Abstract
The introduction of synthetic polypropylene mesh in hernia repair has improved the results of herniorrhaphy. To introduce mesh is to introduce foreign bodies that can impact the human body and may lead to inflammation, infection, fibrosis, calcification, seromas, or adhesions to vital organs such as the bowel. Bioprosthetic meshes, generated from source organs source such as the dermis or small intestine, have emerged as commercially available products for use in hernia repair. The authors discuss the ideal mesh, tissue engineering and hernia repair, bioprosthetic mesh and use in the contaminated field, and porcine acellular lung matrix (PALM) as a natural scaffold capable of cell attachment, while maintaining cell viability was investigated as a novel prosthetic for repair and has demonstrated enhanced incorporation and short-term mechanical stability in a chronic ventral incisional hernia model with bridging repair.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Hernias are among the most common conditions of surgical care with more than 300,000 surgeries occurring annually in the USA alone [1,2,3]. The volume and complexity of hernia surgery continues to remain a challenge because of increasing life expectancy, a proportionally older surgical population, and a growing population of morbidly obese and diabetic patients with other comorbidities, which influence native strength, and perfusion of tissues [4, 5]. Hernias necessitating emergent surgery are on the rise [6]. Five to 27% of people will develop a hernia over the course of their lifetime [7, 8]. Although hernia repairs are performed very frequently, they remain vulnerable to numerous complications such as infection and recurrence.
This socioeconomic burden would greatly benefit from a reduction in these complications; by reducing the rate of recurrence alone, the healthcare system would save $32 million for every 1% reduction in repeat operations [1]. Many of these issues are due to the lack of ideal materials used as mesh in the site of herniation. Reinforcing a repair mesh is the standard of treating incisional hernias given the high likelihood of recurrence with suture repair alone; however the material may migrate from the hernia site, become infected, or erode into adjacent structures [9,10,11,12,13]. Chronic pain due to complications related to mesh is an issue and is generally relieved by surgery to remove it [14,15,16]. To try and overcome these shortcomings and combat the problems plaguing the industry of synthetic mesh, manufacturers and researches have created acellular biological prosthetics to be used in hernia operations [4, 17, 18]. Although expensive, bioprosthetic meshes—constructed from human, bovine, or porcine tissue—have yielded promising results so far, particularly in contaminated fields and have therefore received much clinical and commercial attention [4, 19, 20].
2 The Ideal Mesh
The introduction of synthetic polypropylene mesh in hernia repair revolutionized abdominal wall repair, winning Natta and Ziegler the Nobel Prize in 1954 [7, 21]. Yet synthetic mesh is far from a perfect solution. To introduce mesh is to introduce foreign bodies that can impact the human body. They may lead to inflammation, infection, fibrosis, calcification, seromas, or adhesions to vital organs such as the bowel [22,23,24,25]. Meshes cause abnormal physiologic wound healing and scar formation, altering the ratio of type I to type III collagen, which decrease mechanical stability regardless of the mesh used [26,27,28]. In order to minimize these complications from an immune response, recognizing synthetic mesh as a foreign body, a new question emerged; would it be possible to transplant already existing architecture into which vessels can regrow and fibroblasts can remodel? To address the problems of synthetic mesh, researchers sought to find acellular matrices for reconstruction.
An ideal mesh is characterized by its strength, flexibility, and host tissue compatibility; no synthetic or biologic mesh has yet to fulfill all these criteria, and currently there is no true gold standard [21, 29, 30]. The metrics of a biological prosthetic mesh’s success is revascularization and cell repopulation in the tissue [31,32,33]. An ideal mesh must cause a significant enough of an inflammatory response to signal fibroblasts to deposit collagen, but tame enough to limit excessive scarring, graft encapsulation, and degradation [4]. Angiogenesis must occur to allow for tissue remodeling; otherwise the graft will be replaced by scar tissue [4]. Vascularization and cell repopulation are signs that demonstrate a mesh will incorporate well, hereby decreasing the odds of recurrence and infection [32]. Through the body’s natural healing processes, biological meshes are exposed to proteinases and collagenases that degrade them over time, thus weakening the repair. Cellular infiltration and angiogenesis of decellularized tissue constructs is not only dictated by the tissue source but inherently the microarchitectural cues innate to that tissue. Thus, much work in the realm of tissue engineering and regenerative medicine has been performed to understand the structure and biochemistry of acellular scaffolds.
3 Tissue Engineering and Hernia Repair
The fields of regenerative medicine and tissue engineering hold the promise of revolutionizing the practice of medicine and surgery. Many patients in need of tissue and replacement organs may stand to benefit from the advancement being made in the field of tissue engineering. By combining the principles of engineering and life sciences, tissue engineers seek to develop biological substitutes capable of not only providing a scaffold for innate cellular infiltration but in some ways facilitating drug delivery and cellular delivery in hopes of promoting enhanced healing by the patient’s own body. Specifically, in the realm of hernia repair, many investigators have sought new tissues or tissue constructs to be adapted to the repair of hernias in hopes of overcoming the adverse effects of synthetic material foreign body reactions that lead to the complications seen following hernia repair. Of those, the use of decellularization technology has emerged to generate acellular tissue constructs capable of maintaining adequate mechanical strength while simultaneously providing a biological scaffold that would allow the body to remodel and replace with its own living tissue.
