Abstract
As a result of increasingly stringent environmental regulations being imposed on the automotive sector, ecofriendly alternative solutions are being sought through the use of next-generation bioplastics and biocomposites as novel vehicle components. Thanks to its renewability, low cost, high strength, and rigidity, poly(lactic acid), PLLA, is considered a key material for such applications. Nevertheless, to compete with traditional petroleum-sourced plastics some of the properties of PLLA must be improved to fulfill the requirements of the automotive industry, such as heat resistance, mechanical performance (especially in terms of ductility and impact toughness), and durability. This review focuses on the properties required for plastics used in the automotive industry and discusses recent breakthroughs regarding PLLA and PLLA-based materials in this field.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Reducing vehicle weight is an important issue for the automotive industry, and plastics are attractive materials for addressing this challenge. Because of their renewable origin, bioplastics help minimize the environmental impact of car production by further reducing CO2 emissions and energy consumption [1, 2]. Besides, it is crucial for the industry to develop sustainable alternatives to products derived from petroleum oil, because the price of petroleum oil is unstable and its feedstock is expected to run out in the near future. Of the bioplastics existing today on the market, some are already suitable for the automotive sector, particularly formulations based on poly(lactic acid) (PLLA)and its composites [3]. Several key points contribute to the success of PLLA, especially its excellent (bio)degradability/recyclability and attractive physical and mechanical properties such as high rigidity, strength, and easy processability using conventional processing techniques such as extrusion and injection molding [4, 5]. However, although PLLA can fulfill environmental regulations regarding the automotive sector, the development of technical PLLA-based materials for automotive applications still encompasses some thresholds that have not yet been achieved. These requirements include high toughness, durability, processability at high temperature, and high production rate at affordable cost [6].
Several strategies such as blending with petroleum-based polymers, plasticizers, impact-modifiers, and micro- and/or nanosized fillers have thereby been proposed in efforts to overcome these drawbacks [7]. This chapter describes the great potential of PLLA-based materials and their future trends, while mentioning their current drawbacks and the improvements required to sustain their successful implementation in the automotive sector. Special emphasis is placed on the current and future use of PLLA-based materials for some automobile parts as both bulk and fibrous materials.
2 (Bio)plastics in the Automotive Industry
The plastics industry plays a significant role in the environmental, societal, and economic dimensions of sustainable development. Plastics meet the societal demands of today’s products in packaging, lightweight components for cars and aircraft, electronic housing, insulating materials for buildings, medical devices, etc. Yet, the plastics industry still has to face up to great challenges concerning improvements in safety, protection of the environment, and energy-saving in plastics manufacturing processes as well as during their lifetime. In the automotive sector, plastics and polymer composites are more and more appealing because their light weight can help decrease the fuel consumption of vehicles, without adversely affecting safety issues. Indeed, automotive manufacturers are tending to replace traditional materials such as metals and metal alloys for lightweight materials such as plastics and composites [8, 9]. As an illustration, the contribution of plastics to the average weight of a vehicle is presented in Fig. 1. Today, plastics typically make up 16% of the average weight of a new vehicle and will account for 18% by 2020, with more than 50% of a modern vehicle’s volume. This renders cars lighter and more fuel efficient, resulting in lower greenhouse gas emissions [10, 11].
Evolution of the percentage of plastics in vehicles by weight. Reproduced with permission from [10] Copyright © 2012, A.T. Kearney, Inc
Furthermore, an important challenge for the twenty-first century is undoubtedly sustainable management of resources, accompanied by new environmental regulations and a strengthening bioeconomy. This latter refers to the sustainable production and conversion of renewable resources from agriculture into energy and a large range of food, health, fiber, and industrial products [12]. This trend is pushing automotive manufacturers to propose renewable alternatives to traditional plastics, while fulfilling current and new specifications for automotive parts, the aim being production of smarter, lighter, greener and, if possible, low cost cars. In that context, bioplastics meet these requirements because they have similar structural and functional characteristics as their petroleum-sourced counterparts. Note that the term “bioplastics” covers materials that are both partially and fully biosourced. Beyond their initial use for packaging applications [13,14,15], bioplastics have reached a very high level of maturity for a large range of automotive applications, offering high performance together with reduced environmental impact. It is therefore not surprising that the use of bioplastics is continuously increasing in technical applications, including for the automotive sector (Fig. 2).
Global production capacities of bioplastics 2017 by market segment. Reproduced with permission from [16]
2.1 Technical Requirements for Plastics Used in Car Applications
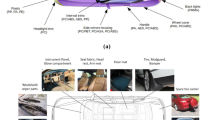
Today, plastics are employed not only to build internal parts in cars but also external components such as bumpers, body panels, laminated safety glass, trims, and many others (Fig. 3). In exterior applications, plastics are not only used for their light weight, but also because they give designers the freedom to create innovative concepts, for instance parts of a complex shape that could not be massively manufactured using other materials such as metals. In other words, plastics are nothing less than revolutionary. They have shown to be great materials for creating comfortable, durable, and aesthetically pleasing interior components, while preserving occupant protection and reducing noise and vibration levels. Furthermore, they have proven to be strong, durable, corrosion-resistant, and able to withstand high temperatures under harsh engine environments. These properties make plastics suitable for electrical, powertrain, fuel, chassis, and engine applications.
Usually, the choice of materials made by vehicle manufacturers depends on a combination of several criteria. Some of the criteria are the result of regulation and legislation on environmental and safety concerns. Other criteria relate to production costs, mechanical and physical properties, and weight reduction. Different characteristics are often mentioned as material selection criteria for the automotive industry (Fig. 4) [17,18,19,20,21]. These criteria vary according to the type of vehicle and component application. In many cases, different factors may conflict with each other and therefore a successful design is only possible through an optimum and balanced solution.
Many types of petroleum-based plastics that can provide the technical requirements for the automotive sector are employed in more than 1,000 different parts of all shapes and sizes. The most important polymers in the automotive industry are polypropylene (PP), used for instance in body panel bumpers and fuel systems; polyamide (PA), used in seats and electrical components; poly(methyl)methacrylate PMMA and polyurethane (PU) used in lighting; and polycarbonate (PC) used in bumpers, dashboards, and interior and exterior trim and usually associated with acrylate-butadiene-styrene (ABS) [22].
2.2 Why PLLA Is Being Viewed as a Key Material for Cars
Together with other bioplastic polymers such as polyhydroxyalkanoate (PHA), polycaprolactone (PCL), polytrimethylene terephthalate (PTT), biopolyamide (BioPA), and biopolyethylene (BioPE) [23], one of most popular alternatives to traditional petroleum-based plastics in automotive applications is poly(l-lactic acid) (PLLA), as currently derived from cornstarch. In spite of intensive R&D on PLLA science and new technology to enable large-scale production, the industrial application of unmodified PLLA is currently limited to short-term goods such as packaging, cold drink cups, bottles, and textiles [24,25,26]. Nevertheless, a number of factors can help extend the application of PLLA to more durable goods: high strength, rigidity, compostability, and recyclability [27]. To assess the strengths and weaknesses of PLLA, its physical and mechanical properties can be compared with those of the most commonly used plastics in automotive applications (see Table 1). The mechanical properties of PLLA appear very attractive, particularly its Young’s modulus (>3.5 GPa), making it an excellent alternative to commonly used stiff polymers.
In the context of designing ecofriendly products, both to satisfy customer demand and increasingly strict legislation [33], PLLA possesses undeniable assets compared with petroleum-based polymers. First, it is derived from bioresources (starch plants), which are abundant and renewable. Moreover, one of the most positive points of PLLA production in comparison with the other hydrocarbon-based polymers is its low CO2 emission. Carbon dioxide is the most important contributor to global climate change. Because CO2 from air is readily transformed when plants grow, the use of PLLA has the potential to reduce the environmental impact of greenhouse gases compared with petroleum-based polymers. In addition, emerging green technologies enable a decrease in greenhouse gas emissions during PLLA production processes. Vink et al. [28] showed that the net greenhouse gas emission of NatureWorks PLLA decreased from 2 kg CO2 equivalents/kg polymer in 2003 to 0.3 kg in 2006. Furthermore, Jamshidian et al. [29] estimated that the net greenhouse gas emissions for next-generation PLLA using wind energy can achieve −0.7 kg CO2/kg polymer (Fig. 5).
Net greenhouse gas emission of commercial PLAs and other polymers. PLA/NG NatureWorks® PLA next-generation, PLA5 NatureWorks® PLA in 2005, PLA6 NatureWorks® in 2006, HIPS high impact poly(styrene), PC poly(carbonate), GPPS general purpose poly(styrene), PET am PET amorphous, PET ssp. PET solid state polycondensed. Reproduced with permission from [29] Copyright © 2010 Institute of Food Technologists®
PLLA has also received some interest from other industrial sectors because of its relatively low price and commercial availability compared with other bioplastics. This is certainly the key point for any successful polymer application. In fact, the average price of commercial PLLA in 2015 was less than 1.8 €/kg, which is sufficiently close to other petroleum-based polymers such as PET and is the lowest among biodegradable polymers [30]. Clearly, the PLLA market is still in its infancy, but it is expected that a decrease in production costs and improvement in product performance will result in a clear breakthrough in PLLA industrial use. Many researchers have developed a more efficient and economic route for cheaper and greener PLA production [31, 32, 34]. It is estimated that PLLA production capacity, currently around 180,000 tons/year, should exceed more than 1 × 106 tons in 2020 [35]. In addition to lower gas emissions, increasingly affordable cost, and appealing mechanical properties, PLLA possesses other assets not generally available with petroleum-based plastics. Among those assets are excellent biocompatibility, (bio)degradability, and recyclability; furthermore, it can be readily processed in large-scale processing equipment such as injection molding units [36, 37]. More particularly, PLLA can be processed by injection molding, sheet-extrusion, blow molding, thermoforming, and film forming and is one of the few plastics suitable for 3D printing [38].
This new technology process for plastics is very important for the automotive industry and aftermarket because it allows the realization of automotive parts of incredibly complex geometry and enables production of spare parts on demand [39, 40]. Recently, an electric Street Scooter C16 short-distance vehicle was built by a team at Aachen University [41]. 3D printing using ABS polymer was used for all the vehicle’s exterior plastic parts, including the large front and back panels, door panels, bumper systems, side skirts, wheel arches, and lamp masks and for interior components such as the retainer instrument board and a host of smaller parts [41]. Local Motors made a car called the “Strati” by building the chassis and body of its cars using giant 3D printers and raw materials [42].
Referring to Table 1 and comparing PLLA with the traditional plastics used in automotive applications, neat PLLA has many appealing properties such us high rigidity but cannot fulfill all the criteria required by the automotive sector, most importantly in terms of brittleness and heat resistance. Several strategies have been studied in efforts to overcome these drawbacks.
