2001, 2003; Jiang et al. 2007). CysLT1 receptors and CysLT2 receptors were found to heterodimerize in primary mast cells, a relatively common feature of GPCRs, which recognize similar ligands (Franco et al. 2007). Thus CysLT2 receptors, by interacting with CysLT1 receptors, limit the surface expression levels and signaling ability of the latter receptors, at least on mast cells. The absence of CysLT2 receptors may also facilitate the formation of CysLT1 receptor homodimers (Lynch et al. 1999) that are strong signaling units for LTD4 (Laidlaw and Boyce 2012).
1.2 Clinical Use of LTRAs
A currently available LTRA is a CysLT1 receptor antagonist. Three types of LTRAs are available: pranlukast hydrate, zafirlukast, and montelukast. LTRAs have a bronchodilator action and inhibit airway inflammation (Minoguchi et al. 2002; Hui and Barnes 1991), resulting in a significant improvement of asthma symptoms, respiratory function, inhalation frequency of as-needed inhaled β2-agonist, airway inflammation, airway hyperresponsiveness, dosage of inhaled corticosteroids (ICSs), asthma exacerbations, and patients’ QOL (Tohda et al. 2002; Tamaoki et al. 1997; Drazen et al. 1999).
In several multicenter, randomized, double-blind trials, the usefulness of LTRAs has been investigated as candidate medications to control chronic asthma.
When estimated by FEV1 and symptoms, montelukast significantly improved asthma control during a 12-week treatment period (Reiss et al. 1998). Note that 90 % of the participants had histories of allergic rhinitis (AR) and exercise-induced asthma (EIA) in this study. Concerning EIA, unlike salmeterol, montelukast showed a sustained bronchoprotective effect throughout 8 weeks of the study (Edelman et al. 2000). Additionally, as compared with placebo, montelukast provided significant protection against EIA over a 12-week period and with neither tolerance to the medication nor a rebound worsening of lung function after discontinuation of the treatment (Leff et al. 1998). Although montelukast provides a rapid improvement in FEV1 to patients with chronic asthma, montelukast alone is less effective compared with low doses of ICSs (Peters et al. 2007; Malmstrom et al. 1999) (Fig. 1). On the other hand, LTRAs are known to be useful as agents used concomitantly with an ICS in patients with asthma that cannot be completely controlled even with a medium dose of an ICS, because the additional administration of LTRAs is as effective as a double dose of an ICS (Wada et al. 2000; Price et al. 2003; Laviolette et al. 1999). Additionally, the effects of LTRAs plus ICSs are reported to be the same as those of LABAs plus ICSs in steroid-naïve asthmatic patients (Peters et al. 2007; Price et al. 2011). There are some reports providing evidence for the advantages of LTRAs plus ICSs. Not only inhaled fluticasone (1000 μg/day) for 2 weeks but also oral prednisolone (60 mg/day) for 1 week did not decrease the LTE4 levels in bronchoalveolar lavage fluids (BALF) (Dworski et al. 1994) and those in urine (O’Shaughnessy et al. 1993). Concerning the effect of LTRA versus LABA added to ICS on asthma controls in patients whose symptoms are inadequately controlled with ICS alone, montelukast used in combination with fluticasone is less effective in improving symptoms and respiratory function and is almost equivalent in preventing exacerbations, when compared with salmeterol in combination with fluticasone (Bjermer et al. 2003; Ilowite et al. 2004). In some patients, respiratory function improves early after the oral administration of an LTRA (in several hours at the earliest, on the following day at the latest) (Hui and Barnes 1991); however, anti-inflammatory effects develop later. Thus, efficacy is generally judged 2–4 weeks after administration.
Distribution of treatment responses for FEV1. The response distributions are shown as histograms for predefined intervals of percentage change in FEV1. Of the montelukast recipients, 42 % had an improvement in FEV1 of at least 11 % from baseline (this was the median response of beclomethasone recipients; that is, 50 % of the beclomethasone recipients had an improvement in FEV1 of at least 11 % from baseline). The proportions of patients who did not show an improvement in FEV1 were 22 % with beclomethasone and 34 % with montelukast. Striped bars represent patients receiving montelukast, 10 mg once daily; white bars represent patients receiving inhaled beclomethasone, 200 μg twice daily. [From reference Malmstrom et al. 1999]
The potential usefulness of LTRAs for relief from acute asthma has been also investigated. In a study that compared the effect of a single dose of intravenous montelukast (7 mg), oral montelukast (10 mg), and placebo on FEV1 in patients with chronic asthma, the onset of action for intravenous montelukast was faster than that for oral montelukast, and the improvement in airway function lasted over the 24 h observation period for both treatments (Dockhorn et al. 2000). It was also reported that intravenous montelukast added to the standard care produced significant and sustained relief of airway obstruction throughout the 2 h after drug administration, with an onset of action as early as 10 min (Camargo et al. 2010).
As a whole, LTRAs are generally useful for long-term management of patients with asthma complicated by AR (Price et al. 2006), EIA (Leff et al. 1998), and AIA (Dahlen et al. 2002).
While more reports have been published about EGPA patients who have received an LTRA than about those who have received other antiasthmatic drugs, no conclusion has been reached as to whether an LTRA can directly cause the onset of EGPA (Beasley et al. 2008; Nathani et al. 2008). LTRAs are generally safe drugs, although zafirlukast should be used with caution because it may cause severe liver dysfunction and interact with other agents, such as warfarin, since it is metabolized by CYP2C9. It was recently reported that CYP2C8, but neither CYP2C9 nor CYP3A4, contributes to the metabolism of montelukast at clinically relevant concentrations (Filppula et al. 2011; Karonen et al. 2012). LTRAs seem to be relatively safe for pregnant women. In 2008, the US Food and Drug Administration (FDA) first issued a safety alert concerning a potential association between montelukast and increased risk of suicide. However, more recent studies found that the use of LTRAs was not associated with an increased risk of suicide attempts in children, adolescents, and young adults with asthma. Further research needs to be conducted to more fully understand the association between LTRAs and suicide (Manalai et al. 2009; Schumock et al. 2012; Philip et al. 2009).
