Abstract
Mesenchymal stroma/stem cells (MSCs) represent a heterogenic cell population that can be isolated from various tissues of the body or can be generated from pluripotent stem cells by in vitro differentiation. Various promising pre-clinical and clinical studies suggest that MSCs might stimulate endogenous regeneration and/or act as anti-inflammatory agents, which could be of high therapeutic relevance for a number of diseases, including graft-versus-host disease after allogeneic hematopoietic stem cell transplantation, inflammatory bowel diseases, or some forms of liver failure. Notably, conflicting results of various studies illustrated that the source of MSCs, the cultivation condition, and the way of administration have important effects on the desired clinical effect. Some of the involved molecular pathways have recently been elucidated and an artificial modulation of these pathways by engineered MSCs might result in superfunctional MSCs for enhanced endogenous regeneration or anti-inflammatory response. In this review, we summarize important findings of conventional MSCs for applications in gastroenterology and we describe the state-of-the-art for the generation of patient-derived iPS cells that eventually might provide genetically engineered superfunctional iPS cells for advanced cell therapies.
Graphical Abstract

Irina Eberle and Mohsen Moslem contributed equally
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cell transplantation
- Induced pluripotent stem cells (iPSC)
- Inflammatory bowel diseases
- Liver diseases
- Mesenchymal stromal (stem) cells (MSC)
1 Introduction
Mesenchymal stromal cells (MSCs) are a heterogeneous cell population that can be obtained from various tissues, such as the bone marrow or adipose tissue. Recently several studies have shown other sources of mesenchymal cells, obtained from amniotic membrane [15], dental pulp [37], and also nonmesodermal tissue origins such as spleen, liver, kidney, and lung (Anker et al. [39] with similar characteristics to bone marrow-derived MSCs, which show a characteristic surface marker profile consisting of CD-45−, CD-31−, and CD-90+ cells. These findings might suggest a possible common niche for all of these cells, in which extracellular matrix compositions, signaling molecules, cell–cell and cell–matrix interactions, and O2 tension would be comparable [15]. By far, the most extensively studied source of MSCs is the bone marrow, because earlier studies addressing hematopoietic stem cells within the bone marrow niche resulted in a profound insight into the biology of these mesenchymal cells. Due to the fact that MSCs harbor clonally expandable cells, which could be differentiated towards adipogenic, chondrogenic, and osteogenic tissues, these cells also could be considered as mesenchymal stem cells. It is noteworthy that the abbreviation “MSC” is not uniformly used for either the term “mesenchymal stromal cells” or the term “mesenchymal stem cells” and often both aspects are not fully distinguished in the respective publication. Considering differentiation processes and further cellular fate changes upon extended in vitro culture, a pure population of mesenchymal stem cells might be hard to obtain or even to propagate and, probably, most cultures of MSCs contain stem cells as well as more differentiated stromal cells.
MSCs are meant to be beneficial in the repair of connective tissue injuries such as wound healing, osteogenic deficiencies, and also cartilage repair [4]. However, some reports suggest that MSCs could be an alternative source to repair a variety of other degenerative tissue lesions and might allow new therapeutic strategies for the treatment of neurological disorders [70], myocardial infarction [64], liver injuries [24, 58], and urological diseases [84]. This list of studies is by far not complete and, more important, the reproducibility as well as clinical impact of these studies is controversially discussed [55] as further described below in the context of gastrointestinal disorders.
Encouraging results from animal models and some clinical trials clearly support the research on MSCs, but also raise concerns about the lack of high cell numbers and the lack of a homogeneous cell population. Interestingly, embryonic stem cells or other pluripotent stem cells harbor the capabilities of unlimited self-renewing and differentiation potential into all somatic cell types [86], including mesenchymal stromal/stem cells. Among the various strategies to obtain MSCs from pluripotent stem cells, most protocols were first evaluated with established human embryonic stem cell lines. However, the generation of patient-derived pluripotent stem cells became feasible after pioneering studies of Shinya Yamanaka, who demonstrated that a set of four transcription factors can convert somatic cells into pluripotent stem cells [83]. Such patient-derived induced pluripotent stem cells [11] offer unique opportunities for applications in personalized medicine and allow the generation of high numbers of pluripotent starting cells, when large-scale cultivation systems were applied [62]. Such pluripotent stem cell lines can be differentiated towards functional MSC-like cells and it remains to be analyzed to which extent those MSC preparations harbor therapeutic effects and consist of a more defined homogeneous cell population.
Obviously, such engineered MSCs need to be investigated in further pre-clinical studies but may possess the potential to overcome some of the limitations that raise profound concerns of the clinical MSC applications at present.
2 Sources and Diversity of MSCs
In spite of data from over 100 trials employing MSCs in different clinical settings, correlation of MSC properties to clinical efficacy is limited due to considerable diversity of the applied MSC populations [88]. Also the broad spectrum of functional activity is not adequately reflected by the internationally agreed minimal set of consensus released criteria [22] and a defined subset of surface molecules that exactly characterize the MSCs’ phenotype does not exist. All spindle-shaped cells attaching to plastic surfaces and expressing surface markers like CD-29, CD-105, CD-73, CD-44, but not hematopoietic markers (CD-34, CD-45, and CD-14) are considered as MSCs, if their ability to differentiate into mesodermal lineages (adipogenic, chondrogenic, and osteogenic differentiation) is provided. It is speculated that slightly different subtypes exist, which vary in their phenotypes due to extra-cellular or intra-cellular signaling. Nevertheless, they still possess multipotentiality as demonstrated by in vitro differentiation and in xenografts [52].
There are several cultivation protocols available that allow a proper in vitro expansion of MSCs, which makes them a readily accessible cell source for stem cell research. Independent of the harvesting sites, namely bone marrow, fat tissue, and umbilical cord blood, MSCs show some in vitro expansion ability even to clinical scale with minimal shortfalls in stemness [15]. But there are contradicting reports concerning karyotype aberrations of in vitro expanded cells, as early as five to nine passages after MSC harvesting [59, 94].