4 Bioprosthetic Mesh
In response to growing evidence of the adverse outcome associated with synthetic mesh repair, physicians and researchers alike sought to investigate alternative approaches to hernia repair with a focus on the prosthetic used. Bioprosthetic meshes, generated from source organs such as the dermis or small intestine, have emerged as commercially available products for use in hernia repair. There are several commercially available bioprosthetic meshes with new products emerging annually. Currently, they are indicated for ventral hernias in contaminated settings but can be used in a variety of scenarios and have been advocated for as an alternative to the more commonly used synthetic meshes [4, 34]. Unfortunately, the lack of high-quality scientifically rigorous studies inhibits our ability to determine a gold standard for bioprosthetic meshes [29]. Further, with the lack of American Society for Testing and Materials (ASTM) International standard for biological tissues, considerable heterogeneity is seen from product to product of the same tissue and even within the same product line [35]. Commercially available biological products include homografts and xenografts from bovine and porcine sources (Table 1). In addition to different species, brands may be classified by organ of origin.
5 Dermis
There have been major advances in the field of bioprosthetics for ventral hernia repair using allogenic and xenogeneic materials. AlloDerm™ (LifeCell Corp, Branchburg, NJ) has been one of the most utilized and most studied homographs available today. Made from human cadaveric acellular dermal matrix (hADM), AlloDerm has been used as a tissue-grafting substitute for decades, although it has not been used for abdominal wall reconstruction and has decreased recently [36]. It has yielded promising results, helping to spur the movement for biomesh [36]. Some authors, however, have described laxity of the material and the propensity to stretch over time, leading to the development of pseudo-recurrences [29, 36]. The processing of bioprosthetics involves decellularization via a variety of methods to influence the native biochemical and biomolecular structure of the collagen scaffold [37]. The processing of hADM is one example of how processing of a tissue may change its architecture [38]. Compared to native hADM, the processed tissue underwent noticeable changes to the ultrastructure (Fig. 1) [38]. The loss of the collagen integrity after processing may lead to an increased inflammatory reaction and increased fibrous capsule [38]. While the mechanical properties may not be ideally suitable for incisional hernia repair where strong mechanical forces are applied to the prosthetic, hADM has been used extensively in breast reconstruction and tissue expansion prior to definitive reconstruction because of its robust ability to neovascularize [36, 39,40,41].
Scanning electron micrograph of out-of-package morphology of hADMs (100×). Native hADM, electron beam-irradiated hADM (e-HADM), γ-irradiated hADM (g-HADM), ethanol-stored hADM (EtOH-hADM) [38]
Porcine dermal tissue has also been a common biological mesh used in abdominal wall repair, Strattice™ (LifeCell Corporation, Bridgewater, NJ, USA) [42]. This non-cross-linked porcine ADM has been shown to have minimal adhesion formation and bowel erosions, complications associated with synthetic mesh repair, but is predisposed to recurrence, particularly when used as a bridge [4]. Butler et al. [43] reported non-cross-linked PADM to have a quicker infiltration with host cells and vessels in comparison to cross-linked PADM, which were encapsulated. Also, non-cross-linked PADMs had weaker intraperitoneal adhesions at repair sites with increasing mechanical strength at an earlier time at the bioprosthesis-musculofascial junction. More recently the use of non-cross-linked porcine ADM has increased as studies continue to demonstrate its ability to resist infection and withstand mechanical forces of the abdominal wall [44, 45]. The rates of recurrence are similar to those with synthetic mesh repair when used in conjunction with component separation. The use of acellular dermal matrices in hernia repair and abdominal wall reconstruction was associated with 11.5–14.6% hernia recurrence rates at 3-–5-years follow-up [46].
Permacol™ (supplemental cross-linked; Covidien, New Haven, CT, USA) is a bioprosthetic made from porcine dermal collagen with post-processing cross-linking of proteins. The cross-linking is performed in order to add strength to the material and reduce its inflammatory response [47]. Traditionally, porcine acellular dermal matrices (PADMs) were noted to induce greater immune response than human acellular dermal matrix (hADM) and thus have been processed to chemically cross-link the collagen fibers [43]. Collagen cross-linking has been noted to have a key role on tissue response to biologic meshes, which alters the extracellular matrix structure and possibly inhibits cellular infiltration, revascularization, and matrix remodeling potential [48]. The structure, similar in structure to human dermis, can support fibroblast infiltration and neovascularization [49]. However, its strengthened cross-linked architecture may actually hinder the material’s success in remodeling and neovascularization [29, 50]. Integration of Permacol™ into host tissue and angiogenesis, though delayed, help to facilitate antibiotic diffusion and help to resist infections [51]. Some studies noted the rate of complications in cross-linked porcine dermal collagen mesh was double than that of their non-cross-linked porcine dermal counterparts [43].
6 Small Intestinal Submucosa
Small intestinal submucosa (SIS) is another tissue for decellularized matrices used in abdominal wall reconstruction. Originally investigated as a bioprosthetic for use in vascular repair, SIS emerged as a potential prosthetic for use in hernia repair [52, 53]. Today, the most commonly placed SIS mesh in abdominal wall repair is constructed from porcine SIS, Surgisis™ (Cook Surgical, Bloomington, IN). It notably has a lower recurrence rate than AlloDerm and Permacol in a comparative study (8.0% rather than 20.8% and 10.9%, respectively) [54]. Some authors note that Surgisis starts strong at first, but loses strength with remodeling [26, 55]. The small intestine is a more vascular organ than the dermis, and neovascularization could play a role. Studies investigating its use in laparoscopic hernia repair were promising, and more recently its use in the contaminated field has been shown to be safe [56, 57].