2 Antiallergic Agents Other Than LTRAs
Antiallergic agents include either mediator-release suppressants or mediator inhibitors and are effective in 30–40 % of the patients with mild-to-moderate atopic asthma, although an administration period of 4–6 weeks or longer is needed to determine their efficacy (Furukawa et al. 1999). The antiallergic agents presented here had been used mainly in Japan, but the use of these agents rapidly decreased after inhaled corticosteroids (ICSs) were introduced in the late 1990s. The safety of oral antiallergic agents has not been demonstrated in fetuses during pregnancy.
2.1 Mediator-Release Suppressants
2.1.1 Pharmacology of Mediator-Release Agents
The anti-inflammatory and antiallergic effect of disodium cromoglycates (DSCG) was shown to work in a dose-dependent fashion through the inhibition of IgE-stimulated mediator release from human mast cells (Netzer et al. 2012; Leung et al. 1988). Recent research has specifically demonstrated that the cromoglycates are potent GPCR35 agonists and that GPCR35 is expressed in human mast cells, basophils, and eosinophils (Kay et al. 1987). GPCR35 mRNA is upregulated upon challenge with IgE antibodies, and cromoglycates may work by dampening the effects of this interaction. DSCG also demonstrated a cell-selective and mediator-selective suppressive effect on macrophages, eosinophils, and monocytes (Yang et al. 2010). DSCG was demonstrated to have anti-inflammatory effects in a study performed using biopsies of bronchial mucosa in nine patients with asthma before and after treatment with inhaled DSCG by a metered-dose inhaler (MDI) (Hoshino and Nakamura 1997). The numbers of eosinophils, mast cells, T-lymphocytes, and macrophages were significantly reduced as a result of DSCG, and the expressions of intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and endothelial leukocyte adhesion molecule-1 (ELAM-1) were also significantly reduced (Hoshino and Nakamura 1997). Head-to-head comparison of DSCG with beclomethasone dipropionate (BDP) revealed similar anti-inflammatory effects between DSCG and BDP in terms of reduced mucosal populations of eosinophils, mast cells, and T-lymphocytes (Hoshino et al. 1998). A review article revealed that DSCG has a protective effect in reducing bronchial hyperreactivity if it is used continuously for longer than 12 weeks (Hoag and McFadden 1991).
2.1.2 Clinical Use of DSCG
The main effect of mediator-release suppressants is inhibiting the release of chemical mediators from mast cells. It is reported that the long-term use of inhaled DSCG inhibits airway inflammation in adult patients with atopic asthma (Hoshino and Nakamura 1997; Hoag and McFadden 1991). Because DSCG has been recommended as a maintenance treatment for children with moderate asthma, it has been mainly used in children with asthma (Tasche et al. 2000). DSCG is supposed to be effective in 60 % of cases (Warner 1989), and no serious side effects have been reported in trials (Tasche et al. 2000). However, the use of DSCG has decreased since the 1990s, while the use of ICS is increasing, even in young children (Price and Weller 1995; Warner 1995).
2.2 Histamine H1 Antagonists
2.2.1 Pharmacology of Histamine and Its Receptors
Histamine was first identified as a mediator of biological functions in the early 1900s, and drugs targeting its receptors have been in clinical use for more than 60 years. It is widely known that histamine is increased in the BALF from patients with allergic asthma, and this increase negatively correlates with airway function (Wenzel et al. 1988; Wardlaw et al. 1988; Liu et al. 1990; Jarjour et al. 1991; Casale et al. 1987; Broide et al. 1991). During inflammation, histamine is released from preformed stores in mast cells and basophils. Histamine acts on vascular smooth muscle cells and endothelial cells, leading to vasodilation and an increase in vascular permeability (Thurmond et al. 2008). All of the receptors for histamine are of the GPCR family. In general, it has been found that many cells involved in inflammatory responses express H1, H2, and H4 receptors. H1 receptors couple to Gq proteins leading to phospholipase C activation, inositol phosphate production, and calcium mobilization (Bakker et al. 2002). H2 receptors activate Gαs and increase cyclic AMP formation (Bakker et al. 2002). Activation of the H4 receptor appears to be mainly coupled to pertussis toxin-sensitive Gαi/o proteins, which signal through increases in the intracellular calcium (Thurmond et al. 2008). H1 receptors are expressed on multiple cell types including endothelial cells and smooth muscle cells, where they mediate vasodilation and bronchoconstriction. H1 histamine receptor antagonists (H1RAs), such as diphenhydramine and loratadine, have been used for many years in the treatment of allergic inflammatory responses. Indeed, airway hyperresponsiveness to histamine is one of the hallmarks of asthma, and the plasma histamine concentrations are elevated during the early and late responses to inhaled allergens and may also increase during spontaneous acute asthma episodes. However, ordinary doses of currently available H1RAs have minimal bronchodilator and bronchoprotective activity, and H1RAs have no significant clinical effect in severe persistent asthma (Simons 1999). To date, it is unlikely that monotherapy with most currently available H1RAs will provide significant clinical benefit in asthma (Lordan and Holgate 2002).