Transcriptome analysis revealed that genes typically expressed in MSCs are cytoskeletal (vimentin and myosin) and cytolytic or extracellular proteins (Collagene I, III, VI, and different matrix metalloproteinases), cell adhesion molecules (fibronectin and integrins), cytokines (IL-11, HGF, TGF-β), and also receptors (IL-1R and IL-10R). However, among the various abilities of MSCs, their homing properties in different tissues and the sectretion of bioactive compounds such as angiogenic (VEGF), antiapoptotic (HGF), and mitogenic (IGF-I) factors [15] are important variables influencing their potential therapeutic applications. The high homing properties of MSCs are dependent on the chemokine receptor CXCR4 expression on the cell surface and its interaction with SDF-1α stimuli from injured tissue in a gradient-dependent manner that attracts MSCs and promotes further cell interactions [4]. Despite all beneficial effects of MSCs in degenerative diseases there are several issues that could interfere with the MSCs’ potential to ameliorate the respective disorder. For instance, aging adversely affects MSCs self-renewal, proliferation, telomerase length, and differentiation capacity [95]. Furthermore, impaired antioxidant activity and the lack of appropriate cytoskeleton properties could lead to malfunction of MSCs in therapeutic settings [43, 45].
However, the effects of MSC therapies are transient and require repeated transplantations and therefore a high number of cells. Due to the fact that MSCs show an impaired growth and increased senescence during in vitro propagation, the proper cell amount for clinical treatment might be a major obstacle. To overcome these obstacles, several groups have reported the derivation of MSC populations from self-renewing human embryonic stem cells by numerous methods [38, 61, 89], which are discussed below.
3 Therapeutic Applications of MSCs in Gastroenterology
3.1 MSCs in Graft-Versus-Host Disease
One of the most critical side effects of allogeneic hematopoietic stem cell transplantation for the treatment of leukemia or other life-threatening hematopoietic diseases is the development of an acute graft-versus-host disease (GvHD) resulting in a high morbidity and mortality [80]. Hereby, graft-derived T cells trigger the induction of GvHD after activation by host-related major histocompatibility class I or II antigens as well as minor antigenic peptides [27]. GvHD mainly targets the skin, intestine, liver, and the hematopoietic system and is routinely treated with immunosuppressive drugs such as cyclosporine or methotrexate [79]. However, various advanced treatment regimes were available using therapeutic antibodies against interleukin-2 [3], tumor necrosis factor α (TNFα; [46], or against CD-147 [20].
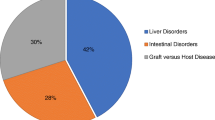
Inspired by various animal studies including baboons [8], third-party MSCs have found ready entry into a series of clinical trials for prevention of severe acute GvHD [69] and striking response rates as high as 50–90 % have been reported with noteworthy resolution of refractory intestinal GvHD [49]. It is also noteworthy that in a randomized trial patients suffering from grade II–IV acute GvHD received two transfusions of a commercially produced MSC preparation (ProchymalTM, Osiris Therapeutics, Columbia, MD, USA). Of the 32 treated patients 94 % showed an initial response and as many as 77 % remained in a complete response state [44]. Despite these promising results some follow-up studies questioned the dramatic effect of MSCs on prevention and treatment of GvHD, as larger clinical trials failed to show a beneficial effect on the most common skin GvHD. However, results from GvHD phenotypes, which are more difficult to treat and which affect mainly the intestine and the liver, showed an improved response rate over placebo [2].
3.2 MSCs in Inflammatory Bowel Disease
Idiopathic inflammatory bowel disorders (IBD) such as Crohn’s disease and ulcerative colitis are highly debilitating diseases of the gut that have remained largely resistant to definitive medical therapy [26, 99]. The pathophysiology of Crohn’s disease includes an exaggerated infiltration of macrophages and neutrophilic granulocytes, which is triggered by activated T-helper cells. These cells produce uncontrolled amounts of inflammatory cytokines and chemokines resulting in tissue destruction of the large intestine. For instance, excessive production of IFN-γ and IL-17 by T cells and IL-12 or IL-23 by monocytes is responsible for an acute inflammation and the production of other cytokines such as TNF-α [81]. Based on the finding that an imbalance of effector T cells and suppressive regulatory T cells causes an expansion of self-reactive T cells [10], there is conclusive evidence that Crohn’s disease is related to a failure of the mucosal immune system. Consequently, the therapeutic challenge applying MSCs for the treatment of IBD is twofold: curbing the inflammatory attack may be considered as the main action, but, secondly, the regeneration of a large organ such as the intestinal mucosa requires additional tissue-trophic measures to re-establish the protective mucosal barrier.
So far, the published literature on clinical evaluation of MSC-based therapy is comparatively sparse with the main evidence stemming from local application to perianal fistulas and i.v. applications pilotized in small numbers of patients [31, 71]. In a phase I clinical trial it was demonstrated that MSCs derived from the bone marrow of refractory Crohn’s disease patients have identical characteristics compared to MSCs from healthy donors and have intact immunomodulatory capacities in vitro. Furthermore, administration of autologous bone marrow-derived MSCs was safe and feasible in the treatment of refractory Crohn’s disease [23]. In addition, a more recent study demonstrated the feasibility of ex vivo expanding autologous bone marrow-derived MSCs and the safety of their intra-fistular injections in patients with Crohn’s disease. Moreover, the authors described a promoting effect of MSCs on in vivo differentiation of regulatory T cells [17]. Osiris Therapeutics (Columbia, MD, USA) also initiated clinical trials for Crohn’s disease using their MSC preparation ProychymalTM. However, the placebo group also showed improvements and the treated arm of the study failed to meet the primary endpoint [2]. In conclusion, the lack of knowledge about the direct and indirect effects of different MSC preparations hampers the evaluation of these early clinical trials and more basic research on paracrine effectors and cellular mechanisms contributing to the MSCs’ immunomodulatory effect are necessary.