3 Development of High-Performance PLLA-Based Materials
As discussed, the major drawbacks of PLLA that hamper its application in the automotive industry are its inherent brittleness, low heat resistance, limited flexibility in design, and poor durability. In order to make PLLA suitable for such applications, many studies have focused on modifications of PLLA, mainly of its bulk properties. These modifications have involved control of crystallinity and processability via blending, plasticizing, stereocomplexing, and other modifications depending on the properties to be improved. There is no unique way to improve PLLA properties and the most interesting solutions are generally a combination of different routes; however, not many have been successful as a result of antagonist effects when combined. Those modifications are described in detail in the next sections, with an emphasis on PLLA-based materials that fit automotive requirements in terms of processing (e.g., injection molding), properties (e.g., stiffness), and durability.
3.1 Tough and/or Ductile PLLA-Based Blends
In cases that require a high level of impact strength and ductility, especially for a vehicle’s exterior parts, the impact toughness and ductility of PLLA in its pristine state is often insufficient. Therefore, there have been tremendous efforts to develop ways to improve these mechanical properties. These approaches are summarized next, with a focus on how the protocols also influence other mechanical properties in the resultant materials.
3.1.1 Plasticized PLLA Blends
Plasticization is a widely used technique for improving the processability of thermoplastics. The main role of plasticizers is to decrease the glass transition temperature (T g). In addition, plasticization frequently opens new possibilities for material processing by lowering degradation rate, which enables processing of materials in equipment with reduced extrusion pressure and mixing time [43]. Furthermore, it can also increase polymer ductility and flexibility, which is related to the decrease in T g. The choice of plasticizer for PLLA is dictated by factors relating to the intended application (e.g., nontoxicity of plasticizer for food and medical applications) as well as by general criteria such as non-volatility to avoid evaporation under the high temperature conditions used during processing, and miscibility for creating homogenous blends with PLLA. Another point is that the plasticizer must migrate as little as possible from the material bulk, otherwise plasticized PLLA blends can rapidly regain the inherent brittle properties of neat PLLA [44].
Many different molecules and classes of plasticizer have been tested for PLLA [45, 46], the three main types being monomeric plasticizers, oligomeric and polymeric plasticizers, and mixed plasticizers. Only plasticizers designed for injection-molded parts are selected here for discussion. Examples of the thermal and mechanical properties of PLLA plasticized with these three classes of plasticizers are given in Table 2 and described in more detail.
Jacobsen and Fritz [47] used glucose monoester (DehydatVPA 1726), partial fatty esters (LoxiolVR GMS95), and polyethylene glycol (PEG) with a molecular weight of 1,500 g/mol (PEG1500), to plasticize PLLA and examined the effect on the tensile strength and unnotched Charpy impact strength of injection-molded PLLA specimens. A significant improvement in both elongation (180%) and impact resistance (unbroken specimens under unnotched Charpy impact tests) was reported with the addition of 10 wt% PEG1500. In the case of glucose monoester and partial fatty acid ester, elongation of PLLA was improved but impact strength was slightly decreased at all concentrations examined (i.e., 2.5–10 wt%). Murariu et al. [48] studied the plasticization of PLLA using three low molecular weight ester-type plasticizers, bis(2-ethyldhexyl) adipate (DOA), glyceryl triacetate (GTA), and acetyltributylcitrate (ATBC). Addition of up to 20 wt% plasticizer led to a gradual decrease in Young’s modulus and increased ductility in the following order of efficiency, GTA>ATBC>DOA. The best notched impact performance was seen in PLLA plasticized with 20 wt% GTA, with unbroken specimens. By comparison, addition of ATBC led to the lowest improvement in the impact strength of the three plasticizers, with “only” an increase of 77% at 20 wt % ATBC.
The main drawback of monomeric plasticizers is their tendency to migrate out of the polymer bulk [51]. This drawback can be overcome by using polymeric plasticizers [47, 49]. Recently, it has been shown that PLLA can be efficiently plasticized and toughened by melt-blending with poly(1,2-propylene glycol adipate) (PPA) [49]. Thermal and dynamic mechanical analysis revealed that PPA was partially miscible with PLLA, and morphological investigation of the blends showed that PPA was compatible with PLLA. As a result, the blends showed a decrease in the tensile strength and Young’s modulus with an increase in PPA content (5–25 wt%) (Table 2). However, the elongation at break and impact strength dramatically increased as a result of plastic deformation. The notched Izod impact strength reached 100 J/m with 25 wt% of PPA. The results show that polymeric plasticizers can cause an additional increase in impact strength. Nevertheless, increasing the molecular mass of plasticizers can lead to poor miscibility with the polymer, causing phase separation [52].
Mixed plasticizers combine an oligomeric or polymeric plasticizer with a small-molecule plasticizer. Therefore, they can lead to a medium drop in T g and more balanced mechanical properties (in terms of elongation, tensile modulus, and strength) than the individual plasticizers. Mixed plasticizers combining low molecular weight triacetin (TAC) and oligomeric poly(1,3-butylene glycol adipate) (PBGA) have been employed to improve the ductility of PLLA, as reported by Ren et al. [50]. They found that this combination led to significant improvement in the elastic properties (for plasticizer content higher than 5 wt%), with a dramatic decrease in tensile strength as the content of plasticizer increased (Table 2).
To enhance mechanical properties without altering the rigidity of PLLA too much and to improve flexibility and toughness, Notta-cuvier et al. [53] had the idea of combining plasticizers with Halloysite nanotubes (HNT) as reinforcing nanofillers, leading to more attractive properties for automotive applications. In particular, this composition achieved the right combination of good rigidity and tensile flexural strength imparted by HNT with better ductility and toughness provided by tributylcitrate (TBC) plasticizer. Tensile behavior and impact results are represented in Fig. 6 and show that the ductility and impact resistance of PLLA/HNT/TBC ternary blends were improved to an extent that depended on the amount of plasticizer. Moreover, a high content of TBC (12.5–15%wt) enhanced the impact resistance of the blend, but led to a drop in rigidity and flexural strength. For a good balance of properties, an optimized PLLA/HNT/TBC composition containing 10 wt% of plasticizer was proposed as a biosourced alternative blend for automotive application.
(a) Nominal behavior of PLLA/TBC/HNT compositions and PP-B in tensile tests at 1 mm min−1. (b) Izod impact resistance (notched specimens) of PLLA/TBC/HNT compositions and PP-B. Sample codes refers to the following weight contents (PLLA/TBC/HNT): N9 (91/0/9); P10 (90/10/0); P15 (85/15/0); P10N9 (81/10/9); P12N9 (78.5/12.5/9); and P15N9 (76/15/9). Reproduced with permission from [53] Copyright © 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
In summary, several studies have demonstrated that plasticizers can play a significant role in tuning the properties of PLLA plastics, mainly to improve the flexibility and ductility of PLLA blends, and could also pave the way to novel applications. However, there are still some limitations associated with plasticization, including leaching during use, lack of thermal stability, and substantial reductions in strength and modulus. This illustrates the need to carefully estimate plasticizer content to reach a better compromise between ductility and strength, especially for automotive applications.
3.1.2 Rubber-Toughened PLLA Blends
Several successful strategies have been proposed to improve the ductility and toughness of PLLA. Of these, melt-blending with rubbers has proved to be efficient, thanks to its remarkable toughening effect and the fact that is based on easy-to-perform and cost-effective techniques. A variety of biodegradable and nonbiodegradable flexible polymers have been used as toughening modifiers for PLLA to improve the balance between stiffness and toughness. Various reviews report the most commonly used polymeric additives to be effective PLLA impact modifiers [45, 54,55,56,57,58,59]. However, this section is strictly limited to recent and noteworthy studies concerned with developing rubber-toughened PLLA blends for engineering applications requiring high ductility and impact toughness.
When aiming to improve PLLA ductility and impact toughness, the use of impact modifiers can be of great interest because they allow an increase in energy dissipation through the material during deformation, without affecting stiffness and thermal stability [60]. Several commercial impact modifiers have been specifically designed to toughen PLLA. When dispersed in the form of rubbery microdomains (with an average size of 0.1–1.0 μm) within the PLLA matrix, they enable a significant increase in energy absorption during impact [61, 62]. However, it is well known that their toughening effect is of varying magnitude, depending on their miscibility with the PLLA matrix, thermal stability under PLLA processing temperatures, interfacial adhesion between the dispersed rubbery phase and continuous PLLA matrix within the blend, etc. Of the impact modifiers compatible with PLLA, Biomax® Strong (BS) from Dupont Company is probably the most investigated because it was specifically designed to improve PLLA toughness. In this regard, BS was used by Taib et al. [63], who noted a significant improvement in notched Izod impact strength of PLLA from 3.6 to 14 kJ/m2 with use of 10% BS, and to 28 kJ/m2 with 20 wt% BS. For more suitable PLLA blends in automotive applications with simultaneously enhanced impact and thermal resistance, ethylene acrylate BS impact modifier was used by Bouzouita et al. [64] to develop a PLLA/PMMA/BS ternary blend capable of competing with commercial ABS/PC blends for the manufacture of injection-molded automotive parts. Thermal mechanical analysis revealed that ternary blends showed a miscible PLLA/PMMA matrix, but the impact modifier was phase-separated and formed immiscible blends. The effect of PMMA content on the notched impact strength and tensile mechanical properties of ternary PLLA-based blends showed that a significant gain in tensile elongation and impact toughness was achieved (116 ± 4% and 44 ± 2.5 kJ/m2, respectively, with addition of 25 wt% PMMA and 17 wt% BS) with only a slight decrease in tensile rigidity and strength (Fig. 7). To understand the evolution of toughness in PLLA/PMMA/BS blends, the average size and distribution of rubber microdomains were analyzed. Results revealed that blend impact toughness and fracture mechanisms depend on rubber particle size, with an optimum of 0.5–0.55 μm achieved at 25 wt% PMMA content. However, PMMA content higher than 50% led to poor mechanical properties and low interfacial adhesion between PLLA/PMMA matrix and BS nodules as a result of the low affinity of impact modifier with PMMA. To conclude the study, the authors selected PLLA/PMMA/BS (58/25/17) as the most promising composition made of at least 50% biosourced polymer in terms of ductility/stiffness balance and characterized by competitive mechanical properties, compared with commercial ABC/PC blends, for use in automotive applications (Table 3).
(a) Tensile stress–strain curves for PLLA and PLLA/PMMA/BS blends. (b) Effect of relative content of PMMA on the notched impact strength of PLLA/PMMA/BS blends. Adapted with the permission from [64] Copyright © 2016 Wiley Periodicals, Inc
Using BS impact modifier, Notta-Cuvier et al. [65] prepared PLLA/plasticizer/impact modifier/nanoclay quaternary compositions designed for automotive applications. Good ductility and toughness were achieved with the binary blend (PLLA/BS) at a resiliency of 16.5 kJ/m2. A synergistic effect of BS (10 wt%), plasticizer TBC (10 wt%), and the nanoclay Cloisite® 25A (1 wt%) was surprisingly seen, with an optimal toughness of 42.8 kJ/m2. Analysis also revealed that a compromise is needed between high tensile rigidity and strength on one hand, and high ductility on the other hand, as illustrated in Fig. 8. In Fig. 8, the mechanical properties of a mineral (talc)-filled polypropylene (PP-talc), commonly used in automotive applications, were taken as reference. The composition PLLA + 10 wt% BS + 10 wt% TBC + 3 wt% CL25A (composition 8 in Fig. 8) has, globally, the most interesting properties compared with PP-talc. In particular, this composition was characterized by an interesting level of ductility, while its rigidity and strength were higher than those of the mineral-filled PP (10 kJ/m2compared with 4.8 kJ/m2 for PP-talc).