2.2.2 Clinical Use of Histamine H1 Antagonists
Specific H1RA completely inhibited histamine-induced bronchoconstriction but failed to completely inhibit inhaled allergen-induced bronchoconstriction (Rafferty et al. 1987). Additionally, singular treatment with H1RA or LTRA caused significant reductions in the mean maximal fall in FEV1 during the early asthmatic reactions (EAR) and late asthmatic reactions (LAR), but the efficacy of H1RA was inferior to that of LTRA (Roquet et al. 1997). H1RAs are not currently a frontline treatment for asthma (Thurmond et al. 2008; Ohta et al. 2014; Global Initiative for Asthma (GINA) 2014). H1RAs are beneficial for asthma accompanied by allergic rhinitis or atopic dermatitis. Adverse effects may include sleepiness and malaise.
2.3 Thromboxane A2 (TXA2) and Its Receptors
2.3.1 Pharmacology of TXA2 and Its Receptors
Like cys-LTs (C4, D4, E4), TXA2 is a lipid mediator that powerfully contributes to the airflow limitation by constricting bronchial smooth muscles, increasing mucous secretion and microvascular leakage, and acting as a chemoattractant for inflammatory cells such as T-lymphocytes, eosinophils, and activated mast cells (Rolin et al. 2006). TXA2 is generated by thromboxane synthase, which belongs to the cytochrome P450 superfamily (Tanabe and Ullrich 1995; Nusing et al. 1990). Due to its prothrombotic and vasoconstrictor effects, this prostanoid is the physiological antagonist of prostacyclin (Rolin et al. 2006). The human TXA2 receptor termed TP was the first eicosanoid receptor cloned (Hirata et al. 1991). Two isoforms of this receptor were described: TPα and TPβ. Both isoforms functionally couple to a Gq protein leading to phospholipase C activation, calcium release, and the activation of protein kinase C (Huang et al. 2004; Shenker et al. 1991; Dorn and Becker 1993). Nevertheless, they couple oppositely to adenylate cyclase. TPα activates adenylate cyclase, while TPβ inhibits this enzyme (Hirata et al. 1996). TXA2 is mainly produced by platelets, monocytes, macrophages, neutrophils, and lung parenchyma (Nusing et al. 1990; Widdicombe et al. 1989; Higgs et al. 1983; Hamberg et al. 1975). TXA2 is a potent stimulator of platelet shape change and aggregation as well as a potent stimulator of smooth muscle constriction and proliferation and bronchial hyperresponsiveness (Kurosawa 1995). TXA2 plays a crucial role in the pathogenesis of bronchial asthma since it is a potent constrictor of bronchial smooth muscles and a stimulator of airway smooth muscle cell proliferation (Tamaoki et al. 2000a; Morris et al. 1980; Devillier and Bessard 1997). It has been shown that TXA2 is increased in the airways of patients suffering from asthma after allergen challenge (Wenzel et al. 1991). Several studies also demonstrated increased concentrations of this mediator and metabolites in BALF, urine, and plasma from asthmatic patients (Kumlin et al. 1992; Oosterhoff et al. 1995; Wenzel et al. 1989). Activation of the prostanoid TP receptors present in bronchial smooth muscle cells by TXA2 leads to intracellular calcium mobilization with bronchoconstriction as a consequence (Capra et al. 2003; Hall 2000). Prostanoid TP receptor activation also contributes to bronchial smooth muscle hyperplasia and airway remodeling, which occur in response to chronic airway inflammation of asthma (Vignola et al. 2003). Using a model of allergen-induced cough in guinea pig, it was demonstrated that airway mucous cells are an important source of TXA2 and that this prostanoid facilitates cough (Rolin et al. 2006). Moreover, this team showed the localization of thromboxane synthase by immunohistochemical detection in the airways, mainly in epithelial goblet cells and tracheal glands (Xiang et al. 2002).
2.3.2 Clinical Use of TXA2 Inhibitors/Antagonists
TXA2 synthesis inhibitors and TXA2 receptor antagonists inhibit airway inflammation, improve airway hyperresponsiveness (Hoshino et al. 1999; Fujimura et al. 1986, 1991), and improve the impaired mucociliary transport (Fujimura et al. 1991). However, like other antiallergic agents described in this chapter, the use of TXA2 antagonists has decreased since ICS and ICS/LABA therapies became widely disseminated worldwide, including in Japan. Their adverse effects include a tendency for increased bleeding; thus, we should be cautious about the concomitant use of other agents with inhibitory effects on platelet aggregation.
2.4 Th2 Cytokine Inhibitor
2.4.1 Pharmacology of Th2 Cytokine Inhibitor
Suplatast tosilate (IPD) is a unique compound that inhibits the release of Th2 cytokines, such as IL-4 and IL-5, and inhibits tissue infiltration by eosinophils (Corry and Kheradmand 2006; Horiguchi et al. 2001; Yamaya et al. 1995; Oda et al. 1999). This orally administered agent is effective in reducing the ECP level in induced sputum, improving the peak expiratory flow (Horiguchi et al. 2001) and airway hyperresponsiveness (Yoshida et al. 2002; Sano et al. 2003). These anti-inflammatory features might be responsible for their beneficial effects on airway function (Corry and Kheradmand 2006).
2.4.2 Clinical Use of Th2 Cytokine Inhibitor
In a randomized, double-blind, placebo-controlled, parallel-group study, IPD improved pulmonary function and symptom control and enabled a decrease in the dose of inhaled corticosteroid without significant side effects in steroid-dependent asthma (Tamaoki et al. 2000b). This promising investigational agent is currently available only in Japan. IPD enables a reduction in the dose of ICS (Tamaoki et al. 2000b).
