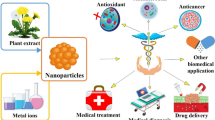
Abstract
The integration of nanotechnology in medicine has had a tremendous impact in the past few decades. The discovery of synthesis of nanomaterials (NMs) and their functions as versatile tools promoted various applications in nano-biotechnology and nanomedicine. Although the physical and chemical methods are still considered as commonly used methods, they introduce several drawbacks such as the use of toxic chemicals (solvent, reducing, and capping agents) and poor control of size, size distribution, and morphology, respectively. Additionally, the NMs synthesized in organic solvents and hydrophobic surfactants rapidly aggregate in aqueous solutions or under physiologic conditions, limiting their applications in medicine. Many of the phase-transfer strategies were developed and applied for the transfer of NMs into aqueous solutions. Although great efforts have been put into phase transfers, they mostly include expensive, time-consuming, intensive labor work, multi steps, and complicated procedures.
Use of plant extracts in the biological synthesis method offers stark advantages over other biomolecules (protein, enzyme, peptide, and DNA). Plant extracts have been commonly used for food, medicine, NM synthesis, and biosensing. There are many viable techniques developed for the production of plant extracts with various contents based on their simplicity, cost, and the type of extract content. In this chapter, we conduct a comparative study for extract preparation techniques, the use of extracts for metallic single and hybrid nanoparticle (NP) synthesis, and their antimicrobial properties against pathogenic and plant-based bacteria.

Graphical Abstract
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In recent years, nanotechnology has been increasingly utilized for the synthesis, engineering, and designing of various nanomaterials (NMs) used as antioxidants, antimicrobials, anticancer agents, therapeutics, and diagnostics agents, and in the fabrication of nanosensors. The NMs have been intensively applied in many different scientific and industrial fields. However, production of biogenic NMs in nanotechnology and their uses in medicine have become the fastest developing and most attractive research. The use of plant extracts is prominent, not only because of their easy production and cheap cost compared to other biomolecules (protein, enzyme, peptide, and DNA), but also because they provide large-scale and environmentally benign NMs, which can widen their medical applications.
2 A Comparative Study of Extraction Methods for Medicinal Plants
Medicinal plants have been practically used as effective and traditional drugs or biocides for various disorders for a long time. The research has focused on the elucidation of the chemical structures of these plant extracts. The physicochemical properties of plants are investigated at the following steps: (1) authentication, (2) extraction, (3) separation, (4) isolation, (5) characterization of isolated compounds, and (6) quantitative evaluation. The methods vary in simplicity, cost, efficiency, and degree of extracted or isolated molecule damage. It is worth mentioning that each extract needs its own characteristic extraction method for production with greater efficiency. For instance, while essential oils as volatile compounds in aromatic plants are extracted using distillation methods, solvent extraction methods are viable and suitable for obtaining other volatile compound-rich extracts.
In addition to conventional extraction methods, some modern extraction methods including microwave-assisted extraction (MAE), ultrasonication-assisted extraction (UAE), supercritical fluid extraction (SFE), and solid-phase micro-extraction (SPME) have been developed and are actively used. They display certain advantages over conventional methods. Although classical methods are fairly simple, standard, and have widespread use, they consume large quantities of organic solvents, cause degradation of heat-labile constituents, produce extracts with a low yield, and have time-consuming and labor-intensive procedures. The use of these classical extraction methods allows the benefits of production efficiency, selectivity, and the elimination of additional steps of modern extraction methods before chromatographic analysis allows them to be used intensively and preferentially. The extraction procedures can also be redesigned to obtain the desired molecules by manipulating experimental parameters. Additionally, the extraction method selection to isolate targeted components with the highest yield and highest purity is dependent upon the plant source [1, 2]. Thus, the development of modern extraction methods plays an important role in the overall effort of ensuring and providing high-quality herbal products [3].
3 Preparation of Extract for Nanoparticle (NP) Synthesis
The extraction of plant contents has received considerable attention owing to the use of plant contents in medicine, nanoparticle (NP) synthesis, and biosensing. Three major extraction methods for NP synthesis are: (1) solvent extraction, (2) microwave-assisted extraction, and (3) maceration extraction. The ideal extraction method should be cost-effective, simple, less time-consuming, and simply conducted in any laboratory.