7 Bovine Pericardium
Bovine pericardium consists of collagenous connective tissue with three-dimensional intertwined fibers. Initial studies showed that the bovine pericardium might not have stood up to the test in hernia repair with either early resorption or poor incorporation in animal models [58]. Tutomesh® (Tutogen Medical GmbH Germany) has been praised for retaining multidirectional strength and keeping the elasticity of the original tissue, yielding good results [29, 59]. The product has less elastin relative to dermal products, resulting in a higher ratio of mature collagen to elastin and reducing pseudo-recurrence [60]. When compared to fascia lata, it was found to be superior in burst strength and adhesion formation [61]. More recently, attention has been paid to its potential use as a prosthetic in contaminated hernia repair. It has been demonstrated to be safe and effective in repair in the contaminated field and particularly in the setting of bowel resection [62].
8 Liver
Biologic mesh has been an available alternative to permanent synthetic mesh for over 20 years. Various biologic meshes, specifically porcine tissue prostheses, have been evaluated. More recently, porcine liver has been decellularized for use in both transplantation science and in decellularized implant tissue engineering (Fig. 2) [63,64,65]. Petro et al. [66] have demonstrated application of porcine liver prosthesis for hernia repair. They utilized a novel prosthetic, Miromesh, a biologic mesh derived from porcine liver. They were able to demonstrate the efficacy of Miromesh in comparison to Strattice in regard to cellular infiltration, acute inflammation, chronic inflammation, granulation tissue, foreign body reaction, and fibrous capsule formation [66]. The advantage in using Miromesh compared to Strattice and other dermal prostheses is its proprietary perfusion decellularization with intact portal triads that provide optimal collagen scaffold for cellular infiltration. The study conducted revealed that Miromesh had greater cellular infiltration with comparable clearance of bacteria. However, there are some weaknesses associated with the use of Miromesh, which include heterogeneity, large porosity, and lower density matrix, which could negatively impact the longtime durability and mechanical ability of the inserted mesh. Further studies are needed to investigate the use of porcine liver prostheses.
Native and decellularized liver. Representative H&E staining of the ultrastructure of native liver (a) and decellularized liver matrix (b). Scale bars = 100 μm. DAPI staining demonstrated a lack of nuclear components suggestive of complete decellularization (c, d). Sirius red staining of different types of collagen and proteoglycan shows retention of important ECM substrates (e, f). Scale bars = 200 μm [63]
9 Use in the Contaminated Field
Since the introduction of biologic mesh material, it has been viewed as a promising alternative to synthetic mesh by providing cellular infiltration, neovascularization, and potentially regeneration into native tissue [67, 68]. These specific properties of biologic material may lead to superior outcomes over synthetic material in the setting of contamination [67]. In addition, biologics have been used with some success to repair complex abdominal wall defects in clean-contaminated and infected fields when synthetic mesh is contraindicated. Management of contaminated ventral hernia repairs has been evaluated over the years; however, there is still no consensus about the most optimal and durable repair. Some authors have argued for a multistage reconstructive approach, which includes delayed definitive reconstruction 6–12 months later with component separation when inflammation and dense adhesions have resolved [69]. Rosen et al. [67], in a retrospective study, were able to demonstrate that biologic mesh reinforcement can be safely performed in the repair of ventral hernias in contaminated fields in a single-stage approach. Despite a high rate of wound morbidity in the study, this did not lead to complete mesh excision nor did it include mesh infections. The study evaluated patients undergoing single-staged ventral hernia repairs in a contaminated field using biologic mesh over a 5-year period. The outcomes included postoperative wound complications in 47.7% and hernia recurrence in 31.3% of the patient population. The high rate of wound morbidity can be accounted for based on the ASA score, recent history of smoking, diabetes mellitus, number of previous abdominal surgeries, number of previous hernia repairs, hernia defect size, bridged defects, and long operative times [67]. Short-term efficacy can be noted with the probability of recurrence at 1 year at 8%. When taken to 24 months, patients who underwent a hernia repair in the contaminated setting had successful repair as a single-stage procedure using porcine ADM [70]. Few randomized controlled comparative studies have compared complication rates on all biomeshes on the market, and currently there is no gold standard [71]. More research is needed to find the most cost-effective mesh with the fewest instances of recurrence. As the source tissue for decellularization continues to expand, we must continue to broaden our view of what is considered a possible tissue. Many organs because of their inherent mechanical strength may not initially be thought of as candidate for hernia repair. Many of the tissues used are connective in nature, but because of this property may not be optimized for the biologic response required for long-term repair. An alternative approach, focusing on the potential for biologic optimization and incorporation, may provide answers as to the expansion of potential sources such as the lung.