3.3 MSCs in Liver Regeneration
Liver, as the second largest organ in the body serves crucial roles in the human homeostasis and its malfunction could be life-threatening. The high mortality rate because of liver deformities that led to 1.4 million deaths annually has not been avoided by liver transplantation which is the most efficient therapy so far [68]. In addition to stem cell mobilizing strategies [56] and bioartificial liver devices (BAL) [78], several alternative cell-based therapies have been investigated to recover unstable conditions in chronic liver disorders as well as during metabolic or acute liver failure. In general the disorders are treated by transplantation of bone marrow hematopoietic, mesenchymal, and mononuclear cell populations (for review see [93] and [74]). The first evidence indicating MSC infusion in mice models could recover liver failure was suggested by Petersen et al. showing the presence of bone marrow-derived hepatic cells from sex-mismatched donors in the recipient mice livers [65]. These data were substantiated by findings of other groups [47, 85], but later analyses questioned the initial hypothesis of a direct transdifferentiation and rather demonstrated that the transplanted cells fuse with host hepatocytes [12, 75, 96]. Nevertheless, some studies described functional integration of MSCs into injured liver after their in vitro specification towards hepatic cells [5, 6]. On the other hand the inhibitory signals of MSCs over hepatic stellate cells (mostly responsible for extracellular matrix accumulation [29]) inhibited the proliferation and triggered their apoptosis [90]. Also secretion of anti-inflammatory cytokines such as TNF-α, IL-1, IL-10, HGF [90], and matrix metalloproteinases regulation [53] could react as an anti-fibrogenic treatment in chronic liver injuries.
Clearly, the clinical relevance of these findings is still very controversially discussed and most pre-clinical and clinical studies indicate that the MSC therapy is a transient treatment, which may have to be applied repeatedly in order to treat the respective disorders. For instance, in the case of chronic fibrogenesis the cell infusion may be crucial for preventing the turnover of new fibers [73]. Therefore, an application of cells with similar characteristics to those of MSCs with a higher self-renewal capacity and a reduced senescence behavior would be an appropriate approach to support more long-term effects and the possibility of providing transplants from the same batch of initially transplanted cells.
4 MSCs from Patient-Derived Pluripotent Stem Cells
4.1 Generation of Patient-Specific Pluripotent Stem Cells
Pluripotent stem cells such as human embryonic stem cells (ESCs) harbor an unlimited self-renewing capability and have the potential to differentiate into all cells of the three germ layers (ectoderm, mesoderm, and endoderm) as well as into germ cells [86]. Various attempts were undertaken to derive pluripotent cells from adult individuals. The early strategies were strongly influenced by the technique of somatic cell nuclear transfer (SCNT) resulting in a cloned embryo, as first demonstrated for mammals by the birth of the sheep “Dolly” [98]. This technique, however, lacks feasibility with human cells and is ethically heavily disputed because the derivation of SCNT-derived cells implicates the destruction of a human embryo. Nevertheless, other strategies were exploited to “re-program” somatic cells towards pluripotent stem cells, either by cell fusion, by application of ESC extracts, or by using a defined set of transcription factors. In the groundbreaking study of Takahashi and Yamanaka in [83], they successfully reprogrammed mouse fibroblast by introducing ectopic defined transcription factors (Oct-4, Sox-2, Klf-4, and c-Myc known as OSKM) into the cells via retroviral transduction [83]. The oncogenic nature of c-Myc and Klf-4 urged other scientists to reprogram cells with other transcription factors such as Oct-4, Sox-2, Nanog, and Lin-28 (OSNL; [104]).
By this direct reprogramming method cell colonies with similar morphology and genetically similar information to ESCs were generated and termed induced pluripotent stem cells. Later on, numerous reprogramming studies used different cell types of ectodermal (keratinocytes [1] and neural progenitor cells [25]), mesodermal (B cells [36], or cord blood [35, 105]), and endodermal (hepatocytes [77]) origin. In addition to the cell type the composition and stoichiometry of the reprogramming factor cocktail affects successful reprogramming. Considering the stoichiometric variability caused by either high or low transgenic expression of each factor in the reprogramming cocktail, several studies ruled out the importance of a dominating Oct4 expression level [63, 87]. Therefore, a polycistronic reprogramming construct that ensures the expression of all four factors in a defined and preferential stoichiometric ratio is of high relevance for the generation of fully reprogrammed iPSC as described by various reports [13, 97]. So far several combinations of these factors along with the other factors such as Esrrb have been used for reprogramming [33]. Furthermore, small molecules, microRNAs [67], and epigenetic modifiers are used to increase reprogramming efficacy. For instance, PD0325901 and CHIR99021 as inhibitors of MEK and GSK3 pathways increased the ratio of pluripotent cells. It has been demonstrated that members of the microRNA-290 cluster are cell cycle regulators of ESCs and could also increase iPSCs colony numbers [41]. In addition other microRNAs, such as microRNA-130b, -301b, and -721 strongly supported the generation of iPSCs [66]. Moreover, DNA methyltransferase inhibitors such as AZA and RG-180 and also histone deacetylase inhibitors such as VPA could increase reprogramming efficacy when used along with OSKM factors [33].
Direct reprogramming has provided a working methodology to induce somatic cells to go back to their embryonic state by viral integration. However, integrative and nonintegrative methods used for reprogramming is a challenging criterion for safe iPSC production. For viral integration methods classic γ-retroviral and newer lentiviral vectors are used. Because of their well-understood biology and high transduction efficacy γ-retroviral vectors are commonly used for gene transfer systems. Despite high transduction efficacy the smallest result is that γ-retroviral vectors just transduce dividing cells. However, lentiviruses, a subclass of retroviruses, can infect both dividing and nondividing cells with high transduction efficacy. Although by using these integrating vectors iPSCs could be generated very efficiently, the viral vectors’ integration in the host cell genome may cause genetic mutagenesis and genomic instability [76, 103]. Nevertheless, several mouse iPSC lines were generated using integrating vectors and further applied to tetraploid embryo aggregation experiments, which resulted in fully iPSC-derived viable mice [9, 100]. To overcome potential issues with integrated reprogramming transgenes several research groups developed nonintegrating reprogramming approaches that could overcome these limitations ([60], [40], [102] and [77]) via transient viral, episomal, modified mRNA, and protein delivery.
However, reprogramming efficiency is extremely low when compared to viral transduction [102]. The same problem exists with the adenoviral delivery system, the efficiency of which is comparable to episomal vector transfection [107]. Most recently viral vectors with floxed transgenes, which could be efficiently removed [92] or piggyback transposone/transposase-based systems [42] were studied to provide clinically applicable iPSC preparations. In this line, delivery of the reprogramming factors with nonintegrating Sendai viruses seems to be a promising alternative, as high reprogramming efficiencies were obtained with this reprogramming setting [30].