3.1 Solvent-based Extraction Methods
The solid-liquid extraction provides soluble components in the solid material to be integrated with the solvent. The mass transfer ratio decreases as the concentration of the active principle in the solvent increases. This process results in the solvent and solid material reaching an equilibrium concentration when a mass transfer of the active components from plant material to solvent occurs. There are different types of this technique: cold percolation, hot percolation, and concentration [4, 5].
3.1.1 Cold Percolation
The extraction of the plant contents is carried out in a percolator that is connected to a condenser and a receiver for removing the solvent from the mixture. The powdered material is in contact with the percolator along with a suitable solvent until equilibrium is reached.
3.1.2 Hot Percolation
The principle of hot percolation is based on increasing the temperature of the solvent, which increases the solubility. The extract is permanently passed into a tubular heat exchanger by steam heating.
3.2 Microwave-assisted Extraction Technique
Microwave-assisted extraction (MAE) allows the materials to reach the given energy that is associated with the dielectric susceptibility of both the solvent and the solid plant material, through rapid heating [6, 7].
3.2.1 Maceration
The maceration process can be completed in three steps: (1) grinding of plant materials into small particles, (2) choosing the appropriate solvent, which is added in a closed vessel, and (3) filtration for the separation of the liquid phase from the plant pulp. The mixture of the plant powder and solvent should be shaken to provide proper extraction with a high efficiency [8, 9].
4 Isolation of Specific Molecules from Plant Extracts Using Appropriate NPs
It is well known that titanium dioxide (TiO2), with a certain crystal form, potentially reacts with phosphorylated biomolecules including peptides, proteins, and glycoproteins, under the proper acidic experimental conditions. The phosphate moieties of those molecules specifically attach to the surface of TiO2 NPs and retain their surfaces, which provides the isolation or enrichment of the corresponding molecules.
Recently, TiO2 NPs have been integrated into plant nanobiology for isolation of specific molecules from plants. In a recent studies, TiO2 NPs acted as nano-harvesting agents to isolate bioactive compounds from living cells. For instance, Kurepa et al. [10] used phosphorylated anatase TiO2 NPs with a 20-nm diameter to capture the specific flavonoids from Arabidopsis plants. This work showed that quercetin and kaempferol as enediol and catechol groups containing flavonoids can successfully bind to the phosphorylated TiO2 NPs, and they were isolated from the plant matrix.
5 Synthesis of NPs Using Plant Extracts
The production of colloidal metallic NPs has become one of the fastest developing and most exciting fields of research and has had an enormous impact on the evolution of nanotechnology over the past decades. The size, shape, and composition-dependent electronic, optical, luminescent, and magnetic features of the NPs with great enhancement have found a wide spectrum of applications in scientific and technical fields [11,12,13,14,15,16]. In general, three major methods, chemical, physical, and biological, have been actively and extensively used for the synthesis of NPs. Although chemical methods are used the most for the synthesis of high-quality NPs with a narrow size distribution, the use of toxic organic solvents as well as reducing and stabilizing agents greatly limits the applications for NPs, especially in biomedicine and bioanalytics [16,17,18,19]. Additionally, in order to use NPs synthesized in organic solvents in biologically-related applications, phase transfer is an indispensable step for introducing the NPs into aqueous solutions. The NPs can be made water-soluble with two common surface-engineering procedures: (1) ligand exchange and (2) ligand polymerization [20,21,22,23,24,25].
Physical methods include simple one-step procedures and provide large-scale production in a short time. However, those methods almost always result in a lack of size, shape, and size distribution of the NPs [26, 27]. To address the drawbacks encountered in chemical and physical methods, researchers have recently focused on biological methods called “green methods” for NP synthesis [28, 29].
The main principle of green methods is to use nontoxic biomolecules including DNA, proteins, enzymes, carbohydrates, and plant extracts for the synthesis of biocompatible metallic NPs through the reduction of metal ions in aqueous solution [30,31,32,33,34,35,36,37,38]. Although DNA, proteins, and enzymes have been employed as scaffolds for nucleation and growth of metallic NPs with unique crystalline structures [39,40,41,42,43], those biomolecules are quite costly, easily decomposable, and can be contaminated. In contrast to those molecules, plant extracts are easily reachable, quite affordable, and very stable against environmental conditions (temperature, pH, and salt concentration).