10 Porcine Acellular Lung Matrix
The generation of decellularized lung tissue began with an effort to address the growing need for tissue-engineered approaches for whole lung regeneration [72]. Of the organs procured for transplantation, the lung is the most sensitive to ischemia and the most damaged as a result of the retrieval and preservation process [73]. While transplantation wait list times continue to go down, the need for organs has not and the need is as great as ever. One approach to this grave problem is the regeneration of whole lung tissue. While other organs have undergone decellularization processes in order to make them suitable for implantation, the lung, with a complex and multifunctional system, had not been investigated for this purpose in humans. In his seminal work, Ott et al. [74] described and perfected the process of lung decellularization and recellularization with autologous cells and implantation (Fig. 3). This work paved the way for more advanced approaches and scaling up of the model for human use.
Decellularized lung is capable of cell adherence and proliferation. (a) A representative stitched image showing endothelial coverage of a HUVEC-hMSC regenerated lung lobe after two-phase culture (CD31, red; laminin, green; DAPI, blue). (b) Interconnected vascular network structures formed by endothelial cells (CD31, red) with individual hMSCs (SM22-α, green) adhering to the network. (c) Establishment of apical-basal polarity shown by localization of PODXL (green) on the luminal surface and COLIV (red) on the basement surface. (d) A representative whole-mount image of decellularized rat lungs perfused with green-fluorescent microspheres (0.2 μm) through the PA. (e) A representative whole-mount image of decellularized rat lungs perfused with green-fluorescent microspheres (0.2 μm) through the PA and red-fluorescent microspheres (0.2 μm) through the PV
Nichols et al. have described porcine acellular lung matrix (PALM) as a natural scaffold capable of cell attachment while maintaining cell viability [72, 75]. Porcine lungs were taken and processed through both mechanical infusion of decreasing gradient of SDS (Fig. 4) [75]. In their work, they were able to demonstrate that PALMs can sustain forces of mechanical ventilation for prolonged periods of time without changes to macro- or microstructure of the tissue due to its predominance of collagen I and elastin [75]. In addition, PALMs were noted to have minimal inflammatory response with minimal apoptosis of mesenchymal stem cells or human alveolar epithelial cells. The extracellular matrix (ECM) proteins play an important role in influencing lung strength, flexibility, and elasticity [72]. It is vital for production of decellularized lung to retain key ECM components while removing cell debris and nucleic acid through exposure to detergents and physical methods. More recently, Dr. Ott’s team also demonstrated the capacity of PALM to sustain human cells, survive implantation in a pig model of lung transplantation, and withstand the forces of mechanical ventilation allowing for gas exchange [76]. Various detergents are used for decellularization of the lung including sodium dodecyl sulfate (SDS), sodium deoxycholate (SDC), and 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS). SDS-based perfusion decellularization has been shown to produce acellular lung scaffold with loss of DNA while preserving ECM composition and architecture [77]. The preservation of this architecture and ECM composition is vital to the success of any tissue-based scaffold, and in this case the PALM. Thus, with a the lack of optimal bioprosthetics material for ventral hernia repair, there is an ongoing search for ideal mesh materials that will provide long-term efficacy with improved biological activity and incorporation into native tissue.
11 PALM in Hernia Repair
Critical to the success of any tissue repair is the robust infiltration of reparative cells and blood vessels. With their rich vascular architecture, the lung theoretically makes an ideal matrix suitable for cell and blood vessel infiltration. Biomedical engineers have long investigated the ideal microarchitecture for angiogenesis. To achieve this, the material must possess a controlled interconnectivity of pores that supports the invasion and proliferation of progenitor cells that will ultimately recapitulate the natural environment [78]. Not only must the porosity be ideal for the migration of cells but also for the diffusion of nutrients and product of cellular activity and to maintain cell-cell and cell-scaffold interaction [78, 79]. Researchers have developed patterned biomaterials mimicking the natural environment with intricate architectures and variable porosity in an attempt to promote angiogenesis [80, 81]. Likewise, investigators have sought out natural materials with high vascularity in order to promote cellularization and neovascularization, two metrics of incorporation [18, 19, 36, 59, 82]. As mentioned above, the dermis, pericardium, submucosa, and liver have all been investigated as tissue sources for enhanced incorporation. With this in mind, we sought to identify another source of highly vascularized tissue to test in the setting of bridging ventral hernia repair.
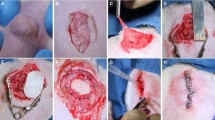
To this end, Fernandez-Moure et al. evaluated the efficacy of PALMs in chronic hernia repair in rat models in comparison to the human acellular dermal matrix (hADM) [83]. In the study, the porcine lungs were processed via a perfusion decellularization process using SDS solution, and decontamination was performed by perfusing streptomycin, penicillin, and amphotericin B prior to dissection. Effectiveness of the decellularization of the porcine lungs was evaluated using hematoxylin and eosin (H&E) staining, which showed loss of cells throughout the tissue. Also, scanning electron microscopy (SEM) showed the highly organized structure of the lung that contributes to its high potential for neovascularization (Fig. 5). Subcutaneous implantation of PALM and hADM showed PALM was capable of robust macroscopic neovascularization compared to hADM (Fig. 6). The animal model was one of a chronic hernia fixed with a bridging repair. The authors feel this approach while not necessarily suitable for human translation is optimal for evaluating mesh under mechanical forces. The rats were divided in two groups, and each animal had hernia repair with bridging repair with implantation of either PALM or hADM. After 6 weeks, the PALMs group demonstrated significantly greater cell infiltration and cell density compared to the hADM group. Also, the PALMs group showed a greater number of vessels per 1 mm2 and up to six times the number of vessels per field of view compared to hADM (Fig. 7). Given the innate architecture of the lung, the authors felt this along with the preserved ECM components contributed to the significant incorporation and the lack of re-herniation or scaffold breakdown. The lung, unlike other bioprosthetics, does not maintain its mechanical properties when decellularized. After processing the lung tissue becomes very soft and fragile. Another critical finding they noted was that PALM implants did not undergo significant bulge or mechanical failure, which is clinically relevant since bulge is a predictor of long-term mechanical failure (Fig. 8) [84]. Based on the characteristics for metrics of incorporation with cell infiltration and vessel formation, the study demonstrated that PALMs have superior surgical outcomes compared to hADM.