3 Conclusion
Although cys-LTs are deeply associated with the pathogenesis of asthma, LTRAs alone are less effective compared with ICS. However, the effects of LTRAs in combination with ICS are the same as those of LABAs in combination with ICS in steroid-naïve asthmatic patients. Currently, the use of LTRAs is mostly limited to asthma patients with AR or EIA. Antiallergic agents other than LTRAs had been used mainly in Japan, but the use of these agents rapidly decreased after ICS was introduced and recommended for the management of asthma in the late 1990s. Further effectiveness studies are needed to determine the true value of these orally administered agents in combination with ICS as an antiasthma treatment.
References
Laidlaw TM, Boyce JA (2012) Cysteinyl leukotriene receptors, old and new; implications for asthma. Clin Exp Allergy 42(9):1313–1320
Kanaoka Y, Boyce AA (2004) Cysteinyl leukotrienes and their receptors: (Cellular distribution and function in immune and inflammatory responses. J Immunol 173(3):1503–1510
Kanaoka Y, Boyce JA (2014) Cysteinyl leukotrienes and their receptors; emerging concepts. Allergy Asthma Immunol Res 6(4):288–295
Clark JD, Milona N, Knopf JL (1990) Purification of a 110-kilodalton cytosolic phospholipase-A2 from the human monocytic cell-line U937. Proc Natl Acad Sci U S A 87(19):7708–7712
Reid GK, Kargman S, Vickers PJ, Mancini JA, Leveille C, Ethier D et al (1990) Correlation between expression of 5-lipoxygenase-activating protein, 5-lipoxygenase, and cellular leukotriene synthesis. J Biol Chem 265(32):19818–19823
Lam BK, Penrose JF, Freeman GJ, Austen KF (1994) Expression cloning of a cDNA for human leukotriene C4 synthase, an integral membrane protein conjugating reduced glutathione to leukotriene A4. Proc Natl Acad Sci U S A 91(16):7663–7667
Maclouf J, Antoine C, Henson PM, Murphy RC (1994) Leukotriene C4 formation by transcellular biosynthesis. Ann N Y Acad Sci 714:143–150, Epub 1994/04/18
Drazen JM, Obrien J, Sparrow D, Weiss ST, Martins MA, Israel E et al (1992) Recovery of leukotriene-E4 from the urine of patients with airway-obstruction. Am Rev Respir Dis 146(1):104–108
Christie PE, Tagari P, Ford-Hutchinson AW, Charlesson S, Chee P, Arm JP et al (1991) Urinary leukotriene E4 concentrations increase after aspirin challenge in aspirin-sensitive asthmatic subjects. Am Rev Respir Dis 143(5 Pt 1):1025–1029
Liu MC, Dube LM, Lancaster J (1996) Acute and chronic effects of a 5-lipoxygenase inhibitor in asthma: a 6-month randomized multicenter trial. Zileuton Study Group. J Allergy Clin Immunol 98(5 Pt 1):859–871
Israel E, Cohn J, Dube L, Drazen JM (1996) Effect of treatment with zileuton, a 5-lipoxygenase inhibitor, in patients with asthma – a randomized controlled trial. JAMA 275(12):931–936
Berges-Gimeno MP, Simon RA, Stevenson DD (2002) The effect of leukotriene-modifier drugs on aspirin-induced asthma and rhinitis reactions. Clin Exp Allergy 32(10):1491–1496
Weiss JW, Drazen JM, Coles N, Mcfadden ER, Weller PF, Corey EJ et al (1982a) Bronchoconstrictor effects of leukotriene-C in humans. Science 216(4542):196–198
Griffin M, Weiss JW, Leitch AG, Mcfadden ER, Corey EJ, Austen KF et al (1983) Effects of leukotriene-D on the airways in asthma. N Engl J Med 308(8):436–439
Lee TH, Austen KF, Corey EJ, Drazen JM (1984) Leukotriene E4-induced airway hyperresponsiveness of guinea pig tracheal smooth muscle to histamine and evidence for three separate sulfidopeptide leukotriene receptors. Proc Natl Acad Sci U S A 81(15):4922–4925
Drazen JM, Venugopalan CS, Austen KF, Brion F, Corey EJ (1982) Effects of leukotriene-E on pulmonary mechanics in the guinea-pig. Am Rev Respir Dis 125(3):290–294
Weiss JW, Drazen JM, Mcfadden ER, Weller P, Corey EJ, Lewis RA et al (1983) Airway constriction in normal humans produced by inhalation of leukotriene-D – potency, time course, and effect of aspirin therapy. JAMA 249(20):2814–2817
Weiss JW, Drazen JM, Mcfadden ER, Lewis R, Weller P, Corey EJ et al (1982b) Comparative bronchoconstrictor effects of histamine and leukotriene-C and leukotriene-D (Ltc and Ltd) in normal human volunteers. Clin Res 30(2):A571
Davidson AB, Lee TH, Scanlon PD, Solway J, Mcfadden ER, Ingram RH et al (1987) Bronchoconstrictor effects of leukotriene-E4 in normal and asthmatic subjects. Am Rev Respir Dis 135(2):333–337
Christie PE, Schmitz-Schumann M, Spur BW, Lee TH (1993) Airway responsiveness to leukotriene C4 (LTC4), leukotriene E4 (LTE4) and histamine in aspirin-sensitive asthmatic subjects. Eur Respir J 6(10):1468–1473
Gauvreau GM, Parameswaran KN, Watson RM, O’Byrne PM (2001) Inhaled leukotriene E(4), but not leukotriene D(4), increased airway inflammatory cells in subjects with atopic asthma. Am J Respir Crit Care Med 164(8 Pt 1):1495–1500