Plant extracts are a rich source of polyphenols, flavonoids, sugars, enzymes, and/or proteins, and can be utilized as reducing and stabling agents for the biosynthesis of metallic NPs. In the potential proposed mechanism, hydroxyl, amine, and thiols groups existing on particular extracts of plants may bind to metal ions, canalize electron flow from the extracts to metal ions, and lead to the completion of the eventual NP synthesis [44,45,46,47,48,49]. Numerous numbers of plant extract-directed metallic NPs have been synthesized and used in various fields [47,48,49]. Using plant extracts for the rapid reduction and formation of metallic NPs was discovered by Sastry and co-workers. They used lemongrass plant extract to synthesize the spherical gold NPs and triangular gold nanoprisms [50]. In addition to the aforementioned unique properties of plant extracts, plant-based biogenic synthesis can provide cost-effective, environmentally-friendly, simple, less labor intensive, and large-scale production procedures.
5.1 Synthesis of Silver and Gold NPs
Silver (Ag) and gold (Au) are two commonly synthesized plasmonic NPs, due to their unique intrinsic properties. Ag NPs have been considered as effective and universal germicidal agents against various microbes. Their use has also been recognized in nanomedical and industrial applications [51,52,53,54]. As stated above, the plant extract-based synthesis method for Ag NPs provides simple, one-step, and rapid procedures compared to other synthesis methods. The extracts produced from different parts of the plant such as leaves, roots, seeds, and fruit act as potential reducing and stabilizing agents to form Ag NPs of various sizes and shapes. For instance, square, spherical, triangular, and hexagonal-shaped Ag NPs with diameters ranging from 10 to 90 nm were synthesized using leaf extracts obtained from plants [55,56,57]. In our view, different plants contain different contents in the extract, which may lead to the formation of Ag NPs of various sizes and shapes. The synthesis of Ag NPs of various morphologies was systematically reported using extracts of roots, seeds, and fruit [58,59,60].
Similar strategies have been used for the synthesis of Au NPs. Au NPs have received considerable attention due to their attractive optical and non-toxic properties, which market them for use in a wide variety of scientific areas including nanomedicine, nanoelectronics, nanobiosensing, and catalysis [61,62,63,64,65,66]. Similar to Ag NPs, the extracts obtained from different parts of plants reacted with Au ions to reduce them and to eventually form Au NPs. For instance, while leaf extract from the Menta piperita plant resulted in the formation of spherical Au NPs with a diameter of 150 nm, triangular-, hexagonal-, and pentagonal-shaped Au NPs with a size ranging from 5 to 500 nm were synthesized from the extracts of Coriandum sativum, Memecylon edule, and Magnolia kobus plants [55, 67, 68].
6 Plant Disease Treatment
Various techniques have been developed and applied to control microorganism-caused diseases in plants. For instance, the antibiotic streptomycin, as part of a chemical technique, was used in the 1950s to prevent the proliferation of Xanthomonas vesicatoria found on plants. However, those bacterial strains developed resistance against streptomycin and thus made it ineffective [69]. Copper-based (Cu) bactericides incorporated ethylene-bis-dithiocarbamate (EBDC) fungicides (e.g., maneb or mancozeb). These fungicides (e.g., maneb or mancozeb) have acted as potential biocides in order to effectively manage the diseases existing on plants. Nevertheless, Cu-resistant bacterial strains have been observed due to their frequent use and the resulting drastic reduction in antimicrobial activity of those biocides [70,71,72].
Research has focused on investigation and development of bacteriophages and systemic-acquired resistance (SAR) inducers as alternative disease-management techniques over the last decade [73, 74]. As an example, acibenzolar-S-methyl (ASM) was used as an SAR inducer agent, to activate and enhance plant defense systems by increasing the transcription of stress-related genes against bacterial tomato spot [73]. Although bacteriophages have been introduced as biological alternatives to Cu-based bactericides, real-time use in the field reduced their viability and then their use was highly limited due to environmental conditions [75, 76]. It is worth mentioning that only very few chemical techniques are available and there is an urgent need to develop effective, biocompatible, and economical materials for disease management.
