Abstract
Thiolated chitosans constitute an integral part of designated “thiomers”, which are thiolated polymers widely investigated for non-invasive drug delivery. In brief, thiomers display thiol-group-bearing ligands on their polymer backbone. Through thiol/disulfide exchange reactions and/or a simple oxidation process, disulfide bonds are formed between such polymers and the cysteine-rich subdomains of mucus glycoproteins, thus building up the mucus gel layer. Most chemical modifications of chitosan are performed at the free amino groups of the glucosamine units. So far, the alkyl thiomers chitosan–cysteine, chitosan–thiobutylamidine, chitosan–thioglycolic acid, chitosan–N-acetylcysteine, and chitosan–thioethylamidine and the aryl thiomers chitosan–6-mercaptonicotinic acid and chitosan–4-mercaptobenzoic acid have been generated. Due to the immobilization of thiol groups on the chitosan backbone, its mucoadhesive, permeation enhancing, in situ gelling, efflux pump inhibitory, and controlled drug release properties are improved. The great benefits of this new generation of chitosans in comparison to the corresponding unmodified polymers has been verified via numerous in vivo studies on various mucosal membranes. A proof of concept for oral, nasal and buccal drug delivery is provided. This chapter includes an overview of the mechanism of adhesion and the design of thiomers as well as of delivery systems comprising thiolated chitosans and their in vivo performance.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Chitosan is a cationic polysaccharide obtained by the alkaline deacetylation of chitin, which is derived from crustaceans. It is composed of randomly distributed β-(1-4)-linked d-glucosamine (deacetylated unit) and N-acetyl-d-glucosamine (acetylated unit). The degree of deacetylation has a direct impact on the solubility of the polymer. Chitosan is a weak base with a pK a value of the d-glucosamine residue of about 6.2–7.0. Therefore, it is not soluble at neutral and alkaline pH values. However, when it is in acid medium such as hydrochloric acid, acetic acid, glutamic acid, and lactic acid, the amine groups of chitosan are protonated and thus promote its solubility [1]. The amino groups on the chitosan backbone undoubtedly play an essential role because they are the main target for the immobilization of thiol groups.
Recently, attention has been paid to chitosan because of its favorable biological properties, such as biodegradability, biocompatibility, and non-toxicity, as well as its physiochemical properties [2, 3]. Moreover, it has been reported that chitosan can accelerate gastric ulcer healing [4], that pretreatment with chitosan prevents ulcerogenic effect in rats [5], and that chitosan displays antimicrobial activity [6, 7].
Chitosan has been widely used as an absorption enhancer [3], drug carrier [8], mucoadhesive, and permeation-enhancing polymer [9] in formulations for buccal/sublingual, nasal, gastrointestinal, vaginal, and colonic drug delivery [2] as well as for gene delivery [10, 11]. For gene delivery, in particular, chitosan nanoparticles offer the advantage of an easy preparation by mixing negatively charged DNA or RNA with the cationic chitosan to produce stable nanoparticles [12]. In the case of oral drug delivery, chitosan offers various advantages. For instance, due to its cohesive properties it can be used in matrix tablets in order to release the drug in a controlled manner [13]. Moreover, being capable of opening tight junctions of mucosal membranes and providing protection towards proteolytic digestion of drugs in the gastrointestinal (GI) tract, it can be used to improve the oral uptake of therapeutic peptides [14].
The mucoadhesive properties of chitosan can be mainly attributed to ionic interactions, in particular between the cationic amino groups of chitosan and the negative substructures of the mucus, such as sialic acid moieties.
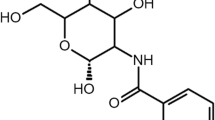
As previously mentioned, the amino groups are target moieties for the modification of the backbone in order to gain certain additional advantageous properties. For instance, various chitosan derivatives such as chitosan–EDTA conjugates [15], N-trimethylated chitosan [16], mono-N-carboxymethyl chitosan [17] and N-sulfo-chitosan [18] have been introduced in the pharmaceutical field in the last decade. A further modification of chitosan is based on the immobilization of thiol groups, leading to so-called thiolated chitosans (Fig. 1).
Thiolated chitosans show various promising properties such as mucoadhesion, efflux pump inhibition, permeation enhancement, in situ gelling capacity, and controlled drug release. In contrast to unmodified chitosans, these novel polymers are capable of forming covalent bonds with the mucus-forming constituents. In fact, through thiol/disulfide exchange reactions and/or a simple oxidation process, disulfide bonds are formed between such polymers and the cysteine-rich subdomains of mucus glycoproteins (Fig. 2). Hence, thiolated chitosans mimic the natural behaviour of secreted mucus glycoproteins, which are also covalently anchored in the mucus layer by disulfide bonds. So far, numerous thiolated chitosan derivatives have been synthesized, including the alkyl thiolated chitosans chitosan–cysteine conjugate (chitosan-Cys) [19], chitosan–4-thio-butyl-amidine conjugate (chitosan-TBA) [20], chitosan–thioglycolic acid conjugate (chitosan-TGA) [19], chitosan– N-acetylcysteine conjugate (chitosan-NAC) [21], chitosan–2-thio-ethyl-amidine conjugate (chitosan-TEA) [22], and chitosan–glutathione conjugate (chitosan-GSH) [23] and the aryl thiolated chitosans chitosan–6-mercaptonicotinic acid (chitosan-MNA) [24] and chitosan–4-mercaptobenzoic acid (chitosan-MBA) [25] (Fig. 3).