Characterization of decellularization. Hematoxylin and eosin staining at (a) day 0 and (b) day 7 confirms complete decellularization following SDS baths (scale bar, 150 μm; inset 40× magnification scale bar 20 μm). (c) Well-formed and cellularized tubular vascular structures are in lung tissue prior to processing (scale bar, 100 μm). (d) Decellularized vascular structures retain tubular morphology and architecture (scale bar, 100 μm)
PALMs demonstrate greater vessel formation. Representative images of Masson’s trichrome-stained implants following (a, c) subcutaneous implantation and (b, d) implanted for hernia repair. PALMs had greater vessel infiltration (white and black arrows) compared with hADM (scale bar, 50 μm) (insert 40× magnification; scale bar, 20 μm)
Conclusions
Abdominal wall hernias continue to be a large socioeconomic burden in the USA. Currently, the most commonly used prosthetics are purely synthetic in nature and carry significant risks with them. To address this issues investigated have looked to nature for biologically derived tissues as prosthetics. Various decellularization technologies have emerged, as have various tissue sources for prosthetic repair. The dermis remains the most commonly used and most studied although it may not be ideal for repair as evidenced by unacceptable long-term failure rates in bridging repair. Thus, research have sought new tissue sources based on the microarchitecture of the substrate organ in order to maximally promote the metrics of incorporation, namely, cell infiltration and vascularization. PALM has been investigated as a novel prosthetic for repair and has demonstrated enhanced incorporation and short-term mechanical stability in a chronic ventral incisional hernia model with bridging repair. We hope this focus of pre-existing microarchitecture and gentle decellularization technologies will foster a new avenue of thought when evaluating current prosthetics and those to come.
References
Poulose BK, Shelton J, Phillips S, Moore D, Nealon W, Penson D, Beck W, Holzman MD (2012) Epidemiology and cost of ventral hernia repair: making the case for hernia research. Hernia 16(2):179–183
Goodenough CJ, Ko TC, Kao LS, Nguyen MT, Holihan JL, Alawadi Z, Nguyen DH, Flores JR, Arita NT, Roth JS, Liang MK (2015) Development and validation of a risk stratification score for ventral incisional hernia after abdominal surgery: hernia expectation rates in intra-abdominal surgery (The HERNIA Project). J Am Coll Surg 220(4):405–413
Martindale RG, Deveney CW (2013) Preoperative risk reduction: strategies to optimize outcomes. Surg Clin North Am 93(5):1041–1055
FitzGerald JF, Kumar AS (2014) Biologic versus synthetic mesh reinforcement: what are the pros and cons? Clin Colon Rectal Surg 27(4):140–148
Garcia A, Baldoni A (2015) Complex ventral hernia repair with a human acellular dermal matrix and component separation: a case series. Ann Med Surg (Lond) 4(3):271–278
Beadles CA, Meagher AD, Charles AG (2015) Trends in emergent hernia repair in the United States. JAMA Surg 150(3):194–200
Basile F, Biondi A, Donati M (2013) Surgical approach to abdominal wall defects: history and new trends. Int J Surg 11(Suppl 1):S20–S23
Primatesta P, Goldacre MJ (1996) Inguinal hernia repair: incidence of elective and emergency surgery, readmission and mortality. Int J Epidemiol 25(4):835–839
Chand M, On J, Bevan K, Mostafid H, Venkatsubramaniam AK (2012) Mesh erosion following laparoscopic incisional hernia repair. Hernia 16(2):223–226
Gandhi D, Marcin S, Xin Z, Asha B, Kaswala D, Zamir B (2011) Chronic abdominal pain secondary to mesh erosion into cecum following incisional hernia repair: a case report and literature review. Ann Gastroenterol 24(4):321–324
Collage RD, Rosengart MR (2010) Abdominal wall infections with in situ mesh. Surg Infect 11(3):311–318
Kuehnert N, Kraemer NA, Otto J, Donker HC, Slabu I, Baumann M, Kuhl CK, Klinge U (2012) In vivo MRI visualization of mesh shrinkage using surgical implants loaded with superparamagnetic iron oxides. Surg Endosc 26(5):1468–1475
Schoenmaeckers EJ, van der Valk SB, van den Hout HW, Raymakers JF, Rakic S (2009) Computed tomographic measurements of mesh shrinkage after laparoscopic ventral incisional hernia repair with an expanded polytetrafluoroethylene mesh. Surg Endosc 23(7):1620–1623
Campanelli G, Bertocchi V, Cavalli M, Bombini G, Biondi A, Tentorio T, Sfeclan C, Canziani M (2013) Surgical treatment of chronic pain after inguinal hernia repair. Hernia 17(3):347–353