No reports have fully explained the mechanism underlying the antimicrobial activity of NPs, and the mechanism is still under debate. Recently, various types of single-component metallic NPs and metal-graphene oxide (GO) NPs have been synthesized and used as novel and effective antimicrobial agents for the management of agricultural crop diseases. The key point in the use of NPs is their toxicity, which can adversely influence environmental and human health [77,78,79,80]). For instance, Paret et al. studied antibacterial properties of light-activated titanium dioxide (TiO2) and metal-doped hybrid TiO2 NPs (TiO2/Ag, TiO2/Zn) against Xanthomonas perforans, which causes bacterial tomato spot disease. This study demonstrated that TiO2 did not show any antimicrobial function under non-illuminated conditions and only TiO2/Ag exhibited some antimicrobial activity due to the intrinsic antimicrobial property of Ag. In contrast, all TiO2-based NPs effectively inhibited bacterial growth when exposed to an incandescent light intensity of 3 × 104 lux. The combination of the photocatalytic activity of TiO2 and the natural germicidal activity of Ag introduced the best antimicrobial activity under illuminated conditions [80].
6.1 Antimicrobial Properties of Silver Nanoparticles
Silver NPs have been considered to be the strongest and most universal biocides compared to other metallic NPs. The one logical proposed mechanism offered is that Ag NP may interact with some functional groups (thiol, carboxyl, hydroxyl, amino, and phosphate groups) existing on bacterial membranes, with membrane degradation then leading to serious structural deformation. In addition to that, some Ag NPs can be internalized through the membranes and may inactivate or distort the working function of enzymes, which may lead to cell death [81, 82]. However, when Ag NPs are aggregated, their antimicrobial activities are weakened and can be lost. Most recent works show that Ag-GO nanocomposites overcome the limitations of bare Ag NPs. Ag-GO nanocomposites display extraordinary antibacterial activity that results in rapid killing [83, 84].
7 Conclusion
The type of extraction method used varies according to the type of content in the extracts. The use of plant extracts has advantages over other biomolecules (proteins, enzymes, peptides, and DNA) in terms of the biosynthesis of metallic NPs, because they are inexpensive, easily producible, and accessible. They provide environmentally friendly NPs with the ability for large-scale production. For these reasons, plant extract-directed NPs can potentially be used in various bioanalytical and biomedical applications as antioxidants, antimicrobial agents, anticancer agents, therapeutics, diagnostic tools, and drug-vehicle agents.
References
Jain D, Daima HK, Kachhwaha S, Kothari SL (2009) Synthesis of plant-mediated silver nanoparticles using papaya fruit extract and evaluation of their anti microbial activities. Dig J Nanomater Biostruct 4:557–563
Gupta A, Naraniwal M, Kothari V (2012) Modern extraction methods for preparation of bioactive plant extracts. IJANS 1:8–26
Huie CW (2002) A review of modern sample-preparation techniques for the extraction and analysis of medicinal plants. Anal Bioanal Chem 373:23–30
Chen S, Sun Y, Chao J, Cheng L, Chen Y, Liu J (2011) Dispersive liquid–liquid microextraction of silver nanoparticles in water using ionic liquid 1-octyl-3 methylimidazolium hexafluorophosphate. J Environ Sci 41:211–217
Nerome H, Machmudah S, Fukuzato R, Higashiura T, Kanda H, Goto M (2016) Effect of solvent on nanoparticle production of β-carotene by a supercritical anti-solvent process. Chem Eng Technol 39:1771–1777