2 Synthesis of Thiolated Chitosans
Most chemical modifications on the chitosan backbone are performed at the free amino groups of the glucosamine units. For example, the formation of amide or amidine bonds between these primary amino groups and the activated carboxylic groups of a low molecular mass thiol-bearing compound can be initiated easily. A typical procedure can be summarized as follows: N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDAC) is added to a solution of the low molecular mass thiol-bearing compound in order to activate the carboxylic groups. After a certain incubation period, the mixture is added to a chitosan solution. The reaction mixture is stirred and incubated at room temperature for several hours. Finally, this is dialyzed to remove unbound reagents and lyophilized [26]. The initial reason for the introduction of thiol groups was based on the hypothesis that these thiol groups would form disulfide bonds with the thiol groups of the mucus, consequently leading to high mucoadhesive properties [20, 27]. In fact, the GI mucus comprises around 80% glycoproteins in which cysteine-rich subdomains occur.
2.1 Alkyl Thiolated Chitosans
Examples of alkyl sulfhydryl-bearing compounds that can covalently attach to a chitosan backbone are cysteine, N-acetylcysteine, thioglycolic acid, and glutathione. These molecules become linked to the chitosan primary amino group via an amide bond.
By contrast, ligands such as thioethylamidine and thiobuthylamidine form amidine bonds with chitosan. Most of these sulfhydryl-bearing compounds have thiol groups whose pK a value is in the range of 8–10 [28]. During the synthesis process, an unintended oxidation of thiol moieties can be avoided by performing the polymer synthesis at a pH < 5. At this pH, the concentration of the reactive form (S−) is marginal. Therefore, low formation of disulfide bonds is observed. The amount of thiol groups remaining on the polymer can be finally determined by Ellman’s method [29].
2.2 Aryl Thiolated Chitosans
It is known that aryl thiols are more reactive than alkyl thiols due to their lower pK a values (5–7) [28]. Aromatic thiols, for example, have already been shown to increase the folding rate of disulfide-containing proteins more than aliphatic thiols [30, 31]. For a pharmaceutical excipient such as thiolated chitosans, the increased reactivity of the thiol group would further increase its beneficial properties over alkyl thiol chitosan and non-thiolated polymers. Examples of aryl sulfhydryl-bearing compounds covalently attached to chitosan via an amide bond are 6-mercaptonicotinic acid and 4-mercaptobenzoic acid. As mentioned for alkyl thiol chitosan, the amount of free and oxidized thiol groups can be determined by Ellman’s method. Examples of developed thiolated chitosans and their degrees of modification are listed in Table 1.
3 Properties of Thiolated Chitosans
3.1 Mucoadhesive Properties
Thiolated chitosans represent an advantage in drug delivery since they are mucoadhesive and therefore able to prolong the residence time of drugs on various mucosa. This allows an increase in the extent of drug absorption. Moreover, thiolated chitosans can improve the specific localization of drug delivery systems [32]. Mucoadhesion is gained by the formation of both non-covalent bonds (such as hydrogen bonds) and ionic interaction or physical interpenetration effects between the mucus gel layers and polymers. In the case of thiolated chitosans, thiol groups on the polymer form disulfide bonds with cysteine-rich subdomains of glycoproteins in the mucus through thiol/disulfide exchange reactions, an oxidation process, and disulfide bond formation within the polymer itself [27]. The formation of disulfide bonds can be determined by both an increase in viscosity of the polymer and a decrease in free thiol groups [20]. The strong mucoadhesive properties of thiolated chitosans in comparison to unmodified chitosans were demonstrated by in vitro studies such as those performed via the rotating cylinder method and/or via tensile studies. The adhesive properties of polymers were expressed either in terms of adhesion time or in terms of total work of adhesion. Examples of the improved mucoadhesion of thiolated chitosans are given in Table 2.
Among these thiolated chitosans, chitosan-MNA showed the highest mucoadhesive features [33]. Even though thiolated chitosans have shown excellent mucoadhesive properties, the adhesion of the polymers is sloughed off by the natural mucus turnover due to a continuous process of mucus secretion. In the human intestine, for instance, the mucus turnover rate is typically about 12–24 h; this is very rapid and makes it difficult for the polymers to interact with the mucosa for a prolonged period of time [34].
3.2 In Situ Gelling Properties
Since the concept of in situ gelling polymers was introduced to the pharmaceutical literature, various formulations showing in situ gelling characteristics have been developed. In fact, a reversible phase shift within a few minutes under physiological conditions is favorable for improving the bioavailability of drugs. The sol–gel transition can be activated by several process changes including pH [35], temperature [36, 37], light [38, 39], and/or ion concentration [40].