4.2 Differentiation of Human iPSCs into MSCs
As outlined above, the therapeutic effect of MSC preparations may depend on the source of MSCs, the in vitro expansion of MSCs, and from batch to batch on preparation variations of MSCs. Therefore, MSCs derived from a self-renewing stem cell source may be a more suitable option. Currently the best investigated source of nontransformed self-renewing stem cells are embryonic stem cells. A number of reports described the in vitro differentiation of human ESCs into mesenchymal cells, which were very similar to primary MSCs. Some reports just applied spontaneous differentiation approaches and basically scraped out differentiating mesenchymal cells from human ESC colonies [61]. Other groups co-cultivated human ESCs with mouse bone marrow stroma cells, namely OP9 cells [7, 89] or isolated migrating cells from embryoid bodies [38]. A more defined MSC-like cell population was obtained after sorting of CD-105+ and CD-24− cells [50]. Also a directed differentiation using the TGFβ inhibitor SB-431542 was successfully described recently [72]. By the inhibition of the SMAD-2/3 pathway the study could show an efficient differentiation of hESCs into MSCs. Mostly, MSCs derived from hESC exhibited a normal karyotype and were very similar if not functionally identical to human bone marrow-derived MSCs concerning their immunophenotype and the thus-far investigated functions [19]. Some groups reported a favorable higher proliferation capability of ESC-derived MSCs compared to human bone marrow-derived MSCs [72, 101]. Moreover, the differentiated cells lacked the expression of remaining pluripotency markers and lost the potential of teratoma formation, when those cells were transplanted into immunodeficient mice. However, the transplanted cells produced homogeneous tissues of mesenchymal appearance [34, 48]. In contrast to bone marrow- or adipose tissue-derived MSCs the hESC-derived MSCs did not show any signs of senescence and grew for multiple passages in vitro [38]. This observation might be the determining aspect for using the cells in future cell- and gene-therapy approaches. Another advantage of ESC-derived over the adipose tissue-derived MSCs might be their increased immunosuppressive properties against T lymphocytes [72]. This observation might be important for studying allograft rejection or applying ESC-derived MSCs in inflammatory bowel diseases.
Another source of pluripotent stem cells are the ethically less concerned induced pluripotent stem cells. As discussed above, iPSCs can be derived from a variety of somatic cell types that are easily obtainable from patients. Recent studies investigated human iPSC-derived MSCs (hiPSC-MSCs) in different degenerative diseases. The first study was done by Lian et al. in [51] in which they generated MSCs from hiPSCs with similar characteristics of human bone marrow-derived MSCs in terms of surface marker expression and differentiation potential. The cells could also substitute the therapeutic ability of classical MSCs in the hind limb ischemia model in mice, where significantly attenuated injury was promoted by increased vascular and muscle regeneration [51]. Additionally, hiPSC-MSCs displayed a remarkable immunosuppressive nature by inhibiting NK-cell proliferation and allograft rejection [32].
Some other studies have used additional supplements in order to differentiate iPSCs in functional MSCs. Villa-Diaz et al. introduced a biocompatible synthetic polymer (PMEDSAH) and xenogene-free culture media for differentiation of human iPSCs towards MSCs. Those cells were then applied in a mouse model with osteogenic calvaria defects, where the integrated cells could significantly recover the defect by regenerating new bone tissue compared to the control group [91]. In another study human ESCs and human iPSCs were differentiated into MSCs by using collagen type I coated plates [54]. In an electrophysiological study, patch clamp analysis demonstrated that hiPSCs-MSCs and human bone marrow-derived MSCs exhibited highly similar ion channel properties [106]. Recently the TGFβ inhibitor SB-431542 was successfully used in order to induce the differentiation of iPSCs into MSCs directly. Cells generated after 10 days of treatment have shown MSC characteristics in terms of immunophenotype and differentiation potential [16].
4.3 Large-Scale Cultivation
Controlled scalable expansion culture and a well-ordered differentiation process are challenges for translational clinical therapies, whenever high amounts of cells need to be transplanted. Although human iPSC-derived MSCs (hiPSC-MSCs) have generated great interest in possible clinical applications using iPSCs in regenerative medicine, the actual number of cells that could be cultivated after differentiation with traditional culture methods would be very low (Cormier et al. [18]. Currently cells aimed to be transplanted to patients are produced and cultured in static flasks. But this cultivation system results in a low amount, heterogeneity of cells, increased risk of contamination, and low cell yield due to the lack of real-time controlled parameters within culture media (including O2 and nutrient concentration, pH, osmolarity, metabolic waste concentration, shear stress, and cell density). One probable resolution to get a sufficient amount of cells is to change the culture conditions towards suspension culture in bioreactors, which might allow the scaling up of the number of these cells in vitro. In this regard all culture parameters must be controlled in a bioreactor in order to get a tangible number of cells [28, 82]. Stirred suspension bioreactors (SSBs) have provided a dynamic condition to produce cell-based products in a safe, robust, and cost-effective manner. SSBs have been developed for many experiments, in which a large amount of cells is required and they were also successfully used for the expansion of undifferentiated pluripotent human stem cells [62, 108]. However, it is unclear if pluripotent cells that were expanded in such a bioreactor system can also be differentiated towards a MSC-like phenotype in a SSB or in another suspension culture system. To investigate these issues, further studies on robust differentiation protocols providing ESC- or iPSC-derived MSCs in suspension cultures or on the amplification of initially differentiated mesenchymal precursor cells in a bioreactor system capable of promoting MSC expansion [21], might be of high impact.
5 Conclusion and Outlook
In spite of data from over 100 trials employing MSCs in different clinical settings correlation of MSC properties to clinical efficacy is limited due to the considerable diversity of the applied MSC-populations [88]. Also the internationally agreed minimal set of consensus criteria for the definition and characterization of MSCs is not able to reflect adequately the broad spectrum of functional activity in the diverse contexts of tissue regeneration and anti-inflammatory therapies. Thus, engineered MSCs from well-characterized iPSC lines may not only solve the problem posed by the principally limited expansion capacity of MSCs, but may also serve as a homogeneous source of MSCs with more defined therapeutic characteristics. In this regard, one could even think of artificial iPSC-derived MSCs that overexpress a distinct therapeutically relevant transgene. One example for such a therapeutic transgene could be indoleamine-2,3-dioxygenase (IDO), which is induced by interferon-gamma (IFN-gamma) and which catalyzes the conversion from tryptophan to kynurenine and has been identified as a T cell inhibitory effector pathway in professional antigen-presenting cells [57]. Engineered MSCs, which were derived from iPSC lines harboring such a constitutively expressed IDO transgene, could serve as artificial MSCs for the treatment of inflammatory disease, where IDO-mediated T cell inhibition could further support the therapeutic effect of MSCs.