Surendra TV, Roopan SM, Arasu MV, Al-Dhabi NA, Rayalu GM (2016) RSM optimized Moringa oleifera peel extract for green synthesis of M. oleifera capped palladium nanoparticles with antibacterial and hemolytic property. J Photochem Photobiol B 162:550–557
Sharma D, Sabela MI, Kanchi S, Mdluli PS, Singh G, Stenström TA, Bisetty K (2016) Biosynthesis of ZnO nanoparticles using Jacaranda mimosifolia flowers extract: synergistic antibacterial activity and molecular simulated facet specific adsorption studies. J Photochem Photobiol B 162:199–207
Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M (2006) Synthesis of gold nanotriangles and silver nanoparticles using Aloevera plant extract. Biotechnol Prog 22:577–583
Azmir J, Zaidul ISM, Rahman MM, Sharif KM, Mohamed A, Sahena F, Omar AKM (2013) Techniques for extraction of bioactive compounds from plant materials: a review. J Food Eng 117:426–436
Kurepa J, Nakabayashi R, Paunesku T, Suzuki M, Saito K, Woloschak GE, Smalle JA (2014) Direct isolation of flavonoids from plants using ultra-small anatase TiO2 nanoparticles. Plant J 77:443–453
Ma X, Zhao Y, Liang X-J (2011) Theranostic nanoparticles engineered for clinic and pharmaceutics. Acc Chem Res 44:1114–1122
Wang H, Yang R, Yang L, Tan W (2009) Nucleic acid conjugated nanomaterials for enhanced molecular recognition. ACS Nano 3:2451–2460
Hu R, Zhang X-B, Kong R-M, Zhao X-H, Jiang J, Tan W (2011) Nucleic acid-functionalized nanomaterials for bioimaging applications. J Mater Chem 21:16323–16334
Shrivas K, Wu H-F (2010) Multifunctional nanoparticles composite for MALDI-MS: Cd2s-doped carbon nanotubes with CdS nanoparticles as the matrix, preconcentrating and accelerating probes of microwave enzymatic digestion of peptides and proteins for direct MALDI-MS analysis. J Mass Spectrom 45:1452–1460
Murray C-B, Norris D-J, Bawendi M-G (1993) Synthesis and characterization of nearly monodisperse CdE (E = Sulfur, selenium, tellurium) semiconductor nanocrystallites. J Am Chem Soc 115:8706–8715
Rosenthal S-J, Chang J-C, Kovtun O, McBride J-R, Tomlinson I-D (2011) Biocompatible quantum dots for biological applications. Chem Biol 18:10–24
Sun S, Zeng H, Robinson DB, Raoux S, Rice PM, Wang SX, Li G (2004) Monodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticles. J Am Chem Soc 126:273–279
Villaraza A-J, Bump A, Brechbiel M-W (2010) Macromolecules, dendrimers, and nanomaterials in magnetic resonance imaging: the interplay between size, function, and pharmacokinetics. Chem Rev 110:2921–2959
Agnihotri S, Mukherji S, Mukherji S (2014) Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv 4:3974
Michalet X, Pinaud F-F, Bentolila L-A, Tsay J-M, Doose S, Li J-J, Sundaresan G, Wu A-M, Gambhir S-S, Weiss S (2005) Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307:538–544
Sperling R-A, Parak W-J (2010) Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos Trans R Soc Lond Ser A 368:1333–1383
Lin C-AJ, Sperling R-A, Li J-K, Yang T-Y, Li P-Y, Zanella M, Chang W-H, Parak W-J (2008) Design of an amphiphilic polymer for nanoparticle coating and functionalization. Small 4:334–341
Chen T, Ocsoy I, Yuan Q, Wang R, You M, Zhao Z, Song E, Zhang X, Tan W (2012) One-step facile surface engineering of hydrophobic nanocrystals with designer molecular recognition. J Am Chem Soc 134:13164–13167
Ocsoy I, Gulbakan B, Shukoor M-I, Xiong X, Chen T, Powell D-H, Tan W (2013) Aptamer-conjugated multifunctional Nnanoflowers as a platform for targeting, apture, and detection in laser desorption ionization mass spectrometry. ACS Nano 7:417–427
Peng L, You M, Wu C, Han D, Öçsoy I, Chen T, Chen Z, Tan W (2014) Reversible phase transfer of nanoparticles based on photoswitchable host–guest chemistry. ACS Nano 8:2555–2561