The in situ gelling properties of thiolated chitosans are significantly better than those of unmodified chitosan. The improvement is based on the formation of inter- and/or intramolecular disulfide bonds within the polymeric matrix under physiological pH. These in situ gelling properties have been characterized in vitro by rheological measurements. For example, it was observed that after 6 h, the elastic modulus of chitosan-TGA was 168-fold increased compared to unmodified chitosan. The elastic modulus of unmodified chitosan remained unchanged over the whole observation period [41]. Nevertheless, the phase transition time of chitosan-TGA is extensively long. When oxidizing agents such as hydrogen peroxide (H2O2) were added to chitosan-TGA, gelation took place within a couple of minutes. The dynamic viscosity value at time zero was not showed in the research article 42 and therefore in Fig. 4. However, as this value is close to 0, a 16,000-fold improvement after 20 minutes was calculated by the author of article 42 [42]. Over an observation period of 20 min, the thiol moieties of the chitosan-TGA/H2O2 system were not completely oxidized; more than 60% of thiol groups remained stable. Moreover, evidence for an increase in the crosslinking of thiolated chitosans as a function of time was provided by frequency sweep measurements.
Comparison of the dynamic viscosity ( η*) of a chitosan-TGA/H2O2 system at pH 6.0 and 37°C. The molar ratios of SH groups:oxidizing agent were 1:0.28, 1:0.63, and 1:1.9 and are represented by white, black, and gray bars, respectively. The means ± SD of three experiments are shown (Adopted from [42])
In conclusion, the capacity of a polymer to achieve an in situ sol–gel transition at pH values above 5 makes the polymer suitable for application to vaginal, nasal, and ocular mucosa.
3.3 Permeation-Enhancing Effect
The use of permeation enhancers in drug delivery systems has gained great importance in overcoming the absorption barrier on mucosal membranes. Auxiliary agents such as low molecular mass sodium salicylates and medium chain glycerides have proven to improve the permeation of drugs across epithelial membranes. However, they can be rapidly absorbed from mucosal tissues, leading to systemic toxic side effects [43]. Thiolated chitosans have demonstrated great benefit over those types of enhancer. They can remain concentrated at the target site because of their mucoadhesive properties and increase the paracellular permeability of drugs due to the opening of tight junctions. This has been shown for various routes of delivery, such as for nasal [44] or intestinal drug delivery [45]. In addition, the systemic side effects of thiolated chitosans are comparatively low, owing to lack of toxicity even after intravenous application [46]. Unabsorbed portions of the polymer may hardly have some side effects.
The influence of thiolated chitosans on the permeation of hydrophilic compounds (such as peptide drugs) across freshly excised rat intestinal mucosa was evaluated in an Ussing-type chamber. For example, 0.5% (m/v) chitosan-TGA combined with the permeation mediator reduced glutathione (GSH) led to a 4.2-fold improvement of rhodamine-123 (Rho-123) permeation across rat intestinal mucosa [48]. In addition, the permeation-enhancing properties of thiolated chitosans was proven by studies on permeation of pituitary cyclase-activating polypeptide (PACAP). It has been reported that 1% (m/v) chitosan-TBA enhanced the permeation of PACAP across buccal tissue up to 38-fold compared to unmodified chitosan. Moreover, with 1% (m/v) chitosan-TBA combined with 2% (m/v) GSH the highest permeation was observed as drug uptake was enhanced approximately 80-fold (Fig. 5) [49]. The underlying mechanism of the permeation-enhancing effect of the system is based on the inhibition of the enzyme tyrosine phosphatase (PTP, 60–65 kDa). This protein is capable of dephosphorylating tyrosine residues of occludine, which is believed to be involved in the opening process of tight junctions. When dephosphorylation occurs, the tight junctions are closed, resulting in a decreased permeation of hydrophilic macromolecules. According to this mechanism, the inhibition of PTP by reduced glutathione avoids the phosphorylation process, with consequent opening of tight junctions.
In vitro permeation profile of PACAP across the buccal mucosa of pigs. Control without polymer (times); 1% (m/v) chitosan (filled triangle); 1% (m/v) chitosan-TBA (open diamond); 1% (m/v) chitosan-TBA and 2% (m/v) GSH (filled circle) (Adopted from [49])
Thiolated chitosans demonstrated the ability to shift the balance between oxidized glutathione and reduced glutathione [50].
Another example of a thiolated chitosan that has permeation-enhancing properties is chitosan-NAC, which showed an improvement in the uptake of tobramycin sulfate on Caco-2 cell monolayers of up to 2.7-fold in comparison to control tobramycin sulphate in buffer. A more pronounced permeation-enhancing effect could be achieved by the combination of 0.5% (m/v) chitosan-NAC and 0.5% GSH, giving an improvement ratio of 3.3 [51].