Aroori S, Spence RAJ (2007) Chronic pain after hernia surgery—an informed consent issue. Ulster Med J 76(3):136–140
Starling JR, Harms BA, Schroeder ME, Eichman PL (1987) Diagnosis and treatment of genitofemoral and ilioinguinal entrapment neuralgia. Surgery 102(4):581–586
Rastegarpour A, Cheung M, Vardhan M, Ibrahim MM, Butler CE, Levinson H (2016) Surgical mesh for ventral incisional hernia repairs: Understanding mesh design. Plast Surg (Oakv) 24(1):41–50
Bellows CF, Alder A, Helton WS (2006) Abdominal wall reconstruction using biological tissue grafts: present status and future opportunities. Expert Rev Med Devices 3(5):657–675
Franklin ME Jr, Gonzalez JJ Jr, Glass JL (2004) Use of porcine small intestinal submucosa as a prosthetic device for laparoscopic repair of hernias in contaminated fields: 2-year follow-up. Hernia 8(3):186–189
Cevasco M, Itani KM (2012) Ventral hernia repair with synthetic, composite, and biologic mesh: characteristics, indications, and infection profile. Surg Infect 13(4):209–215
Bringman S, Conze J, Cuccurullo D, Deprest J, Junge K, Klosterhalfen B, Parra-Davila E, Ramshaw B, Schumpelick V (2010) Hernia repair: the search for ideal meshes. Hernia 14(1):81–87
Gandolfo L, Donati M, Palmeri S, Brancato G, Donati A (2006) Late cutaneous fistula after inguinal hernia repair. A case report. Ann Ital Chir 77(5):447–450
Klinge U, Klosterhalfen B, Müller M, Schumpelick V (1999) Foreign body reaction to meshes used for the repair of abdominal wall hernias. Eur J Surg 165(7):665–673
Bowman KL, Birchard SJ, Bright RM (1998) Complications associated with the implantation of polypropylene mesh in dogs and cats: a retrospective study of 21 cases (1984-1996). J Am Anim Hosp Assoc 34(3):225–233
Aguirre DA, Santosa AC, Casola G, Sirlin CB (2005) Abdominal wall hernias: imaging features, complications, and diagnostic pitfalls at multi–detector row CT. Radiographics 25(6):1501–1520
Brown C, Finch J (2010) Which mesh for hernia repair? Ann R Coll Surg Engl 92(4):272–278
Junge K, Klinge U, Rosch R, Mertens PR, Kirch J, Klosterhalfen B, Lynen P, Schumpelick V (2004) Decreased collagen type I/III ratio in patients with recurring hernia after implantation of alloplastic prostheses. Langenbeck’s Arch Surg 389(1):17–22
Klosterhalfen B, Junge K, Klinge U (2005) The lightweight and large porous mesh concept for hernia repair. Expert Rev Med Devices 2(1):103–117
Cavallaro A, Lo Menzo E, Di Vita M, Zanghì A, Cavallaro V, Veroux PF, Cappellani A (2010) Use of biological meshes for abdominal wall reconstruction in highly contaminated fields. World J Gastroenterol 16(15):1928–1933
Bilsel Y, Abci I (2012) The search for ideal hernia repair; mesh materials and types. Int J Surg 10(6):317–321
Ferzoco SJ (2013) A systematic review of outcomes following repair of complex ventral incisional hernias with biologic mesh. Int Surg 98(4):399–408
Baumann DP, Butler CE (2012) bioprosthetic mesh in abdominal wall reconstruction. Semin Plast Surg 26(1):18–24
Holton LH 3rd, Kim D, Silverman RP, Rodriguez ED, Singh N, Goldberg NH (2005) Human acellular dermal matrix for repair of abdominal wall defects: review of clinical experience and experimental data. J Long-Term Eff Med Implants 15(5):547–558
Primus FE, Harris HW (2013) A critical review of biologic mesh use in ventral hernia repairs under contaminated conditions. Hernia 17(1):21–30
Deeken CR, Melman L, Jenkins ED, Greco SC, Frisella MM, Matthews BD (2011) Histologic and biomechanical evaluation of crosslinked and non-crosslinked biologic meshes in a porcine model of ventral incisional hernia repair. J Am Coll Surg 212(5):880–888
Misra S, Raj PK, Tarr SM, Treat RC (2008) Results of AlloDerm use in abdominal hernia repair. Hernia 12(3):247–250
Novitsky YW, Rosen MJ (2012) The biology of biologics: basic science and clinical concepts. Plast Reconstr Surg 130(5 Suppl 2):9s–17s
Sandor M, Leamy P, Assan P, Hoonjan A, Huang LT, Edwards M, Zuo W, Li H, Xu H (2017) Relevant In vitro predictors of Human Acellular Dermal Matrix-associated inflammation and capsule formation in a nonhuman primate subcutaneous tissue expander model. Eplasty 17:e1
Zenn MR, Salzberg CA (2016) A direct comparison of alloderm-ready to use (RTU) and DermACELL in immediate breast implant reconstruction. Eplasty 16:e23
Weiss SR, Tenney JM, Thomson JL, Anthony CT, Chiu ES, Friedlander PL, Woltering EA (2010) The effect of AlloDerm on the initiation and growth of human neovessels. Laryngoscope 120(3):443–449
Newman MI, Samson MC, Berho M (2009) AlloDerm in breast reconstruction: 2 years later. Plast Reconstr Surg 123(6):205e–206e
