Abstract
The combination of rubber elasticity and anisotropic liquid crystalline order of liquid crystalline elastomers (LCEs) gives rise to exceptional physical properties, unknown for conventional solid-state materials. By selecting suitable chemistry of the liquid crystalline moieties, the liquid crystalline phase structure, and the chemistry of the macromolecular network structure, these physical properties can be optimized for specific applications like mechanics, optics, diffusional or electronic transport, etc. In this chapter we outline basic aspects to be considered when synthesizing LCEs, including some basic characterization techniques. We give an overview of the chemistry involved in synthesizing LCEs and the different techniques of chemical crosslinking developed for this purpose. The unique coupling of the polymer chain conformation and the anisotropic LC order in LCEs can be exploited to induce a macroscopic orientation of the LC phase structure by applying mechanical fields. By performing chemical crosslinking in the aligned state, the uniform macroscopic orientation can be fixed permanently. Different strategies are introduced to synthesize such liquid single-crystal elastomers (LSCEs), and illustrated by a wide range of examples. Orientation techniques based on external magnetic and electric fields or surface treatment are also included. We emphasize practical aspects and give advice for successful preparation of this fascinating class of materials. A promising future for LSCEs is expected because of their unique and as yet largely unexplored abilities to mimic living systems.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Chain conformation
- Cholesteric
- Elastomer
- Liquid crystal
- Liquid crystal polymer
- Nematic
- Polymer networks
- Smectic
1 Introduction
Understanding the chemistry and physics of macromolecules is a prerequisite for the development and application of new materials in the present age of plastics. One of the major challenges will be the development of responsive materials with the ability to mimic biological systems. Such properties require a high level of mobility combined with shape retention that cannot be found in the glassy or (semi-)crystalline state of conventional polymers.
As early as 1908 Otto Lehmann [1] speculated in his article on “Scheinbar lebende Kristalle, Pseudopodien, Cilien und Muskeln” that liquid crystals might be the origin of the tension in natural muscles. However, liquid crystals are fluids and any internal or external tension is usually eliminated by flow. The same holds for linear and branched macromolecules in the liquid crystalline state. In three-dimensional networks of macromolecules (elastomers), on the contrary, the macro-Brownian motions of the polymer chains are prevented and a form-retaining material results. Because the micro-Brownian motions of the chain segments are hardly influenced by the network structure of the polymer chains, a liquid crystalline state of order of the chain segments can exist [2].
In 1975 P.G. de Gennes recognized that the interplay between liquid crystalline order and the macromolecular network structure generates new physical properties that also resemble those of biological systems and muscles [3]. In the following years Otto Lehmann’s early ideas were actually demonstrated with liquid crystalline elastomers although at that time the basic concepts of macromolecular chemistry were still unknown.
Just as for biological beings, the liquid crystalline phase structure and simultaneously the functionality of liquid crystalline elastomers are strictly limited to a defined temperature regime. Similar to low molar mass liquid crystals and LC polymers this temperature regime is determined by the chemical constitution of the polymer networks. For the synthesis and investigation of liquid crystalline elastomers the basic concepts of liquid crystals, LC polymers, and polymer networks have to be brought together.
Research on liquid crystal elastomers has attracted scientists from very different fields, such as theoretical and experimental physics, organic and physical chemistry, biology, material science, and engineering. Theories and experiments have always gone hand in hand and there are several examples where theoretical predictions have actually led to the development of new materials.
In this chapter we outline the basic aspects to be considered when working with liquid crystal elastomers (LCEs), including techniques for their synthesis and characterization. For readers new to the field – who may not have a strong background in macromolecular chemistry – we shall introduce strategies for a successful approach. We start with an introduction to the synthesis of LC polymer networks and their basic characterization (Sect. 2). Subsequently their mechanical orientation behavior will be discussed (Sect. 3). Techniques to prepare elastomers that are permanently oriented to form a single crystal are the subject of Sect. 4. Finally a brief outlook is given in Sect. 5.
2 Synthesis of LC Polymer Networks
To obtain the liquid crystalline state in a polymer network, several strategies are conceivable. They are all based on well known principles evaluated during the last few decades for linear liquid crystalline polymers. The monomer units of the network have to consist of mesogenic moieties, which are either rigid rods or discs in the case of thermotropic polymorphism or amphiphiles in the case of lyotropic polymorphism. The mesogenic units can be attached either as side chains to the monomer units yielding “side chain elastomers” (Fig. 1a, b) or directly linked together within the polymer backbone yielding “main chain elastomers” (Fig. 1c, d). An additional variation of the architecture of the network is given by connecting the mesogenic groups via different geometries, e.g., for rods via their long axis (“end-on”) as shown in Fig. 1a, c or via their short axis (“side-on”) (Fig. 1b, d). All these different geometries directly and specifically affect the liquid crystalline state of order and the polymorphism.
Compared to low molar mass liquid crystals having a similar chemical constitution as the monomer units of the networks, two principal tendencies have to be considered that determine the LC phase regime. First, the specific volume of the mesogenic monomers normally exceeds the specific volume of the network. This reduction of the free volume shifts the liquid crystalline phase regime of the networks towards higher temperatures and may modify the polymorphic liquid crystalline variants. The second fact concerns the translational diffusion and the degree of rotational freedom of the mesogenic monomer units incorporated into or attached to the polymer backbone. Compared to low molar mass liquid crystals, both are elementary restricted and determine the liquid crystalline state and order. While, for example, end-on side chain polymers (or elastomers) (Fig. 1a) tend towards the formation of smectic phases, the lateral linkage of the mesogenic units (Fig. 1b) often prevents long range positional ordering and favors the formation of nematic phases. Furthermore, side-on linkage directly restricts the rotation of the mesogenic unit along their long molecular axis and may convert a uniaxial nematic or smectic phase into the corresponding biaxial phase [4–9].
Bearing in mind these basic aspects, the synthesis of LCEs is straightforward and offers two strategies. The first strategy is to start the synthesis of a network with a polymerization process of a new mesogenic monomer. Here all the factors mentioned above have to be considered and the knowledge of the liquid crystalline phase behavior of the corresponding linear polymer has to be elucidated. The consolidated findings of intensive research on linear LC polymers will help to evaluate a chemical constitution of a new monomer that successfully yields an LCE. The second strategy avoids all these problems by already starting with a linear LC polymer with known LC phase behavior. A crosslinking process – either via suitable functionalization of the linear polymer or by γ-irradiation – hardly modifies the LC phase behavior.
For the synthesis as well as for the usability of the networks the transition temperatures are most important. They determine the regime of use and hence the functionality of the LC structure. These are the liquid crystalline to isotropic phase transformation temperature T LC,i and the glass transition temperature T g, where the material transfers from the glassy state into the LC state. A transformation from the LC into the crystalline phase at T c,LC is mainly suppressed due to the complex structure of LCEs and should be avoided as it might destroy the network.
T LC,i should be above the temperature-regime of interest in cases where the physical properties of the elastomers within the LC phase, such as ferroelectricity or optical properties, are to be used. If changes in the physical properties are to be exploited, such as length changes at the phase transition, T LC,i must be adjusted to the desired transition temperature. For polymers with discotic or calamitic mesogenic groups, the phase transition temperatures are determined by the structure of the mesogenic monomer and of the main chain. The principles of their manipulation are well known from work on linear LC polymers. Additionally T LC,i can be systematically modified by copolymerization with different mesogenic or non-mesogenic co-monomers.
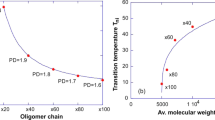
T g of LC elastomers is determined by the flexibility of the main chain, by interactions between the mesogens and the main chain, as well as by interactions between the mesogens. In the case of elastomers, the crosslinking density also plays an important role for the glass transition. This is rather different depending on the flexibility of the crosslinker used. If a flexible crosslinker is used, T g decreases with respect to the linear polymer for low crosslinking densities. Here the crosslinker acts as softening agent. If the crosslinking density is further increased, T g rises due to increasing immobilization of the network strands. For a rigid, mesogenic crosslinker, T g increases continuously with the concentration of the crosslinker. A high crosslinking density offers the chance to freeze-in the LC structures into anisotropic glasses, yielding duromers with highly interesting properties. Those materials have been investigated, for example, in the work of Broer (for a review, see [10]). For systems where dynamic processes such as electromechanical behavior or photo-mechanics are of interest, a T g below 20 °C is favorable. Strategies to obtain such low T g will be described below.
Another very important aspect for the synthesis of LC elastomers is the functionality Φ of the crosslink. Φ determines the number of chains that meet at a junction. The local topology of the crosslink determines the properties of the network and may be considered as a local defect within the LC phase structure of the network. This topology is not only determined by the chemical constitution of the multifunctional molecule, e.g., rod-like or flexible, but it is also affected by the local order imprinted during the crosslinking procedure. A network synthesized in the liquid crystalline state differs from a network with the same chemistry that was synthesized within the isotropic state. To minimize the effect of crosslinking molecules, their concentration has to be minimized. The influence of Φ can be shown using an elementary molecular theory of amorphous polymeric networks introduced by Flory in 1953. This ideal network theory does not consider dangling chain ends (i.e., a network chain that is connected to a junction of the network at only one end) or loops (i.e., a network chain, both ends of which are attached to the same polymer chain) and assumes that all junctions of the network have a functionality of Φ > 2. Such an ideal network can of course not be achieved in reality. In the ideal network the number of network chains is denoted as v and the number of junctions as μ. The formation of a perfect network can then be imagined as a process of end-linking of network chains with a crosslink. Herby, the number of chain-ends 2ν has to be equal to the number of functional groups Φμ, so that \( \mu = 2\nu /\phi \). Fewer junctions are needed when the functionality of the crosslink is higher [11, 12]. A higher functionality thus leads to fewer local defects in LC elastomers.
In the following sections some selected examples of the chemistry of LC networks will be summarized. While the synthesis of LC side chain elastomers mainly follows the radical polymerization technique and the polymer analogous addition reaction, LC main chain elastomers are exclusively synthesized by polycondensation or polyaddition reactions.
2.1 Side Chain Elastomers
In side chain elastomers, the mesogenic moieties, which can be rods, discs, or amphiphiles, are attached as side-groups to a polymer main chain via a flexible, aliphatic spacer. The existence of this spacer is crucial for the formation of LC phases as it lowers the tendency for crystallization and allows for a sufficient decoupling of the mesogenic units from the polymer backbone. This partial decoupling is necessary to allow the polymer chains to gain some entropy while the mesogenic side chains can exhibit orientational (and positional) long-range order of the LC phase. Concepts to change the LC phase behavior by modifying the chemical constitution are well known from linear polymers and crosslinking usually does not change the phase behavior dramatically. Rod-like mesogenic units are typically composed of two aromatic rings which are linearly connected via ester or ether bonds. For short aliphatic spacers and short tails of the mesogenic units, nematic phases are observed. However, with increasing spacer or tail length the stability of smectic phases increases. Mesogens based on three aromatic rings show preferably nematic phases but suffer from high transition temperatures. For the preparation of cholesteric elastomers part of the nematogenic side chains is replaced by chiral dopands, e.g., cholesteryl derivatives that exhibit high helical twisting power. In such co-elastomers of chiral and non-chiral side chains, the phase behavior and the helical pitch which define the optical properties of the material can easily be tuned. Smectic-A phase behavior can be induced reliably by using perfluorinated tails, which are much stiffer compared to alkyl chains and may further promote the segregation into a lamellar phase structure. The incorporation of polar ethylene oxide (EO) side chains or of mesogenic units carrying EO tails can cause lyotropic phase behavior when the elastomer is swollen with water.
In the following we will outline two basic methods to synthesize LC side chain elastomers. As a starting point for the synthesis of LC elastomers a mixture of mesogenic monomers and bi- or multi-functional crosslinker molecules may serve. This will be discussed in the first part of the section. Alternatively, polymer analogous reactions, where the mesogenic moieties are attached to a polymer backbone, can be employed, which will be discussed in the second part of the section.
If LC monomers are used as starting materials, it is very important to consider that monomer and polymer networks can differ in their phase behavior as previously mentioned. This is a particular issue for nematic elastomers. Only very few examples are known in which the temperature regime of a nematic phase of a monomer overlaps with the nematic temperature regime of the polymer. The systematic that was hereby obtained for the LC phase behavior of linear polymers also holds for LC polymer networks because for elastomers the concentration of the mesogens is much higher than that of the crosslink. The chemistry that can be used for the crosslink is determined by the chemistry of the polymerization technique.
The only practicable chemical reaction to prepare LC elastomers from functional monomers is radical polymerization. Acrylates or methacrylates are mainly used as starting materials. It has to be ensured that the mesogenic group and the crosslinker have the same reactivity, so that a statistical arrangement along the chains can be achieved and a change in concentration ratio with advancing reaction is avoided. In principal, radical polymerization can be carried out in bulk. However, only if the polymerization temperature is within the overlapping temperature regime where monomer and polymer exhibit the same LC phase a homogeneous reaction can take place. Otherwise de-mixing occurs, which causes an uncontrolled network formation. Furthermore, the large reaction enthalpy of the polymerization restricts this method to the preparation of thin films only, in which a suitable heat transfer and control is guaranteed. To avoid these problems, the reaction can be carried out in solution. When the reaction is completed, the solvent has to be carefully removed in a de-swelling process. In some cases, especially if macroscopically ordered LCE are to be synthesized (see Sect. 4), low molar mass LCs of appropriate phase behavior can also serve as a solvent and have to be removed in an extraction process after synthesis [13].