3.4 Efflux Pump Inhibition
In the human body, the multidrug resistance proteins (MDRs) and the multidrug resistance-associated proteins MRP1 and MRP2, members of the ATP-binding cassette (ABC) transporter superfamily, are membrane-associated proteins that carry out a detoxification role. In fact, they are responsible for molecule efflux, especially xenobiotics, out of the cells thus limiting drug accumulation within cells. These proteins are efflux pumps located in several tissues such as kidney, brain, liver, and intestine [52]. Although their physiological function guarantees protection of the body from harmful compounds, the efflux pump transporters also reduce the bioavailability of a variety of drugs.
The most important transporter in this group is probably P-glycoprotein (P-gp), which is primarily expressed at the apical side of epithelial cells of the intestine and in tumor cells. A broad range of compounds like β-adrenergic agonists, vinca alkaloids, digoxin, and antracyclines are known to be P-gp substrates [50]. Efflux pumps can be inhibited through several mechanisms; however, blocking the drug-binding site both via competition and allosteric regulation is the most important [53]. In the case of thiolated chitosans, the inhibition of efflux pumps is based on the interaction of the thiol moieties expressed on the polymeric backbone with the cysteine-rich subunit of the transmembrane region of P-gp [54].
The P-gp inhibition effect of chitosan-TBA was shown in an in vitro study using Rho-123 as representative substrate. It has been reported that chitosan-TBA improves the transport of substrates from the apical to basolateral site of freshly excised guinea pig ileal mucosa and that it decreases the basolateral to apical transport. Furthermore, chitosan-TGA/GSH, labeled with cationic fluorescence marker in order to avoid unintended ionic interaction between the polymer and the marker, showed higher permeation-enhancing properties than the unmodified chitosan control. The permeation of Rho-123 across freshly excised rat small intestinal mucosa in the presence of 0.5% (m/v) chitosan-TGA was 4.2-fold improved in comparison to unmodified chitosan [48].
The efficacy of chitosan-TGA as permeation enhancer was also proven in vivo. Oral administration to rats of enteric-coated tablets containing chitosan-TBA/GSH significantly increased the area under the plasma concentration versus time curve (AUC0–12) of Rho-123 compared to Pluronic P85 and Myrj 52, as shown in Fig. 6 [55].
Plasma concentration of Rho-123 after oral administration of 1.5 mg Rho-123 in Pluronic P85-tablets (filled square), Myrj 52-tablets (filled circle), and chitosan-TBA/GSH-tablets (times). Indicated values are means ± SD of five experiments. Values for Rho-123 at 4.5, 6, 7.5, 9, 10.5, and 12 h differ from corresponding values for Myrj 52-tablets with significances of p < 0.001, p < 0.001, p < 0.001, p < 0.007, p < 0.006, and p < 0.01, respectively (Adopted from [55])
3.5 Transfection-Enhancing Properties
In gene delivery, a site-specific carrier system has to release active compounds such as nucleic acids in a way that they are readily available for translation or transcription. Viral vectors have been widely used because of their high transfection efficacy. However, they cause oncogenic and inflammatory responses of the human body. To overcome the drawbacks of viral vectors, non-viral gene delivery systems such as cationic phospholipids and cationic polymers are currently adopted. Polyplexes, complexes of DNA and polymer, are one example of delivery systems that offer advantages such as high stability and high transfection efficiencies [56]. Regarding thiolated chitosans, it was demonstrated that the complexation of chitosan-TGA with pDNA leads to a transfection rate in Caco-2 cells that was fivefold higher than that gained with unmodified chitosan/pDNA [57]. Thiolated chitosan/pDNA complexes were prepared at pH 4.0 and 5.0 and were resistant to degradation during incubation with DNase I for 30 min. This result was consistent with a previous study with thiolated chitosan/DNA nanocomplexes [58]. The transfection efficiency of chitosan-TGA/DNA nanocomplexes was assessed in HEK293, MDCK, and Hep-2 cell lines, in which the nanocomplexes induced significantly higher green fluorescent protein gene expression in comparison to unmodified chitosan. In particular, when chitosan-TGA/pDNA nanoparticles were crosslinked by oxidation with H2O2, the expression of green fluorescence protein was improved in comparison to non-crosslinked nanoparticles [58].
3.6 Controlled Drug Release Properties
Controlled drug delivery technology represents one of the most rapidly advancing areas of science. Such delivery systems offer numerous advantages over conventional dosage forms, including improved efficacy, reduced toxicity, improved patient compliance, and convenience. Therefore, all controlled release systems aim to improve the effectiveness of drug therapy and can be achieved via temporal and/or distribution control of drug release.
Controlled release over an extended duration is highly beneficial for drugs that are rapidly metabolized and eliminated from the body after administration. Requirements for these delivery systems are the cohesion and the stability of the drug formulation. Chitosan is a polymer that can fulfill such requirements, especially in its thiolated form. In fact, model compounds like salmon calcitonin and pDNA have been formulated in controlled drug release systems using thiolated chitosans as a carrier matrix [59–61].