Romain B, Story F, Meyer N, Delhorme JB, Brigand C, Rohr S (2016) Comparative study between biologic porcine dermal meshes: risk factors of postoperative morbidity and recurrence. J Wound Care 25(6):320–325
Butler CE, Burns NK, Campbell KT, Mathur AB, Jaffari MV, Rios CN (2010) Comparison of cross-linked and non-cross-linked porcine acellular dermal matrices for ventral hernia repair. J Am Coll Surg 211(3):368–376
Broyles JM, Abt NB, Sacks JM, Butler CE (2013) Bioprosthetic tissue matrices in complex abdominal wall reconstruction. Plast Reconstr Surg Glob Open 1(9):e91
Patel KM, Albino FP, Nahabedian MY, Bhanot P (2013) Critical analysis of Strattice performance in complex abdominal wall reconstruction: intermediate-risk patients and early complications. Int Surg 98(4):379–384
Garvey PB, Giordano SA, Baumann DP, Liu J, Butler CE (2017) Long-term outcomes after abdominal wall reconstruction with Acellular Dermal Matrix. J Am Coll Surg 224(3):341–350
Li J, Ren N, Qiu J, Jiang H, Zhao H, Wang G, Boughton RI, Wang Y, Liu H (2013) Carbodiimide crosslinked collagen from porcine dermal matrix for high-strength tissue engineering scaffold. Int J Biol Macromol 61:69–74
Butler CE (2006) The role of bioprosthetics in abdominal wall reconstruction. Clin Plast Surg 33(2):199–211
Dieterich M (2013) Biological matrices and synthetic meshes used in implant-based breast. Geburtshilfe Frauenheilkd 73(11):1100–1106
Fernandez-Moure JS, Van Eps JL, Peterson LE, Shirkey BA, Menn ZK, Cabrera FJ, Karim A, Tasciotti E, Weiner BK, Ellsworth WA 4th (2017) Cross-linking of porcine acellular dermal matrices negatively affects induced neovessel formation using platelet-rich plasma in a rat model of hernia repair. Wound Repair Regen 25(1):98–108
Parker DM, Armstrong PJ, Frizzi JD, North JH Jr (2006) Porcine dermal collagen (Permacol) for abdominal wall reconstruction. Curr Surg 63(4):255–258
Lantz GC, Badylak SF, Coffey AC, Geddes LA, Blevins WE (1990) Small intestinal submucosa as a small-diameter arterial graft in the dog. J Investig Surg 3(3):217–227
Sandusky GE, Lantz GC, Badylak SF (1995) Healing comparison of small intestine submucosa and ePTFE grafts in the canine carotid artery. J Surg Res 58(4):415–420
Beale EW, Hoxworth RE, Livingston EH, Trussler AP (2012) The role of biologic mesh in abdominal wall reconstruction: a systematic review of the current literature. Am J Surg 204(4):510–517
Annor AH, Tang ME, Pui CL, Ebersole GC, Frisella MM, Matthews BD, Deeken CR (2012) Effect of enzymatic degradation on the mechanical properties of biological scaffold materials. Surg Endosc 26(10):2767–2778
Edelman DS (2002) Laparoscopic herniorrhaphy with porcine small intestinal submucosa: a preliminary study. JSLS 6(3):203–205
Petter-Puchner AH, Fortelny RH (2010) Use of porcine small intestine submucosa as a prosthetic material for laparoscopic hernia repair in infected and potentially contaminated fields: long-term follow up assessment; Surg Endosc. (2008) 22: 1941-1946. Surg Endosc 24(1):230–231
James NL, Poole-Warren LA, Schindhelm K, Milthorpe BK, Mitchell RM, Mitchell RE, Howlett CR (1991) Comparative evaluation of treated bovine pericardium as a xenograft for hernia repair. Biomaterials 12(9):801–809
Limpert JN, Desai AR, Kumpf AL, Fallucco MA, Aridge DL (2009) Repair of abdominal wall defects with bovine pericardium. Am J Surg 198(5):e60–e65
Jin J, Rosen MJ, Blatnik J, McGee MF, Williams CP, Marks J, Ponsky J (2007) Use of acellular dermal matrix for complicated ventral hernia repair: does technique affect outcomes? J Am Coll Surg 205(5):654–660
Kapan S, Kapan M, Goksoy E, Karabicak I, Oktar H (2003) Comparison of PTFE, pericardium bovine and fascia lata for repair of incisional hernia in rat model, experimental study. Hernia 7(1):39–43
Gurrado A, Franco IF, Lissidini G, Greco G, De Fazio M, Pasculli A, Girardi A, Piccinni G, Memeo V, Testini M (2015) Impact of pericardium bovine patch (Tutomesh((R))) on incisional hernia treatment in contaminated or potentially contaminated fields: retrospective comparative study. Hernia 19(2):259–266
Wang Y, Bao J, Wu X, Wu Q, Li Y, Zhou Y, Li L, Bu H (2016) Genipin crosslinking reduced the immunogenicity of xenogeneic decellularized porcine whole-liver matrices through regulation of immune cell proliferation and polarization. Sci Rep 6:24779
Hussein KH, Park KM, Kim HM, Teotia PK, Ghim JH, Woo HM (2015) Construction of a biocompatible decellularized porcine hepatic lobe for liver bioengineering. Int J Artif Organs 38(2):96–104