As the systemic application of MSCs might be hampered by an impaired pulmonary passage, MSCs could also be genetically modified to overcome such a limitation. For example, Rap1 [14], a member of the GTPase family of proteins with regulatory effects on multiple adhesion molecules, could be knocked-down in engineered MSCs, which then should gain an enhanced bioavailability after intravenous administration due to an improved pulmonary passage.
In conclusion, engineering MSCs from pluripotent stem cells such as iPSCs could generate advanced cellular therapies for the treatment of a variety of diseases, including intestinal GvHD and inflammatory bowel diseases, as well as some forms of liver failure.
References
Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, Vassena R, Bilic J, Pekarik V, Tiscornia G, Edel M, Boue S, Izpisua Belmonte JC (2008) Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol 26(11):1276–1284
Allison M (2009) Genzyme backs Osiris, despite Prochymal flop. Nat Biotechnol 27(11):966–967
Anasetti C, Hansen JA, Waldmann TA, Appelbaum FR, Davis J, Deeg HJ, Doney K, Martin PJ, Nash R, Storb R et al (1994) Treatment of acute graft-versus-host disease with humanized anti-Tac: an antibody that binds to the interleukin-2 receptor. Blood 84(4):1320–1327
Augello A, Kurth TB, De Bari C (2010) Mesenchymal stem cells: a perspective from in vitro cultures to in vivo migration and niches. Eur Cell Mater 20:121–133
Aurich H, Sgodda M, Kaltwasser P, Vetter M, Weise A, Liehr T, Brulport M, Hengstler JG, Dollinger MM, Fleig WE, Christ B (2009) Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut 58(4):570–581
Aurich I, Mueller LP, Aurich H, Luetzkendorf J, Tisljar K, Dollinger MM, Schormann W, Walldorf J, Hengstler JG, Fleig WE, Christ B (2007) Functional integration of hepatocytes derived from human mesenchymal stem cells into mouse livers. Gut 56(3):405–415
Barberi T, Willis LM, Socci ND, Studer L (2005) Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med 2(6):e161
Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R (2002) Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 30(1):42–48
Boland MJ, Hazen JL, Nazor KL, Rodriguez AR, Gifford W, Martin G, Kupriyanov S, Baldwin KK (2009) Adult mice generated from induced pluripotent stem cells. Nature 461(7260):91–94
Bouma G, Strober W (2003) The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol 3(7):521–533
Cantz T, Martin U (2010) Induced pluripotent stem cells: characteristics and perspectives. Adv Biochem Eng Biotechnol 123:107–126
Cantz T, Sharma AD, Jochheim-Richter A, Arseniev L, Klein C, Manns MP, Ott M (2004) Reevaluation of bone marrow-derived cells as a source for hepatocyte regeneration. Cell Transp 13(6):659–666
Carey BW, Markoulaki S, Hanna JH, Faddah DA, Buganim Y, Kim J, Ganz K, Steine EJ, Cassady JP, Creyghton MP, Welstead GG, Gao Q, Jaenisch R (2011) Reprogramming factor stoichiometry influences the epigenetic state and biological properties of induced pluripotent stem cells. Cell Stem Cell 9(6):588–598
Carmona G, Gottig S, Orlandi A, Scheele J, Bauerle T, Jugold M, Kiessling F, Henschler R, Zeiher AM, Dimmeler S, Chavakis E (2009) Role of the small GTPase Rap1 for integrin activity regulation in endothelial cells and angiogenesis. Blood 113(2):488–497
Chamberlain G, Fox J, Ashton B, Middleton J (2007) Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25(11):2739–2749
Chen YS, Pelekanos RA, Ellis RL, Horne R, Wolvetang EJ, Fisk NM (2012) Small molecule mesengenic induction of human induced pluripotent stem cells to generate mesenchymal stem/stromal cells. Stem Cells Trans Med 1:83–95
Ciccocioppo R, Bernardo ME, Sgarella A, Maccario R, Avanzini MA, Ubezio C, Minelli A, Alvisi C, Vanoli A, Calliada F, Dionigi P, Perotti C, Locatelli F, Corazza GR (2011) Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut 60(6):788–798
Cormier JT, zur Nieden NI, Rancourt DE, Kallos MS (2006) Expansion of undifferentiated murine embryonic stem cells as aggregates in suspension culture bioreactors. Tissue Eng 12 (11):3233–3245
de Peppo GM, Svensson S, Lenneras M, Synnergren J, Stenberg J, Strehl R, Hyllner J, Thomsen P, Karlsson C (2010) Human embryonic mesodermal progenitors highly resemble human mesenchymal stem cells and display high potential for tissue engineering applications. Tissue Eng Part A 16(7):2161–2182
Deeg HJ, Blazar BR, Bolwell BJ, Long GD, Schuening F, Cunningham J, Rifkin RM, Abhyankar S, Briggs AD, Burt R, Lipani J, Roskos LK, White JM, Havrilla N, Schwab G, Heslop HE (2001) Treatment of steroid-refractory acute graft-versus-host disease with anti-CD147 monoclonal antibody ABX-CBL. Blood 98(7):2052–2058
Diederichs S, Roker S, Marten D, Peterbauer A, Scheper T, van Griensven M, Kasper C (2009) Dynamic cultivation of human mesenchymal stem cells in a rotating bed bioreactor system based on the Z RP platform. Biotechnol Prog 25(6):1762–1771
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8(4):315–317
Duijvestein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW, Kooy-Winkelaar EM, Koning F, Zwaginga JJ, Fidder HH, Verhaar AP, Fibbe WE, van den Brink GR, Hommes DW (2010) Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut 59(12):1662–1669
El-Ansary M, Abdel-Aziz I, Mogawer S, Abdel-Hamid S, Hammam O, Teaema S, Wahdan M (2011) Phase II trial: undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Rev 8(3):972–981
Eminli S, Utikal J, Arnold K, Jaenisch R, Hochedlinger K (2008) Reprogramming of neural progenitor cells into induced pluripotent stem cells in the absence of exogenous Sox2 expression. Stem Cells 26(10):2467–2474