It was reported that in simulated gastric fluids the release profile of salmon calcitonin out of mucoadhesive tablets based on chitosan-TBA was about 87% after 3 h [59]. Over the whole observation period, a pseudo-zero-order release profile of salmon calcitonin in artificial gastric fluid was detected. Tablets exhibited good cohesiveness and released the active agent via a controlled diffusion process.
Additionally, controlled release profiles of pDNA out of a chitosan-TEA matrix system have been demonstrated. The study was performed under simulated physiological conditions and the results showed that within 10 h pDNA was totally released from chitosan/pDNA particles, whereas only 12% of it was liberated from the chitosan-TEA/pDNA particles [62].
3.7 Safety and Stability
To investigate the safety of thiolated chitosans, several studies have been performed measuring its potential cytotoxicity on various cell lines. The toxicity profiles of chitosan-TBA, for example, were assessed using the red blood cell lysis test. The results showed that thiolated chitosan had a lower membrane damaging effect than the corresponding unmodified control. The safety of thiolated chitosan was further confirmed by metabolic activity (cleavage of dimethyl-thiazolyl-diphenyltetrazolium bromide) (MTT) and DNA synthesis (incorporation of 5-bromo-2′-deoxyuridine [BrdU]) assay on L-929 mouse fibroblast cells. It was found that the toxicity of chitosan-TBA was concentration-dependent [63]. Another thiolated chitosan investigated for cytotoxicity was chitosan-MBA, which was found to be nontoxic because Caco-2 cell viability was around 100% after 4 and 24 h of incubation [25].
The stability of thiolated chitosans was tested under different storage conditions. Chitosan-TGA was chosen as a model polymer and stored in the form of freeze-dried powders and matrix tablets for 6 months. The free thiol groups of chitosan-TGA were found to be stable against oxidation processes when stored at −20°C and at 4°C. In the case of storage at 20°C and 70% relative humidity, a decrease of 25% of the initial amount of free thiol groups was found after 6 months [64].
4 Formulations
4.1 Microparticles and Nanoparticles
For non-invasive drug administration, particulate delivery systems offer the advantage of providing a prolonged residence time on mucosal membranes [65] and the possibility to reach greater mucosal surface areas, leading to a comparatively higher drug uptake. Delivery systems such as liposomes, and micro- and nanoparticles have attracted great attention in the last few decades. For example, nanoparticulate delivery systems based on polymers such as polyacrylates, poly(lactic-co-glycolic acid), or chitosans have been developed and widely investigated. Among these polymers, chitosan has shown numerous advantages and, due to the presence of thiol groups on their polymeric backbone, micro- and nanoparticles guarantee strong mucoadhesive properties and a high permeation-enhancing effect [66]. Micro- and nanoparticulate thiolated chitosans can be produced via different techniques such as ionic gelation, emulsification/solvent evaporation, radical emulsion polymerization, and air jet milling.
The mucoadhesive features of chitosan- and chitosan-TBA-coated poly(isobutylcyanoacrylate) (PIBCA) nanoparticles were evaluated under physiological conditions. The results obtained by ex vivo studies showed that the presence of chitosan-TBA on the particle surface significantly enhanced the mucoadhesive properties of carrier systems [67]. In particular, the higher the amount of thiol groups immobilized on the surface of particles, the stronger the mucoadhesive properties and the stability of the particles [68]. The underlying mechanism is based on disulfide bond formation between mucus glycoproteins and the polymer, as already explained [27]. In addition, the permeation-enhancing effect of chitosan- and thiolated-chitosan-coated PIBCA as a potential carrier for mucosal administration was evaluated using the Ussing chamber technique [69]. Results showed that the paracellular permeability of intestinal epithelium was more pronounced in the presence of thiolated-chitosan-coated PIBCA than in the presence of chitosan-coated core–shell nanoparticles. The thiolated-chitosan-coated nanoparticulate core–shell structure offers advantageous effects because of its biocompatibility and harmless features.
Furthermore, chitosan-GSH-coated poly(hydroxyethyl methacrylate) nanoparticles prepared by radical polymerization showed an improvement in mucoadhesion and in the apparent permeability coefficient (P app) compared to unmodified chitosan [70]. Finally, thiolated chitosan nanoparticles significantly enhanced the anti-inflammatory effect of theophylline, as evidenced by the decrease in parameters such as eosinophilis in bronchoalveolar lavege fluid and bronchial damage [71].
4.2 Matrix Tablets
Mucoadhesive matrix tablets are useful for intraoral, peroral, ocular, and vaginal local or systemic delivery. In oral administration, for example, approximately 40% of products on the market are tablets due to the convenience of such a dosage form [43]. Thiolated chitosan can be easily compressed to matrix tablets by simply incorporating the active pharmaceutical ingredient in the polymer. In addition, the in situ crosslinking properties of thiolated chitosans guarantee the cohesiveness and, subsequently, the stability of the swollen carrier matrix. Disintegration studies, for instance, performed with tablets comprising unmodified chitosan revealed a stability of less than 6 h, whereas tablets based on chitosan-MBA were stable for over 12 h [25].