Barakat O, Abbasi S, Rodriguez G, Rios J, Wood RP, Ozaki C, Holley LS, Gauthier PK (2012) Use of decellularized porcine liver for engineering humanized liver organ. J Surg Res 173(1):e11–e25
Petro CC, Prabhu AS, Liu L, Majumder A, Anderson JM, Rosen MJ (2016) An in vivo analysis of Miromesh—a novel porcine liver prosthetic created by perfusion decellularization. J Surg Res 201(1):29–37
Rosen MJ, Krpata DM, Ermlich B, Blatnik JA (2013) A 5-year clinical experience with single-staged repairs of infected and contaminated abdominal wall defects utilizing biologic mesh. Ann Surg 257(6):991–996
Xu H, Wan H, Sandor M, Qi S, Ervin F, Harper JR, Silverman RP, McQuillan DJ (2008) Host response to human acellular dermal matrix transplantation in a primate model of abdominal wall repair. Tissue Eng Part A 14(12):2009–2019
Jernigan TW, Fabian TC, Croce MA, Moore N, Pritchard FE, Minard G, Bee TK (2003) Staged management of giant abdominal wall defects: acute and long-term results. Ann Surg 238(3):349–355
Itani KM, Rosen M, Vargo D, Awad SS, Denoto G 3rd, Butler CE, RICH Study Group (2012) Prospective study of single-stage repair of contaminated hernias using a biologic porcine tissue matrix: the RICH Study. Surgery 152(3):498–505
Finan KR, Kilgore ML, Hawn MT (2009) Open suture versus mesh repair of primary incisional hernias: a cost-utility analysis. Hernia 13(2):173–182
Nichols JE, Niles JA, Cortiella J (2012) Production and utilization of acellular lung scaffolds in tissue engineering. J Cell Biochem 113(7):2185–2192
Chen F, Date H (2015) Update on ischemia-reperfusion injury in lung transplantation. Curr Opin Organ Transplant 20(5):515–520
Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP (2010) Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med 16(8):927–933
Nichols JE, Niles J, Riddle M, Vargas G, Schilagard T, Ma L, Edward K, La Francesca S, Sakamoto J, Vega S, Ogadegbe M, Mlcak R, Deyo D, Woodson L, McQuitty C, Lick S, Beckles D, Melo E, Cortiella J (2013) Production and assessment of decellularized pig and human lung scaffolds. Tissue Eng Part A 19(17–18):2045–2062
Zhou H, Kitano K, Ren X, Rajab TK, Wu M, Gilpin SE, Wu T, Baugh L, Black LD, Mathisen DJ, Ott HC (2017) Bioengineering human lung grafts on porcine matrix. Ann Surg. In publication
Gilpin SE, Guyette JP, Gonzalez G, Ren X, Asara JM, Mathisen DJ, Vacanti JP, Ott HC (2014) Perfusion decellularization of human and porcine lungs: bringing the matrix to clinical scale. J Heart Lung Transplant 33(3):298–308
Salerno A, Guarnieri D, Iannone M, Zeppetelli S, Netti PA (2010) Effect of micro- and macroporosity of bone tissue three-dimensional-poly(epsilon-caprolactone) scaffold on human mesenchymal stem cells invasion, proliferation, and differentiation in vitro. Tissue Eng Part A 16(8):2661–2673
Guaccio A, Guarino V, Perez MA, Cirillo V, Netti PA, Ambrosio L (2011) Influence of electrospun fiber mesh size on hMSC oxygen metabolism in 3D collagen matrices: experimental and theoretical evidences. Biotechnol Bioeng 108(8):1965–1976
Nomi M, Atala A, Coppi PD, Soker S (2002) Principals of neovascularization for tissue engineering. Mol Asp Med 23(6):463–483
Bramfeldt H, Sabra G, Centis V, Vermette P (2010) Scaffold vascularization: a challenge for three-dimensional tissue engineering. Curr Med Chem 17(33):3944–3967
Campbell KT, Burns NK, Ensor J, Butler CE (2012) Metrics of cellular and vascular infiltration of human acellular dermal matrix in ventral hernia repairs. Plast Reconstr Surg 129(4):888–896
Fernandez-Moure JS, Van Eps JL, Rhudy JR, Cabrera FJ, Acharya GS, Tasciotti E, Sakamoto J, Nichols JE (2016) Porcine acellular lung matrix for wound healing and abdominal wall reconstruction: a pilot study. J Tissue Eng 7:2041731415626018
Schoenmaeckers EJ, Wassenaar EB, Raymakers JT, Rakic S (2010) Bulging of the mesh after laparoscopic repair of ventral and incisional hernias. JSLS 14(4):541–546
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Chegireddy, V., Caplan, K.D., Fernandez-Moure, J.S. (2018). Porcine Acellular Lung Matrix in Wound Healing and Hernia Repair. In: Shiffman, M., Low, M. (eds) Chronic Wounds, Wound Dressings and Wound Healing. Recent Clinical Techniques, Results, and Research in Wounds, vol 6. Springer, Cham. https://doi.org/10.1007/15695_2017_102
Download citation
DOI: https://doi.org/10.1007/15695_2017_102
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-10697-3
Online ISBN: 978-3-030-10698-0
eBook Packages: MedicineMedicine (R0)