Engel MA, Neurath MF (2010) New pathophysiological insights and modern treatment of IBD. J Gastroenterol 45(6):571–583
Ferrara JL, Levy R, Chao NJ (1999) Pathophysiologic mechanisms of acute graft-vs.-host disease. Biol Blood Marrow Transpl 5(6):347–356
Fridley KM, Fernandez I, Li MT, Kettlewell RB, Roy K (2010) Unique differentiation profile of mouse embryonic stem cells in rotary and stirred tank bioreactors. Tissue Eng Part A 16(11):3285–3298
Friedman SL (2007) Reversibility of hepatic fibrosis and cirrhosis—is it all hype? Nat Clin Pract Gastroenterol Hepatol 4(5):236–237
Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M (2009) Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci 85(8):348–362
Garcia-Bosch O, Ricart E, Panes J (2010) Review article: stem cell therapies for inflammatory bowel disease—efficacy and safety. Aliment Pharmacol Ther 32(8):939–952
Giuliani M, Oudrhiri N, Noman ZM, Vernochet A, Chouaib S, Azzarone B, Durrbach A, Bennaceur-Griscelli A (2011) Human mesenchymal stem cells derived from induced pluripotent stem cells down-regulate NK-cell cytolytic machinery. Blood 118(12):3254–3262
Gonzalez F, Boue S, Izpisua Belmonte JC (2011) Methods for making induced pluripotent stem cells: reprogramming a la carte. Nat Rev Genet 12(4):231–242
Gruenloh W, Kambal A, Sondergaard C, McGee J, Nacey C, Kalomoiris S, Pepper K, Olson S, Fierro F, Nolta JA (2011) Characterization and in vivo testing of mesenchymal stem cells derived from human embryonic stem cells. Tissue Eng Part A 17(11–12):1517–1525
Haase A, Olmer R, Schwanke K, Wunderlich S, Merkert S, Hess C, Zweigerdt R, Gruh I, Meyer J, Wagner S, Maier LS, Han DW, Glage S, Miller K, Fischer P, Scholer HR, Martin U (2009) Generation of induced pluripotent stem cells from human cord blood. Cell Stem Cell 5(4):434–441
Hanna J, Saha K, Pando B, van Zon J, Lengner CJ, Creyghton MP, van Oudenaarden A, Jaenisch R (2009) Direct cell reprogramming is a stochastic process amenable to acceleration. Nature 462(7273):595–601
Hung CN, Mar K, Chang HC, Chiang YL, Hu HY, Lai CC, Chu RM, Ma CM (2011) A comparison between adipose tissue and dental pulp as sources of MSCs for tooth regeneration. Biomaterials 32(29):6995–7005
Hwang NS, Varghese S, Lee HJ, Zhang Z, Ye Z, Bae J, Cheng L, Elisseeff J (2008) In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proc Natl Acad Sci U S A 105(52):20641–20646
in ‘t Anker PS, Noort WA, Scherjon SA, Kleijburg-van der Keur C, Kruisselbrink AB, van Bezooijen RL, Beekhuizen W, Willemze R, Kanhai HH, Fibbe WE (2003) Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica 88(8):845–852
Jia F, Wilson KD, Sun N, Gupta DM, Huang M, Li Z, Panetta NJ, Chen ZY, Robbins RC, Kay MA, Longaker MT, Wu JC (2010) A nonviral minicircle vector for deriving human iPS cells. Nat Methods 7(3):197–199
Judson RL, Babiarz JE, Venere M, Blelloch R (2009) Embryonic stem cell-specific microRNAs promote induced pluripotency. Nat Biotechnol 27(5):459–461
Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K (2009) Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature 458(7239):771–775
Kasper G, Mao L, Geissler S, Draycheva A, Trippens J, Kuhnisch J, Tschirschmann M, Kaspar K, Perka C, Duda GN, Klose J (2009) Insights into mesenchymal stem cell aging: involvement of antioxidant defense and actin cytoskeleton. Stem Cells 27(6):1288–1297
Kebriaei P, Isola L, Bahceci E, Holland K, Rowley S, McGuirk J, Devetten M, Jansen J, Herzig R, Schuster M, Monroy R, Uberti J (2009) Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transpl 15(7):804–811
Kemp K, Gray E, Mallam E, Scolding N, Wilkins A (2010) Inflammatory cytokine induced regulation of superoxide dismutase 3 expression by human mesenchymal stem cells. Stem Cell Rev 6(4):548–559
Kobbe G, Schneider P, Rohr U, Fenk R, Neumann F, Aivado M, Dietze L, Kronenwett R, Hunerliturkoglu A, Haas R (2001) Treatment of severe steroid refractory acute graft-versus-host disease with infliximab, a chimeric human/mouse antiTNFalpha antibody. Bone Marrow Transpl 28(1):47–49
Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M (2000) Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med 6(11):1229–1234
Laurila JP, Laatikainen L, Castellone MD, Trivedi P, Heikkila J, Hinkkanen A, Hematti P, Laukkanen MO (2009) Human embryonic stem cell-derived mesenchymal stromal cell transplantation in a rat hind limb injury model. Cytotherapy 11(6):726–737
Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringden O (2008) Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371(9624):1579–1586
Lian Q, Lye E, Suan Yeo K, Khia Way Tan E, Salto-Tellez M, Liu TM, Palanisamy N, El Oakley RM, Lee EH, Lim B, Lim SK (2007) Derivation of clinically compliant MSCs from CD105+, CD24− differentiated human ESCs. Stem Cells 25(2):425–436
Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, Lam FF, Kang S, Xia JC, Lai WH, Au KW, Chow YY, Siu CW, Lee CN, Tse HF (2010) Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation 121(9):1113–1123
Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, Marshak DR, Flake AW (2000) Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med 6(11):1282–1286
Liu CH, Hwang SM (2005) Cytokine interactions in mesenchymal stem cells from cord blood. Cytokine 32(6):270–279
Liu Y, Goldberg AJ, Dennis JE, Gronowicz GA, Kuhn LT (2012) One-step derivation of mesenchymal stem cell (MSC)-like cells from human pluripotent stem cells on a fibrillar collagen coating. PLoS One 7(3):e33225
Locke M, Feisst V, Dunbar PR (2011) Concise review: human adipose-derived stem cells: separating promise from clinical need. Stem Cells 29(3):404–411