In the case of using thiolated chitosans as drug carrier matrixes, the release rate of the active ingredient is mainly controlled by hydration and diffusion processes [9]. An enteric coating is beneficial for matrix tablets to protect them from the harsh environment in the gastrointestinal tract (e.g., the low pH) and from degradation mediated by enzymes, and to guarantee the swelling behavior of tablets on the intestinal mucosa.
5 In Vivo Studies: Proof of Concept
Several in vivo studies have demonstrated the potential of thiolated chitosans to improve the bioavailability of therapeutic compounds. In particular, thiolated chitosans have been used to develop carrier matrices for oral, nasal, and buccal delivery systems.
5.1 Oral Drug Delivery
Since the 1970s, a considerable number of publications in the field of peptide and protein delivery have been established [72]. Peptides and proteins are generally administered via parenteral routes, which involve the pain, fear, and risks associated with these types of administration. A conversion from “injectable formulation” to “oral formulations” is therefore in high demand [43]. The efficacy of thiolated chitosans in term of peptide delivery systems via oral administration has been demonstrated. In fact, after oral administration of chitosan-TBA matrix tablets containing salmon calcitonin to rats, the calcium plasma level as a primary indicator was measured and it was found that unmodified chitosan tablets showed no significant effect on plasma levels. By contrast, the calcium plasma level obtained with chitosan-TBA tablets containing calcitonin decreased by over 5% [59]. In addition, oral insulin delivery systems based on chitosan-TBA tablets have been developed. Blood glucose levels of non-diabetic rats were determined after oral administration of chitosan-TBA/insulin tablets. After 24 h, the relative pharmacological efficacy for chitosan-TBA/insulin tablets was 1.7 + 0.4% (Table 3) [72].
Recently, it has been shown that the AUC of human insulin plasma levels after oral administration of chitosan-MNA nanoparticles was increased fourfold compared to that after administration of unmodified chitosan nanoparticles (Fig. 7) [73].
Serum concentrations of human insulin (HI) after oral administration of formulations containing unmodified chitosan (open square) and chitosan-MNA (closed square). Indicated values are means ± SD of at least five rats (Adopted from [73])
Oral administration of the peptide drug antide in a liquid dosage form showed no detectable concentration of the drug in plasma at all. When a thiolated chitosan was introduced into the formulation, however, an improved uptake of the drug was found. The absolute and relative bioavailability of these formulations were calculated to be 1.1% and 3.2% increased, respectively [74].
A further benefit of oral drug uptake based on thiolated chitosan has been documented. The efflux pump inhibitory capacity of chitosan-TBA combined with GSH was proven by the significant improvements in Rho-123 plasma levels in rats. The improvement was higher than that obtained with tablets based on poloxamer or Myrj 52, which are well-known P-gp inhibitors [75]. Indeed, the applicability of thiolated chitosans for oral administration of other high molecular mass compounds would appear to be an interesting subject for ongoing studies.
5.2 Nasal Drug Delivery
Dealing with nasal applications means that obstacles such as low membrane permeability, short local residence time, and high turnover rate of secretion in nasal cavities have to be overcome. These limitations lead the bioavailability of nasally administered drugs to be less than than 80% [76]. The most common approach to improve nasal drug absorption is to use permeation enhancers. The potential of thiomers in nasal drug delivery has already been demonstrated [77].
Thiolated chitosans, in particular, seem to be suitable for overcoming the above-mentioned drawbacks owing to their permeation-enhancing effect. To prove the efficacy of thiolated chitosans for nasal drug targeting, insulin was chosen as model drug. Insulin-loaded chitosan-TBA microparticles were administered to the nostrils of male Wister rats, using an intravenous injection of insulin as a positive control. It was found that the absolute bioavailability of insulin loaded in chitosan-TBA microparticles was 7.24 ± 0.76%, which was more than 3.5-fold higher than the absolute bioavailability obtained from the unmodified chitosan/insulin microparticles [78].
Moreover, intranasally delivered theophylline incorporated into thiolated chitosan nanoparticles as carrier matrix was developed and investigated for its capacity to relieve allergic asthma. In a mouse model of allergic asthma, the combination of theophylline with thiolated chitosan nanoparticles accelerated the efficacy of the drug in comparison to theophylline alone [70].
5.3 Buccal Drug Delivery
Bypassing degradation of drugs in the GI tract and the hepatic first-pass metabolism, the buccal route is an alternative choice for distributing drugs to the application site. Although highly accepted by patients as route of application, buccal administration has the drawback of poor drug permeation across the buccal mucosa [79]. With thiolated chitosans, however, a successful nasal drug delivery has been achieved. In fact, the capacity of chitosan-TBA combined with GSH to liberate a peptide via buccal administration has been demonstrated [80]. Pituitary adenylate-cyclase-activating polypeptide (PACAP), a peptide drug for treatment of type 2 diabetes, was chosen as model peptide. The absolute bioavailability of PACAP was evaluated after buccal administration and results revealed that the combination of chitosan-TBA/GSH led to an absolute bioavailability of 1%, whereas no systemic drug uptake was achieved at all with unmodified chitosan formulations. In the case of the thiolated chitosan system, the plasma level of glucose was maintained in the therapeutic range over the whole 6 h experimental period.