(a) Summary of all mechanical properties compared with those of PP-B. Numbers refer to PLLA/TBC/BS/CL25A composition codes: 1 (100/0/0/0); 2 (90/0/10/0); 3 (90/10/0/0); 4 (80/10/10/0); 5 (90/0/10/1); 6 (90/0/10/3); 7 (80/10/10/1); 8 (80/10/10/3), in weight percentages. Values presented are ratios of properties of compositions 1–8 divided by those of PP-talc, so that qualitative comparisons are immediate (a value higher than 1.0 stands for a mechanical property higher than that of PP-talc). (b) Summary of all mechanical properties compared with those of PP-B (focus on lower values). Adapted with permission from [65] Copyright © 2016 Elsevier Ltd
Zhang et al. [66] developed super-toughened PLLA multiphase reactive blends using a commercial class of renewable elastomeric copolymers. Polyether block amide PEBA (Pebax®) offered high impact resistance and excellent elasticity, and an ethylene-methyl acrylate-glycidyl methacrylate (EMA-GMA) terpolymer impact modifier (commercialized under the name of Lotader® AX8900; Arkema Ltd.) improved the interfacial adhesion of PLLA/PEBA blends and enhanced toughness. As shown in Fig. 9b, only limited improvement in impact strength was achieved for binary PLLA/PEBA blends but significant enhancement of PLLA impact toughness, together with higher elongation at break, thanks to addition of the impact modifier. Elongation at break increased with the EMA-GMA content, reaching almost 73% with 20 wt% of impact modifier (20 times higher than that of the neat PLLA). Nevertheless, a decrease in tensile strength and modulus for ternary blends was noticed with an increase in EMA-GMA content (Fig. 9a). This result can be attributed to the presence of soft PEBA and EMA-GMA elastomers.
(a) Tensile properties of PLLA/EMA-GMA/PEBA ternary blends as a function of the weight fraction. (b) Notched Izod impact strength and percentage elongation at break of PLLA/EMA-GMA/PEBA ternary blends as a function of the weight fraction: (A) neat PLLA; (B) PLLA/PEBA (80/20); (C) PLLA/EMA-GMA (80/20); (D) PLLA/EMA-GMA/PEBA (70/10/20); (E) PLLA/EMA-GMA/PEBA (70/15/15); and (F) PLLA/EMA-GMA/PEBA (70/20/10). Adapted with the permission from [66] Copyright © 2014 American Chemical Society
Good interfacial adhesion was achieved by addition of Lotader AX8900. A supertough PLLA ternary blend was thus developed, exhibiting an impact strength of 500 J/m with only partial break of impact specimens, therefore allowing this blend to be used in automotive interior parts.
Although many of the solutions investigated for achieving a decrease in PLLA brittleness and/or an improvement in PLLA impact strength are not fully ecofriendly, blending PLLA with ecofriendly rubber modifiers [67,68,69,70,71,72], elastomers [73, 74], and biodegradable polymers [75, 76] has gained momentum in recent years. Zhang et al. [67] reported an improvement in the impact strength of PLLA with the incorporation of 20 wt% of epoxidized natural rubber containing 20 mol% (ENR20) and 50 mol% (ENR50) of epoxidation content. ENR20 was found to impart higher impact strength to the PLLA matrix than ENR50 (Fig. 10). Moreover, the higher content of epoxy groups in ENR50 led to an increase in viscosity and therefore decreased deformability of the blends. Interchain crosslinking reactions and molecular entanglements were more pronounced in PLLA/ENR50 blends and, in turn, increased the tensile strength.
Impact strength of pure PLLA, ENR20/PLLA, and ENR50/PLLA blends. Reproduced with permission from [67] Copyright © 2012 Elsevier Ltd
3.1.3 Annealing Process
Of the additional approaches related to process modification, the literature shows that annealing is an effective method for improving the mechanical properties of PLLA blends (rigidity and impact strength), in particular for injection-molded parts. Annealing modifys the inherent crystalline structure and crystal polymorphism, which have a deep impact on the mechanical properties of PLLA [77,78,79,80,81,82,83,84].
Perego et al. [83] studied the effect of crystallinity on the mechanical properties of PLLA of different molecular weights. To promote further crystallization, PLLA injection-molded specimens were annealed at 105°C for 90 min under nitrogen. DSC analysis demonstrated that the crystallinity degree of PLLA reached 42–65 J/g, instead of 3–13 J/g for nonannealed PLLA, as a function of molecular weight (\( \overline{M} \) v). Annealing PLLA samples also led to some increase in impact strength, depending on the evolution of crystallinity and molecular weight. Values of notched impact strength ranged from 66 to 70 J/m, following the molecular weight of PLLA. The highest tensile elastic modulus attained was 4.15 GPa, resulting from the higher crystallinity degree of annealed materials (Table 4).
From a general viewpoint, in the automotive industry, annealing is mainly used for semifinished components after shaping or cold-forming, with the aims of producing a uniform material structure that offers softness and removing any residual stresses for both alloy and plastic components [85, 86]. Annealing is carried out after heating the material at a specific temperature for a definite period of time, followed by slow cooling to room temperature. This process is used to control the degree of crystallinity and/or orientation of the material and to remove any internal stresses within the product that were created during primary processing. Annealing can also improve impact resistance and reduce the tendency to crazing and cracking during service. It can also offer additional value to the final alloy, such as improved welding properties, better corrosion resistance, and good dimensional and shape accuracy. Unfortunately, this post-process is expensive and involves longer production times. Moreover, annealing can induce some structural defects within the part, making it difficult to control shrinking and complicating part ejection. Therefore, it cannot be seen as a promising solution for mass-production processes currently used in the automotive industry.
3.2 Heat-Resistant PLLA-Based Blends
In some applications, heat resistance over extended periods of time is compulsory. Yet, heat resistance is an important issue for plastics used in engineering applications. Indeed, all polymers exhibit a wide variation in mechanical and physical properties as a function of temperature. Apart from the extreme temperature attained in combustion chambers, automotive parts may face service temperatures ranging from −40°C for a car parked outdoors in a cold country to 85°C for the driver interior, and even 125°C under the bonnet [87]. With increasing temperature, polymer strength and rigidity tend to decrease dramatically. Above a given temperature, rigidity drops in a more pronounced way and becomes too low to enable the use of the material in these technical parts. In this regard, the heat deflection temperature (HDT) is defined as the temperature at which a standardized test bar deflects a specified distance under an imposed load value (see ASTM D648 procedure). HDT is therefore an effective way to evaluate the thermal stability, or heat resistance, of plastics [88]. Many researchers have studied the improvement in heat-resistance of PLLA by manufacturing, for example, PLLA-based green composites containing reinforcing fibers. Fibers from wood [89], banana [90], kenaf [91], bamboo [92], and cellulose [93] can all increase HDT, but the resulting composites is brittle.
By contrast, nanofilled PLLA/petroleum-based thermoplastic blends can offer a good balance between thermal stability and ductility, allowing their use in the automotive sector.
3.2.1 PLLA-Based Nanocomposites with High Thermal Properties
Polymer nanocomposites are commonly defined as the combination of a polymer matrix and nanofillers that have at least one dimension in the nanometer range. The nanofillers can be one-dimensional (1D; nanofibers or whiskers), two-dimensional (2D; plate-like nanofillers) or three-dimensional (3D; nanoparticles). The use of nanocomposites in vehicle parts and systems is expected to enhance manufacturing speed, improve thermal stability, and reduce weight. Applying this technology only to structurally noncritical parts such as front and rear panels, cowl ventilator grids, and valve/timing covers could allow a reduction in weight. Indeed, nanocomposite plastic parts offer a 25% weight savings on average compared with highly (micro)filled plastics and as much as 80% compared with steel [94, 95]. As important as the process advantages and weight and energy savings are, nanocomposites can also offer enhanced physical properties. Depending on composition, nanocomposites show stiffness and strength comparable to, or even better than, metals [96,97,98,99,100,101,102]. Nanofillers can also improve the corrosion resistance, noise dampening, thermal stability, and dimensional stability of a material. However, as a relatively new approach, it is still unknown whether the cost/performance ratio of nanocomposites is superior to that of materials currently used by the automotive industry.
Layered silicate (clay) nanocomposites (PLS) are among the best-known nanocomposites. Their interesting properties at low nanofiller content make them appealing in both academic and industrial realms [103]. Three main methods have been proposed for preparation of nanocomposites: in situ intercalative polymerization, solution intercalation, and melt intercalation [104, 105]. Clay materials can be dispersed and exfoliated into polymers by conventional melt-compounding or solution methods. Toyota Motor Company successfully pioneered an in situ intercalation polymerization method to create a Nylon–clay hybrid (NCH) for manufacture of an automotive timing belt cover [106]. Although in situ intercalative polymerization is the most efficient technique for obtaining an exfoliated structure, it is not the most viable option for current industrial challenges [104, 107]. Alternatively, the melt-intercalation technique is more versatile and less environmentally harmful. Therefore, it is the most efficient method for the preparation of polymer nanocomposites from an industrial viewpoint. The main advantages in comparison with other approaches are the utilization of elevated shearing force and the absence of solvent during preparation. The applied shear force during mixing readily promotes the diffusion of polymer chains from the bulk to the clay gallery spacing, resulting in further nanoplatelet delamination and improved nanofiller distribution and dispersion [105].
Addition of layered silicates can potentially achieve enhanced barrier properties, high heat deflection temperatures, improved rate of biodegradation, good optical properties, and antimicrobial properties; therefore, numerous research studies have focused on the development of PLLA-based nanocomposite blends for food packaging and medical applications [96,97,98,99,100,101,102]. However, only limited studies deal with PLLA-based nanocomposites for automotive applications, for instance in the form of PLLA nanocomposite foams and injection-molded PLLA nanocomposites [61, 108,109,110,111,112,113,114,115,116,117]. Liu et al. [61] developed a nanocomposite of thermoplastic polyurethane (TPU)-toughened PLLA/talc/organic modified clay (OMC) and demonstrated that the annealing process is necessary for improving the heat resistance of PLLA. As shown in Table 5, the differences between compositions of injection-molded specimens were negligible before annealing, with HDT values of about 60°C. Annealing increased HDT values to more than 120°C, mostly due to the increase in crystallinity resulting from thermal treatment and, to a lesser extent, to some interactions between PLLA and TPU molecular chains.