An example for the synthesis of a side chain elastomer using radical polymerization of acrylates is presented in Scheme 1 and was demonstrated by Thomsen and co-workers [14]. Here, the mesogenic groups are attached side-on to the polymer-backbone. This attachment geometry is useful for a number of applications, because it offers larger length changes at T LC,i as compared to elastomers with end-on attached mesogens. In this example, a bifunctional, flexible crosslinker was used and the elastomer can thus be synthesized in a one-pot reaction.
Synthesis of poly(acrylate) LC elastomers with side-on attached mesogenic moieties [14]
Another method is based on the synthesis of functional linear prepolymers. This can be achieved by copolymerization of the mesogenic monomer with a functional co-monomer, e.g., co-monomers containing a reactive hydroxy group. In a second reaction step, these functional linear polymers can be crosslinked, e.g., with a diisocyanate crosslinker [15].
Ionic polymerization and especially anionic polymerization offers the opportunity to achieve a very narrow molar mass distribution of the polymer chains and would therefore be ideal for the synthesis of well-defined polymer networks. However, the method is strongly limited due to the sensitive chemistry of this reaction. Most LC side chain polymers synthesized so far are based on acrylates and methacrylates as the involved chemistry works well for most of the commonly used mesogenic building blocks. In contrast, many mesogens are sensitive to nucleophilic attack, e.g., phenyl benzoate moieties. Ionic polymerization works rather reliably for mesogens with azo-groups and when a bulky initiator is used [16]. Polymer networks have not yet been synthesized by ionic polymerization techniques, but their properties would certainly be interesting. Stereoregular ionic polymerization using proper initiators could also open up the possibility to analyze the impact of polymer tacticity on the LC phase behavior.
Polymer analogous reactions can be carried out in two general ways: either functionalized linear polymers are crosslinked or the mesogenic monomer, the crosslinker, and a functional prepolymer are reacted in a one-pot synthesis. The first method offers the advantage that the crosslinking process can be performed in the LC state of the linear polymer, e.g., the LC phase structure of the linear polymer can be ordered macroscopically by techniques well known from low molar mass LCs (surface effects, electric or magnetic fields) or the polymer can be brought into a casting mold [17].
Functionalized linear polymers were synthesized by Schuring et al. [18]. With linear poly(siloxanes) obtained by a hydrosilylation reaction of a poly(hydrogenmethylsiloxane) and olefinic mesogenic side-groups and side-groups with additional acrylate functionality as crosslinker they were able to produce free-standing films. The crosslinkers can be activated by UV-irradiation that causes degradation of dissolved initiator molecules. A drawback of this procedure is the rather complex synthesis of the functional, linear polymers and spontaneous crosslinking reactions that can occur at elevated temperatures.
Another very useful approach was introduced by Komp et al., who attached a photo-crosslinkable benzophenone side-group to a polymer-backbone. This system can be crosslinked with UV-light via a diradical state (Scheme 2). The linear polymers are thermally stable and can be processed in the liquid state at elevated temperatures without undergoing a crosslinking reaction. The photophore absorbs a photon and promotes one electron from a non-bonding sp2-orbital on the oxygen in the carbonyl group to an antibonding π*-orbital. The electrophilic oxygen n-orbital can interact with any weak C–H σ-bond, abstracting an H-atom. The residual ketyl radical recombines with the C-radical. If this reaction occurs between two different polymer chains a crosslinking reaction takes place [19]. Hence, it should be considered that the benzophenone unit needs to be separated from neighboring mesogens. This can be done via a long flexible spacer or by inserting a long mesogen-like moiety between the photophore and the polymer backbone.
Mechanism of photo-crosslinking of a benzophenone unit via a diradical state [19]
Without the use of functional side-groups, the network formation can also be realized by γ-irradiation as demonstrated by Vazilets and co-workers. A benefit of this method is that good control of the crosslink distribution can be achieved and that the crosslinking works with non-functionalized and thus thermally stable polymers that are not light sensitive. However, for this method an irradiation source is required that is not usually readily available [20, 21].
More recently, ring opening metathesis polymerization (ROMP) has been used to prepare telechelic liquid crystalline polymers that carry azide end groups which can be crosslinked with a triacetylene species using the well known copper catalyzed click chemistry [22].
Carrying out the synthesis of LC elastomers in a polymer-analogous one-pot reaction is chemically much simpler. Poly(hydrogenmethylsiloxanes) have been proven to be very useful prepolymers as the Si–H bond can be easily functionalized in a platinum catalyzed hydrosilylation reaction with vinyl terminated mesogens and crosslinkers, respectively. Moreover, the resulting elastomers show low glass transition temperatures. In contrast to polyacrylates or polymethacrylates, LCEs are accessible which are liquid crystalline at room temperature. Furthermore, this reaction can be easily interrupted at a certain point, yielding a lightly crosslinked polymer gel that can be oriented before the reaction is completed (see Sect. 4). Typical components are shown in Scheme 3. An advantage of this method is that the components can be easily synthesized and are stable towards light and air. What is more, statistical copolymers can be easily obtained to modify phase transformation temperatures or induce the desired phase behavior of the final network. The crosslinking molecules can be bi- or multi-functional, they can be isotropic or mesogenic rods, and can even be light-sensitive. In earlier works the crosslinker was often functionalized with a vinyl group at one end and a methacrylate group at the other. These groups exhibit very different reaction speeds toward the hydrosilylation reaction, so that the synthesis of the elastomers can be carried out in two relatively well-defined steps. The length of the spacer that links the mesogen to the polymer backbone can be varied to induce different polymer chain conformations (see Sect. 3). The mesogens can also be attached side-on when they carry a vinyl-terminated lateral spacer.
2.2 Main Chain Elastomers
The chemistry of main chain elastomers is limited to step-growth reactions, i.e., polycondensation and polyaddition reactions, which demand the highest purity of the starting materials and experimental conditions which exclude side reactions. Compared to side chain elastomers the preparation of main chain elastomers with suitable transition temperatures is rather challenging. What is more, due to the rigid rod-like mesogenic moieties in the polymer main chain, most linear main chain liquid crystalline polymers exhibit high clearing temperatures and tend to crystallize [23], making them unfavorable for many LC elastomer applications.
Due to the regular arrangement of the mesogens along the polymer backbone main chain polymers tend to form smectic or higher ordered mesophases [23], so that, especially if nematic phase behavior is required, strategies to overcome these problems have to be employed. The ability to do this is well known from linear LC polymers and has been discussed extensively in the literature [24, 25]: kinks can be incorporated in the mesogens, laterally attached side chains can act as plasticizers, or flexible spacers can be introduced, separating the rigid mesogenic moieties.
Despite their more complex chemistry, main chain LC elastomers have gained much interest in recent years due to the direct coupling of the liquid crystalline order and the polymer backbone conformation. The ground breaking predications of de Gennes were also based on main chain elastomers [3].
The chemistry used for the synthesis of main chain elastomers is usually based on poly-esterification, hydrosilylation reactions or epoxy resins. Here we shall discuss the possibilities for realizing main chain elastomers depending on three well-established preparation techniques. These include: (1) crosslinking of terminally functionalized LC polymers with a suitable multifunctional crosslinker, (2) photo-crosslinking of LC main chain polymers containing a photo-crosslinkable group, and (3) one-pot synthesis of main chain LC elastomers, usually starting from a mesogen, a flexible chain-extender, and a crosslinker.
Crosslinking of terminally functionalized LC polymers with a suitable multifunctional crosslinker was introduced by Zentel et al. [26]. They crosslinked allyl side-groups of a liquid crystalline polyester with an oligo-siloxane crosslinker, yielding elastomers with SA and SB phase behavior and rather high transition temperatures.
Bergmann [27] was able to synthesize nematic elastomers following a similar approach. As precursor, a main chain polyether with a comparatively low LC-isotropic phase transformation temperature of about 120 °C developed by Percec et al. [28] was used (Scheme 4). The free rotation around the C–C bond between the biphenyl unit and the phenyl ring of the mesogenic moiety allows for the formation of two different conformations of the mesogen, which not only lowers T ni but also suppresses the formation of smectic phases. The ethyl-substituent creates chirality within the repeating units causing four different constitutional possibilities. This additionally hinders crystallization, improves solubility in organic solvents, and decreases both T g and T ni. The mesogenic moieties are separated from each other by a rather long C10 spacer to allow for the formation of a low-temperature nematic phase. For the synthesis of the elastomer, a prepolymer with vinyl end groups was prepared and successively crosslinked with a cyclo-siloxane crosslinker in a hydrosilylation reaction (Scheme 4). This approach offers the advantage that the phase behavior of the prepolymer is only slightly changed by the crosslinking so that the properties of the resulting elastomer can be easily predicted.
One of the difficulties of this method is that both the content of end-groups of the polymer and the amount of volatile crosslinker is hard to measure and weigh, respectively, so that it is extremely difficult to keep to the exact stoichiometry. This often results in high soluble contents of the elastomers and imperfect comparability of the samples.
It is also possible to use the vinyl-terminated pre-polymer to crosslink a side chain LC polymer. This was demonstrated by Wermter et al. [29] who crosslinked a side chain end-on polymer similar to that shown in Scheme 3 with a main chain polymer carrying vinyl end-groups (Scheme 5). The side chain component can be understood as a multifunctional crosslinker for the main chain elastomer and also acts as a plasticizer, further decreasing the transition temperature to about 90 °C. Such combined main chain/side chain elastomers show, if oriented to a permanent monodomain (Sect. 4) extremely high length changes at the phase transformation to the isotropic phase and have been very useful in examining the influence of the elastomers chemical constitution on its physical properties [30].
Combined main chain side chain elastomer synthesized by Wermter et al. [29]
Photo-crosslinking of LC main chain polymers containing photo-crosslinkable groups was successfully performed by Beyer et al. [31]. They synthesized SA elastomers with an LC polyester as a precursor (Scheme 6). By introducing photo-crosslinkable side chains, they were able to crosslink thin prepolymer films in bulk using UV-irradiation. Compared to the elastomers synthesized by Bergmann, this approach allows much better control of the crosslinking reaction. This is a rare example of main chain elastomers showing SA phase behavior. Usually main chain polymers tend to form nematic or smectic-C phases, the latter especially when the mesogenic units are based on aromatic esters or ethers. In most cases SA phase behavior can only be induced by using biphenyl derivatives which, however, suffer from high transition temperatures and a tendency to crystallize. Here an LC polymer where 50 mol% of the biphenyl units are laterally substituted with bromine to suppress crystallization was developed, yielding an SA elastomer with suitable transition temperatures (T S,I ≈ 60 °C). Krause et al. [32] used a similar concept with photo-crosslinkable units introduced into the main chain of an LC polymer containing flexible poly(ethyleneoxide) spacers and two-ring mesogens to yield nematic elastomer films and fibers.
Photo-crosslinkable main chain polymer with SA phase behavior [31]
The one-pot synthesis of main chain LC elastomers is a less complicated approach for the synthesis of main chain LC elastomers and was introduced by Donnio [33] based on the chemistry of linear polymers investigated by Aguilera et al. [34]. Instead of starting from pre-synthesized main chain polymers, this synthetic approach is based on a polyaddition reaction in solution via hydrosilylation of a divinyl substituted mesogen with tetramethyl dihydro disiloxane as additional flexible spacer to lower the phase-transition temperatures. As a crosslinker a cyclic pentasiloxane was used (Scheme 7) which is less volatile than the cyclic tetrasiloxane used by Bergmann.
Components for the synthesis of main chain elastomers (MCLCEs) in a one-pot reaction. The two-ring mesogen yields smectic and nematic phases, depending on the spacer-length; the three-ring mesogen yields broad nematic phases with low transition temperatures [30]
Brandt and Krause [30] continued these investigations by varying the mesogenic shape and the length of the siloxane units. Using mesogenic units consisting of two aromatic rings lowers the phase transition temperatures significantly. The formation of smectic phases mainly caused by the tendency of the siloxane units to phase-separate can be avoided by using short vinyl-chains and short siloxane units. Copolymerization of mesogens with different spacer length or the introduction of lateral (alkyl) substituents at the central aromatic core can additionally decrease the tendency for the formation of smectic phases.
In the early stages, main chain elastomers that were synthesized with the one-pot method exhibited a considerable amount of soluble content, caused by an incomplete conversion of the polyaddition reaction. To overcome this problem Krause [30] developed a new mesogenic unit based on Donnio’s three-ring core with a laterally attached ethyl-substituent that successfully suppresses smectic phases. The terminal vinyloxy groups (Scheme 7) lead to an exceptionally clean polyaddition reaction and prevent side-reactions such as double-bond migration. This allows for the synthesis of elastomers with very low crosslink content and low elastic moduli. The vinyloxy groups also significantly lower the T ni of the elastomers. However, the vinyloxy chemistry requires a lot of effort, as the end groups are sensitive to acids and not stable towards air. With respect to clean polyaddition reactions one nowadays has recourse to the more robust vinyl spacers as the according precursors can be commercially purchased in excellent purity.
A very similar chemical approach with a trisiloxane co-monomer and a tetracyclic crosslinker was used to realize main chain LCEs with pentaphenyl mesogens incorporated side-on into the polymer main chain [35]. Such elastomers have an SA phase behavior due to the segregation of the aromatic and the siloxane moieties.