Herzer G (1989) Grain structure and magnetism of nanocrystalline ferromagnets. IEEE Trans Magn 25:3327–3329
Skorvánek I, O’Handley R-C (1995) Fine-particle magnetism in nanocrystalline Fe-CuNb-Si-B at elevated temperatures. J Magn Magn Mater 140–144:467–468
Raveendran P, Fu J, Wallen S-L (2003) Completely “green” synthesis and stabilization of metal nanoparticles. J Am Chem Soc 125:13940–13941
Iravani S (2011) Green synthesis of metal nanoparticles using plants. Green Chem 13:50–2638
De La Rica R, Matsui H (2008) Urease as a nanoreactor for growing crystalline ZnO nanoshells at room temperature. Angew Chem Int Ed 47:5415–5417
Ocsoy I, Gulbakan B, Chen T, Zhu G, Chen Z, Sari M-M, Peng L, Xiong X, Fang X, Tan W (2013) DNA-guided metal-nanoparticle formation on graphene oxide surface. Adv Mater 25:2319–2325
Ocsoy I, Paret M-L, Ocsoy M-A, Kunwar S, Chen T, You M, Tan W (2013) Nanotechnology in plant disease management: DNA-directed silver nanoparticles on graphene oxide as an antibacterial against xanthomonas perforans. ACS Nano 7:8972–8980
Li C, Chen T, Ocsoy I, Zhu G, Yasun E, You M, Wu C, Zheng J, Song E, Huang C-Z, Tan W (2014) Gold-coated Fe3O4 nanoroses with five unique functions for cancer ell targeting, imaging and therapy. Adv Funct Mater 24:1772–1780
Leng Y, Fu L, Ye L, Li B, Xu X, Xing X, He J, Song Y, Leng C, Guo Y, Ji X, Lu Z (2016) Protein-directed synthesis of highly monodispersed, spherical gold nanoparticles and their applications in multidimensional sensing. Sci Rep 6:28900
Strayer A-L, Ocsoy I, Tan W, Jones J, Paret M-L (2016) Low concentrations of a silver-based nanocomposite to manage bacterial spot of tomato in the greenhouse. Plant Dis 100:1460–1465
Duman F, Ocsoy I, Kup F-O (2016) Chamomile flower extract-directed CuO nanoparticle formation for its antioxidant and DNA cleavage properties. Mat Sci Eng C 60:333–338
Demirbas A, Welt B-A, Ocsoy I (2016) Biosynthesis of red cabbage extract directed Ag NPs and their effect on the loss of antioxidant activity. Mater Lett 179:20–23
Sun Q, Cai X, Li J, Zheng M, Chen Z, Yu C-P (2014) Green synthesis of silver nanoparticles using tea leaf extract and evaluation of their stability and antibacterial activity. Colloids Surf A Physicochem Eng Asp 444:226–231
Wei H, Wang Z, Zhang J, House S, Gao Y-G, Yang L, Robinson H, Tan L-H, Xing H, Hou C, Robertson I-M, Zuo J-M, Lu Y (2011) Time-dependent, protein-directed growth of gold nanoparticles within a single crystal of lysozyme. Nat Nanotechnol 6:93–97
Ma X, Huh J, Park W, Lee L-P, Kwon Y-J, Sim S-J (2016) Gold nanocrystals with DNA-directed morphologies. Nat Commun 7:12873
Rodríguez-Lorenzo L, De La Rica R, Álvarez-Puebla R-A, Liz-Marzán L-M, Stevens M-M (2012) Plasmonic nanosensors with inverse sensitivity by means of enzyme-guided crystal growth. Nat Mater 11:604–607
Tikhomirov G, Hoogland S, Lee P-E, Fischer A, Sargent E-H, Kelley S-O (2011) DNA-based programming of quantum dot valency, self-assembly and luminescence. Nat Nanotechnol 6:485–490
Ma N, Sargent E-H, Kelley S-O (2009) One-step DNA-programmed growth of luminescent and biofunctionalized nanocrystals. Nat Nanotechnol 4:121–125
Karatoprak G-Ş, Aydin G, Altinsoy B, Altinkaynak C, Koşar M, Ocsoy I (2017) The effect of pelargonium Endlicherianum fenzl. Root extracts on formation of nanoparticles and their antimicrobial activities. Enzyme Microb Technol 97:21–26
Katircioğlu Z, Şakalaka H, Ulaşan M, Gören A-C, Yavuz M-S (2014) Facile synthesis of “green” gold nanocrystals using cynarin in an aqueous solution. Appl Surf Sci 318:191–198