Thermal stability, mechanical performance, and added-value properties such as flame retardancy are among the most targeted properties for PLLA materials intended for the automotive sector. Sinha Ray et al. [110] reported the flexural properties of neat PLLA and various PLLA nanocomposites prepared with organically modified layered silicate (OMLS) (injection-molded samples). The flexural modulus, flexural strength, and distortion at break of neat PLLA and various PLLA nanocomposites were measured at 25°C and the results showed a significant increase in flexural modulus for PLLA nanocomposites with 4 wt% of OMLS (5.5 GPa) compared with that of neat PLLA (4.8 GPa). This was followed by a much slower increase with OMLS content, and a maximum at 5.8 GPa (increase of 21%) for 7 wt% OMLS. In addition, the flexural strength and distortion at break remarkably increased for 4 wt% OMLS, then gradually decreased with increased OMLS loading. The study revealed that a high content of OMLS leads to brittleness in the material and that there is an optimal amount of OMLS in nanocomposites, which must be carefully adjusted to achieve the most significant improvement in mechanical properties. Murariu et al. [109] developed a calcium sulfate-containing PLLA-based nanocomposite with flame-retardant properties for technical applications requiring rigidity, heat resistance, and dimensional stability. Accordingly, composites of PLLA and β-anhydrite II (AII) characterized by specific end-use flame-retardant properties were added together with selected OMLS. Co-addition of AII and OMLS led to PLLA nanocomposites characterized by good nanofiller dispersion, thermal stability, and adequate mechanical resistance. The flame-retardant properties, as measured by cone calorimetry (Table 6), displayed a significant increase in ignition time (TTI) compared with neat PLLA and a substantial decrease (about 40%) in the maximum (peak) rate of heat release (pHRR). The composite successfully passed the UL94 HB standard flammability test in terms of nondripping effect and extensive char formation (Fig. 11).
Behavior of specimens during UL94 HB testing: (a) PLLA and (b) PLLA-43% AII burned with drips; (c) PLLA-40% AII-3% C30B and (d) PLLA-40% AII-3% B104 burned without drips. Reproduced with permission from [109] Copyright © 2009 Elsevier Ltd
In summary, PLLA-based nanocomposites show very interesting properties that should enable their more widespread use in automotive applications in the near future, in particular for reduced energy consumption resulting from weight reduction. The high strength and rigidity of these materials are also undeniable assets. However, nanofilled PLLA blends still have low heat resistance in the absence of additional heat processes such as annealing.
3.2.2 PLLA/Petroleum-Sourced Polymer Blends of High Thermal Stability
The comparatively high thermal stability of commonly used petroleum-based thermoplastics, as indicated by their HDT values (Table 1), has encouraged interest in compounding PLLA with petroleum- or biosourced polymers to improve the HDT [118, 119]. In some cases, compounding in association with toughening modifiers also achieved high impact properties, leading to a competitive partially biobased material with enhanced thermal stability and mechanical performance that is suitable for use in vehicle parts. PLLA/PC blends have been widely reported [120,121,122,123,124,125] as a simple binary blends or with addition of some additives such as chain extenders and compatibilizers to significantly enhance toughness and heat resistance, while minimizing the reduction in stiffness. Recently, Srithep et al. [124] developed a PLLA/PC blend with the addition of epoxy-based chain extender (CE) to improve compatibility of the blend by reaction between the epoxide groups in the CE and the hydroxyl/carboxylic end groups of PLLA and PC. The HDT value of PLLA/PC blends without and with addition of CE was improved from 62°C for PLLA + 50%PC to 106°C after mixing with CE (Table 7).
PMMA is often viewed as an excellent polymer partner for PLLA, resulting in blends exhibiting high miscibility, excellent thermal stability, increased HDT, high sustainability, and good ageing behavior [126,127,128,129]. Recently, Samuel et al. [128] prepared miscible PLLA/PMMA blends of enhanced thermomechanical properties and confirmed that the addition of even a moderate amount of PMMA can deeply modify the thermal properties of PLLA (in terms of T g and HDT values), which were easily adjusted between those of neat PLLA and neat PMMA. More particularly, the HDT progressively increased from 51.5 to 54.8°C with 20 wt% PMMA and up to 61.9°C with 50 wt% PMMA. Similarly, Bouzouita et al. [64] reported the design of PLLA/PMMA blends with incorporation of impact modifier. The authors confirmed that the addition of impact modifier led to a supertough ternary blend (33 kJ/m2 with 50 wt% PMMA) but also noticed that the impact modifier had a slight influence on HDT, which reached 63°C for ternary PLLA/PMMA/BS blends with 50 wt% PMMA.
3.2.3 Heat-Resistant PLLA-Based Stereocomplexes
Together with the development of PLLA/petroleum-sourced polymer associations, other studies have focused on making PLA stereocomplexes (sc-PLA) [130], as stereocomplexing is generally judged to be an effective method for increasing material crystallinity. Poly(l-lactic acid) (PLLA) and poly(d-lactic acid) (PDLA) readily form stereocomplexed crystallites with a distinct crystal structure that leads to a high melting point in the range of 220–230°C, that is, values that are significantly higher than those of PLLA homocrystallites (approximately 170°C) [131, 132]. Stereocomplexing can therefore enhance PLLA properties in terms of resistance to heat and hydrolysis, in particular. A drawback of stereocomplexing is that it can lead to brittle PLLA-based materials, which could dramatically reduce their industrial implementation, as shown by Torres et al. [133]. Other research has aimed at simultaneously improving the HDT and toughness of PLLA using stereocomplexing [134,135,136]. Similarly, Nam et al. [137] prepared sc-PLA by extruding PLLA with various amounts of PDLA and 10–20 wt% of two types of commercial impact modifiers (BioStrong 120, Elvaloy) to enhance both thermal (Table 8) and mechanical properties.
As shown in Table 8, HDT dramatically increased to over 100°C as a result of incorporation of different amounts of PDLA. Nevertheless, impact strength decreased with addition of PDLA (18 J/m for neat PLLA to 11 J/m with 15 wt% of PDLA). Once impact modifiers were added, HDT decreased with the increase in impact modifier content (Table 8). Taking all results into account, a well-balanced composition of toughened sc-PLA with 10 wt% of impact modifier could compete with petroleum-based polymers. In this context, Corbion Purac [138] created a developmental grade of highly heat-resistant PLLA (not commercialized yet) that in a given stereocomplex form could replace polystyrene (PS), PP, and ABS-type materials in applications where heat performance is compulsory.
As shown in Fig. 12, the key driver behind the improvement of HDT in blend A is the presence of PLLA homopolymers that are nucleated with a small amount of PDLA homopolymers and a traditional nucleating agent. The improved heat performance of blend A was obtained without adding a significant amount of filler. To achieve a higher modulus, and an even higher temperature resistance, talc was added to blend A to form blend B (4 GPa and 120°C for blend B instead of 3 GPa and 105°C for blend A), leading to better performance than those of PP and PS blends. To achieve an impact resistance comparable to that of ABS, blend A was impact-modified and, to minimize the drop in modulus, talc was added to obtain blend C, which was characterized by a good balance of properties (33 kJ/m2 for impact resistance, 3.5 GPa for tensile modulus, and 95°C for HDT).
Typical results of using PLLA homopolymer blends, showing heat performances similar to those of PS, PP, and ABS. Reproduced with permission from [139]© Copyright 2015 Corbion
Sections 3.1 and 3.2 presented the most efficient way to improve PLLA impact strength, ductility, and thermal stability in order to obtain PLLA-based materials with properties compatible with their implementation as automotive components. Another key point when developing materials for use in the automotive industry is to ensure their good processability through mass production methods, typically by injection molding. This subject is discussed in the next section.
3.3 Mold-Injected PLLA Materials for Automotive Applications
In the automotive industry, PLLA components are generally produced by injection molding, similarly to other thermoplastics. The advantage of injection molding is continuous production capability with minimal maintenance and labor, allowing significant economies for large-scale production. Several factors are involved in the injection molding process and have a great influence on the injected part; these include material formability, characteristics of the molding machine, mold design, and process conditions [139, 140].
The typical cycle for an injection molding process has been detailed by Lim et al. [141] (Fig. 13). The authors described the different steps of the cycle and gave some advice on how to avoid defects in injection-molded products. Although the cooling time must be sufficient to ensure a dimensionally stable injected part, cycle time should be minimized to maximize production throughput.
Typical cycle for an injection molding process. Reproduced with permission from [141] Copyright ©2008 Elsevier Ltd
The slow crystallization rate of PLLA compared with that of commonly used injection-molded thermoplastics is a major obstacle to its use in the automotive industry (i.e., at high production rate). Indeed, the injection molding cycle time in the automotive industry is typically within the range of 20–60 s [142], which implies a high cooling rate. However, the crystallization half-time, t ½, of a pure sample of PLLA was reported in the literature as being in the range of 17–45 min, depending on crystallization temperature, stereochemistry, and molecular weight [143]. Therefore, injection molding of PLLA for automotive parts is limited to the manufacture of parts for which a high degree of crystallinity is not required. Indeed, using a post-annealing step or longer cycle time to obtain sufficient crystallinity for PLLA components would be impractical for high volume automotive use. In addition, the slow crystallization rate of PLLA also causes difficulties during part ejection, again increasing molding cycle duration.
An efficient way to accelerate crystallization of PLLA is by incorporation of nucleating agents. In polymers, nucleating agents provide additional sites for initiation of crystallization and influence crystalline morphology and crystallization kinetics [144,145,146]. For example, blending PLLA with 5 wt% talc can fundamentally change the crystalline morphology of the blend (Fig. 14). Judging from polarized optical micrographs of PLLA formulations, spherulite concentration increases after nonisothermal crystallization from the melt, whereas spherulite size decreases in PLLA/talc compared with neat PLLA. Therefore, talc addition can lead to much more heterogeneous nuclei and can reduce the size of spherulites [147]. Nucleating agents have a remarkable effect on the kinetics of crystallization by reducing t ½, leading to better processability of PLLA. In some cases, they may also lead to enhanced mechanical properties [116].
Polarized optical micrographs of neat PLLA (left) and PLLA containing 5 wt% talc (right) at 122°C after quenching from 180°C. Adapted with permission from [147] Copyright ©The Royal Society of Chemistry 2013
Shi et al. [148] demonstrated that the addition of 1 wt% aromatic sulfonate derivative LAK-301 (Takemoto Oil & Fat Co) to PLLA as an effective nucleating agent led to a dramatic reduction in t ½ of up to 2 min, with a crystallinity content reaching 27%. Kolstad [149] showed that talc can be added to PLLA to effectively modify the crystallization rate of the polymer. The t ½ of the polymer reduced from 3 min at 110°C to approximately 25 s with the addition of 6% talc to PLLA. At the same content of talc, for 3% mesolactide copolymerized with l-lactide, the t ½ value reduced from 7 to 1 min. The stereocomplexation of PLLA and PDLA was previously mentioned as an effective way to increase PLLA HDT (see Sect. 3.2.3). It can also be regarded as a potential tool for self-nucleation of PLLA. Schmidt and Hillmyer [150] investigated self-nucleation of PLLA, in which small crystallites of the stereocomplex were formed by blending up to 15% PDLA into PLLA. They compared the effectiveness of self-nucleation with the heterogeneous nucleation obtained after addition of talc, finding self-nucleation to be more efficient. Self-nucleation reduced t ½ by nearly 40-fold, in the best case, whereas a similar loading of talc only decreased t ½ by twofold under the same conditions. The majority of works dealing with nucleation of PLLA are focused on the study of crystallization kinetics, morphology, and some mechanical properties, but Harris and Lee [143] pushed forward the study of nucleated PLLA using talc and ethylene bis-stearamide (EBS) by optimizing the parameters for injection molding and post-processing in order to increase crystallinity within the finished injected part. Furthermore, HDT and flexural strength were both analyzed. The authors showed that the addition of 2% of nucleating agent (talc or EBS) improved the crystallization rate by over 20 times and 65 times for EBS and talc, respectively, compared with neat PLLA. The authors then worked on the optimization of processing conditions, especially for nucleated samples, aiming to increase PLLA crystallinity and mechanical performance.