Ishige and co-workers [36–38] produced SCA main chain elastomers with transition temperatures of about 180 °C also utilizing a one-pot method, by a melt trans-esterification chemistry to obtain polyesters. They did not use a flexible chain-extender like the short tetrasiloxane in the examples discussed above, but started from a flexible dialcohol, an aromatic di-ester as a mesogenic group, and a trifunctional aromatic ester as a crosslinker.
Components for the synthesis of main chain elastomers (MCLCEs) in a solvent-free one-pot reaction employing a photo-initialized thio-ene polycondensation [39]
A new and elegant solvent-free approach to main chain LCEs was found by Yang et al. [39] who, based on the work on linear LC polymers by Lub et al. [40–42], made use of the photo-induced addition of thiols and olefins (click-chemistry) to synthesize nematic polymer networks. Starting from a mixture of the mesogen, a tetrafunctional crosslinker and a photo-initiator networks with a T ni around 170 °C were obtained by UV crosslinking (Scheme 8).
2.3 Basic Characterization of LC Networks
The first question that has to be addressed after the synthesis of a polymer network is whether the crosslinking reaction was successful and is reproducible. Therefore, it is advisable to measure the soluble content of the elastomer, that is, the weight loss in percent after the extraction of unreacted monomers, oligomers, or polymers. For the extraction the elastomer is placed in a poor solvent of the linear polymer (e.g., isohexane). Subsequently the solvent quality is increased by slowly adding a good solvent (e.g., toluene). The swelling of the elastomers has to be carried out very carefully as inhomogeneous or too fast swelling can produce local mechanical stress which might cause the sample to break. This is especially important for macroscopically oriented samples (LSCEs) for which the LC-isotropic phase transformation that occurs upon swelling is accompanied with large length-changes of the sample. The extraction is usually done over about 1 day for nematic main chain elastomers; for the less sensitive side chain elastomers or for smectic elastomers this can be done faster. After the extraction of unreacted material is completed the elastomers have to be deswollen again. This is done by the reverse procedure by adding poor solvent. Afterwards the elastomer is dried at elevated temperatures to evaporate any remaining solvent. This extraction procedure is crucial to obtain samples with reproducible properties.
Information on the crosslinking density of the synthesized elastomer can be obtained using various methods. Mechanical characterization, especially stress–strain measurements, thermoelastic measurements in the isotropic phase, and swelling experiments yield information about the molecular weight of the network chains M c. In a first approach, the affine network model, introduced by Kuhn, may be used to correlate the experimental results with the chemical constitution of the networks. This network model assumes that the average squared distance of the chain ends of a network chain is the same as for an uncrosslinked polymer of the same length. Furthermore, a deformation of the network does not change the sample’s volume so that two polymer segments are separated about the same factor λ that is determined by the macroscopic deformation. Finally, the enthalpy of network chains does not change under deformation. Network defects like loops or dangling chain ends are not considered in this theoretical description. Nevertheless, within a homologous series of networks that only differ with respect to the crosslinking density, some relative quantities can be obtained that help to control the chemistry of the network formation.
The E-modulus in the isotropic phase can be determined from both stress–strain and thermoelastic measurements and M c can be calculated according to \( {M_{\text{c}}} = 3\frac{{\rho \cdot\,R \cdot\,T}}{E} \) when the density ρ of the elastomer is known. The degree of swelling Q in a certain solvent can yield M c according to
The second virial coefficient χ can be determined for the linear, uncrosslinked polymer, for example, in osmosis measurements [11, 12, 43].
The LC phase behavior of the elastomers can be analyzed with conventional techniques also used for low molar mass LCs. Polarizing optical microscopy as well as X-ray scattering provide information about the phase structure of the networks and about T LC,i and T c,LC. In addition to the determination of phase transformation temperatures, DSC experiments can be used to measure the glass transition temperature T g. To obtain reliable DSC results, it is necessary to measure at various heating and cooling rates (usually about four heating and cooling runs, with heating and cooling rates between ~10 and ~40 K min−1) and to plot the obtained transition temperatures as a function of the square-root of the heating and cooling rate. The transition temperatures are then obtained by extrapolating to zero heating rates.
3 Mechanical Orientation Behavior
When LCEs are synthesized in the absence of external fields, so-called polydomain LC elastomers are obtained, which show macroscopically isotropic properties similar to polycrystalline materials. This resembles, e.g., bulk material of a low molar mass liquid crystal, where thermal fluctuations prevent a uniform director orientation over the whole sample. For nematic elastomers the overall isotropic behavior also indicates an overall isotropic conformation of the polymer chains, which is the consequence of the maximization of the chain entropy.
However, it is well known that a mechanical deformation of a conventional, isotropic polymer network causes anisotropy. Under deformation the chain segments become oriented according to the symmetry of the external field and the state of order of the network can be characterized by an order parameter similar to that of nematic liquid crystals. Very early mechanical experiments on nematic polydomain elastomers actually demonstrate that a uniaxial deformation of a nematic elastomer converts the polydomain structure into a macroscopically uniformly ordered monodomain network [44]. This is shown in Fig. 2, where the opaque polydomain becomes optically transparent and converts into a monodomain network by stretching. The experiment unambiguously indicates that the state of order of the network chains is directly connected with the liquid crystalline state of order. Obviously, with respect to the orientation of the LC phase structure of the elastomer, a mechanical field acts very similar to an electric or magnetic field on low molar mass LCs. This opens new perspectives for macroscopically uniformly ordered LC networks.
To get a systematic insight into the interplay between mechanical deformation of an LC elastomer and the orientation behavior of the LC phase structure, the knowledge of the chain conformation of a polymer backbone in the liquid crystalline state is necessary. This chain conformation has been investigated in detail on linear LC polymers in the past and will be briefly summarized in Sect. 3.1. In Sect. 3.2 it will be described how the global state of order of polydomain elastomers can easily be manipulated using mechanical fields. We will hereby only focus on some principal aspects and concentrate on symmetry arguments between the local and the global chain conformation. While the local chain conformation of the network strands originates from the chemical constitution of the monomer units and their interaction with the anisotropic LC order, the global chain conformation is induced by the external mechanical field. These considerations are the basis for many synthetic concepts to prepare liquid single crystal elastomers (LSCEs). A more detailed description of various orientation strategies is given in Sect. 4.1.
3.1 Chain Conformation of Linear Polymers
In the isotropic melt of macromolecules the undisturbed dimensions of the chains can be well described with random flight statistics. On average the chains form a statistical coil with spherical shape (Fig. 3a) [11]. The radius of gyration of the coil is given by \( \overline {R_0^2} = NP \). N is the number and P the length of the statistical chain segments. In the LC state, however, the monomer units become spontaneously aligned due to their mesogenic character. This can cause a significant deformation of the statistical coil towards a prolate or oblate shape and has been directly proven by small-angle neutron scattering (SANS) on deuterated polymers (Fig. 3b, c) [45]. It is obvious that the chemical constitution of the mesogenic monomer units as well as their position and linkage within the polymer chain play an important role. The knowledge of the chemical constitution of the polymer chain and the corresponding chain conformation is the key to the understanding of the orientation behavior of LC elastomers. In the following we restrict the discussion to polymers having rod like mesogens.
Schematic representation of the polymer chain conformation: spherical with R x = R y = R z (a), prolate with R z > R x , R y (b), and oblate with R z < R x , R y (c). R z and R x , R y are the radii of gyration parallel and perpendicular to the axis of highest priority (nematic director, smectic layer normal, or helix axis), respectively
For main chain polymers in the LC state the coil is strongly extended along the director (prolate chain conformation) showing an anisotropy of the radii of gyration parallel and perpendicular to the director of up to R ||/R ⊥ ≈ 5 [46–53]. On a local scale the polymer chain is fully extended which is in agreement with 2H-NMR experiments [54] as well as theoretical predictions of Yoon and Flory [55]. For large flexible spacers separating the mesogenic groups the existence of hairpins, i.e., local backfolding of the polymer main chain, has been proven by SANS experiments [50, 51, 53, 56]. Investigations on smectic-C fluctuations existing in the nematic phase showed a strong orientational coupling of the mesogenic units and the global chain conformation, so that the whole polymer chain becomes inclined in the layers of the SC short-range order [53]. Prolate chain conformations also exist for smectic main chain polymers. However, the tendency of backbone backfolding might be stronger in lamellar mesophases [36–38].
For side chain polymers with side-on attached mesogenic units (Fig. 1b) very similar results are obtained. For short flexible linkages between the polymer backbone and the mesogenic units, prolate chain conformations comparable to main chain polymers are found (R ||/R ⊥ ≈ 5). However, with increasing spacer length the chain anisotropy decreases. The limit is reached using a spacer length of x = 11 (x is the number of spacer atoms), where a nearly spherical coil is obtained (R ||/R ⊥ ≈ 1) [57–62]. This can be explained by the so-called “jacketed effect”: as a consequence of the lateral attachment to the polymer chain, the rod-like units and the polymer backbone are oriented parallel to each other on a local scale. For short spacers lengths the polymer backbone is stretched significantly parallel to the mesogenic rods. Although the polymer main chain, e.g., a polysiloxane or polyacrylate, is intrinsically flexible, it forms a highly elongated polymer coil. As the spacer length increases this effect becomes less pronounced until these steric interactions between side-groups and the polymer backbone are completely decoupled [45].
Side chain polymers with end-on attached mesogenic units (Fig. 1a) show a more complex behavior, as the coupling between the LC order and the polymer backbone depends on the chemical constitution of the flexible spacer and the phase structure [2, 63–68]. Nematic polymers having an odd number x of atoms in the spacer have the tendency to adopt a weak globally prolate shape with R ||/R ⊥ ≈ 1.5. Here the polymer backbone and the mesogenic units are oriented parallel on average (Fig. 4b). Slightly oblate chain conformations, where the polymer backbone and nematic director are oriented perpendicular to each other, have been observed for nematic polymers having a short spacer with an even number of atoms of x < 7 (Fig. 4a). Oblate chain conformations are also observed for nematic polymers that exhibit smectic short-range order. Here the chain conformation results from the local confinement of the polymer backbone between the layers of the smectic fluctuations (see below).
Smectic side chain polymers show an oblate equilibrium conformation of the polymer melt where the polymer backbone is partially confined between the smectic layers independent of the attachment geometry. For SA polymers the confinement depends not only on the smectic order parameter but also on the type of SA phase structure. For the monolayer phase structure SA1 the anisotropy of the radii of gyration is R ||/R ⊥ ≈ 0.3 while for the less densely packed partially bilayer structure SAd the confinement of the backbone is less pronounced (R ||/R ⊥ ≈ 0.7) [63, 64, 69–71].
Simple mechanical experiments can provide information about the local orientation of the mesogenic units and the polymer chain and can give some indications about the existing chain anisotropy [2, 65–68]. If a polymeric fiber is drawn from the isotropic melt, the polymer chains are expanded in the stretching direction. The mechanical deformation induces an orientation of the LC phase structure which can easily be analyzed using X-ray scattering. When the mesogens are aligned along the stretching direction, locally a prolate chain exists. In the opposite case the mesogenic units and the stretching direction are aligned perpendicular to each other, indicating locally an oblate chain conformation. In order to obtain conclusive information about the local orientation of mesogenic units with respect to the stretching direction, equilibrium conditions have to be ensured. Sometimes this can be problematic, as for high drawing speeds and high cooling rates non-equilibrium orientations or non-uniform orientations within the fiber cross-section may be frozen-in.
3.2 Orientation of Polydomain Networks
As a direct consequence of the interaction between LC order and polymer chain conformation, the global state of order in polydomain LCEs can be manipulated by mechanical stretching. It is well known from conventional rubbers that mechanical deformation induces changes in the macroscopic chain conformation of the network strands. Uniaxial elongation causes the formation of prolate chain conformations while biaxial stretching, which is equivalent to uniaxial compression, establishes global oblate chain conformations. This has important consequences for LC elastomers. By changing the macroscopic chain conformation so that it is consistent with the phase symmetry of the LC state, macroscopic alignment can be induced. Uniaxial stretching a polydomain nematic elastomer with locally prolate chain conformation, e.g., a main chain polymer, a side chain side-on polymer or a side chain end-on polymer with an odd number of spacer atoms, produces a transparent polymer network with uniform orientation of the nematic director along the stress axis above a characteristic strain. We shall refer to this alignment of the director in the film plane as homogeneous orientation. The formation of a monodomain is shown for a nematic side chain elastomer in Fig. 2. Above the characteristic threshold strain, the directors of the individual domains rotate towards the mechanical stress axis. The resulting macroscopic orientation of the director can be easily proven by X-ray scattering or IR dichroism. This process is completely reversible. When the mechanical stress is released, the sample relaxes back into the polydomain equilibrium state.
The concept of mechanical field induced orientation can easily be transferred to nematic elastomers with oblate chain conformation, i.e., side chain end-on elastomers with an even number of spacer atoms. In order to achieve a monodomain structure, a globally oblate chain conformation has to be established. This can be achieved by uniaxial compression or biaxial stretching of the polydomain elastomer which induces a uniform homeotropic alignment of the nematic director perpendicular to the film plane. Up to now, this orientation technique has only been realized experimentally for chiral nematic elastomers [72].