Christie PE, Hawksworth R, Spur BW, Lee TH (1992) Effect of indomethacin on leukotriene4-induced histamine hyperresponsiveness in asthmatic subjects. Am Rev Respir Dis 146(6):1506–1510
Mollerup J, Jorgensen ST, Hougaard C, Hoffmann EK (2001) Identification of a murine cysteinyl leukotriene receptor by expression in Xenopus laevis oocytes. Biochim Biophys Acta 1517(3):455–459
Hui YQ, Yang GC, Galczenski H, Figueroa DJ, Austin CP, Copeland NG et al (2001) The murine cysteinyl leukotriene 2 (CysLT(2)) receptor – cDNA and genomic cloning, alternative splicing, and in vitro characterization. J Biol Chem 276(50):47489–47495
Mellor EA, Maekawa A, Austen KF, Boyce JA (2001) Cysteinyl leukotriene receptor 1 is also a pyrimidinergic receptor and is expressed by human mast cells. Proc Natl Acad Sci U S A 98(14):7964–7969
Lynch KR, O’Neill GP, Liu Q, Im DS, Sawyer N, Metters KM et al (1999) Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature 399(6738):789–793
Heise CE, O’Dowd BF, Figueroa DJ, Sawyer N, Nguyen T, Im DS et al (2000) Characterization of the human cysteinyl leukotriene 2 receptor. J Biol Chem 275(39):30531–30536
Mellor EA, Frank N, Soler D, Hodge MR, Lora JM, Austen KF et al (2003) Expression of the type 2 receptor for cysteinyl leukotrienes (CysLT2R) by human mast cells: functional distinction from CysLT1R. Proc Natl Acad Sci U S A 100(20):11589–11593
Jiang Y, Borrelli LA, Kanaoka Y, Bacskai BJ, Boyce JA (2007) CysLT2 receptors interact with CysLT1 receptors and down-modulate cysteinyl leukotriene dependent mitogenic responses of mast cells. Blood 110(9):3263–3270
Franco R, Casado V, Cortes A, Ferrada C, Mallol J, Woods A et al (2007) Basic concepts in G-protein-coupled receptor homo- and heterodimerization. ScientificWorldJournal 7:48–57
Minoguchi K, Kohno Y, Minoguchi H, Kihara N, Sano Y, Yasuhara H et al (2002) Reduction of eosinophilic inflammation in the airways of patients with asthma using montelukast. Chest 121(3):732–738
Hui KP, Barnes NC (1991) Lung function improvement in asthma with a cysteinyl-leukotriene receptor antagonist. Lancet 337(8749):1062–1063
Tohda Y, Fujimura M, Taniguchi H, Takagi K, Igarashi T, Yasuhara H et al (2002) Leukotriene receptor antagonist, montelukast, can reduce the need for inhaled steroid while maintaining the clinical stability of asthmatic patients. Clin Exp Allergy 32(8):1180–1186
Tamaoki J, Kondo M, Sakai N, Nakata J, Takemura H, Nagai A et al (1997) Leukotriene antagonist prevents exacerbation of asthma during reduction of high-dose inhaled corticosteroid. The Tokyo Joshi-Idai Asthma Research Group. Am J Respir Crit Care Med 155(4):1235–1240
Drazen JM, Israel E, O’Byrne PM (1999) Treatment of asthma with drugs modifying the leukotriene pathway. N Engl J Med 340(3):197–206
Reiss TF, Chervinsky P, Dockhorn RJ, Shingo S, Seidenberg B, Edwards TB (1998) Montelukast, a once-daily leukotriene receptor antagonist, in the treatment of chronic asthma: a multicenter, randomized, double-blind trial. Montelukast Clinical Research Study Group. Arch Intern Med 158(11):1213–1220
Edelman JM, Turpin JA, Bronsky EA, Grossman J, Kemp JP, Ghannam AF et al (2000) Oral montelukast compared with inhaled salmeterol to prevent exercise-induced bronchoconstriction. A randomized, double-blind trial. Exercise Study Group. Ann Intern Med 132(2):97–104
Leff JA, Busse WW, Pearlman D, Bronsky EA, Kemp J, Hendeles L et al (1998) Montelukast, a leukotriene-receptor antagonist, for the treatment of mild asthma and exercise-induced bronchoconstriction. N Engl J Med 339(3):147–152
Peters SP, Anthonisen N, Castro M, Holbrook JT, Irvin CG, Smith LJ et al (2007) Randomized comparison of strategies for reducing treatment in mild persistent asthma. N Engl J Med 356(20):2027–2039
Malmstrom K, Rodriguez-Gomez G, Guerra J, Villaran C, Pineiro A, Wei LX et al (1999) Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma. A randomized, controlled trial. Montelukast/Beclomethasone Study Group. Ann Intern Med 130(6):487–495
Wada K, Minoguchi K, Adachi M (2000) Effect of a leukotriene receptor antagonist, pranlukast hydrate, on airway inflammation and airway hyper responsiveness in patients with moderate to severe asthma. Allergol Int 49:63–68
Price DB, Hernandez D, Magyar P, Fiterman J, Beeh KM, James IG et al (2003) Randomised controlled trial of montelukast plus inhaled budesonide versus double dose inhaled budesonide in adult patients with asthma. Thorax 58(3):211–216
Laviolette M, Malmstrom K, Lu S, Chervinsky P, Pujet JC, Peszek I et al (1999) Montelukast added to inhaled beclomethasone in treatment of asthma. Montelukast/Beclomethasone Additivity Group. Am J Respir Crit Care Med 160(6):1862–1868
Price D, Musgrave SD, Shepstone L, Hillyer EV, Sims EJ, Gilbert RFT et al (2011) Leukotriene antagonists as first-line or add-on asthma-controller therapy. N Engl J Med 364(18):1695–1707