Post-annealing processing of both nucleated and neat PLLA materials was found to increase the crystallinity of PLLA to a maximum level of 42% (Fig. 15a). Interestingly, annealing is significantly faster in the presence of nucleating agent. Injection molding the PLLA materials into a preheated mold (110°C) was found to significantly increase the crystalline content of the molded specimens to their maximum level (41–43%) in both neat and nucleated PLLA materials (Fig. 15b). Furthermore, the same crystallinity was reached at a lower temperature in the nucleated samples than for neat PLLA; for instance, a crystallinity ratio of 35% was reached at a mold temperature of about 80°C for nucleated samples, compared with about 100°C for neat PLLA. The combination of nucleating agents and process optimization therefore resulted in an increase in crystallinity level in the final injection-molded part, together with a decrease in processing time. Moreover, an increase of 30°C in the HDT (Fig. 15c) and an improvement in flexural modulus by over 25% were achieved thanks to material nucleation and process optimization.
(a) Crystallinity versus annealing time at 80°C for neat PLLA, PLLA + talc, and PLLA + EBS samples. (b) Crystallinity as a function of injection molding mold temperature for neat PLLA and PLLA + talc samples. (c) Dependence of HDT on annealing time at 80°C for neat PLLA, PLLA + talc, and PLLA + EBS samples. Adapted with permission from [143] Copyright © 2007 Wiley Periodicals, Inc
Previous research has demonstrate that PLLA crystallization kinetics can be improved to make PLLA-based materials suitable for high-rate production processes by injection molding, which is a typical process in the automotive industry. Furthermore, the increase in crystallization rates achieved in these works and discussed in this section can result in reductions in both cycle time and energy consumption during injection molding. Moreover, materials with higher crystallinity degree show increased mechanical properties, especially in terms of rigidity and strength (and sometimes thermal resistance) and are thought to have better durability. The issue of the durability of PLLA blends is the subject of the next section.
3.4 Durable PLLA/Polymer Blends
The plastics and polymer composites developed recently can provide mechanical performances that can withstand the stresses related to many applications, including those applied to loaded parts. However, some operating conditions, such as high temperature, corrosive chemicals in fluids and lubricants, electric currents, weather variations, or minerals from roadways, can be too harsh to be sustained by some plastics and polymer composites over a vehicle’s lifetime. Both interior and exterior parts can be exposed to a large range of temperatures (−40 to 125°C), together with high humidity levels, throughout the vehicle’s lifetime (possibly more than 10 years). Obviously, these conditions can have long-term detrimental effects on the durability, performance, and aesthetics of the materials in automotive components. The suitability of a material for automotive application must therefore be evaluated regarding long-term performance.
Under specific conditions, PLLA presents a fast degradation rate, which makes it appealing for disposable applications but inadequate for applications requiring long-term durability. This explains why the majority of current commercial applications for PLLA blends are oriented toward clothing and linens and disposable packaging and objects (e.g., water cups). Unfortunately, very few research studies or applications concern the use of PLLA in durable goods [151, 152], and even less in cases of the severe environmental conditions that can be encountered in automotive applications. Among the few available works, Harris and Lee [153] investigated the durability of a commercial injection molding grade of PLLA through its crystallization behavior. Commercially available injection molding grade PLLA and annealed PLLA were conditioned at a temperature of 50°C and 90% relative humidity for 12 weeks, which corresponds to a simulated environment for automotive interiors. Moisture absorption, molecular weight, and mechanical performances were investigated. Both amorphous and crystalline PLLA showed significant moisture absorption, allowing hydrolysis to occur. The linear regression of average molecular weight was more accentuated for amorphous PLLA than for crystalline PLLA (Fig. 16a, b). The effects of moisture and heat-conditioning on mechanical performance were examined through the evolution of flexural strength (Fig. 16c). This decreased significantly for both amorphous and annealed PLLA. In conclusion, the properties of the conditioned samples were not maintained, even for crystallized PLLA, and commercial PLLA grades remain inadequate for durable use in automotive parts.
(a) Moisture absorption as a function of conditioning time for amorphous and crystalline PLLA. (b) Dependence of molecular weight on conditioning time for amorphous and crystalline PLLA. (c) Dependence of flexural strength on conditioning time for amorphous and crystalline PLLA. Adapted with permission from [153] Copyright © 2009 Wiley Periodicals, Inc
However, different approaches have proved to improve the durability of PLLA-based materials. A possible approach is to blend PLLA with resins that are not susceptible to hydrolysis and could therefore prevent contact between PLLA and water by acting as moisture barrier [154, 155]. In this regard, Harris and Lee [155] studied the durability of PLLA/PC blends (100, 45, 30, and 25 wt% PLLA) for use in injection-molded automotive interior parts. Harsh conditions were set up to study the durability of the blends, namely 70°C and 90% relative humidity for 62 days, corresponding to 10 years of in-field exposure for an automotive interior component in the climate of southern Florida. The durability of ABS/PC was used as a basis for comparison. Results demonstrated that the presence of PC can improve the long-term performance of blends compared with neat PLLA (durability increase for one in-field exposure year). Nevertheless, all samples containing PLLA exhibited extreme degradation after 14 conditioning days, resulting in a drop in mechanical performance (in terms of flexural strength; see Fig. 17a) together with the formation of a large amount of brown liquid residue at the sample surface as a result of hydrolysis of the PC phase (accompanied by the appearance of potentially health-harmful bisphenol-A (BPA); see Fig. 17b, c). Although PC was initially blended to stabilize PLLA and improve overall durability, the final durability of the blend was only marginally improved because of the hydrolytic degradation of PC. It should be noted that PC suffered more from hydrolysis when blended with PLLA than with ABS.
(a) Flexural strength as a function of conditioning time of PLLA/PC blends. (b) Hydrolysis of PC. (c) Chemical structure of bisphenol-A (BPA). Adapted with permission from [155] Copyright© 2012 Wiley Periodicals, Inc
In conclusion, the formation of PC/PLLA blends has not yet achieved a durability level high enough for automotive applications, regardless of PC content. However, blending PLLA with more durable polymer resins in order to enhance the durability of blends constitutes a promising research field.
4 PLLA in the Automotive Industry: Current Applications
Corporate environmental responsibility has to become reconciled with business economics. Therefore, original equipment manufacturers (OEMs) must adopt the use of biobased materials. However, before biobased materials can replace conventional nonrenewable materials in the automotive industry, different criteria must be fulfilled, in addition to environmental benefits, namely cost, suitable physical and mechanical properties, processability, and continuous supply. The willingness for a more widespread use of biobased materials by the automotive OEMs is heavily influenced by those criteria, as is already the case for currently used conventional materials.
The previous sections showed PLLA to be an environmentally friendly polymer that can respond to the requirements of car manufacturers for some applications. In particular, PLLA-based materials can be tailor-made for different manufacturing processes, including injection molding, sheet extrusion, blow molding, thermoforming, film forming, and fiber-spinning. This is an undeniable asset for the use of PLLA in products of different forms and applications [141, 156].
4.1 PLLA-Based Plastics and Reinforced Plastics for Automotive Interior Parts
Thanks to technological breakthroughs in recent years, significant progress has been made in the development of PLLA-based blends, not only at the laboratory scale but also at industrial scale, which makes PLLA biopolymers suitable for a growing number of automotive components, in particular vehicle interior parts. Indeed, biobased PLLA blends are used today by many automobile manufacturers, including Mazda, Toyota, Ford, and Hyundai Motors for the manufacture of various components (see Table 9). Some car manufacturers (or their suppliers) have developed their own blends based on PLLA or some other biobased or petroleum-based thermoplastics or fibers for use in interior automotive parts. For instance, Teijin has developed in cooperation with Mazda a new brand of high-performance stereocomplexed PLA, called BIOFRONT, by utilizing superior polymerization techniques and molecular structure control techniques (stereocomplexation). Major quality improvements were achieved compared with conventional PLLA, and BIOFRONT can be found in car dashboards and door tread plates [160].
Another major revolution in biobased materials for automotive application was the introduction by Toyota of a PLLA/kenaf fiber biocomposite in the Raum mini-MPV [157]. Specifically, Toyota substituted the PP matrix used within its kenaf fiber-based composite with a PLLA resin, thanks to a unique technology that optimizes the raw material mixture and molding conditions to achieve a high level of heat and shock resistance. This 100% plant-derived composite is particularly used in door trims. It is worth noting that increasing use of composites made of natural fibers coincides with attempts to reduce the use of expensive glass [168], aramid, and carbon fibers and also lighten the car body considerably by taking advantage of the low density of most natural fibers. Renewable fibers as reinforcements are increasingly used in composites for the interior parts of many passenger and commercial vehicles. When regrouping different studies, it is noticeable that in most cases “green composites” made of PLA, semicrystalline PLLA, and natural fibers such as flax, ramie, or jute have mechanical properties that can compete with those of the traditional PP/glass fiber composites (Fig. 18 shows performance in terms of tensile strength and Young’s modulus) [169].
Mechanical performance of several fiber-based composites. Reproduced with permission from [169] Copyright © 2012 Elsevier Ltd
Following the pioneering work by Toyota, many automotive OEMs and suppliers have launched research aimed at introducing biocomposites into their vehicles. For instance, Röchling Automotive produces a wide range of automotive plastic parts such as air filter box and interior trim parts using a PLLA-based biopolymer called Planutra™ [162]. This biopolymer, developed by Corbion Purac, is an ecological and economic alternative to traditional thermoplastics and could replace PP, ABS, and polyamide. Composites made of modified PLA Planutra™ and wood fiber reinforcement are already undergoing tests and are being exposed to temperatures well above 100°C. These materials are claimed to show improved hydrolysis and thermal resistance up to 140°C, as well as a good scratch and UV resistance.