Mechanical fields can also be deployed to achieve a macroscopic orientation of the helicoidal z-axis of cholesteric elastomers. As discussed above, nematic side chain polymers with odd spacer length exhibit a locally prolate chain conformation with respect to the local director. In the helicoidal phase structure of chiral nematic elastomers, however, this orientation corresponds to an overall oblate chain conformation with respect to the helix axis as the local director rotates continuously along this axis (Fig. 5). Therefore a globally oblate chain conformation has to be established in order to achieve a cholesteric monodomain. Using biaxial stretching or uniaxial compression, elastomers with homeotropically aligned helix axes can be obtained [72]. In the case of a chiral nematic polymer with even spacer length and thus locally oblate chain conformation with respect to the local director, a prolate chain conformation exists with respect to the helix axis. Uniaxial mechanical stretching induces a macroscopic orientation of the helix axis along the stretching direction in the film plane [73].
Smectic side-chain polymers prefer locally oblate chain conformations, independent of the spacer length or attachment geometry. Analogous to oblate nematic polydomain elastomers, biaxial mechanical stretching or uniaxial compression can be used to orient SA polydomain elastomers. This achieves a simultaneous orientation of the director and the smectic layer normal in a uniform homeotropic fashion [74].
Upon uniaxial stretching a polydomain elastomer in the smectic-A phase, the layer planes usually couple to the mechanical field. This process does not produce a monodomain but causes a reorientation of the polydomain structure consistent with the induced globally prolate chain conformation. The layer normals align perpendicular to the stretching direction (z-direction) but are randomly distributed in the xy-plane [75]. The orientational behavior of smectic elastomers can change drastically if a uniaxial mechanical field is applied in the isotropic state followed by slowly cooling into the LC phase. If an intermediate nematic phase occurs before reaching the smectic state, a uniaxial mechanical field can be sufficient to induce a macroscopic orientation [75–77]. This will be discussed in more detail in Sect. 4.1.1 when we will address the synthesis of SA LSCEs.
While the partial decoupling of the LC order and the polymer backbone in side chain elastomers allows for some flexibility in their relative orientation, the much stronger coupling in SA main chain elastomers induces only prolate chain conformations [31, 36–38]. Therefore, uniaxial mechanical stretching macroscopically aligns the director, which has also been observed for main chain elastomers with laterally attached mesogenic units [35, 78]. However, the stretching speed can have an impact on the induced orientation. For high stretching rates a coupling of the layer planes has been observed which should lead to a polydomain structure similar to that discussed above for side chain elastomers under uniaxial mechanical stress [79].
For smectic-C polydomain elastomers the situation becomes more complex as the director is inclined at an angle theta from the layer normal. A simultaneous orientation of both is usually not possible when simple mechanical deformations are deployed. This can only be achieved using more complex deformations in a multi-step procedure which will be discussed in Sect. 4.2.2. Uniaxial mechanical fields only cause reorientations of the LC phase structure yielding a polydomain which is consistent with the induced macroscopic chain conformation. This usually induces a macroscopic orientation of the director and leaves a conical distribution of the layer normal around the stress axis. The attendant angle θ between n and k corresponds to the tilt angle of the smectic-C phase (Fig. 6). The formation of this special polydomain structure has been observed for side chain elastomers [80–84] as well as for main chain elastomers [33, 79, 85–88]. For side chain elastomers the orientation can be strongly influenced by the crosslinking topology. Upon cooling from the SA phase to the SC phase under uniaxial mechanical stress, elastomers with rod-like crosslinkers form a conical layer distribution, while for more flexible crosslinkers the mesogenic side-chains tilt at an angle of ±θ with respect to the layer normal which is oriented parallel to the mechanical stress [89].
Schematic representation of the conical layer distribution of polydomain SC elastomers exposed to uniaxial mechanical deformation (a) and corresponding X-ray pattern (b). θ (denoted φ in this figure) is the SC tilt angle, d the smectic layer spacing, and l the length of the mesogenic units. The layer normal k is conically distributed around the stress axis z. Reprinted with permission from [87]. Copyright (2008) American Chemical Society
In conclusion, simple symmetry considerations allow for a successful orientation of polydomain elastomers using mechanical fields. In principle knowledge is only needed of the local chain conformation of the LC polymer on which the elastomer is based, and the consistent mechanical deformation must be applied. Nevertheless, the chemical constitution of the whole polymer network has to be considered. Often, the orientational behavior is strongly influenced by the crosslinking topology. As a rule of thumb, prolate chain conformations are increasingly preferred when the crosslinker concentration is increased and when the crosslinker molecules are more rod-shaped [90, 91].
4 Liquid Single Crystal Elastomers
For a detailed understanding of the desired physical properties of liquid crystalline elastomers as well as for many applications, elastomers are desired with a permanently stable macroscopic orientation of the LC phase structure. Such monodomain polymer networks show anisotropic physical properties comparable to single crystals and the term “LSCEs” has been coined. As discussed in Sect. 3.2, the synthesis of LCEs basically leads to polydomain samples, analogous to polycrystalline materials. However, the presence of external fields, e.g., magnetic, electric, and mechanical fields, or surface effects, can induce a macroscopic orientation of the LC phase structure. As a direct consequence of the coupling of the liquid crystalline order and the chain conformation of the network strands, macroscopic orientation of the LC phase structure leads to a macroscopically anisotropic chain conformation and vice versa. As demonstrated by Küpfer et al. in 1991, a suitable synthetic concept to fix this global orientation and chain conformation permanently yielding LSCEs, is a chemical crosslinking reaction in the aligned state (Fig. 7) [92].
In the first part of this section we will discuss several strategies developed in the last decades to prepare LSCEs with a permanently stable orientation of the main axis, i.e., the director n for nematic elastomers, the helix axis for cholesteric elastomers, and the layer normal k for smectic polymer networks, respectively (Sect. 4.1). In some cases, however, alignment of the main axes is not sufficient to achieve a uniform orientation of the whole phase structure and more complex procedures have to be applied. This is the case for the mechanical field induced orientation of SC elastomers where two separate steps are necessary to orient both the layer normal and the director. For biaxial nematic and biaxial SA phases the mesogens exhibit orientational long range order in all three spatial directions, and the minor director m is used in addition to the major director n to denote the orientation of the minor molecular axes. Here, a second orientation step is necessary to produce monodomains with a macroscopic 3D orientation of the LC phase structure. These two cases will be discussed in Sect. 4.2.
4.1 Permanent Orientation of the Main Axis
For the preparation of monodomains with respect to the main axis, two principal strategies can be deployed (Fig. 8). The first approach takes advantage of the anisotropy of the polymer chain conformation of the network strands and is based on considerations discussed in Sect. 3.2. Mechanical deformation of polydomain LCEs leads to a globally anisotropic chain conformation and can induce a macroscopic orientation of the LC phase structure above a certain threshold stress. By introducing a globally anisotropic chain conformation a priori during synthesis, LSCEs are accessible. This can be realized by chemical crosslinking after or during orientation by means of mechanical or viscous flow fields and will be discussed in detail in Sect. 4.1.1.
Following the second route, the anisotropic physical properties of the LC phase structure, such as the anisotropy of the diamagnetic or dielectric susceptibility, can be utilized to orient the mesogenic units in a magnetic or electric field, respectively. Macroscopic alignment can also be achieved by surface effects, a technique well known from low molar weight LCs. As the mesogenic units are covalently linked to the polymer chains, the orientation of the LC phase structure leads directly to the formation of a globally anisotropic chain conformation. Both are fixed permanently by chemical crosslinking in the aligned state, which will be discussed in Sect. 4.1.2.
If the first route is chosen, large-area elastomers can be produced with a wide variety of film thicknesses. As precursors, only polymeric materials, for example, photo-crosslinkable polymers or lightly crosslinked polymer gels, can be used. The second route is usually limited by the available field strength to thin film geometries. Typically photo-crosslinkable LC polymers or monomer/crosslinker mixtures (usually containing additional photo-initiators and/or inert LCs as solvents) can serve as starting materials. The latter offer the advantage that molding techniques can be applied and elastomeric materials of more complex geometries can be realized.
Before synthesizing LSCEs, it has to be considered that the preparation routes can have significant impact on the material properties as it influences the crosslinking topology as well as the global chain conformation. The application of strong mechanical fields during synthesis that are used when employing the first route leads to a strong deformation of the polymer backbone. This results in a non-Gaussian chain conformation as stated recently by Martinoty et al. In contrast, elastomers that have been prepared employing the second route show a lower anisotropy of polymer chain conformation and can be described well with the Gaussian rubber elasticity theory. Both LSCEs will therefore differ strongly, e.g., in their mechanical properties, their swelling behavior, and their spontaneous length change at the LC-isotropic phase transformation [93, 94].
4.1.1 Coupling to the Chain Anisotropy
The orientation of polydomain polymers by mechanical or viscous flow fields can be achieved easiest if a macroscopic chain anisotropy that coincides with the local symmetry of the LC phase structure is induced and fixed by chemical crosslinking. For nematic or SA main chain polymers which locally show a prolate (see Sect. 3) chain anisotropy, a uniaxial deformation leads to a globally prolate chain conformation. If the chain conformation of the LC polymer is locally oblate, a globally oblate chain conformation can be induced by either uniaxial compression or – equivalently – biaxial stretching of the sample (Fig. 9).
In some cases it is also possible to induce a global chain conformation of the LSCE opposite to the local chain conformation of the polymeric starting material which will be discussed for cholesteric and smectic-A side chain elastomers.
In the following, several examples of the preparation of LSCEs will be given. For selected preparation techniques we will also discuss some practical aspects which are important to produce high quality elastomer samples. The examples are ordered according to their different phase structures.
4.1.1.1 Nematic LSCEs
The simplest way to prepare nematic LSCEs from locally prolate polymers which bear, e.g., photo-crosslinkable side-groups, is to draw manually a fiber from the polymer melt and to photo-crosslink afterwards [95]. A more sophisticated version of this approach is, for example, the electrospinning of LC fibers from a polymer solution with subsequent crosslinking [32]. A one-step network formation during the spinning process has so far only been reported for thermoplastic LC elastomers, in which the fast occurring phase separation of crystalline segments drives the physical crosslinking [96]. LSCEs that can be obtained from fiber spinning/drawing methods are usually restricted in their dimensions. Larger elastomer samples have only been described for uniaxially drawn films of a very viscous and robust photo-crosslinkable main chain polymer [32]. However, comparatively thin samples can be well obtained with the described methods and can be useful for some applications.
A reliable route to globally prolate, large-area LSCEs of variable film thickness provides the two-step crosslinking procedure introduced by Küpfer et al. [92], in which a lightly crosslinked gel is oriented in the nematic state by applying a uniaxial mechanical field followed by a second crosslinking step in order to fix the induced orientation. Figure 10 shows a stretched monodomain elastomer prepared with the Küpfer method and an unoriented polydomain elastomer as well as the corresponding X-ray patterns.
Photographs of oriented and unoriented nematic elastomers (a) and corresponding X-ray patterns of the monodomain (b) and the polydomain (c) sample [92]
This robust synthetic approach has frequently been used to produce LSCEs in the last few years as it works well for side chain as well as main chain elastomers. It is also applicable for polymer networks of different LC phase structures (smectic, cholesteric, lyotropic hexagonal) as long as they exhibit prolate chain conformations. We will therefore give a more detailed description in the following paragraphs.
In the first step a lightly crosslinked polymer gel is prepared using a one-pot reaction in isotropic solution, e.g., a hydrosilylation reaction to yield side chain or main chain elastomers (see Sects. 2.1 and 2.2). A spin-casting technique can be deployed in order to prepare elastomer films of homogenous thickness. This is necessary for detailed investigations of the mechanical properties of LSCEs as local variations in the film thickness can lead to non-uniform stress distributions and the formation of neckings or ruptures. Such uniform films cannot usually be obtained by simply casting the reaction mixture into a mold (early attempts to cast elastomers on the very flat surface of liquid mercury were discarded for obvious reasons). With a typical spin-casting setup, films of about 15 cm length, 1–2 cm width, and 80–900 mm thickness are accessible.
In order to produce LSCEs, the crosslinking/casting reaction is interrupted before completion. At that time a lightly crosslinked gel is obtained which is swollen with organic solvent and thus isotropic. A macroscopic orientation can now be induced by deswelling the gel under uniaxial mechanical load at room temperature. The gel is removed from the casting setup, hung vertically, and has a load attached (Fig. 11). Upon deswelling the elastomer slowly becomes liquid crystalline and the uniaxial mechanical stress induces a macroscopic orientation of the nematic director. After complete alignment, the crosslinking reaction is finished at elevated temperatures. This “second crosslinking step” has to be carried out deeply enough in the nematic phase, as the crosslinking temperature has significant impact on the crosslink topology and in the vicinity of T n,i a more disordered state is fixed.
Compared to approaches based on crosslinking of aligned LC prepolymers (Sect. 4.1.2), LSCEs with rather complex crosslinking histories are obtained. This is important to consider when discussing chain anisotropies, effects of random disorder, or pretransformational effects.
In the following, a detailed description of the two-step crosslinking procedure, including many practical details that proved to be useful in order to obtain high quality samples will be presented. Readers not interested in experimental details can proceed below.