Dworski R, Fitzgerald GA, Oates JA, Sheller JR (1994) Effect of oral prednisone on airway inflammatory mediators in atopic asthma. Am J Respir Crit Care Med 149(4 Pt 1):953–959
O’Shaughnessy KM, Wellings R, Gillies B, Fuller RW (1993) Differential effects of fluticasone propionate on allergen-evoked bronchoconstriction and increased urinary leukotriene E4 excretion. Am Rev Respir Dis 147(6 Pt 1):1472–1476
Bjermer L, Bisgaard H, Bousquet J, Fabbri LM, Greening AP, Haahtela T et al (2003) Montelukast and fluticasone compared with salmeterol and fluticasone in protecting against asthma exacerbation in adults: one year, double blind, randomised, comparative trial. BMJ 327(7420):891
Ilowite J, Webb R, Friedman B, Kerwin E, Bird SR, Hustad CM et al (2004) Addition of montelukast or salmeterol to fluticasone for protection against asthma attacks: a randomized, double-blind, multicenter study. Ann Allergy Asthma Immunol 92(6):641–648
Dockhorn RJ, Baumgartner RA, Leff JA, Noonan M, Vandormael K, Stricker W et al (2000) Comparison of the effects of intravenous and oral montelukast on airway function: a double blind, placebo controlled, three period, crossover study in asthmatic patients. Thorax 55(4):260–265
Camargo CA Jr, Gurner DM, Smithline HA, Chapela R, Fabbri LM, Green SA et al (2010) A randomized placebo-controlled study of intravenous montelukast for the treatment of acute asthma. J Allergy Clin Immunol 125(2):374–380
Price DB, Swern A, Tozzi CA, Philip G, Polos P (2006) Effect of montelukast on lung function in asthma patients with allergic rhinitis: analysis from the COMPACT trial. Allergy 61(6):737–742
Dahlen SE, Malmstrom K, Nizankowska E, Dahlen B, Kuna P, Kowalski M et al (2002) Improvement of aspirin-intolerant asthma by montelukast, a leukotriene antagonist: a randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med 165(1):9–14
Beasley R, Bibby S, Weatherall M (2008) Leukotriene receptor antagonist therapy and Churg-Strauss syndrome: culprit or innocent bystander? Thorax 63(10):847–849
Nathani N, Little MA, Kunst H, Wilson D, Thickett DR (2008) Churg-Strauss syndrome and leukotriene antagonist use: a respiratory perspective. Thorax 63(10):883–888
Filppula AM, Laitila J, Neuvonen PJ, Backman JT (2011) Reevaluation of the microsomal metabolism of montelukast: major contribution by CYP2C8 at clinically relevant concentrations. Drug Metab Dispos 39(5):904–911
Karonen T, Neuvonen PJ, Backman JT (2012) CYP2C8 but not CYP3A4 is important in the pharmacokinetics of montelukast. Br J Clin Pharmacol 73(2):257–267
Manalai P, Woo JM, Postolache TT (2009) Suicidality and montelukast. Expert Opin Drug Saf 8(3):273–282
Schumock GT, Stayner LT, Valuck RJ, Joo MJ, Gibbons RD, Lee TA (2012) Risk of suicide attempt in asthmatic children and young adults prescribed leukotriene-modifying agents: a nested case–control study. J Allergy Clin Immunol 130(2):368–375
Philip G, Hustad C, Noonan G, Malice MP, Ezekowitz A, Reiss TF et al (2009) Reports of suicidality in clinical trials of montelukast. J Allergy Clin Immunol 124(4):691–696, e6
Furukawa C, Atkinson D, Forster TJ, Nazzario K, Simpson B, Uryniak T et al (1999) Controlled trial of two formulations of cromolyn sodium in the treatment of asthmatic patients > or = 12 years of age. Intal Study Group. Chest 116(1):65–72
Netzer NC, Kupper T, Voss HW, Eliasson AH (2012) The actual role of sodium cromoglycate in the treatment of asthma—a critical review. Sleep Breath 16(4):1027–1032
Leung KB, Flint KC, Brostoff J, Hudspith BN, Johnson NM, Lau HY et al (1988) Effects of sodium cromoglycate and nedocromil sodium on histamine secretion from human lung mast cells. Thorax 43(10):756–761
Kay AB, Walsh GM, Moqbel R, MacDonald AJ, Nagakura T, Carroll MP et al (1987) Disodium cromoglycate inhibits activation of human inflammatory cells in vitro. J Allergy Clin Immunol 80(1):1–8
Yang Y, Lu JY, Wu X, Summer S, Whoriskey J, Saris C et al (2010) G-protein-coupled receptor 35 is a target of the asthma drugs cromolyn disodium and nedocromil sodium. Pharmacology 86(1):1–5
Hoshino M, Nakamura Y (1997) The effect of inhaled sodium cromoglycate on cellular infiltration into the bronchial mucosa and the expression of adhesion molecules in asthmatics. Eur Respir J 10(4):858–865
Hoshino M, Nakamura Y, Sim JJ, Tomioka H (1998) A comparative study of the effects of ketotifen, disodium cromoglycate, and beclomethasone dipropionate on bronchial mucosa and asthma symptoms in patients with atopic asthma. Respir Med 92(7):942–950
Hoag JE, McFadden ER Jr (1991) Long-term effect of cromolyn sodium on nonspecific bronchial hyperresponsiveness: a review. Ann Allergy 66(1):53–63
Tasche MJA, Uijen JHJM, Bernsen RMD, de Jongste JC, van der Wouden JC (2000) Inhaled disodium cromoglycate (DSCG) as maintenance therapy in children with asthma: a systematic review. Thorax 55(11):913–920