Ocsoy I, Temiz M, Celik C, Altinsoy B, Yilmaz V, Duman F (2017) A green approach for formation of silver nanoparticles on magnetic graphene oxide and highly effective antimicrobial activity and reusability. J Mol Liq 227:147–152
Mittal A-K, Chisti Y, Banerjee U-C (2013) Synthesis of metallic nanoparticles using plant extracts. Biotechnol Adv 31:346–356
Akhtar M-S, Panwar J, Yun Y-S (2013) Biogenic synthesis of metallic nanoparticles by plant extracts. ACS Sustain Chem Eng 1:591–602
Park Y, Hing Y-N, Weyers A, Kim Y-S, Linhardt R-J (2011) Polysaccharide and phytochemicals: a natural reservoir for the green synthesis of gold and silver nanoparticles. IET Nanobiotechnol 5:69–78
Shankar S-S, Rai A, Ankamwar B, Singh A, Ahmad A, Sastry M (2004) Biological synthesis of triangular gold nanoprisms. Nat Mater 3:482
Jiang H, Manolache S, Wong ACL, Denes FS (2004) Plasmaenhanced deposition of silver nanoparticles onto polymer and metal surfaces for the generation of antimicrobial characteristics. J Appl Polym Sci 93(3):1411–1422
Sondi I, Salopek-Sondi B (2004) Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for gram-negative bacteria. J Colloid Interface Sci 275:177–182
Kim K-J, Sung W, Suh B, Moon S-K, Choi J-S, Kim J, Lee D (2009) Antifungal activity and mode of action of silver nano-particles on Candida albicans. Biometals 22:235–242
Zodrow K, Brunet L, Mahendra S, Li D, Zhang A, Li Q, Alvarez PJJ (2009) Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res 43:715–723
Elavazhagan T, Arunachalam KD (2011) Memecylon edule leaf extract mediated green synthesis of silver and gold nanoparticles. Int J Nanomedicine 6:1265–1278
Philip D, Unni C, Aromal SA, Vidhu VK (2011) Murraya koenigii leaf-assisted rapid green synthesis of silver and gold nanoparticles. Spectrochim Acta Part A 78(2):899–904
Phillip D (2011) Mangifera indica leaf-assisted biosynthesis of welldispersed silver nanoparticles. Spectrochim Acta Part A 78(1):327–331
Bar H, Bhui DK, Sahoo GP, Sarkar P, Pyne S, Misra A (2009) Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Colloids Surf A Physicochem Eng Asp 348:212–216
Ahmad N, Sharma S, Alam MK, Singh VN, Shamsi SF, Mehta BR, Fatma A (2010) Rapid synthesis of silver nanoparticles using dried medicinal plant of basil. Colloids Surf B Biointerfaces 81(1):81–86
Dubey SP, Lahtinen M, Sillanpaa M (2010) Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem 45(7):1065–1071
Aromal SA, Philip D (2012) Green synthesis of gold nanoparticles using Trigonella foenum-graceum and its size-dependent catalytic activity. Spectrochim Acta Part A 97:1–5
Liping Q, Tao C, Ismail Ö, Emir Y, Wu C, Guizhi Z, Mingxu Y, Da H, Jianhui J, Ruqin Y, Weihong T (2015) A cell-targeted, size-photocontrollable, nuclear-uptake nanodrug delivery system for drug-resistant cancer therapy. Nano Lett 15:457–463
Yasun E, Gulbakan B, Ocsoy I, Yuan Q, Shukoor MI, Li C, Tan W (2012) Enrichment and detection of rare proteins with aptamer-conjugated gold nanorods. Anal Chem 84:6008–6015
Shukoor MI, Altman MO, Han D, Bayrac AT, Ocsoy I, Zhu Z, Tan W (2012) Aptamer-nanoparticle assembly for logic-based detection. ACS Appl Mater Interfaces 4:3007–3011
Ocsoy I, Arslan Ocsoy M, Yasun E, Tan W (2013) Nucleic acid-funtionaized nanomaterials. Nano Life 03:1–10
McLamore ES, Convertino M, Ocsoy I, Vanegas DC, Taguchi M, Rong Y, Gomes C, Chaturvedi P, Claussen JC (2016) Biomimetic fractal nanometals as a transducer layer in electrochemical biosensing. Semiconductor-based sensors. World Scientific Publishing, Singapore, pp 35–67. https://doi.org/10.1142/9789813146730_0002
Narayanan KB, Sakthivel N (2008) Coriander leaf mediated biosynthesis of gold nanoparticles. Mater Lett 62(30):4588–4590