The spin casting process can be carried out in a heatable centrifuge using a spinning cell of the desired sample dimensions. It is advisable to cover the inner wall of the cell with a strip of poly(tetrafluoroethylene) foil (Teflon) in order to avoid adherence between the casted elastomer film and the wall and allow for easy removal. The ends of the strip should not overlap and they should be marked so that they can be recognized easily after synthesis.
For side chain elastomers, the reactants – mesogens, crosslinker, and prepolymer – are dissolved in a minimum of solvent. Toluene is a good choice as it evaporates slowly at room temperature and thus leaves enough time for the orientation of the swollen polymer gel. For a hydrosilylation reaction the solvent needs to be thiophene-free because otherwise the platinum catalyst is inhibited. Before use, toluene has to be washed with concentrated sulfuric acid and distilled several times. If one of the components is insoluble in toluene, dichloromethane, or tetrahydrofuran also give good results. Both can be used at analytical purity without prior distillation. However, the orientation process usually becomes more difficult for solvents with low vapor pressure. If polymeric crosslinkers are used, it is advisable to dissolve the mixtures overnight. To start the hydrosilylation reaction, the catalyst is added to the clear solution of the starting materials. Next, the mixture is transferred into the centrifuge cell through a millipore filter in order to remove dust particles or other insoluble impurities. As a catalyst, a solution of dichloro(1,5-cyclooctadiene)platinum-II in dichloromethane (5–15 μL, 1.0 wt%) is used. The catalyst has been developed for the insertion of terminal C=C double bonds into the Si–H bond for vinylic spacers (except for allylic spacers). It provides a fast conversion at moderate temperatures (T = 60 °C) with a minimum of side reactions. Higher quantities of catalyst or aged catalyst solutions result in a brown color of the elastomers. If a catalyst with a higher reactivity is required, solutions of hexachloroplatinum acid can be used.
For main chain elastomers, a proper preparation of the reaction mixture is crucial as volatile reactants are often involved and the exact stoichiometry has to be kept in order to obtain high molecular weight products. Therefore, non-volatile reactants are weighed first before adding more volatile starting materials. For the synthesis of main chain elastomers based on a hydrosilylation reaction, 1,2-dihydrotetramethyldisiloxane has often been used as co-monomer, as it acts as flexible chain extender. Owing to its very low vapor pressure it should be added quickly to the glass vessel after the mesogen and crosslinker. It is used in slight excess and then left to evaporate until the excess to stoichiometric conditions is about 2%. Then the solvent is added and after all components are dissolved the mixture is quickly transferred to the centrifuge cell (it is often better to do that without prior filtration). The slight excess of the chain extender is needed to compensate for the loss due to evaporation during the transfer of the solution. If there are problems in dissolving the mesogen quickly in the solvent used, it is advisable to recrystallize it in an appropriate solvent and cooling rapidly using an ice-bath so that small, fluffy crystals are obtained.
After the reaction mixture has been transferred into the centrifuge cell, it is spun at 5,000 rpm and an interior cell temperature of 60 °C. For the reaction time of the first crosslinking step, the minimum time to obtain a stable gel film at the cell’s wall is chosen. This minimizes the number of crosslinks formed in the isotropic state. The film has to be mechanically stable enough to be removed from the Teflon support and to be loaded with a small weight, e.g., a paper clip. It should not be too sticky and it should not be possible to draw fibers from the elastomer film. The reaction time usually has to be optimized for each system and can range from 45 min to 24 h. At a given temperature it basically depends on the purity of the starting materials and the crosslinker concentration used. Especially after longer storage times it is advisable to recrystallize mesogens and crosslinkers followed by drying in vacuo in order to remove impurities and humidity.
In order to interrupt the crosslinking/casting reaction the cell is removed from the centrifuge and cooled for about 30 s in liquid nitrogen to room temperature. This is preferable to water cooling since traces of water can prevent the proceeding of the reaction when they happen to get into the gel. If no stable gel is obtained the crosslinking reaction under spinning is continued. One has to be very careful to add more catalyst to the reaction mixture, as close to the gel state this can cause heterogeneous reactions.
Once the reaction is successful, the elastomer strip is loosened from the centrifuge wall with the non-cutting side of a thin lancet. The elastomer film is carefully removed from the cell together with the Teflon support and cut into smaller (usually two or three) pieces. For cutting the swollen polymer gel the best method is to use a carpet knife and a hammer. That way, a clean cut of both the gel and the Teflon band is achieved. It is useful, especially if working with sticky side chain elastomers, to keep some toluene at the preparation table to clean the tools before reuse. If possible, both long rims of the film should also be cut with a carpet knife, because small defects at the rim can cause the elastomer to break when later a mechanical load is applied during the orientation process.
In order to remove the cast elastomer film from the Teflon support, a strip of Kapton based adhesive tape is fixed at one end of the swollen gel. Kapton is used as it can withstand high temperatures and is stable towards organic solvents. The film is now removed from the support by slowly pulling the tape. In some cases it is useful to rinse the space between the elastomer film and the Teflon foil with toluene. The elastomer film is fixed at a metal holder and hung vertically. At the lower end of the film a small load, e.g., a paper clip, is attached and held steady with Kapton tape from both sides (Fig. 10).
Some elastomers cannot be prepared as described above because they are too sticky or mechanically not stable enough. This often holds for side chain side-on elastomers, for elastomers with mesogens carrying ethylene oxide or fluorinated chains, or for very low crosslinking densities. In that case the gels can be prepared over a liquid nitrogen bath. The gel is frozen to the glassy state while the Teflon still remains flexible at this temperature, allowing for an easier removal. For this procedure, a small Dewar is filled with liquid nitrogen and a cork ring is placed inside. On the cork ring a PVC Petri dish is placed with the rim facing downwards. The elastomer film is put on the Petri dish with the Teflon foil facing the Petri dish. After the gel is frozen (test with tweezers) the sample ist turned over and the Teflon foil is removed using a pair of tweezers. In the case where the electrostatic interaction is too strong, one can earth oneself during preparation. Then two strips of Kapton are attached to the ends of the film and thereby warmed with one’s thumbs, because they are not sticky in the cold. One of the Kapton strips should already have a paper clip attached because the Kapton tends to curl in the cold and it is difficult to attach the clip afterwards. The film is then attached to the holder and fixed with additional Kapton strips as described above.
For the orientation process, the load is increased successively. For nematic elastomers one typically waits until the gel becomes slightly turbid before more load is applied. For nematic side chain elastomers the load needed to orient the film ranges between 300 mg and about 4 g. For nematic main chain elastomers, loads of about 100 g are often used. It is useful to leave nematic elastomers under load overnight so that they become stable enough for further crosslinking in the oven. Before putting the elastomers in an oven to perform the second crosslinking step, the load should be reduced to the minimum necessary to induce a uniform orientation. The elastomers are typically crosslinked in an oven at T = 60 °C, the optimum temperature for the catalyst. However, one should be careful not to crosslink at temperatures too close to T ni because this leads to disorder in the elastomer. The elastomers are usually left in the oven for 1 week to ensure complete reaction and good reproducibility.
After complete crosslinking, the elastomer is carefully extracted with a good solvent as described in Sect. 2.3.
To orient nematic elastomers showing locally an oblate chain conformation, the same basic ideas presented above can be applied. In order to produce a monodomain, a global chain conformation consistent with the local phase symmetry has to be induced. Therefore, a biaxial mechanical deformation is deployed, which causes uniaxial compression of the film thickness and produces a homeotropically aligned sample with a globally oblate chain conformation. Biaxial mechanical stretching is, however, experimentally very complicated. It is difficult to stretch an elastomer strip uniformly in two directions. Only small sample sizes are accessible as quadratic elastomer sheets have to be deformed. Moreover, the strong boundary conditions at the edges allow for a uniform orientation only in the very center of the sample. Not to mention that it is a very delicate operation to clamp a swollen elastomer sample for such a procedure. A more accessible technique to realize uniaxial compression based on anisotropic deswelling has been introduced for cholesteric elastomers and will be discussed in the next section (Fig. 12).
4.1.1.2 Cholesteric LSCEs
Since a cholesteric phase is a twisted nematic phase, the local director n is not constant in space but helically arranged perpendicular to an axis, usually referred to as the z-axis. For a nematic polymer with locally a prolate chain conformation, the helicoidal arrangement of n in the z-direction will cause an overall oblate network conformation. As described above for nematic elastomers, a uniaxial compression of the elastomer film can be used to achieve a global network conformation that is consistent with the helicoidal structure of the cholesteric phase. This deformation can be realized experimentally by using an anisotropic deswelling technique as described by Kim et al. (Fig. 12) [72]. Analogous to the classical two-step crosslinking procedure, a lightly crosslinked elastomer film swollen with a solvent is produced by a spin-casting technique. In a second step the solvent is slowly evaporated under centrifugation. Ordinarily, such a deswelling process would be isotropic. The network deswells simultaneously in all dimensions and the spherical shape of the chain conformation of the network strands is not affected. However, during centrifugation in a confined cell the film can only deswell in one direction – the film thickness (z-direction) – while the other two dimensions are defined by the experimental setup and remain unchanged. This anisotropic deswelling effectively exposes the elastomer film to a uniaxial compression and induces a uniform homeotropic orientation of the cholesteric helix, while finishing the crosslinking process.
Schematic representation of deswelling processes of LC gels. Figure taken from [72]
In the following box a detailed description of this preparation technique is presented, including some practical details.
In the first step a lightly crosslinked gel is prepared according to the spin-casting procedure described above for nematic elastomers. Instead of removing the elastomer strip from the centrifuge cell and applying a uniaxial load, two screws in the cover plate of the cell are removed after 2 h of reaction time in order to create small windows through which the solvent can slowly evaporate. The spinning is continued at elevated temperatures (T = 65 °C) and the crosslinking process proceeds during the deswelling of the elastomer film for more than 5 h. Afterwards the film is removed from the centrifuge cell and cured at elevated temperature in the LC state for several days to complete the crosslinking reaction. Usually the dry polymer networks stick strongly to the Teflon support foil but can be removed carefully when cooled below the glass transition temperature above a liquid nitrogen bath (see above).
A significant drawback of this procedure is that the orientation process strongly depends on the swelling properties of the polymer networks. Usually the experimental conditions have to be varied when the chemical constitution of the elastomer is changed. A precondition for a successful orientation is a sufficiently high degree of swelling (q = q x · q y · q z > 3) in the used solvent. Toluene has been shown to be a good solvent for most LC polymers and it is especially useful for the anisotropic deswelling procedure as it possesses a high vapor pressure. However, using this technique for some LC polymers, e.g., smectic side chain polymers carrying perfluorinated tails, the degree of swelling in toluene appears to be too low to obtain a good orientation. In those cases the utilization of more polar solvents (CHCl3 or THF) might be reasonable. Besides the solvent, the temperature used for the deswelling process plays a crucial role. On one hand the evaporation of the solvent has to be sufficiently slow in order to achieve a uniform orientation over the whole sample. Especially at high reaction temperatures (T > 70 °C) gradients in the orientation become more severe. On the other hand the crosslinking reaction speeds up upon deswelling and the swelling capacity of the gel decreases. If the solvent does not evaporate fast enough to match the equilibrium degree of swelling, the uniformly formed elastomer film may collapse under the mechanical stress. This becomes an issue especially for the synthesis of elastomers with high crosslinking densities. Finding the right reaction parameters can therefore be a time-consuming and challenging task.
Cholesteric polymers with locally oblate chain conformation can be obtained when a short flexible spacer between backbone and rigid mesogenic moiety consists of an even number of atoms. In this case, an allyloxy-substituted mesogenic group is normally used for the hydrosilylation reaction. To get a uniform orientation of the helicoidal z-axis of the cholesteric structure, an overall prolate chain conformation is necessary. This can easily be achieved by applying a uniaxial mechanical field in direct analogy to the preparation of nematic LSCEs having a locally prolate chain conformation. Cholesteric LSCEs are obtained with the helix axis parallel to the stretching direction, as directly observable by the selective reflection of the elastomer’s cross-section (Fig. 13).
Cholesteric LSCE having a locally oblate chain conformation. The helicoidal z-axis is ordered parallel to the elongation axis [73].
4.1.1.3 Smectic-A LSCEs
In the smectic-A phase, classical side chain polymers with end-on attached mesogenic units prefer an oblate chain conformation, where the polymer chains are on average located between the smectic layers. Consequently a uniaxial stretching is usually insufficient to achieve a simultaneous orientation of the director and the layer normal (see Sect. 3). Nishikawa et al. demonstrated that the anisotropic deswelling technique can be used to synthesize SA LSCEs with homeotropic alignment, i.e., a uniform orientation of the layer normal and the director perpendicular to the film plane [74]. A detailed description of this preparation technique as well as some experimental remarks have been given in the previous section. It should be pointed out that this orientation procedure is rather complicated and involves a lot of work in optimizing the experimental conditions. However, it allows the synthesis of rather thick bulk samples (up to 600 μm) whereas conventional strategies to prepare homeotropically aligned elastomers are limited to thin film geometries.
As already discussed for nematic elastomers in Sect. 3.2, for a successful orientation using mechanical fields it is important to consider the chemical constitution of the whole polymer network. While the equilibrium conformation of smectic side-chain elastomers is oblate, this local symmetry and therefore the orientational behavior can change drastically for high crosslinking densities. If the length of the crosslinking unit exceeds the length of the siloxane backbone between two network junctions, the crosslinker itself becomes part of the polymer main chain. This changes the local orientation between polymer main chain and mesogenic units, and uniaxial mechanical fields become sufficient to induce a macroscopic orientation.