Warner JO (1989) The place of Intal in paediatric practice. Respir Med 83 Suppl A:33–37
Price JF, Weller PH (1995) Comparison of fluticasone propionate and sodium cromoglycate for the treatment of childhood asthma (an open parallel group study). Respir Med 89(5):363–368
Warner JO (1995) Review of prescribed treatment for children with asthma in 1990. BMJ 311(7006):663–666
Wenzel SE, Fowler AA 3rd, Schwartz LB (1988) Activation of pulmonary mast cells by bronchoalveolar allergen challenge. In vivo release of histamine and tryptase in atopic subjects with and without asthma. Am Rev Respir Dis 137(5):1002–1008
Wardlaw AJ, Dunnette S, Gleich GJ, Collins JV, Kay AB (1988) Eosinophils and mast cells in bronchoalveolar lavage in subjects with mild asthma. Relationship to bronchial hyperreactivity. Am Rev Respir Dis 137(1):62–69
Liu MC, Bleecker ER, Lichtenstein LM, Kagey-Sobotka A, Niv Y, McLemore TL et al (1990) Evidence for elevated levels of histamine, prostaglandin D2, and other bronchoconstricting prostaglandins in the airways of subjects with mild asthma. Am Rev Respir Dis 142(1):126–132
Jarjour NN, Calhoun WJ, Schwartz LB, Busse WW (1991) Elevated bronchoalveolar lavage fluid histamine levels in allergic asthmatics are associated with increased airway obstruction. Am Rev Respir Dis 144(1):83–87
Casale TB, Wood D, Richerson HB, Trapp S, Metzger WJ, Zavala D et al (1987) Elevated bronchoalveolar lavage fluid histamine levels in allergic asthmatics are associated with methacholine bronchial hyperresponsiveness. J Clin Invest 79(4):1197–1203
Broide DH, Gleich GJ, Cuomo AJ, Coburn DA, Federman EC, Schwartz LB et al (1991) Evidence of ongoing mast cell and eosinophil degranulation in symptomatic asthma airway. J Allergy Clin Immunol 88(4):637–648
Thurmond RL, Gelfand EW, Dunford PJ (2008) The role of histamine H1 and H4 receptors in allergic inflammation: the search for new antihistamines. Nat Rev Drug Discov 7(1):41–53
Bakker RA, Timmerman H, Leurs R (2002) Histamine receptors: specific ligands, receptor biochemistry, and signal transduction. Clin Allergy Immunol 17:27–64
Simons FER (1999) Is antihistamine (H-1-receptor antagonist) therapy useful in clinical asthma? Clin Exp Allergy 29:98–104
Lordan JL, Holgate ST (2002) H1-antihistamines in asthma. Clin Allergy Immunol 17:221–248
Rafferty P, Beasley R, Holgate ST (1987) The contribution of histamine to immediate bronchoconstriction provoked by inhaled allergen and adenosine 5′ monophosphate in atopic asthma. Am Rev Respir Dis 136(2):369–373
Roquet A, Dahlen B, Kumlin M, Ihre E, Anstren G, Binks S et al (1997) Combined antagonism of leukotrienes and histamine produces predominant inhibition of allergen-induced early and late phase airway obstruction in asthmatics. Am J Respir Crit Care Med 155(6):1856–1863
Ohta K, Ichinose M, Nagase H, Yamaguchi M, Sugiura H, Tohda Y et al (2014) Japanese Guideline for Adult Asthma 2014. Allergol Int 63(3):293–333
Global Initiative for Asthma (GINA) (2014) The global strategy for asthma management and prevention. http://www.ginasthma.org/
Rolin S, Masereel B, Dogné J (2006) Prostanoids as pharmacological targets in COPD and asthma. Eur J Pharmacol 533:89–100
Tanabe T, Ullrich V (1995) Prostacyclin and thromboxane synthases. J Lipid Mediat Cell Signal 12(2–3):243–255
Nusing R, Lesch R, Ullrich V (1990) Immunohistochemical localization of thromboxane synthase in human tissues. Eicosanoids 3(1):53–58
Hirata M, Hayashi Y, Ushikubi F, Yokota Y, Kageyama R, Nakanishi S et al (1991) Cloning and expression of cDNA for a human thromboxane A2 receptor. Nature 349(6310):617–620
Huang J, Ramamurthy S, Lin X, Le Breton G (2004) Cell signalling through thromboxane A2 receptors. Cell Signal 16:521–533
Shenker A, Goldsmith P, Unson CG, Spiegel AM (1991) The G protein coupled to the thromboxane A2 receptor in human platelets is a member of the novel Gq family. J Biol Chem 266(14):9309–9313
Dorn GW 2nd, Becker MW (1993) Thromboxane A2 stimulated signal transduction in vascular smooth muscle. J Pharmacol Exp Ther 265(1):447–456
Hirata T, Ushikubi F, Kakizuka A, Okuma M, Narumiya S (1996) Two thromboxane A2 receptor isoforms in human platelets. Opposite coupling to adenylyl cyclase with different sensitivity to Arg60 to Leu mutation. J Clin Invest 97(4):949–956
Widdicombe JH, Ueki IF, Emery D, Margolskee D, Yergey J, Nadel JA (1989) Release of cyclooxygenase products from primary cultures of tracheal epithelia of dog and human. Am J Physiol 257(6 Pt 1):L361–L365
Higgs GA, Moncada S, Salmon JA, Seager K (1983) The source of thromboxane and prostaglandins in experimental inflammation. Br J Pharmacol 79(4):863–868
Hamberg M, Svensson J, Samuelsson B (1975) Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A 72(8):2994–2998
Kurosawa M (1995) Role of thromboxane A2 synthetase inhibitors in the treatment of patients with bronchial asthma. Clin Ther 17(1):2–11, discussion 1