Song JY, Jang HK, Kim BS (2009) Biological synthesis of gold nanoparticles using Magnolia kokus and Diopyros kaki leaf extracts. Process Biochem 44:1133–1138
Thayer PL, Stall RE (1962) A survey of xanthomonas vesicatoria resistance to streptomycin. Proc Fla State Hort Soc 75:163–165
Jones JB, Jones JP (1985) The effect of bactericides, tank mixing time and spray schedule on bacterial leaf spot of tomato. Proc Fla State Hort Soc 98:244–247
Marco GM, Stall RE (1983) Control of bacterial spot of pepper initiated by strains of xanthomonas xampestris Pv. vesicatoria that differ in sensitivity to copper. Plant Dis 67:779–781
Jones JB, Woltz SS, Jones JP, Portier KL (1991) Population dynamics xanthomonas campestris Pv. vesicatoria on tomato leaflets treated with copper bactericides. Phytopathology 81:714–719
Obradovic A, Jones JB, Momol MT, Olson SM, Jackson LE, Balogh B, Guven K, Iriarte FB (2005) Integration of biological control agents and systemic acquired resistance inducers against bacterial spot on tomato. Plant Dis 89:712–716
Huang C-H, Vallad GE, Zhang S, Wen A, Balogh B, Figueiredo JFL, Behlau F, Jones JB, Momol MT, Olson SM (2012) Effect of application frequency and reduced rates of acibenzolar-S-methyl on the field efficacy of induced resistance against bacterial spot on tomato. Plant Dis 96:221–227
Neal A (2008) What can be inferred from bacterium-nanoparticle interactions about the potential consequences of environmental exposure to nanoparticles? Ecotoxicology 17:362–371
Yoon K-Y, Hoon Byeon J, Park J-H, Hwang J (2007) Susceptibility sonstants of escherichia coli and bacillus subtilis to silver and copper nanoparticles. Sci Total Environ 373:572–575
Mallick S, Sharma S, Banerjee M, Ghosh SS, Chattopadhyay A, Paul A (2012) Iodine-stabilized Cu nanoparticle chitosan composite for antibacterial applications. ACS Appl Mater Interfaces 4:1313–1323
Karlsson HL, Cronholm P, Gustafsson J, Möller L (2008) Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and arbon nanotubes. Chem Res Toxicol 21:1726–1732
Hu W, Peng C, Luo W, Lv M, Li X, Li D, Huang Q, Fan C (2010) Graphene-based antibacterial paper. ACS Nano 4:4317–4323
Paret LM, Vallad EG, Averett RD, Jones BJ, Olson MS (2013) Photocatalysis: effect of light-activated nanoscale formulations of TiO2 on xanthomonas perforans, and control of bacterial spot of tomato. Phytopathology 103:228–236
Panácek A, Kvítek L, Prucek R, Kolář M, Večeřová R, Pizurová N, Sharma VK, Nevěčná T j, Zbořil R (2006) Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem B 110:16248–16253
Xiu Z, Zhang Q, Puppala HL, Colvin VL, Alvarez PJJ (2012) Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett 12:4271–4275
Xu W-P, Zhang L-C, Li J-P, Lu Y, Li H-H, Ma Y-N, Wang W-D, Yu S-H (2011) Facile synthesis of silver@graphene oxide nanocomposites and their enhanced antibacterial properties. J Mater Chem 21:4593–4597
Das MR, Sarma RK, Saikia R, Kale VS, Shelke MV, Sengupta P (2011) Synthesis of silver nanoparticles in an aqueous suspension of graphene oxide sheets and its antimicrobial activity. Colloids Surf B Biointerfaces 83:16–22
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Ocsoy, I., Tasdemir, D., Mazicioglu, S., Tan, W. (2018). Nanotechnology in Plants. In: Varshney, R., Pandey, M., Chitikineni, A. (eds) Plant Genetics and Molecular Biology. Advances in Biochemical Engineering/Biotechnology, vol 164. Springer, Cham. https://doi.org/10.1007/10_2017_53
Download citation
DOI: https://doi.org/10.1007/10_2017_53
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-91312-4
Online ISBN: 978-3-319-91313-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)