6 Conclusions
Despite the beneficial properties of chitosan in drug delivery, various thiolated chitosans have been synthesized and found to exhibit improved features in comparison to unmodified chitosan. This improvement is due to the covalent attachment of thiol-bearing compounds on the polymeric backbone.
In detail, thiolated chitosans, which constitute a group of the so-called thiomers, have shown strong mucoadhesive, in situ gelling, and controlled drug release properties. In addition, these biopolymers proved efficacy as efflux pump inhibitors and permeation enhancers. The efficacy of this new generation of chitosan derivatives has already been demonstrated by various in vivo studies and their combination with innovative technologies such as micro- and nanotechnology is a promising choice in drug delivery.
References
Hejazi R, Amiji M (2003) J Control Release 89:151
Chandy T, Sharma C (1990) Biomater Artif Cells Artif Org 18:1
Felt O, Buri P, Gurny R (1998) Drug Dev Ind Pharm 24:979
Ito M, Ban A, Ishihara M (2000) Jpn J Pharmacol 82:218
Anandan R, Nair PG, Mathew S (2004) J Pharm Pharmacol 56:265
Roller S, Covill N (1999) Int J Food Microbiol 47:67
Rhoades J, Roller S (2000) Appl Environ Microbiol 66:80
Agnihotri S, Mallikarjuna N, Aminabhavi T (2004) J Control Release 100:5
Werle M, Takeuchi H, Bernkop-Schnürch A (2008) J Pharm Sci 98:1643
Bernkop-Schnürch A (2000) Int J Pharm 194:1
Borchard G (2001) Adv Drug Deliv Rev 52:145
Leong KW, Mao HQ, Truong-Le VL, Roy K, Walsh SM, August JT (1998) J Control Release 53:183
Kawashima Y, Lin SY, Kasai A, Handa T, Takenaka H (1985) Chem Pharm Bull 33:2107
Dodane V, Amin Khan M, Merwin JR (1999) Int J Pharm 182:21
Bernkop-Schnürch A, Krauland A, Valenta C (1998) J Drug Target 6:207
Thanou M, Florea B, Langemeyer M, Verhoef J, Junginger H (2000) Pharm Res 17:27
Thanou M, Nihot M, Jansen M, Verhoef J, Junginger H (2001) J Pharm Sci 90:38
Baumann H, Faust V (2001) Carbohydr Res 331:43
Bernkop-Schnürch A, Brandt U, Clausen A (1999) Sci Pharm 67:197
Bernkop-Schnürch A, Hornof M, Zoidl T (2003) Int J Pharm 260:229
Schmitz T, Hombach J, Bernkop-Schnürch A (2008) Drug Deliv 15:245
Kafedjiiski K, Krauland AH, Hoffer MH, Bernkop-Schnürch A (2005) Biomaterials 26:819
Kafedjiiski K, Föger F, Werle M, Bernkop-Schnürch A (2005) Pharm Res 22:1480
Millotti G, Samberger C, Fröhlich E, Bernkop-Schnürch A (2009) Biomacromolecules 10:3023
Millotti G, Samberger C, Fröhlich E, Sakloetsakun D, Bernkop-Schnürch A (2010) J Mater Chem 20:2432
Kafedjiiski K, Hoffer MH, Werle M, Bernkop-Schnürch A (2006) Biomaterials 27:127
Leitner VM, Walker GF, Bernkop-Schnürch A (2003) Eur J Pharm Biopharm 56:207
Wilson JM, Bayer RJ, Hupe D (1977) J Am Chem Soc 99:7922
Bernkop-Schnürch A, Schwarz V, Steininger S (1999) Pharm Res 16:876
Gough JD, Gargano JM, Donofrio AE, Lees WJ (2003) Biochemistry 42:11787
Gough JD, Lees WJ (2005) Bioorg Med Chem Lett 15:777
Dodou D, Breedveld P, Wieringa P (2005) Eur J Pharm Biopharm 60:1–16
Millotti G, Hoyer H, Engbersen JFJ, Bernkop-Schnürch A (2010) J Drug Deliv Sci Tech 20:181
Madara J, Stafford J, Dharmsathaphorn K, Carlson S (1987) Gastroenterology 92:1133
Gupta H, Jain S, Mathur R, Mishra P, Mishra AK, Velpandian T (2007) Drug Deliv 14:507
Hou Q, Chau DY, Pratoomsoot C, Tighe PJ, Dua HS, Shakesheff KM, Rose FR (2008) J Pharm Sci 97:3972
Edsman K, Carlfors J, Petersson R (1998) Eur J Pharm Sci 6:105
Ishihara M, Obara K, Nakamura S, Fujita M, Masuoka K, Kanatani Y, Takase B, Hattori H, Morimoto Y, Maehara T, Kikuchi M (2006) J Artif Organs 9:8