Tamaoki J, Kondo M, Nakata J, Nagano Y, Isono K, Nagai A (2000a) Effect of a thromboxane A(2) antagonist on sputum production and its physicochemical properties in patients with mild to moderate asthma. Chest 118(1):73–79
Morris HG, Sherman NA, Shepperdson FT, Selner JC (1980) Radioimmunoassay of thromboxane B2 in plasma of normal and asthmatic subjects. Adv Prostaglandin Thromboxane Res 8:1759–1764
Devillier P, Bessard G (1997) Thromboxane A2 and related prostaglandins in airways. Fundam Clin Pharmacol 11(1):2–18
Wenzel SE, Westcott JY, Larsen GL (1991) Bronchoalveolar lavage fluid mediator levels 5 minutes after allergen challenge in atopic subjects with asthma: relationship to the development of late asthmatic responses. J Allergy Clin Immunol 87(2):540–548
Kumlin M, Dahlen B, Bjorck T, Zetterstrom O, Granstrom E, Dahlen SE (1992) Urinary excretion of leukotriene E4 and 11-dehydro-thromboxane B2 in response to bronchial provocations with allergen, aspirin, leukotriene D4, and histamine in asthmatics. Am Rev Respir Dis 146(1):96–103
Oosterhoff Y, Kauffman HF, Rutgers B, Zijlstra FJ, Koeter GH, Postma DS (1995) Inflammatory cell number and mediators in bronchoalveolar lavage fluid and peripheral blood in subjects with asthma with increased nocturnal airways narrowing. J Allergy Clin Immunol 96(2):219–229
Wenzel SE, Westcott JY, Smith HR, Larsen GL (1989) Spectrum of prostanoid release after bronchoalveolar allergen challenge in atopic asthmatics and in control groups. An alteration in the ratio of bronchoconstrictive to bronchoprotective mediators. Am Rev Respir Dis 139(2):450–457
Capra V, Habib A, Accomazzo MR, Ravasi S, Citro S, Levy-Toledano S et al (2003) Thromboxane prostanoid receptor in human airway smooth muscle cells: a relevant role in proliferation. Eur J Pharmacol 474(2–3):149–159
Hall IP (2000) Second messengers, ion channels and pharmacology of airway smooth muscle. Eur Respir J 15(6):1120–1127
Vignola AM, Mirabella F, Costanzo G, Di Giorgi R, Gjomarkaj M, Bellia V et al (2003) Airway remodeling in asthma. Chest 123(3 Suppl):417S–422S
Xiang A, Uchida Y, Nomura A, Iijima H, Sakamoto T, Ishii Y et al (2002) Involvement of thromboxane A(2) in airway mucous cells in asthma-related cough. J Appl Physiol (1985) 92(2):763–770
Hoshino M, Sim J, Shimizu K, Nakayama H, Koya A (1999) Effect of AA-2414, a thromboxane A2 receptor antagonist, on airway inflammation in subjects with asthma. J Allergy Clin Immunol 103(6):1054–1061
Fujimura M, Sakamoto S, Saito M, Miyake Y, Matsuda T (1991) Effect of a thromboxane A2 receptor antagonist (AA-2414) on bronchial hyperresponsiveness to methacholine in subjects with asthma. J Allergy Clin Immunol 87(1 Pt 1):23–27
Fujimura M, Sasaki F, Nakatsumi Y, Takahashi Y, Hifumi S, Taga K et al (1986) Effects of a thromboxane synthetase inhibitor (OKY-046) and a lipoxygenase inhibitor (AA-861) on bronchial responsiveness to acetylcholine in asthmatic subjects. Thorax 41(12):955–959
Corry DB, Kheradmand F (2006) Control of allergic airway inflammation through immunomodulation. J Allergy Clin Immunol 117(2):S461–S464
Horiguchi T, Tachikawa S, Handa M, Hanazono K, Kondo R, Ishibashi A et al (2001) Effects of suplatast tosilate on airway inflammation and airway hyperresponsiveness. J Asthma 38(4):331–336
Yamaya H, Basaki Y, Togawa M, Kojima M, Kiniwa M, Matsuura N (1995) Down-regulation of Th2 cell-mediated murine peritoneal eosinophilia by antiallergic agents. Life Sci 56(19):1647–1654
Oda N, Minoguchi K, Yokoe T, Hashimoto T, Wada K, Miyamoto M et al (1999) Effect of suplatast tosilate (IPD-1151T) on cytokine production by allergen-specific human Th1 and Th2 cell lines. Life Sci 65(8):763–770
Yoshida M, Aizawa H, Inoue H, Matsumoto K, Koto H, Komori M et al (2002) Effect of suplatast tosilate on airway hyperresponsiveness and inflammation in asthma patients. J Asthma 39(6):545–552
Sano Y, Suzuki N, Yamada H, To Y, Ogawa C, Ohta K et al (2003) Effects of suplatast tosilate on allergic eosinophilic airway inflammation in patients with mild asthma. J Allergy Clin Immunol 111(5):958–966
Tamaoki J, Kondo M, Sakai N, Aoshiba K, Tagaya E, Nakata J et al (2000b) Effect of suplatast tosilate, a Th2 cytokine inhibitor, on steroid-dependent asthma: a double-blind randomised study. Lancet 356(9226):273–278
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Tamada, T., Ichinose, M. (2016). Leukotriene Receptor Antagonists and Antiallergy Drugs. In: Page, C., Barnes, P. (eds) Pharmacology and Therapeutics of Asthma and COPD. Handbook of Experimental Pharmacology, vol 237. Springer, Cham. https://doi.org/10.1007/164_2016_72
Download citation
DOI: https://doi.org/10.1007/164_2016_72
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-52173-2
Online ISBN: 978-3-319-52175-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)