4.2 PLLA-Based Fabrics and Nonwovens Products
The use of fabrics in cars is increasing as a promising strategy for production of lighter vehicles that consume less fuel. In that context, more and more applications using tissues and nonwoven composites are being developed in the automotive industry. Depending on the formulation, such materials can offer flexibility, low weight, sound absorption, improved aesthetics, cost-efficiency, and ecofriendliness. From nonwoven interior and trunk carpets to knitted or woven upholstery, package trays, and dash panels, textile producers are finding innovative ways to keep the momentum going. There are now more than 40 applications for inner automobile seat upholstery, belts, airbags, cladding, filters, and insulating materials. More than 35 m2 of flat textile surfaces can be found inside one of today’s cars [170]. Recently, there has been a gradual switch in many automotive applications toward the use of recycled materials or materials made of natural fibers, instead of conventional fabrics. Many current applications are described in Table 9. Mazda Motor Corporation, with the cooperation of Teijin Fibers Limited, has developed a heat-resistant PLA-based fabric (commercialized under the name BIOFRONT, see Sect. 4.1), which was initially used for the manufacture of a high-quality and highly durable car-seat fabric for the new Premacy Hydrogen RE Hybrid vehicle [163]. During manufacture of BIOFRONT, the molecular architecture of the polymer is controlled to improve fiber strength until the fabric attains sufficient resistance to abrasion and light damage. Recently, the project “Nature Wins” succeeded in producing a 100% biobased composite with biopolymer PLLA as matrix and mix of hemp and flax fibers as reinforcement [164]. The fibers were assembled into various structures such as yarn and woven and nonwoven fabrics and the flax/PLLA nonwoven composite used to make a molded demonstration model of a race car seat. Recently, the BIOFIBROCAR project has focused on the development of PLLA-based fibers to be used as an alternative to PET and PP fibers in car interiors [167]. The developed PLLA compound was shown to fulfill the requirements of automotive interior applications in terms of thermal resistance, antimicrobial resistance, and odor emission. Its conversion into fabric and nonwoven samples allowed fabrication of a prototype of a 100% biobased molded door panel, with the association of two nonwoven layers and a woven fabric.
5 Conclusions and Perspectives
The automotive industry is one of the mainsprings for the development of bioplastics and biocomposites in durable applications, due to its well-structured network of car manufacturers, OEMs, and raw material suppliers. All have shown significant need for bioplastics and biocomposites to meet joint objectives in terms of reduction of vehicle weight combined with increasing demands for environmentally friendly materials.
In this review, the technical requirements for plastics used in automotive applications are presented, with a special emphasis on renewable PLLA-based materials. Attention is paid to the development of PLLA-based blends and the different modifications that make PLLA suitable for automotive applications in terms of mechanical properties (ductility combined with high rigidity and strength), high heat resistance, durability, and good processability, even under high-rate production. Some of these modifications can provide relevant PLLA-based materials for intensive use in the automotive sector, allowing car makers to invest in ecofriendly vehicles using developed PLLA-based bioplastics and biocomposites. For instance, Koronis et al. [169] judiciously mentioned that “the application of green composites in automobile body panels seems to be feasible as far as green composites have comparable mechanical performances with the synthetic ones.” However, up to now, automotive application of tailored PLLA compounds has been limited to interior parts, particularly because of long-term durability issues under the severe environmental conditions that exterior parts have to withstand. However, in near future, research efforts can offer PLLA-based blends suitable for the exterior parts of vehicles. One issue to be investigated now concerns the recyclability and to some extent biodegradability of these novel parts in the end-life scenario. A final question here is, why not using 3D printing processes to print full size cars? PLLA is proven to be a good candidate for use in 3D printing, and it would improve the need for storage facilities in the case of spare parts.
Abbreviations
- ABS:
-
Acrylate-butadiene-styrene
- ATBC:
-
Acetyltributylcitrate
- BioPA:
-
Bio-polyamide
- BioPE:
-
Bio-polyethylene
- BS:
-
Biomax® Strong (Commercial impact modifier)
- CE:
-
Chain extender
- CL25A:
-
Cloisite® 25A
- DEHA:
-
Bis(2-ethylhexyl) adipate
- DOA:
-
Dioctyl adipate
- DSC:
-
Differential scanning calorimetry
- EBS:
-
Ethylene bis-stearamide (nucleating agent)
- EMA-GMA:
-
Ethylene-methyl acrylate-glycidyl methacrylate
- ENR:
-
Epoxidized natural rubber
- GTA:
-
Glyceryl triacetate
- HDT:
-
Heat deflection temperature
- HNT:
-
Halloysite nanotube
- NCH:
-
Nylon-clay hybrid
- OEM:
-
Original equipment manufacturer
- OMC:
-
Organic modified clay
- OMLS:
-
Organically modified layered silicate
- PA:
-
Polyamide
- PBGA:
-
Oligomericpoly(1,3-butylene glycol adipate)
- PC:
-
Polycarbonate
- PCL:
-
Polycaprolactone
- PDLA:
-
Poly(d-lactic acid)
- PEBA:
-
Polyether block amide
- PEG:
-
Polyethylene glycol
- PET:
-
Polyethylene terephtalate
- PHA:
-
Polyhydroxyalkanoate
- PLLA:
-
Poly(l,d-lactic acid)
- PLS:
-
Polymer-layered silicate nanocomposites
- PMMA:
-
Poly(methyl methacrylate)
- PP:
-
Polypropylene
- PPA:
-
Poly(1,2-propylene glycol adipate)
- PS:
-
Polystyrene
- PTT:
-
Polytrimethylene terephtalate
- PU:
-
Polyurethane
- RH:
-
Relative humidity
- sc-PLA:
-
Stereocomplex of polylactide
- TAC:
-
Triacetin
- TBC:
-
Tributylcitrate
- TPU:
-
Thermoplastic polyurethane
References
Brostow W, Datashvili T (2016) Environmental impact of natural polymers. In: Natural polymers. Springer, Berlin, pp 315–338
Akampumuza O et al (2016) Review of the applications of biocomposites in the automotive industry. Polym Compos. doi:10.1002/pc.23847
Niaounakis M (2015) Automotive applications. In: Biopolymers: applications and trends. William Andrew, Oxford, pp. 257–289
Garlotta DA (2001) Literature review of poly(lactic acid). J Polym Environ 9(2):63–84
Avérous L (2008) Polylactic acid: synthesis, properties and applications. In: Gandini MNB (ed) Monomers, polymers and composites from renewable resources. Elsevier, Amsterdam, pp. 433–450
Maxwell J (1994) Plastics in the automotive industry. Elsevier, Amsterdam
Auras RA et al (2011) Poly(lactic acid): synthesis, structures, properties, processing, and applications, vol 10. Wiley, Hoboken
Reynolds N, Ramamohan AB (2013) High-volume thermoplastic composite technology for automotive structures. In: Advanced composite materials for automotive applications. Wiley, Hoboken, pp 29–50
Rowe J (2012) Advanced materials in automotive engineering. Elsevier, Amsterdam
Götz Klink GR, Znojek B, Wadivkar O (2012) Plastics. The future for automakers and chemical companies. A.T. Kearney, Korea
Swift TK, Moore M, Sanchez E (2015) Plastics and polymer composites in light vehicles. Economics and Statistics Department/American Chemistry Council
Philp JC et al (2013) Biobased plastics in a bioeconomy. Trends Biotechnol 31(2):65–67
Thielen M (2012) Bioplastics: basics, applications, markets. Polymedia, Mönchengladbach
Pilla S (2011) Handbook of bioplastics and biocomposites engineering applications, vol 81. Wiley, Hoboken
Siracusa V et al (2008) Biodegradable polymers for food packaging: a review. Trends Food Sci Technol 19(12):634–643
European Bioplastics (2013) Applications for bioplastics. European Bioplastics, Berlin. http://www.european-bioplastics.org/market/applications-sectors/
Lee E, Flanigan C (2012) Automotive plastics and composites. In: Encyclopedia of polymer science and technology. Wiley, Hoboken
Szeteiová K (2010) Automotive materials: plastics in automotive markets today. Institute of Production Technologies, Slovak University of Technology, Bratislava
Andrea DJ, Brown WR (1993) Material selection processes in the automotive industry. OSAT, Michigan
Ghassemieh E (2011). Materials in automotive application, state of the art and prospects. In: Chiaberge M (ed) New trends and developments in automotive industry. Intech, Rijeka, pp 366–394. doi:10.5772/13286
Weber M, Weisbrod J (2003) Requirements engineering in automotive development-experiences and challenges. In: IEEE joint international conference on requirements engineering, 2002. IEEE, pp 331–340. doi:10.1109/MS.2003.1159025
Biron M (2012) Thermoplastics and thermoplastic composites. William Andrew, Oxford
Babu RP et al (2013) Current progress on bio-based polymers and their future trends. Prog Biomater 2(1):1–16
Fiori S (2014) Industrial uses of PLA. In: Jiménez A, Peltzer M, Ruseckaite R (eds) Poly(lactic acid) science and technology: processing, properties, additives and applications. Royal Society of Chemistry, London, pp 315–333. doi:10.1039/9781782624806-00315
Lunt J (1998) Large-scale production, properties and commercial applications of polylactic acid polymers. Polym Degrad Stab 59(1–3):145–152
Signori F et al (2009) Thermal degradation of poly(lactic acid) (PLA) and poly(butylene adipate-co-terephthalate) (PBAT) and their blends upon melt processing. Polym Degrad Stab 94(1):74–82
Song J et al (2009) Biodegradable and compostable alternatives to conventional plastics. Philos Trans R Soc Lond B Biol Sci 364(1526):2127–2139
Vink ET et al (2007) Original research: the eco-profiles for current and near-future NatureWorks® polylactide (PLA) production. Ind Biotechnol 3(1):58–81
Jamshidian M et al (2010) Poly-lactic acid: production, applications, nanocomposites, and release studies. Compr Rev Food Sci Food Saf 9(5):552–571
Sin LT et al (2012) Polylactic acid: PLA biopolymer technology and applications. William Andrew, Oxford
Dusselier M et al (2015) Shape-selective zeolite catalysis for bioplastics production. Science 349(6243):78–80
Achmad F et al (2009) Synthesis of polylactic acid by direct polycondensation under vacuum without catalysts, solvents and initiators. Chem Eng J 151(1–3):342–350
Gerrard J, Kandlikar M (2007) Is European end-of-life vehicle legislation living up to expectations? Assessing the impact of the ELV directive on ‘green’ innovation and vehicle recovery. J Clean Prod 15(1):17–27
Madhavan Nampoothiri K et al (2010) An overview of the recent developments in polylactide (PLA) research. Bioresour Technol 101(22):8493–8501
Nova Institute (2012) Growth in PLA bioplastics: a production capacity of over 800,000 tonnes expected by 2020. Bio-based News, 7 Aug 2012
Nair LS, Laurencin CT (2007) Biodegradable polymers as biomaterials. Prog Polym Sci 32(8–9):762–798