Mark AL, Sun Z, Warren DS, Lonze BE, Knabel MK, Melville Williams GM, Locke JE, Montgomery RA, Cameron AM (2010) Stem cell mobilization is life saving in an animal model of acute liver failure. Ann Surg 252(4):591–596
Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D (2004) Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 103(12):4619–4621
Mohamadnejad M, Namiri M, Bagheri M, Hashemi SM, Ghanaati H, Zare Mehrjardi N, Kazemi Ashtiani S, Malekzadeh R, Baharvand H (2007) Phase 1 human trial of autologous bone marrow-hematopoietic stem cell transplantation in patients with decompensated cirrhosis. World J Gastroenterol 13(24):3359–3363
Mohseny AB, Szuhai K, Romeo S, Buddingh EP, Briaire-de Bruijn I, de Jong D, van Pel M, Cleton-Jansen AM, Hogendoorn PC (2009) Osteosarcoma originates from mesenchymal stem cells in consequence of aneuploidization and genomic loss of Cdkn2. J Pathol 219(3):294–305
Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S (2008) Generation of mouse induced pluripotent stem cells without viral vectors. Science 322(5903):949–953
Olivier EN, Rybicki AC, Bouhassira EE (2006) Differentiation of human embryonic stem cells into bipotent mesenchymal stem cells. Stem Cells 24(8):1914–1922
Olmer R, Lange A, Selzer S, Kasper C, Haverich A, Martin U, Zweigerdt R (2012) Suspension culture of human pluripotent stem cells in controlled, stirred bioreactors. Tissue Eng Part C Methods. doi:10.1089/ten.tec.2011.0717 [Epub ahead of print]
Papapetrou EP, Tomishima MJ, Chambers SM, Mica Y, Reed E, Menon J, Tabar V, Mo Q, Studer L, Sadelain M (2009) Stoichiometric and temporal requirements of Oct4, Sox2, Klf4, and c-Myc expression for efficient human iPSC induction and differentiation. Proc Natl Acad Sci U S A 106(31):12759–12764
Penn MS, Ellis S, Gandhi S, Greenbaum A, Hodes Z, Mendelsohn FO, Strasser D, Ting AE, Sherman W (2012) Adventitial delivery of an allogeneic bone marrow-derived adherent stem cell in acute myocardial infarction: phase I clinical study. Circ Res 110(2):304–311
Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP (1999) Bone marrow as a potential source of hepatic oval cells. Science 284(5417):1168–1170
Pfaff N, Fiedler J, Holzmann A, Schambach A, Moritz T, Cantz T, Thum T (2011) miRNA screening reveals a new miRNA family stimulating iPS cell generation via regulation of Meox2. EMBO Rep 12(11):1153–1159
Pfaff N, Moritz T, Thum T, Cantz T (2012) miRNAs involved in the generation, maintenance, and differentiation of pluripotent stem cells. J Mol Med 90(7):747–752
Poynard T, Yuen MF, Ratziu V, Lai CL (2003) Viral hepatitis C. Lancet 362(9401):2095–2100
Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, Marschall HU, Dlugosz A, Szakos A, Hassan Z, Omazic B, Aschan J, Barkholt L, Le Blanc K (2006) Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation 81(10):1390–1397
Saito F, Nakatani T, Iwase M, Maeda Y, Hirakawa A, Murao Y, Suzuki Y, Onodera R, Fukushima M, Ide C (2008) Spinal cord injury treatment with intrathecal autologous bone marrow stromal cell transplantation: the first clinical trial case report. J Trauma 64(1):53–59
Salas A, Ricart E, Panes J (2009) Cell therapies for inflammatory bowel diseases. Expert Rev Gastroenterol Hepatol 3(4):321–324
Sanchez L, Gutierrez-Aranda I, Ligero G, Rubio R, Munoz-Lopez M, Garcia-Perez JL, Ramos V, Real PJ, Bueno C, Rodriguez R, Delgado M, Menendez P (2011) Enrichment of human ESC-derived multipotent mesenchymal stem cells with immunosuppressive and anti-inflammatory properties capable to protect against experimental inflammatory bowel disease. Stem Cells 29(2):251–262
Sato Y, Araki H, Kato J, Nakamura K, Kawano Y, Kobune M, Sato T, Miyanishi K, Takayama T, Takahashi M, Takimoto R, Iyama S, Matsunaga T, Ohtani S, Matsuura A, Hamada H, Niitsu Y (2005) Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood 106(2):756–763
Sharma AD, Cantz T, Manns MP, Ott M (2006) The role of stem cells in physiology, pathophysiology, and therapy of the liver. Stem Cell Rev 2(1):51–58
Sharma AD, Cantz T, Richter R, Eckert K, Henschler R, Wilkens L, Jochheim-Richter A, Arseniev L, Ott M (2005) Human cord blood stem cells generate human cytokeratin 18-negative hepatocyte-like cells in injured mouse liver. Am J Pathol 167(2):555–564
Stadtfeld M, Hochedlinger K (2010) Induced pluripotency: history, mechanisms, and applications. Genes Dev 24(20):2239–2263
Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K (2008) Induced pluripotent stem cells generated without viral integration. Science 322(5903):945–949
Stockmann HB, Hiemstra CA, Marquet RL, JN IJ (2000) Extracorporeal perfusion for the treatment of acute liver failure. Ann Surg 231(4):460–470
Storb R, Deeg HJ, Pepe M, Appelbaum F, Anasetti C, Beatty P, Bensinger W, Berenson R, Buckner CD, Clift R et al (1989) Methotrexate and cyclosporine versus cyclosporine alone for prophylaxis of graft-versus-host disease in patients given HLA-identical marrow grafts for leukemia: long-term follow-up of a controlled trial. Blood 73(6):1729–1734
Storb R, Thomas ED (1985) Graft-versus-host disease in dog and man: the Seattle experience. Immunol Rev 88:215–238
Strober W, Fuss I, Mannon P (2007) The fundamental basis of inflammatory bowel disease. J Clin Invest 117(3):514–521
Taiani JT, Krawetz RJ, Zur Nieden NI, Elizabeth WuY, Kallos MS, Matyas JR, Rancourt DE (2010) Reduced differentiation efficiency of murine embryonic stem cells in stirred suspension bioreactors. Stem Cells Dev 19(7):989–998
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676
Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S, Sun X, Chen J, Yang S, Cai J, Gao X, Pileggi A, Ricordi C (2012) Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA 307(11):1169–1177
Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause DS (2000) Liver from bone marrow in humans. Hepatology 32(1):11–16
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282(5391):1145–1147
Tiemann U, Sgodda M, Warlich E, Ballmaier M, Schöler HR, Schambach A, Cantz T (2011) Optimal reprogramming factor stoichiometry increases colony numbers and affects molecular characteristics of murine induced pluripotent stem cells. Cytom Part A 79A:426–435
Tolar J, Le Blanc K, Keating A, Blazar BR (2010) Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells 28(8):1446–1455
Trivedi P, Hematti P (2008) Derivation and immunological characterization of mesenchymal stromal cells from human embryonic stem cells. Exp Hematol 36(3):350–359
van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, Tilles AW, Yarmush ML (2008) Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology 47(5):1634–1643
Villa-Diaz LG, Brown SE, Liu Y, Ross AM, Lahann J, Parent JM, Krebsbach PH (2012) Derivation of mesenchymal stem cells from human induced pluripotent stem cells cultured on synthetic substrates. Stem Cells 30(6):1174–1181
Voelkel C, Galla M, Maetzig T, Warlich E, Kuehle J, Zychlinski D, Bode J, Cantz T, Schambach A, Baum C (2010) Protein transduction from retroviral Gag precursors. Proc Natl Acad Sci U S A 107(17):7805–7810
Vosough M, Moslem M, Pournasr B, Baharvand H (2011) Cell-based therapeutics for liver disorders. Br Med Bull 100:157–172
Wagner W (2010) Senescence is heterogeneous in mesenchymal stromal cells: kaleidoscopes for cellular aging. Cell Cycle 9(15):2923–2924
Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V, Ho AD (2008) Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One 3(5):e2213
Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M (2003) Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature 422(6934):897–901
Warlich E, Kühle J, Cantz T, Brugman MH, Mätzig T, Galla M, Filipczyk A, Halle S, Klump H, Schöler HR, Baum C, Schroeder T, Schambach A (2011) Lentiviral vector design and imaging approaches to visualize the early stages of cellular reprogramming. Mol Ther 19(4):782–789
Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH (1997) Viable offspring derived from fetal and adult mammalian cells. Nature 385(6619):810–813
Wolters FL, Russel MG, Sijbrandij J, Schouten LJ, Odes S, Riis L, Munkholm P, Langholz E, Bodini P, O’Morain C, Katsanos K, Tsianos E, Vermeire S, Van Zeijl G, Limonard C, Hoie O, Vatn M, Moum B, Stockbrugger RW (2006) Disease outcome of inflammatory bowel disease patients: general outline of a Europe-wide population-based 10-year clinical follow-up study. Scand J Gastroenterol Suppl 243:46–54
Wu G, Liu N, Rittelmeyer I, Sharma AD, Sgodda M, Zaehres H, Bleidissel M, Greber B, Gentile L, Han DW, Rudolph C, Steinemamm D, Schambach A, Ott M, Schöler HR, Cantz T (2011) Generation of healthy mice from gene-corrected disease-specific induced pluripotent stem cells. PLoS Biol 9(7):e1001099
Yen ML, Hou CH, Peng KY, Tseng PC, Jiang SS, Shun CT, Chen YC, Kuo ML (2011) Efficient derivation & concise gene expression profiling of human embryonic stem cell-derived mesenchymal progenitors (EMPs). Cell Transpl. doi:10.3727/096368910X564067 [Epub ahead of print]
Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA (2009) Human induced pluripotent stem cells free of vector and transgene sequences. Science 324(5928):797–801
Yu J, Thomson JA (2008) Pluripotent stem cell lines. Genes Dev 22(15):1987–1997
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318(5858):1917–1920
Zaehres H, Kogler G, Arauzo-Bravo MJ, Bleidissel M, Santourlidis S, Weinhold S, Greber B, Kim JB, Buchheiser A, Liedtke S, Eilken HM, Graffmann N, Zhao X, Meyer J, Reinhardt P, Burr B, Waclawczyk S, Ortmeier C, Uhrberg M, Schöler HR, Cantz T, Wernet P (2010) Induction of pluripotency in human cord blood unrestricted somatic stem cells. Exp Hematol 38(9):809–818
Zhang J, Chan YC, Ho JC, Siu CW, Lian Q, Tse HF (2012) Regulation of cell proliferation of human induced pluripotent stem cell-derived mesenchymal stem cells via ether a go–go 1 (hEAG1) potassium channel. Am J Physiol Cell Physiol
Zhou W, Freed CR (2009) Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells. Stem Cells 27(11):2667–2674
Zweigerdt R, Olmer R, Singh H, Haverich A, Martin U (2010) Scalable expansion of human pluripotent stem cells in suspension culture. Nat Protoc (in press)
Acknowledgments
We are very grateful to the members of our lab providing intensive discussions and much critical input, which contributed to this review. M.M. and T.C. receive funding from the cluster of excellence REBIRTH (REgenerative BIology and Reconstructive THerapy), which is funded by the German Research Foundation (DFG; EXC 62/1). I.E. receives funding from the Centre for Cell and Gene Therapy of the LOEWE initiative. Further funding is provided by the Federal Ministry of Education and Research through grants 01GM1110A, 01GP1007C, as well as by the José Carreras leukemia foundation (DJCLS R11/22f).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 2013
About this chapter
Cite this chapter
Eberle, I., Moslem, M., Henschler, R., Cantz, T. (2012). Engineered MSCs from Patient-Specific iPS Cells. In: Weyand, B., Dominici, M., Hass, R., Jacobs, R., Kasper, C. (eds) Mesenchymal Stem Cells - Basics and Clinical Application II. Advances in Biochemical Engineering/Biotechnology, vol 130. Springer, Berlin, Heidelberg. https://doi.org/10.1007/10_2012_156
Download citation
DOI: https://doi.org/10.1007/10_2012_156
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-37943-7
Online ISBN: 978-3-642-37944-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)