The alignment of SA side chain elastomers with end-on attached mesogenic units becomes much simpler if a nematic phase exists in a temperature range between the smectic and the isotropic state. Nishikawa et al. reported the synthesis of an SA LSCE by applying a uniaxial mechanical deformation to a coelastomer, which showed a narrow nematic phase region at high temperature [76]. Upon deswelling under uniaxial load analogous to Küpfer’s procedure, the elastomer becomes initially nematic and the deformation induces a macroscopic orientation of the director. Subsequently, transition to the smectic state occurs and uniformly aligned layers are built up. By deploying a second crosslinking step this macroscopic orientation can be fixed permanently. In contrast to the anisotropic deswelling technique, this subsequent orientation of the director and the layer normal induces globally a prolate chain conformation, which is inconsistent with the equilibrium conformation of the smectic polymer melt. By variation of the composition in coelastomers of smectogenic and nematogenic side-chains, it is possible to induce systematically this specific phase behavior. With increasing fraction of nematogenic side chains, the stability of the smectic phase decreases and for a certain composition range the phase sequence smectic-A, nematic, isotropic is observed. Inducing a macroscopic orientation then becomes easy by applying uniaxial mechanical stretching. Of course, the mesogenic side chains must possess a flexible spacer with an odd number of spacer atoms or a long spacer (x > 7) to give locally a prolate chain conformation in the nematic state. This synthetic concept is very robust and works for a wide range of chemical constitutions, including smectogenic side chains carrying perfluorinated tails [75, 77].
In contrast to classical side chain elastomers, smectic-A main chain elastomers exhibit prolate chain conformations. Consequently, macroscopically oriented samples can be prepared according to the method of Küpfer et al. utilizing a second crosslinking step under uniaxial deformation [31]. Analogous, SA LSCEs based on side chain elastomers with side-on attached mesogenic units can be prepared [97].
In general the practical aspects presented in detail for nematic elastomers can be used for the synthesis of SA LSCEs as well. However, a few additional remarks will be given in the following box.
While deswelling under uniaxial mechanical load is performed at room temperature for nematic elastomers, this procedure is usually not applicable for smectic side-chain elastomers. Especially for elastomers carrying partially fluorinated mesogenic side-chains, the deswelling occurs too fast to allow for a good orientation of the layer normal and the director. As soon as the smectic state is reached the layer planes can couple to the mechanical field and a uniaxial mechanical deformation becomes insufficient to produce a monodomain elastomer (see Sect. 3.2). It is therefore advisable to perform the deswelling at elevated temperature. The elastomer is heated to the isotropic state under uniaxial load until most of the solvent is evaporated. Afterwards it is slowly cooled to a temperature slightly below T n,i, detectable by a spontaneous length change. The load is successively increased until a perfectly transparent sample is obtained. After 2 h of crosslinking in the nematic state, the elastomer is cooled to the smectic state and the crosslinking reaction is completed over several days. In the smectic phase the load can be further increased if necessary, as the elastomers can now stand much higher mechanical stress.
Alternatively, the orientation can be carried out at room temperature in a chamber saturated with toluene vapor. This is achieved by placing a few drops of toluene in a chamber of suitable size. After the chamber is filled with toluene vapor the loaded elastomer samples are placed inside. By slightly opening the chamber the deswelling time can easily be controlled. After the orientation is completed, the second crosslinking step is performed at elevated temperature, preferably in the smectic phase.
4.1.1.4 Lyotropic Elastomers
For the macroscopic orientation of lyotropic elastomers in mechanical fields the same principles as for thermotropic elastomers can be applied. In some ways the situation is even simpler as the rod-like micelles of the hexagonal phase are compatible with an overall prolate network chain conformation. In addition, the lamellar phase built up from disk-like micelles requires an overall oblate chain conformation to form a liquid single-crystal hydrogel (LSCH) [98].
Locally oblate lyotropic elastomers with lamellar phase structure (Lα-phase) can be oriented by uniaxial compression, as outlined above for thermotropic smectic-A elastomers. Fischer et al. synthesized crosslinked polysiloxane elastomers carrying non-ionic amphiphilic side-groups attached with their hydrophobic end to the polymer backbone. They were able to compress elastomer samples between Teflon half-cylinders to about half of their original thickness. The orientation of the phase structure – except for some unoriented domains – was demonstrated by means of 2H-NMR spectroscopy on the directly deuterated samples as well as by X-ray scattering. The preferred orientation of the director, and hence the amphiphilic side chains, was found to be parallel to the axis of compression with the amphiphilic bilayers aligned perpendicularly [98, 99].
Permanently aligned lamellar networks were prepared by fixing the ordered structure in a second crosslinking step employing the anisotropic deswelling technique. The globally oblate orientation of the polymer chains was fixed in the isotropic state in the two-step crosslinking procedure. Subsequent swelling of the fully crosslinked elastomer with water allowed for the formation of well-ordered disc-like micelles as proven by X-ray scattering [100].
Lyotropic elastomers with an H1 phase exhibit locally a prolate chain conformation in which cylindrical micelles are hexagonally packed. They can be oriented, for example, by placing a strip of the dry elastomer in a cylindrical glass tube and adding a defined amount of water from both sides. The capillary is then sealed and annealed for several days to achieve equilibrium conditions. The elastomer strip can only swell in one direction. As this uniaxial deformation is consistent with the uniaxial phase structure of the hexagonal phase a uniform orientation of the long micellar axis in the direction of the applied field (parallel to the glass walls) can be achieved. This has been directly proven by X-ray scattering as presented by Löffler et al. [101].
Weiss et al. were able to orient a hexagonal epoxy-amine elastomer by applying uniaxial strain yielding high degrees of order. The elastomer was crosslinked in the isotropic state and successively swollen with water to give a hexagonal phase [102].
4.1.2 Coupling to LC Properties
In this section some selected examples for the orientation due to surface effects and magnetic and electric fields will be presented. Elastomers oriented using the anisotropic physical properties of the liquid crystalline phase structure are usually restricted to thin films, except for lyotropic samples and when extremely high fields are available. Typically, a thin film of a linear LC polymer or a reactive mixture of low molar mass LC and crosslinker is aligned by using surface effects, magnetic fields, or electric fields and subsequently photo-crosslinked. This method has been widely used for side chain polymers, which show low viscosities, allowing good orientation at moderate field strengths. Main chain polymers exhibit much higher viscosities and thus it is usually very difficult to achieve good alignment.
An advantage with respect to the methods discussed in Sect. 4.1.1 is that a homogeneous or homeotropic orientation of the mesogens can be chosen independently of the chain conformation. For the orientation in an electric or magnetic field it has to be considered that the mesogens must have a suitable anisotropy of the dielectric constant and the diamagnetic susceptibility, respectively.
The orientation achieved by the methods discussed in this section is sometimes, especially in the case of cholesteric elastomers, much better than for the thick films prepared by coupling to a mechanical field.
The orientation of the LC polymer films is usually carried out between treated glass slides. Consequently the orientation process, the existence of defect structures and variations in orientation, as well as boundary effects can be directly visualized by means of polarizing optical microscopy.
4.1.2.1 Orientation Due to Surface Effects
In order to minimize the energy at its surface, thin films of monomeric and polymeric material often form nicely ordered liquid crystalline monodomains on surfaces. This happens spontaneously especially for smectic-A and cholesteric films and can be promoted by coating of the surfaces and by annealing the sample. Annealing is usually done a few Kelvin below T LC,i, often after slowly cooling from the isotropic phase, so that the mesogens are mobile enough to organize themselves at the surface.
Urayama et al. were able to synthesize a nematic elastomer with homeotropic orientation of the mesogens employing surface effects. They started from a mixture of a reactive acrylate-monomer, the crosslinker 1,6-hexanediol diacrylate, a photoinitiator, and a non-reactive nematogen that is necessary to broaden the temperature range of the nematic phase. This mixture was sandwiched between two polyimide coated glass plates that were separated by spacers and induce a normal alignment of the mesogens. After photo-polymerization the resulting film was detached from the glass substrate by immersing the cell in dichloromethane. The swelling in dichloromethane also washes out unreacted and nonreactive material. To deswell the film, methanol was gradually added and the film was subsequently dried in air [13].
Homogeneously planar oriented nematic elastomers have been prepared using a procedure very similar to that presented above. As alignment layers, unidirectionally rubbed polyimide or rubbed poly(vinyl alcohol) were used [14].
Cholesteric films that were prepared using surface interactions show substantially improved optical properties in comparison with samples obtained by the anisotropic deswelling method (Sect. 4.1.1). This is indicated by much lower threshold intensities in lasing experiments [103].
A widely used preparation method has been introduced by Komp et al. and consists in annealing a photo-crosslinkable polymer in the vicinity of the cholesteric to isotropic phase transition temperature T ci between glass plates coated with a sacrificial layer of water-soluble polymer over two days. Thereafter a well-ordered Grandjean texture is obtained [19]. The content of mesogens with odd and even spacers can be changed to vary systematically the main chain conformation from prolate to oblate [104]. The orientation via surface effects is especially suitable for SA elastomers. In very thin films the lamellar phase structure prefers homeotropic alignment with the layer planes parallel to the solid substrates. In early experiments, linear side chain polymers were spin-coated onto a sodium chloride substrate and were found to align spontaneously. Photo-initiated radical crosslinking in the smectic-A phase and subsequent dissolving of the substrate in water produced thin free-standing elastomer films of 2–3 μm thickness [105]. Subsequently, films with locally varying film thickness in the sub-micron range were drawn from the isotropic melt on a metal frame, followed by UV crosslinking in the smectic phase. Complex sample geometries have also been realized, e.g., free-standing elastomer balloons by expanding thin smectic polymer films (2–3 μm) from a glass capillary [106].
A rather different approach to utilize surface effects for the orientation of smectic elastomers is radical crosslinking of liquid crystalline polymers using a dispersion or miniemulsion technique in a mixture of solvent and surfactants. Crosslinked smectic-A colloids were prepared in sizes of 100–200 nm showing highly oriented phase structures that could be visualized directly in transmission electron microscopy [107].
4.1.2.2 Orientation in a Magnetic Field
Nematic photo-crosslinkable polymers [108] and reactive monomer mixtures [109] can be easily oriented in a magnetic field. Due to their diamagnetism the mesogens usually orient parallel to the magnetic field in the LC phase.
Figure 14 shows an example of the preparation of a homogeneously oriented free-standing polymer film. A microscope slide is coated with a water-soluble sacrificial layer such as poly(2-ethyl-3-oxazolin) (PEOx) from an ethanol solution. The polymer and two spacers of the desired thickness are placed on the glass slide and successively covered with a second glass slide that is also coated with PEOx. In that state, it is a bit delicate to avoid the formation of air-bubbles, which of course complicate measurements on the films later. It usually helps to lower the second glass slide from one side of the film and to keep the temperature rather high so that the polymer is relatively fluent. It is also advisable to heat the LC polymer for one night in vacuo prior to preparation in order to remove solute gases. However, this should not be done too often as the photo-crosslinker is sensitive to thermal degradation. The sandwiched polymer is placed in a magnetic field (1.8 T, produced by an electromagnet; for a reactive monomer mixture a strong permanent magnet can be sufficient) and slowly cooled from the isotropic to the nematic phase. Subsequent annealing in the magnetic field for several days minimizes surface defects that usually occur.
After removing the sample from the magnetic field the polymer is photo-crosslinked from both sides (a few minutes are sufficient) using a UV-lamp. If T g is relatively low it can be helpful to photo-crosslink under cooling with an ice-bath so that the mesogens do not reorient due to the heat produced by the UV-lamp. The sandwiched film should now be transparent and can be removed from the glass slide after dissolving the sacrificial layer in water.
Lyotropic liquid crystalline elastomers can be oriented in a magnetic field when they contain rod-like amphiphiles with an aromatic core. This structural element is usually needed to achieve a sufficiently large diamagnetic anisotropy. It has been demonstrated that reactive mixtures of methacrylate monomers, bifunctional crosslinkers, and (deuterated) water in the strong field of an NMR-magnet (7–11 T) can be oriented by slowly cooling from the isotropic phase to the lamellar mesophase and subsequent annealing in the biphasic region. The process of orientation can thereby be nicely followed by NMR spectroscopy [110, 111].
4.1.2.3 Orientation in an Electric Field
Free-standing nematic LSCE films can be prepared analogous to the procedure shown in Fig. 14 utilizing electric fields. Glass substrates covered with translucent indium tin oxide (ITO) are used to apply alternating electric fields (3 V/μm, 1 kHz). This is sufficient to induce homeotropic alignment of the LC phase structure in elastomer films of thicknesses up to 50 μm. A precondition for this method is the availability of photo-crosslinkable polymers that contain mesogens with a positive anisotropy of the dielectric constant, e.g., with terminal cyano groups. It is important to use relatively long spacers for these polar mesogens in order to achieve sufficient decoupling from the polymer backbone, allowing them to follow the electric field [93].
Electric fields have also been used to prepare freestanding chiral smectic-A* LSCEs with homogeneous alignment of the layer normal and the director in the film plane. An ITO covered glass cell was coated with rubbed polyimide and rubbed polyvinyl alcohol as sacrificial layer and filled with a eutectic mixture of smectic acrylate monomers and bifunctional acrylate crosslinker. The mixture was heated to the isotropic state and cooled to the S *A phase, while applying an alternating electric field (6 V/μm, 0.5 Hz, square wave). After successful alignment, the electric field was turned off followed by UV photo-polymerization. Dissolving the sacrificial layer in water yielded a free-standing film of 60 μm thickness [112].