Ta HT, Dass CR, Dunstan DE (2008) J Control Release 126:205
Paulsson M, Hagerstrom H, Edsman K (1999) Eur J Pharm Sci 9:99
Hornof MD, Kast CE, Bernkop-Schnürch A (2003) Eur J Pharm Biopharm 55:185
Sakloetsakun D, Hombach J, Bernkop-Schnürch A (2009) Biomaterials 30:6151
Bernkop-Schnürch A, Krauland A, Leitner V, Palmberger T (2004) Eur J Pharm Biopharm 58:253
Illum L, Farraj NF, Davis SS (1994) Pharm Res 11:1186
Artursson P, Lindmark T, Davis SS, Illum L (1994) Pharm Res 11:1358
Rao SB, Sharma CP (1997) J Biomed Mater Res 34:21
Muranishi S (1990) Crit Rev Ther Drug Carrier Syst 7:1
Greindl M (2008) University of Innsbruck
Langoth N, Bernkop-Schnürch A, Kurka P (2005) Pharm Res 22:2045
Werle M (2008) Pharm Res 25:500
Hombach J, Hoyer H, Bernkop-Schnürch A (2008) Eur J Pharm Sci 33:1
Cordon-Cardo C, O’Brien J, Boccia J, Casals D, Bertino J, Melamed M (1990) J Histochem Cytochem 38:1277
Varma M, Ashokrai J, Dey C, Panchagnula R (2003) Pharmacol Res 48:347
Ferté J (2000) Eur J Biochem 267:277
Föger F, Hoyer H, Kafedjiiski K, Bernkop-Schnürch A (2006) Biomaterials 27:5855
De Smedt S, Demeester J, Hennink W (2000) Pharm Res 17:113
Martien R, Loretz B, Thaler M, Majzoob S, Bernkop-Schnürch A (2007) J Biomed Mater Res A 82:1
Lee D, Zhang W, Shirley S, Kong X, Hellermann G, Lockey R, Mohapatra S (2007) Pharm Res 24:157
Guggi D, Krauland A, Bernkop-Schnürch A (2003) J Control Release 92:125
Bernkop-Schnürch A, Kast C, Guggi D (2003) J Control Release 93:95
Chesnut CH, Azria M, Silverman S, Engelhardt M, Olson M, Mindeholm L (2008) Osteoporos Int 19:479
Schmitz T, Bravo-Osuma I, Vauthier C, Ponchel G, Loretz B, Bernkop-Schnürch A (2007) Biomaterials 28:524
Guggi D, Langoth N, Hoffer M, Wirth M, Bernkop-Schnürch A (2004) Int J Pharm 278:353
Bernkop-Schnürch A, Hornof M, Kast C (2002) Sci Pharm 70:331
Coupe A, Davis S, Wilding I (1991) Pharm Res 8:360
Albrecht K, Bernkop-Schnürch A (2007) Nanomedicine 2:41
Bravo-Osuna I, Vauthier C, Farabollini A, Palmieri G, Ponchel G (2007) Biomaterials 28:2233
Bernkop-Schnürch A, Weithaler A, Albrecht K, Greimel A (2006) Int J Pharm 317:76
Bravo-Osuna I, Vauthier C, Chacun H, Ponchel G (2008) Eur J Pharm Biopharm 69:436
Moghaddam F, Atyabi F, Dinarvand R (2009) Nanomedicine 5:208
Lee D, Shirley S, Lockey R, Mohapatra S (2006) Respir Res 7:1
Krauland A, Guggi D, Bernkop-Schnürch A (2004) J Control Release 95:547
Millotti G, Perera G, Vigl C, Pickl K, Sinner F, Bernkop-Schnürch A (2011) Drug Deliv 18:190
Bernkop-Schnürch A, Pinter Y, Guggi D, Kahlbacher H, Schöffmann G, Schuh M, Schmerold I, Del Curto MD, D’Antonio M, Esposito P, Huck C (2005) J Control Release 106:26
Föger F, Schmitz T, Bernkop-Schnürch A (2006) Biomaterials 27:4250
Illum L (2003) J Control Release 87:187
Leitner V, Guggi D, Bernkop-Schnürch A (2004) J Pharm Sci 93:1682
Krauland A, Guggi D, Bernkop-Schnürch A (2006) Int J Pharm 307:270
Veuillez F, Kalia Y, Jacques Y, Deshusses J, Buri P (2001) Eur J Pharm Biopharm 51:93
Langoth N, Kahlbacher H, Schöffmann G, Schmerold I, Schuh M, Franz S, Kurka P, Bernkop-Schnürch A (2006) Pharm Res 23:573
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Sarti, F., Bernkop-Schnürch, A. (2011). Chitosan and Thiolated Chitosan. In: Jayakumar, R., Prabaharan, M., Muzzarelli, R. (eds) Chitosan for Biomaterials I. Advances in Polymer Science, vol 243. Springer, Berlin, Heidelberg. https://doi.org/10.1007/12_2011_109
Download citation
DOI: https://doi.org/10.1007/12_2011_109
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-23113-1
Online ISBN: 978-3-642-23114-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)