Bhardwaj R, Mohanty AK (2007) Advances in the properties of polylactides based materials: a review. J Biobased Mater Bioenergy 1(2):191–209
Canessa E et al (2013) Low-cost 3D printing for science, education and sustainable development. ICPT, Trieste. http://sdu.ictp.it/3D/book.html
Campbell T et al (2011) Could 3D printing change the world. Technologies, potential, and implications of additive manufacturing. Atlantic Council, Washington
Reeves P, Mendis D (2015) The current status and impact of 3D printing within the industrial sector: an analysis of six case studies. Intellectual Property Office, Newport
Staff S (2014) Revolutionary new electric car built and tested in one year with Objet1000 multi-material 3D production system. Stratasys. http://blog.stratasys.com/2014/11/18/streetscooter-3d-printing/. Accessed 14 Jan 2016
Bunkley N (2014) “World’s first” 3D printed car created and driven by Local Motors. Automotive News. http://www.autonews.com/article/20141027/OEM06/310279987/auto-industry-uses-3-d-printing-heavily-in-product-development. Accessed 14 Jan 2016
Wypych G (2012) Handbook of plasticizers, 2nd edn. William Andrew, Boston
Ljungberg N, Wesslén B (2005) Preparation and properties of plasticized poly(lactic acid) films. Biomacromolecules 6(3):1789–1796
Liu H, Zhang J (2011) Research progress in toughening modification of poly(lactic acid). J Polym Sci B Polym Phys 49(15):1051–1083
Ruellan A et al (2015) Plasticization of poly(lactide). In: Jiménez A, Peltzer M, Ruseckaite R (eds) Poly(lactic acid) science and technology: processing, properties, additives and applications. The Royal Society of Chemistry, London, pp. 124–170
Jacobsen S, Fritz HG (1999) Plasticizing polylactide—the effect of different plasticizers on the mechanical properties. Polym Eng Sci 39(7):1303–1310
Murariu M et al (2008) Polylactide (PLA) designed with desired end-use properties: 1. PLA compositions with low molecular weight ester-like plasticizers and related performances. Polym Adv Technol 19(6):636–646
Zhang H et al (2013) Thermal, mechanical, and rheological properties of polylactide/poly(1,2-propylene glycol adipate). Polym Eng Sci 53(1):112–118
Ren Z et al (2006) Dynamic mechanical and thermal properties of plasticized poly(lactic acid). J Appl Polym Sci 101(3):1583–1590
Wojciechowska P (2012) The effect of concentration and type of plasticizer on the mechanical properties of cellulose acetate butyrate organic-inorganic hybrids. In: Luqman M (ed) Recent advances in plasticizers. Intech, Rijeka, pp. 141–164
Suvorova AI et al (1993) Chemical structure of plasticizers, compatibility of components and phase equilibrium in plasticized cellulose diacetate. Makromol Chem Rapid 194(5):1315–1321
Notta-Cuvier D et al (2015) Tailoring polylactide properties for automotive applications: effects of co-addition of halloysite nanotubes and selected plasticizer. Macromol Mater Eng. doi:10.1002/mame.201500032
Kfoury G et al (2013) Recent advances in high performance poly (lactide): from “green” plasticization to super-tough materials via (reactive) compounding. Front Chem 1:1
Sangeetha VH et al (2016) State of the art and future prospectives of poly(lactic acid) based blends and composites. Polym Compos. doi:10.1002/pc.23906
Odent J et al (2015) Highly toughened polylactide-based materials through melt-blending techniques. In: Biodegradable polyesters. Wiley-VCH, Weinheim, pp. 235–274
Anderson KS et al (2008) Toughening polylactide. Polym Rev 48(1):85–108
Hongzhi L, Jinwen Z (2012) Toughening Modification of Poly(lactic acid) via melt blending. In: Biobased monomers, polymers, and material. ACS Symposium Series, vol 1105, pp 27–46
Detyothin S et al (2010) Poly(lactic acid) blends. In: Poly(lactic acid). Wiley, Hoboken, pp. 227–271
Perkins WG (1999) Polymer toughness and impact resistance. Polym Eng Sci 39(12):2445–2460
Liu Z-W et al (2014) Mechanical and thermal properties of thermoplastic polyurethane-toughened polylactide-based nanocomposites. Polym Compos 35(9):1744–1757
NatureWorks (2007) Technology focus report: toughened PLA. NatureWorks, Minnetonka
Taib RM et al (2012) Thermal, mechanical, and morphological properties of polylactic acid toughened with an impact modifier. J Appl Polym Sci 123(5):2715–2725
Bouzouita A et al (2016) Design of highly tough poly(l-lactide)-based ternary blends for automotive applications. J Appl Polym Sci. doi:10.1002/app.43402
Notta-Cuvier D et al (2014) Tailoring polylactide (PLA) properties for automotive applications: effect of addition of designed additives on main mechanical properties. Polym Test 36(0):1–9
Zhang K et al (2014) Supertoughened renewable PLA reactive multiphase blends system: phase morphology and performance. ACS Appl Mater Interface 6(15):12436–12448u
Zhang C et al (2013) Thermal, mechanical and rheological properties of polylactide toughened by expoxidized natural rubber. Mater Des 45:198–205
Pattamaprom C et al (2016) Improvement in impact resistance of polylactic acid by masticated and compatibilized natural rubber. Iran Polym J 25(2):169–178
Pongtanayut K et al (2013) The effect of rubber on morphology, thermal properties and mechanical properties of PLA/NR and PLA/ENR blends. Energy Procedia 34:888–897
Desa M et al (2015) Mechanical and thermal properties of rubber toughened poly(lactic acid). In: Advanced materials research, vol 1125. Trans Tech Publications, pp 222–226
Sun Y, He C (2013) Biodegradable “core–shell” rubber nanoparticles and their toughening of poly(lactides). Macromolecules 46(24):9625–9633
Odent J et al (2013) Toughening of polylactide by tailoring phase-morphology with P[CL-co-LA] random copolyesters as biodegradable impact modifiers. Eur Polym J 49(4):914–922
Meyva Y, Kaynak C (2015) Toughening of polylactide by bio-based and petroleum-based thermoplastic elastomers. Int Polym Process 30(5):593–602
Li Y, Shimizu H (2007) Toughening of polylactide by melt blending with a biodegradable poly(ether) urethane elastomer. Macromol Biosci 7(7):921–928
Todo M, Takayama T (2014) Fracture mechanisms of biodegradable PLA and PLA/PCL blends. In: Pignatello R (ed) Biomaterials – physics and chemistry. InTech, Rijeka, pp 375–394
Todo M, Takayama T (2007) Toughening of bioabsorbable polymer blend by microstructural modification. In: Watanabe M, Okuno O, Sasaki K, Takahashi N, Suzuki O, Takada H (eds) Proceedings of the 2nd international symposium for Interface oral health science, Sendai, Japan, 18–19 February, 2007. Springer Japan, Tokyo, pp. 95–104
Turng L-S, Srithep Y (2014) Annealing conditions for injection molded poly (lactic acid). Society of Plastics Engineers (SPE). doi:10.2417/spepro.005392. http://www.4spepro.org/pdf/005392/005392.pdf
Grijpma DW et al (2002) Improvement of the mechanical properties of poly(D,L-lactide) by orientation. Polym Int 51(10):845–851
Carrasco F et al (2010) Processing of poly(lactic acid): characterization of chemical structure, thermal stability and mechanical properties. Polym Degrad Stab 95(2):116–125
Nascimento L et al (2010) Effect of the recycling and annealing on the mechanical and fracture properties of poly(lactic acid). J Polym Environ 18(4):654–660
Gámez-Pérez J et al (2011) Fracture behavior of quenched poly (lactic acid). Express Polym Lett 5(1):82–91
Yang G et al (2012) Toughening of poly (L-lactic acid) by annealing: the effect of crystal morphologies and modifications. J Macromol Sci B 51(1):184–196
Perego G et al (1996) Effect of molecular weight and crystallinity on poly(lactic acid) mechanical properties. J Appl Polym Sci 59(1):37–43
Cocca M et al (2011) Influence of crystal polymorphism on mechanical and barrier properties of poly(l-lactic acid). Eur Polym J 47(5):1073–1080
Edenhofer B et al (2006) The flexible heat treatment of automotive components in a novel type of pusher furnace. La Metallurgia Italiana (2):39–45. http://www.fracturae.com/index.php/aim/article/download/633/602
Funatani K (2004) Heat treatment of automotive components: current status and future trends. Trans Indian Inst Metals 57(4):381–396
Johnson RW et al (2004) The changing automotive environment: high-temperature electronics. IEEE Trans Electron Packag Manuf 27(3):164–176
ASTM (2016) ASTM standard test method for deflection temperature of plastics under flexural load in the edgewise position. ASTM. http://www.astm.org/Standards/D648.htm
Huda MS et al (2006) Wood-fiber-reinforced poly(lactic acid) composites: evaluation of the physicomechanical and morphological properties. J Appl Polym Sci 102(5):4856–4869
Shih Y-F, Huang C-C (2011) Polylactic acid (PLA)/banana fiber (BF) biodegradable green composites. J Polym Res 18(6):2335–2340
Lee B-H et al (2009) Bio-composites of kenaf fibers in polylactide: role of improved interfacial adhesion in the carding process. Compos Sci Technol 69(15–16):2573–2579
Shi QF et al (2012) Influence of heat treatment on the heat distortion temperature of poly(lactic acid)/bamboo fiber/talc hybrid biocomposites. J Appl Polym Sci 123(5):2828–2836
Ganster J, Fink H-P (2006) Novel cellulose fibre reinforced thermoplastic materials. Cellulose 13(3):271–280
Chanda M, Roy SK (2008) Industrial polymers, specialty polymers, and their applications, vol 74. CRC, Boca Raton
Coelho MC et al (2012) Nanotechnology in automotive industry: research strategy and trends for the future—small objects, big impacts. J Nanosci Nanotechnol 12(8):6621–6630
Mai Y-W, Yu Z-Z (2006) Polymer nanocomposites. Woodhead, Cambridge
Huang J-W et al (2009) Polylactide/nano and microscale silica composite films. I. Preparation and characterization. J Appl Polym Sci 112(3):1688–1694
Frone AN et al (2011) Cellulose fiber-reinforced polylactic acid. Polym Compos 32(6):976–985
Li ZQ et al (2010) Preparation and characterization of bacterial cellulose/polylactide nanocomposites. Polym-Plast Technol Eng 49(2):141–146
Shameli K et al (2010) Silver/poly(lactic acid) nanocomposites: preparation, characterization, and antibacterial activity. Int J Nanomedicine 5:573–579
Xu X et al (2006) Biodegradable electrospun poly(l-lactide) fibers containing antibacterial silver nanoparticles. Eur Polym J 42(9):2081–2087
González A et al (2012) Fire retardancy behavior of PLA based nanocomposites. Polym Degrad Stab 97(3):248–256
Sinha Ray S, Okamoto M (2003) Polymer/layered silicate nanocomposites: a review from preparation to processing. Prog Polym Sci 28(11):1539–1641
Alexandre M, Dubois P (2000) Polymer-layered silicate nanocomposites: preparation, properties and uses of a new class of materials. Mater Sci Eng R Rep 28(1–2):1–63
Sinha Ray S, Bousmina M (2005) Biodegradable polymers and their layered silicate nanocomposites: in greening the 21st century materials world. Prog Mater Sci 50(8):962–1079
Kurauchi T et al (1991) Nylon 6-clay hybrid-synthesis, properties and application to automotive timing belt cover. SAE Technical Paper 910584. doi:10.4271/910584
Paul M-A et al (2003) Exfoliated polylactide/clay nanocomposites by In-situ coordination–insertion polymerization. Macromol Rapid Commun 24(9):561–566