Orientation in electric fields is in particular useful for SC* elastomers as their mesogenic units usually possess a strong dipole moment. LC polymers with crosslinkable acrylate side-groups were filled into commercially available ITO cells with 10 μm electrode separation and heated into the SC* phase. A macroscopic orientation was induced by applying an alternating electric field (100–200 V, 1–5 Hz) and performing several heating and cooling cycles around the S *C –S *A phase transformation. In order to fix the monodomain structure permanently, a DC electric field was applied before UV-initiated radical crosslinking of the sample [113]. Using this method, ferroelectric elastomers have been prepared with a nearly perfect homogeneous orientation and a polar axis perpendicular to the glass substrate [15, 22].
4.2 More Complex Orientation Methods
If LSCEs are prepared using one of the methods described in Sect. 4.1, a monodomain may be obtained with respect to the main director but not necessarily for the whole phase structure. In the case of SC elastomers an orientation of the director by mechanical stretching or by external fields yields a polydomain with respect to the layer normal. The additional orientation steps that are necessary for a full orientation of this phase are outlined in Sect. 4.2.2.
Biaxial nematic or SA elastomers lack rotational symmetry around their molecular long axes and the orientation of the three axes is described by the additional minor director m. A third director l is thereby automatically defined. Figure 15 gives a schematic representation of a uniaxial and biaxial nematic phase. The orientation methods discussed in the previous section yield polydomains with respect to the minor director. Additional orientation steps are necessary to obtain elastomers with a 3D orientation of the mesogenic axes. Such a 3D monodomain of a biaxial elastomer has not yet been reported, but some potential strategies to obtain such an elastomer will be outlined in Sect. 4.2.1.
Schematic representation of the uniaxial (a) and biaxial (b) nematic phase [4]
4.2.1 Orientation of the Second Director
Over the last few years, significant evidence has been obtained that nematic [4] as well as SA [9] elastomers can show phase biaxiality over a broad temperature range. A biaxial-nematic phase from 15 °C to about 70 °C was found by means of 2H-NMR spectroscopy on a directly deuterated side chain end-on elastomer prepared by the Küpfer-procedure [4].
After preparation, the minor directors form a polydomain, which can be oriented in the magnetic field of the NMR-experiment. A reorientation of the major director does not occur because this orientation is fixed in the polymer network. If a nematic or SA elastomer is prepared to yield a homeotropic film, the differently oriented domains can be directly observed by optical methods. Strain-dependent optical investigations give evidence that the minor director can be oriented using a mechanical field.
To produce a monodomain with respect to the orientation of all three molecular axes, an additional orientation step is required. If the elastomer is prepared by orienting the major director of a photocrosslinkable polymer in an electric field, one could think about simultaneously orienting the second director by surface effects, e.g., with a rubbed PI-surface. For orientation by biaxial deswelling an SA elastomer, an additional crosslinking step under uniaxial strain on the not yet completely crosslinked elastomer could also lead to the orientation of the minor director.
Such a monodomain has as yet not been produced and it is also not clear whether the orientation of the minor director could be permanently fixed in the polymer network or whether it would relax to a polydomain state after some time. Another question is the behavior after heating to the isotropic phase.
4.2.2 Orientation of the Layer Normal in SC LSCEs
Smectic-C elastomers and especially chiral SC* elastomers have attracted a lot of interest as they can respond to electric stimuli or transfer mechanical stress into electrical signals. Owing to the C2-symmetry, chiral smectic-C* phases show effects like ferroelectricity, piezoelectricity, pyroelectricity, and second harmonic generation [114]. However, a macroscopic polarization represents a high energy state, which – in the absence of external fields – is cancelled out by the formation of helical superstructure. During the external field induced orientation process of SC elastomers this helix can easily be unwound. With the preparation of SC LSCEs the macroscopic C2-symmetry can be fixed permanently and materials are obtained that can be used as electro-mechanical actuators or sensors.
As shown in the previous section, SC elastomers can be well aligned in electric fields. These preparation techniques are limited to thin film geometries, while mechanical orientation offers the possibility to prepare uniformly oriented bulk samples.
In Sect. 3.2 it was shown that uniaxial stretching or compression of SC elastomers does not produce macroscopically oriented samples. Due to the SC symmetry, uniaxial deformation only induces a uniform orientation of the director but leaves the smectic layer normals conically distributed around the stress axis. Usually such elastomers remain opaque. For the preparation of SC LSCEs a more complex orientation strategy is necessary which can be realized by deploying two successive orientation processes.
In a first step a monodomain sample with respect to the director is produced. This can be done completely analogous to Küpfer’s procedure described for nematic elastomers in Sect. 4.1.1. For this a lightly crosslinked elastomer gel that is swollen with solvent is slowly deswollen under uniaxial mechanical load for several hours. Then in the second orientation step a mechanical field has to be applied that induces a reorientation of the smectic layer structure in order to produce a uniform orientation of both the director and the layer normal.
Semmler et al. have shown for SC* side chain elastomers that a second uniaxial stretching under an angle of θ ±90° with respect to the first deformation axis (θ is the SC tilt angle) produces a layer reorientation towards the uniform C2 symmetry of the SC* phase (Fig. 16). The tilt angle of uniaxially elongated SC elastomers can easily be analyzed by using X-ray scattering. The smectic layer normals reorient and take up a position more or less perpendicular to the second deformation: Layers which enclose large angles with the mechanical stress axis realign towards orientations enclosing small angles with the axis. However, by this method no real monodomain elastomers have been obtained. The mechanical deformation only leads to an anisotropic distribution of the layer normals on the cone causing a non-centrosymmetric phase structure but cannot induce a completely uniform orientation of both the layer normal and the director. Nevertheless, the partially oriented phase structure can be fixed permanently by chemical crosslinking, yielding highly transparent elastomers which show piezoelectric properties and second harmonic generation [80–83, 115]. The same orientation procedure has also been deployed to align main chain SC elastomers [116].
A mechanical deformation that is consistent with the biaxial SC phase symmetry and that is able to induce a macroscopic orientation of the layer normal and the director is a shear mechanical field. Hiraoka et al. prepared side chain SC* LSCEs by applying a shear deformation perpendicular to the director followed by chemical crosslinking [117, 118]. For this, the uniaxially aligned elastomer with uniformly oriented director but conical layer distribution is mounted into a mechanical shear setup (Fig. 17a). The elastomer is sheared stepwise at room temperature until the shear angle is consistent with the tilt angle of the SC phase structure. Between each shear step a sufficiently high relaxation time should be maintained to avoid damaging the sample. The mechanical field induces a continuous reorientation of the SC phase structure where the director rotates towards the shear diagonal, while the layer normal takes up the position perpendicular to the shear mechanical field (Fig. 17b). This multi-step orientation process can take up to several hours. It must be avoided that the chemical crosslinking reaction is completed before a macroscopic orientation is induced. If a hydrosilylation reaction is used, the reaction speed is rather slow at ambient temperature. However, it might be advisable to cool the sample during the orientation process to slow down the crosslinking process and to provide enough time for a successful alignment. The sheared state which corresponds to an SC monodomain structure can be fixed permanently by completing the chemical crosslinking reaction at elevated temperatures – completely analogous to nematic elastomers. Following this procedure, main chain LSCEs showing SC and chiral SC* phase structures have also been realized by Sanchez et al. and Heinze et al., respectively [86, 87, 119].
Smectic-C elastomer with uniformly aligned director but conical layer distribution subjected to shear strain perpendicular to the director [117] (a) and corresponding reorientation process that produces an SC monodomain (b). Reprinted with permission from [87]. Copyright (2008) American Chemical Society
5 Conclusions and Outlook
The preparation of LCEs comprises sophisticated chemistry that brings together the broad areas of research on macromolecular networks and on liquid crystals. For a successful synthesis it is not sufficient to combine only conventional principles and methods of both fields. In addition specific new features emerge that have to be considered.
Conventional principles and methods concern the synthetic routes for macromolecular networks and the realization of the liquid crystalline state by mesogenic monomer units. Network chemistry has to consider the reactivity and functionality of the monomer units. In most cases, this excludes ionic polymerization techniques and reduces utilizable methods to radical polymerization and polymer analog reactions for side chain networks, and to polycondensation or polyaddition reactions for main chain elastomers. The chemistry of the crosslinking process and the chemical constitution of the crosslinker have to be adapted to the polymerization process. Applying photo-chemistry of suitable functional monomer units opens an additional, versatile pathway to build up the network structure.
The liquid crystalline state can be systematically introduced by rigid anisometric building blocks or by amphiphilic moieties. According to their chemical constitution and basically following the same systematic as known from low-molar-mass liquid crystals, all types of LC phase structure are accessible. Additional aspects to be considered arise from modification of the state of order due to the decreased specific volume of the macromolecules (compared to that of the monomers) and the reduced translational and rotational mobility of the mesogenic units due to their linkage within the macromolecule.
New features to be taken into account for the synthesis of LCEs are (1) the interaction between the LC order and the conformation of the polymer backbone, and (2) the effect of the chemical constitution of the crosslinker on both the local topology of the network and the LC order. The interaction between the LC order and the conformation of the polymer backbone causes a deviation from a conventional statistical spherical coil to an oblate or prolate chain conformation. Without any precautions during the synthesis, LCEs are obtained in a polydomain structure due to maximization of the entropy of the overall chain conformation. The crucial interplay of LC order and conformation of the polymer backbone, however, offers the chance to induce macroscopically an overall anisotropic chain conformation by an external mechanical field. If the symmetry of the mechanical field is consistent with the symmetry of the LC phase structure, a uniform orientation is produced. Introducing an additional chemical crosslinking process, the macroscopic uniform alignment becomes locked-in permanently and a liquid single-crystal elastomer (LSCE) is realized. Alternatively, uniform alignment of LC monomers or linear pre-polymers can be induced by electric or magnetic fields or surface effects. Subsequently, a chemical crosslinking reaction performed in the aligned state yields LSCEs. However, these conventional orientation techniques are limited to very small sample thicknesses.
The macroscopically aligned state of LSCEs is imprinted to the network structure during chemical crosslinking. After heating to the isotropic state and subsequently cooling down to the LC state again, the monodomain structure turns out to be completely reversible. Furthermore, even complex LC structures like biaxial, untwisted, and thus ferroelectric smectic-C LSCEs are accessible if a suitable shear deformation is performed before the final crosslinking step.
The effect of the chemical constitution of the crosslinker on the local topology of the network is the second new feature to be considered. If the crosslinker molecule is flexible it can behave like an isotropic solvent. In that case, essentially only the phase transition and phase transformation temperatures of the LC phase are affected [90]. If, however, the chemical constitution resembles that of a mesogen of the constituent polymer backbone, the history of the crosslinking process becomes important. Under these conditions the crosslinker adopts the state of order in which the final crosslink process of the network occurs and thus determines the local topology of the crosslink [120, 121]. The mechanical properties and the reorientational behavior are considerably modified for networks with the same chemical constitution but crosslinked either in the isotropic or in the liquid crystalline state [122–124]. Other important aspects of the local topology at the crosslink concern the phase transformation behavior [125] as well as the positional ordering in smectic systems [126].
LSCEs offer numerous new aspects to the field of polymer science due to their exceptional physical properties. With respect to mechanical behavior, LSCEs are unique “soft crystals.” Mechanical fields not only bias the state of LC order but also cause exceptional reorientation behavior, which impressively modifies the mechanical response. If the LC phase structure additionally exhibits one-dimensional positional long-range order, conventional entropic elasticity exists solely in two dimensions, while the third dimension is determined by the enthalpic elasticity of the LC order.
It is not only the mechanical properties of LSCEs that offer new perspectives for their application and basic research. In fact, all physical properties directly related to the LC order can be influenced by the interaction between mechanics and the state of order of the LC networks. A completely open field is to mimic biological systems in which collective molecular order is responsible for functionality. Lyotropic LSCEs provide one step in this direction. These networks enable ion conductivity [127], which opens the possibility to perform chemical reactions within the network. If, e.g., for a redox reaction, reactants and products modify the LC order differently, a mechanical response will be directly connected. It remains a challenging task for chemists to synthesize new LSCEs that are adapted for such problems.
Abbreviations
- 3D:
-
Three-dimensional
- EO:
-
Ethylene oxide
- k :
-
Smectic layer normal
- LC:
-
Liquid crystal
- LCE:
-
Liquid crystalline elastomer
- LCP:
-
Liquid crystalline polymer
- LSCE:
-
Liquid single crystal elastomer
- n :
-
Nematic director
- SA :
-
Smectic-A
- SANS:
-
Small-angle neutron scattering
- SC :
-
Smectic-C
- T g :
-
Glass transition temperature
- T LC, i :
-
Clearing temperature, i.e., LC – isotropic phase transformation temperature
References
Lehmann O (1908) Biologisches Centralblatt 28:31
Schätzle J, Finkelmann H (1987) Mol Cryst Liq Cryst 142:85
de Gennes PG (1975) C R Acad Sci B Phys 281:101
Brömmel F, Stille W, Finkelmann H, Hoffmann A (2011) Soft Matter 7:2387
Leube HF, Finkelmann H (1990) Makromol Chem 191:2707
Leube HF, Finkelmann H (1991) Makromol Chem 192:1317
Severing K, Saalwächter K (2004) Phys Rev Lett 92:125501
Severing K, Stibal-Fischer E, Hasenhindl A, Finkelmann H, Saalwächter K (2006) J Phys Chem B 110:15680
Storz R, Komp A, Hoffmann A, Finkelmann H (2009) Macromol Rapid Commun 30:615
Crawford GP, Broer D, Zumer S (2011) Cross-Linked Liquid Crystalline Systems: From Rigid Polymer Networks to Elastomers. CRC Press
Flory PJ (1953) Principles of polymer chemistry. Cornell University Press, Ithaca, p 399
Treloar LRG (1975) The physics of rubber elasticity. Clarendon, Oxford
Urayama K, Mashita R, Kobayashi I, Toshikazu T (2007) Macromolecules 40:7665
Thomsen DL, Keller PN, Pink JR, Jeon H, Senoy D, Ratna BR (2001) Macromolecules 34:5868
Gebhard E, Zentel R (2000) Macromol Chem Phys 201:902
Bohnert R, Finkelmann H (1994) Macromol Chem Phys 195:689
Amigó-Melchior A, Finkelmann H (2002) Colloid Polym Sci 280:207
Schuring H, Stannarius R, Tolksdorf C, Zentel R (2001) Macromolecules 34:3962
Komp A, Rühe J, Finkelmann H (2005) Macromol Rapid Commun 26:813
Vasilets VN, Kovalchuk AV, Yuranova TI, Ponomarev AN, Talroze RV, Zubarev ER, Plate NA (1996) Polym Adv Technol 7:173
Vasilets VN, Kovalchuk AV, Yuranova TI, Ponomarev AN, Talroze RV, Plate NA (2000) Polym Adv Technol 11:330
Xia Y, Verduzco R, Grubbs RH, Kornfield JA (2008) J Am Chem Soc 130:1735
Ober CK, Jin J-I, Lenz WR (1998) Adv Polym Sci 68:387
Greiner A, Schmidt HW (1998) Aromatic Main Chain Liquid Crystalline Polymers. In: Demus D, Goodby J, Grey GW, Spiess H-W, Vill V (eds). Handbook of Liquid Crystals, Wiley-VCH, Weinheim, p 1
Sirigu A (1991) In: Ciferri A (ed) Liquid crystallinity in polymers. VCH Publishers, New York, p 261
Zentel R, Reckert G (1986) Macromol Chem Phys 187:1915
Bergmann GHF, Finkelmann H, Percec V, Zhao MY (1997) Macromol Rapid Commun 18:353
Percec V, Asandei AD (1997) Macromolecules 30:7701
Wermter H, Finkelmann H (2001) e-Polymers 13:1
Krause S, Zander F, Bergmann G, Brandt H, Wertmer H, Finkelmann H (2008) C R Chimie 12:85
Beyer P, Terentjev EM, Zentel R (2007) Macromol Rapid Commun 28:1485
Krause S, Dersch R, Wendorff JH, Finkelmann H (2007) Macromol Rapid Commun 28:2062
Donnio B, Wermter H, Finkelmann H (2000) Macromolecules 33:7724
Aguilera C, Bartulin J, Hisgen B, Ringsdorf H (1983) Makromol Chem 184:253
Ren W, McMullan PJ, Griffin AC (2008) Macromol Chem Phys 209:1896
Ishige R, Osada K, Tagawa H, Niwano H, Tokita M, Watanabe J (2008) Macromolecules 41:7566
Ishige R, Tokita M, Funaoka S, Kang S, Watanabe J (2011) Macromol Chem Phys 212:48
Ishige R, Tokita M, Naito Y, Zhang CY, Watanabe J (2008) Macromolecules 41:2671
Yang H, Buguin A, Taulemesse J-M, Kaneko K, Méry S, Bergeret A, Keller P (2009) J Am Chem Soc 131:15000
Lub J, Broer DJ, Van Den Broek N (1997) Liebigs Ann 2281
Lub J, Broer DJ, Martinez Antonio ME, Mol GN (1998) Liq Cryst 24:375
Lub J, Broer DJ, Allan JF (1999) Mol Cryst Liq Cryst 332:259
Kuhn W (1936) Kolloid Zeitschrift 76:258
Finkelmann H, Kock HJ, Gleim W, Rehage G (1985) Makromol Chem Rapid Commun 5:287
Cotton JP, Hardouin F (1997) Prog Polym Sci 22:795
D’Allest JF, Maissa P, ten Bosch A, Sixou P, Blumstein A, Blumstein RB, Teixera J, Noirez L (1988) Phys Rev Lett 61:2562
D’Allest JF, Sixou P, Blumstein A, Blumstein RB, Teixera J, Noirez L (1988) Mol Cryst Liq Cryst 155:581
Arrighi V, Higgins JS, Weiss RA, Cimecioglu AL (1992) Macromolecules 25:5297
Abis J, Arrighi V, Cimecioglu AL, Higgins JS, Weiss RA (1993) Eur Polym J 29:175
Li MH, Bralet A, Keller P, Strazielle C, Cotton JP (1993) Macromolecules 26:119
Li MH, Brûlet A, Cotton JP, Davidson P, Strazielle C, Keller P (1994) J Phys II 4:1843
Li MH, Brûlet A, Keller P, Cotton JP (1996) J Mol Struct 383:11
Hardouin F, Sigaud G, Achard MF, Brulet A, Cotton JP, Yoon DY, Percec V, Kawasumi M (1995) Macromolecules 28:5427
Sherwood HH, Sigaud G, Yoon DY, Wade CG, Kawasumi M, Percec V (1994) Mol Cryst Liq Cryst 254:455
Yoon DY, Flory PJ (1989) MRS Symp Proc 134:11
Li J, Brulet A, Davidson P, Keller P, Cotton JP (1993) Phys Rev Lett 70:2297
Hardouin F, Leroux N, Mery S, Noirez L (1992) J Phys II 2:271
Leroux N, Keller P, Achard MF, Noirez L, Hardouin F (1993) J Phys II 3:1289
Hardouin F, Leroux N, Noirez L, Keller P, Mauzac M, Achard MF (1994) Mol Cryst Liq Cryst 254:267
Noirez L, Hardouin F, Lecommandoux S, Richard H, Achard MF, Strazielle C (1996) J Phys II 6:225
Lecommandoux S, Achard MF, Hardouin F, Brulet A, Cotton JP (1997) Liq Cryst 22:549
Lecommandoux S, Noirez L, Achard MF, Brulet A, Cotton JP, Hardouin F (1997) Physica B 234:250
Noirez L, Keller P, Cotton JP (1995) Liq Cryst 18:129
Noirez L, Cotton JP, Hardouin F, Keller P, Moussa F, Pépy G, Strazielle C (1988) Macromolecules 21:2889
Finkelmann H, Kock HJ, Gleim W, Rehage G (1984) Makromol Chem Rapid Commun 5:287
Finkelmann H, Gleim W, Hammerschmidt K, Schätzle J (1989) Makromol Chem Macromol Symp 26:67
Hammerschmidt K, Finkelmann H (1989) Makromol Chem Macromol Chem Phys 190:1089
Mitchell GR, Coulter M, Davis FJ, Guo W (1992) Journal de Physique II 2:1121
Noirez L, Davidson P, Schwarz W, Pépy G (1994) Liq Cryst 16:1081
Noirez L, Boeffel C, Daoud-Aladine A (1998) Phys Rev Lett 80:1453
Lecommandoux S, Noirez L, Achard MF, Hardouin F (2000) Macromolecules 33:67
Kim ST, Finkelmann H (2001) Macromol Rapid Commun 22:429
Potdevin A (2003) Diploma Thesis, University of Freiburg
Nishikawa E, Yamamoto J, Yokoyama H, Finkelmann H (2004) Macromol Rapid Commun 25:611
Kramer D (2010) PhD thesis, University of Freiburg
Nishikawa E, Finkelmann H, Brand HR (1997) Macromol Rapid Commun 18:65
Kramer D, Finkelmann H (in preparation)
Lu XH, He CB, Liu PW, Griffin AC (2005) J Polym Sci A Polym Chem 43:3394
Patil HP, Liao J, Hedden RC (2007) Macromolecules 40:6206
Semmler K, Finkelmann H (1994) Polym Adv Technol 5:231
Benné I, Semmler K, Finkelmann H (1994) Macromol Rapid Commun 15:295
Semmler K, Finkelmann H (1995) Macromol Chem Phys 196:3197
Benné I, Semmler K, Finkelmann H (1995) Macromolecules 28:1854
Eckert T, Finkelmann H, Keck M, Lehmann W, Kremer F (1996) Macromol Rapid Commun 17:767
Rousseau IA, Mather PT (2003) J Am Chem Soc 125:15300
Sánchez-Ferrer A, Finkelmann H (2009) Mol Cryst Liq Cryst 508:357
Sanchez-Ferrer A, Finkelmann H (2008) Macromolecules 41:970
Sánchez-Ferrer A, Finkelmann H (2011) Macromol Rapid Commun 32:309
Hiraoka K, Tagawa N, Baba K (2008) Macromol Chem Phys 209:298
Greve A, Finkelmann H (2001) Macromol Chem Phys 202:2926
Kramer D, Finkelmann H (2007) Macromol Rapid Commun 28:2318
Küpfer J, Finkelmann H (1991) Makromol Chem Rapid Commun 12:717
Rogez D, Martinoty P (2011) Eur Phys J E 34:69
Rogez D, Brömmel F, Finkelmann H, Martinoty P (2011) Macromol Chem Phys 212:2667
Naciri J, Srinivasan A, Jeon H, Nikolov N, Keller P, Ratna BR (2003) Macromolecules 36:8499
Ahir S, Tajbakhsh AR, Terentjev EM (2006) Adv Funct Mater 16:556
Komp A, Finkelmann H (2007) Macromol Rapid Commun 28:55
Fischer P, Schmidt C, Finkelmann H (1994) Macromol Rapid Commun 16:435
Fischer P, Finkelmann H (1998) Prog Colloid Polym Sci 111:127
Brillault J (2009) PhD thesis, University of Freiburg
Löffler R, Finkelmann H (1990) Macromol Rapid Commun 11:321
Weiss F, Finkelmann H (2004) Macromolecules 37:6587
Schmidtke J, Kniesel S, Finkelmann H (2005) Macromolecules 38:1357
Bourgerette C, Chen B, Finkelmann H, Mitov M, Schmidtke J, Stille W (2006) Macromolecules 39:8163
Gebhard E, Zentel R (1998) Macromol Rapid Commun 19:341
Schüring H, Stannarius R, Tolksdorf C, Zentel R (2001) Macromolecules 34:3962
Vennes M, Zentel R, Rossle M, Stepputat M, Kolb U (2005) Adv Mater 17:2123
Müller S (2006) PhD thesis, University of Freiburg
Buguin A, Li MH, Silberzan P, Ladoux B, Keller P (2006) J Am Chem Soc 128:1088
Kleinschmidt F, Hickl M, Saalwächter K, Schmidt C, Finkelmann H (2005) Macromolecules 38:9772
Amigó-Melchior A, Finkelmann H (2002) Polym Adv Technol 13:363
Spillmann CM, Ratna BR, Naciri J (2007) Appl Phys Lett 90:021911
Brehmer M, Zentel R, Wagenblast G, Siemensmeyer K (1994) Macromol Chem Phys 195:1891
Meyer RB, Liebert L, Strzelecki L, Keller P (1975) J Phys Lett Paris 36:69
Hiraoka K, Stein P, Finkelmann H (2004) Macromol Chem Phys 205:48
Heinze P (2010) PhD thesis, University of Freiburg
Hiraoka K, Finkelmann H (2001) Macromol Rapid Commun 22:456
Hiraoka K, Sagano W, Nose T, Finkelmann H (2005) Macromolecules 38:7352
Heinze P, Finkelmann H (2010) Macromolecules 43:6655
Küpfer J, Finkelmann H (1994) Macromol Chem Phys 195:1353
Zander F, Finkelmann H (2010) Macromol Chem Phys 211:1167
Finkelmann H, Kundler I, Terentjev EM, Warner M (1997) J Phys II France 7:1059
Kundler I, Finkelmann H (1998) Macromol Chem Phys 199:677
Urayama K, Kohmon E, Kojima M, Takigawa T (2009) Macromolecules 42:4084
Lebar A, Cordoyiannis G, Kutnjak Z, Zalar B (2012) Adv Polym Sci. doi: 10.1007/12_2010_103
de Jeu WH, Ostrovskii BI (2012) Adv Polym Sci. doi: 10.1007/12_2010_105
Ramón-Gimenez L, Storz R, Haberl J, Finkelmann H, Hoffmann A (2012) Macromol Rapid Commun 33:353
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Brömmel, F., Kramer, D., Finkelmann, H. (2012). Preparation of Liquid Crystalline Elastomers. In: de Jeu, W. (eds) Liquid Crystal Elastomers: Materials and Applications. Advances in Polymer Science, vol 250. Springer, Berlin, Heidelberg. https://doi.org/10.1007/12_2012_168
Download citation
DOI: https://doi.org/10.1007/12_2012_168
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-31581-7
Online ISBN: 978-3-642-31582-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)