Khankrua R et al (2013) Thermal and mechanical properties of biodegradable polyester/silica nanocomposites. Energy Procedia 34:705–713109
Murariu M et al (2010) New trends in polylactide (PLA)-based materials: “green” PLA–calcium sulfate (nano)composites tailored with flame retardant properties. Polym Degrad Stab 95(3):374–381
Sinha Ray S et al (2003) New polylactide-layered silicate nanocomposites. 2. Concurrent improvements of material properties, biodegradability and melt rheology. Polymer 44(3):857–866
Pilla S et al (2010) Microcellular processing of polylactide–hyperbranched polyester–nanoclay composites. J Mater Sci 45(10):2732–2746
Ameli A et al (2014) Development of high void fraction polylactide composite foams using injection molding: mechanical and thermal insulation properties. Compos Sci Technol 90:88–95
Pilla S et al (2007) Solid and microcellular polylactide-carbon nanotube nanocomposites. Int Polym Process 22(5):418–428
Kramschuster A et al (2007) Injection molded solid and microcellular polylactide compounded with recycled paper shopping bag fibers. Int Polym Process 22(5):436–445
Seo J-H et al (2012) Combined effects of chemical and microcellular foaming on foaming characteristics of PLA (poly lactic acid) in injection molding process. Polym-Plast Technol Eng 51(5):455–460
Harris AM, Lee EC (2006) Injection molded polylactide (PLA) composites for automotive applications. SPE ACCE Paper Draft 62906
Najafi Chaloupli N (2015) Development of polylactide (PLA) and PLA nanocomposite foams in injection molding for automotive applications. Doctoral dissertation, École Polytechnique de Montréal
NatureWorks (2007) Technology focus report: blends of PLA with other thermoplastics. NatureWorks, Minnetonka
Guo X et al (2015) Poly(lactic acid)/polyoxymethylene blends: morphology, crystallization, rheology, and thermal mechanical properties. Polymer 69:103–109
Hashima K et al (2010) Structure-properties of super-tough PLA alloy with excellent heat resistance. Polymer 51(17):3934–3939
Wang Y et al (2012) Improvement in toughness and heat resistance of poly(lactic acid)/polycarbonate blend through twin-screw blending: influence of compatibilizer type. J Appl Polym Sci 125(S2):E402–E412
Lin L et al (2015) Improving the impact property and heat-resistance of PLA/PC blends through coupling molecular chains at the interface. Polym Adv Technol 26(10):1247–1258
Lin L et al (2015) Super toughened and high heat-resistant poly(lactic acid) (PLA)-based blends by enhancing interfacial bonding and PLA phase crystallization. Ind Eng Chem Res 54(21):5643–5655
Srithep Y et al (2014) Processing and characterization of poly(lactic acid) blended with polycarbonate and chain extender. J Polym Eng 34:665–672
Liu J (2014) Heat resistant, flame retardant polylactic acid compounds. Patent WO2014113453 A1
Eguiburu JL et al (1998) Blends of amorphous and crystalline polylactides with poly(methyl methacrylate) and poly(methyl acrylate): a miscibility study. Polymer 39(26):6891–6897
Li S-H, Woo EM (2008) Immiscibility–miscibility phase transitions in blends of poly(L-lactide) with poly(methyl methacrylate). Polym Int 57(11):1242–1251
Samuel C et al (2013) PLLA/PMMA blends: a shear-induced miscibility with tunable morphologies and properties? Polymer 54(15):3931–3939
Zhang G et al (2003) Miscibility and phase structure of binary blends of polylactide and poly(methyl methacrylate). J Polym Sci B Polym Phys 41(1):23–30
Tsuji H (2007) Poly(lactide) stereocomplexes: formation, structure, properties, degradation, and applications. Macromol Biosci 7(12):1299–1299
Ikada Y et al (1987) Stereocomplex formation between enantiomeric poly(lactides). Macromolecules 20(4):904–906
Tsuji H et al (1991) Stereocomplex formation between enantiomeric poly(lactic acid)s. 4. Differential scanning calorimetric studies on precipitates from mixed solutions of poly(D-lactic acid) and poly(L-lactic acid). Macromolecules 24(20):5657–5662
Torres L et al (2016) Effect of multi-branched PDLA additives on the mechanical and thermomechanical properties of blends with PLLA. J Appl Polym Sci 133(1). doi:10.1002/app.42858
Zou J et al (2012) Effects of poly (D-lactide acid) on the properties of crystallization and thermal behavior of poly (L-lactide acid). Adv Info Sci Service Sci 4(10):382
Oyama HT, Abe S (2015) Stereocomplex poly(lactic acid) alloys with superb heat resistance and toughness. ACS Sustain Chem Eng 3(12):3245–3252
Sun B et al (2016) Enhanced toughness and strength of poly (d-lactide) by stereocomplexation with 5-arm poly (l-lactide). J Appl Polym Sci 133(1). doi:10.1002/app.42857
Nam B-U, Lee B-S (2012) Toughening of PLA stereocomplex by impact modifiers. J Korea Acad Industr Coop Soc 13(2):919–925
Corbion Purac (2015) high heat PLA: unlocking bioplastic potential for durable applications. http://www.corbion.com/media/77166/corbion-puracpla-high-heat-themesheet.pdf. Accessed Jan 2016
Min BH (2003) A study on quality monitoring of injection-molded parts. J Mater Process Technol 136(1–3):1–6
Spina R (2004) Injection moulding of automotive components: comparison between hot runner systems for a case study. J Mater Process Technol 155–156:1497–1504
Lim LT et al (2008) Processing technologies for poly(lactic acid). Prog Polym Sci 33(8):820–852
Mazumder SK (ed) (2002) Composites manufacturing, materials, product and process engineering. CRC Taylor & Francis, London. ISBN 0-8493-0585-3
Harris AM, Lee EC (2008) Improving mechanical performance of injection molded PLA by controlling crystallinity. J Appl Polym Sci 107(4):2246–2255
Li M et al (2010) Nonisothermal crystallization kinetics of poly(lactic acid) formulations comprising talc with poly(ethylene glycol). Polym Eng Sci 50(12):2298–2305
Urayama H et al (2003) Controlled crystal nucleation in the melt-crystallization of poly(l-lactide) and poly(l-lactide)/poly(d-lactide) stereocomplex. Polymer 44(19):5635–5641
Petchwattana N et al (2014) Influence of talc particle size and content on crystallization behavior, mechanical properties and morphology of poly (lactic acid). Polym Bull 71(8):1947–1959
Zhou J et al (2013) Synthesis and characterization of triblock copolymer PLA-b-PBT-b-PLA and its effect on the crystallization of PLA. RSC Adv 3(40):18464–18473
Shi X et al (2015) Synergistic effects of nucleating agents and plasticizers on the crystallization behavior of poly (lactic acid). Molecules 20(1):1579–1593
Kolstad JJ (1996) Crystallization kinetics of poly(L-lactide-co-meso-lactide). J Appl Polym Sci 62(7):1079–1091
Schmidt SC, Hillmyer MA (2001) Polylactide stereocomplex crystallites as nucleating agents for isotactic polylactide. J Polym Sci B Polym Phys 39(3):300–313
NEC (2006) NEC & UNITIKA Realize bioplastic reinforced with Kenaf fiber for mobile phone use. http://www.nec.co.jp/press/en/0603/2001.html
Fujitsu Limited, Fujitsu Laboratories Ltd.,Toray Industries, Inc. (2005) Fujitsu and Toray develop world’s first environmentally-friendly large-size plastic housing for notebook PCs [Press release]. Retrieved from http://www.fujitsu.com/global/about/resources/news/press-releases/2005/0113-01.html
Harris AM, Lee EC (2010) Heat and humidity performance of injection molded PLA for durable applications. J Appl Polym Sci 115(3):1380–1389
Finniss A (2014) Polylactic acid-based polymer blends for durable applications. West Virginia University, Morgantown
Harris AM, Lee EC (2013) Durability of polylactide-based polymer blends for injection-molded applications. J Appl Polym Sci 128(3):2136–2144
Jiménez A et al (2014) Poly (lactic acid) science and technology: processing, properties, additives and applications, vol 12. Royal Society of Chemistry, London
Toyota Motor Corporation (2000) Utilising plant-derived materials in automotive interior parts. http://www.toyota-boshoku.com/global/about/development/eco/index.html. Accessed 19 Feb 2016
Toyota Motor Corporation (2008) Toyota to increase ‘ecological plastic’ in vehicle interiors. http://www.toyota.co.jp/en/news/08/1217.html. Accessed 21 Feb 2016
Thielen M (2010) Hyundai Blue-Will concept to feature PLA and PA 11. Bioplastics Magazine, Jan/Feb 2010
Teijin Ltd. (2007) Highly heat-resistant bioplastic. http://www.teijin.com/rd/technology/bioplastic/. Accessed 22 Feb 2016
Motors M (2006) Mitsubishi Motors develops plant-based green plastic floor mat. http://www.mitsubishi-motors.com/en/corporate/pressrelease/corporate/detail1475.html. Accessed 20 Feb 016
Corbion Purac (2013) PLA bioplastics: a driving force in automotive. Corbion Purac. http://www.corbion.com/bioplastics/pla-markets/automotive. Accessed 05 Jan 2016
Kitamura H (2007) Teijin Launches BIOFRONT heat-resistant bioplastic - 100% BIOFRONT car seat fabrics developed with Mazda
Ramaswamy S, Aslan B, Gries T, Urbanus M (2015) Biobased thermoplastic composites for automotive interiors. Bioplastics Magazine, Jan/Feb 2015
Aslan B et al (2012) Bio-composites: processing of thermoplastic biopolymers and industrial natural fibres from Staple fibre blends up to fabric for composite applications. J Textile Eng 19(85)
Ichhaporia PK (2008) Composites from natural fibers. Dissertation, North Carolina State University, Raleigh
Ampro Verdù Solis A (2015) New biobased fibres for automotive interior applications. Bioplastics Magazine, Sept/Oct 2015
Wambua P et al (2003) Natural fibres: can they replace glass in fibre reinforced plastics? Compos Sci Technol 63(9):1259–1264
Koronis G et al (2013) Green composites: a review of adequate materials for automotive applications. Compos Part B 44(1):120–127
Edana (2013) Nonwovens in automotive applications. Edana, Brussels. http://www.edana.org/discover-nonwovens/products-applications/automotive
Acknowledgements
LAMIH authors are grateful to CISIT, the Nord-Pas-de-Calais Region, the European Community, the Regional Delegation for Research and Technology, the Ministry of Higher Education and Research, and the National Center for Scientific Research for their financial support. UMONS and Materia Novaauthors are grateful to the “RegionWallonne” and the European Community (FEDER, FSE) in the frame of “Pole d’ExcellenceMateria Nova” INTERREG IV—NANOLAC project and in the excellence program OPTI2MAT for their financial support. CIRMAP thanks the “Belgian Federal Government Office Policy of Science (SSTC)” for general support in the frame of the PAI-7/05. J.O. thanks F.R.I.A. for its financial support thesis grant. J.-M. Raquez is a “Chercheur Qualifié” by the F.R.S.-FNRS (Belgium).
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Bouzouita, A., Notta-Cuvier, D., Raquez, JM., Lauro, F., Dubois, P. (2017). Poly(lactic acid)-Based Materials for Automotive Applications. In: Di Lorenzo, M., Androsch, R. (eds) Industrial Applications of Poly(lactic acid). Advances in Polymer Science, vol 282. Springer, Cham. https://doi.org/10.1007/12_2017_10
Download citation
DOI: https://doi.org/10.1007/12_2017_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-75458-1
Online ISBN: 978-3-319-75459-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)