Summary
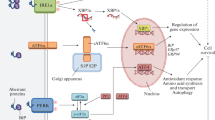
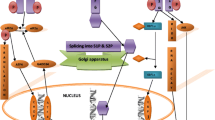
The endoplasmic reticulum (ER) is a subcellular compartment playing a central role in calcium storrage and calcium signalling. Furthermore, all newly synthesized membrane and secretory proteins are folded and processed in this subcellular compartment. These are strictly calcium-dependent processes that need for correct functioning a calcium activity in the range close to that of the extracellular space. Under conditions associated with ER dysfunction, unfolded proteins accumulate in the lumen of the ER. This is the warning signal for activation of highly conserved stress responses, including the unfolded protein response (UPR), necessary to restore normal functioning of the ER, and the ER-associated degradation (ERAD) to degrade unfolded proteins at the proteasome. In acute pathological states of the brain, such as stroke, neurotrauma, epileptic seizures, and in degenerative diseases ER functioning is impaired in multiple ways. These include disturbances of ER calcium homeostasis, impairment of ERAD and UPR, and insufficient proteasome functioning which triggers secondary ER dysfunction. Therapeutic interventions designed to suppress the pathological process culminating in neuronal cell death in acute and degenerative diseases of the brain could therefore focus on strategies aimed at improving the capability of neurons to withstand conditions associated with ER dysfunction
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Siesjö BK (1978) Brain Energy Metabolism. New York, John Wiley
Siesjö BK (1981) Cell damage in the brain: a speculative synthesis. J Cereb Blood Flow Metabol 1:155–185
Sattler R, Charlton MP, Hafner M, Tymianski M (1998) Distinct influx pathways, not calcium load, determine neuronal vulnerability to calcium neurotoxicity. J Neurochem 71:2349–2364
Paschen W (1996) Disturbances in calcium homeostasis within the endoplasmic reticulum may contribute to the development of ischemic cell damage. Med Hypoth 47:283–288
Paschen W, Doutheil J (1999) Disturbances of the functioning of endoplasmic reticulum: a key mechanism signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional underlying neuronal cell injury? J Cereb Blood Flow Metabol 19:1–18
Paschen W, Frandsen A (2001) Endoplasmic reticulum dysfunction — a common denominator for cell injury in acute and degenerative diseases of the brain? J Neurochem 79:719–725
Berridge MJ (1998) Neuronal calcium signaling. Neuron 21:13–26
Kaufman RJ (1999) Stress and translational controls. Genes Dev 13:1211–1233
Hofer AM, Machen TE (1993) Technique for in situ measurement of calcium in intracellular inositol 1, 4, 5-triphosphate-sesitive stores using the fluorescent indicator mag-fura-2. Proc Natl Acad Sci USA 90:2598–2602
Galione A, Churchill GC (2002) Interactions between calcium release pathways: multiple messengers and multiple stores. Cell Calcium 35:343–354
Kaufman RJ (2002) Orchestrating the unfolded protein response in health and disease. J Clin Invest 110:1389–1398
Hampton RY (2002) ER-asociated degradation in protein quality control and cellular regulation. Curr Opinion Cell Biol 14:476–482
Doutheil J, Althausen S, Treiman M, Paschen W (2000) Effect of nitric oxide on endoplasmic reticulum calcium homeostasis, protein synthesis and energy metabolism. Cell Calcium 27:107–115
Kohno K, Higuchi T, Ohta S, Kohno K, Kumon Y, Sakaki S (1997) Neuroprotective nitric oxide synthase inhibitor reduces intracellular calcium accumulation following transient global ischemia in the gerbil. Neurosci Lett 224:17–20
Parsons JT, Churn SB, Delorenzo RJ (1997) Ischemia-induced inhibition of calcium uptake into rat brain microsomes mediated by Mg2+/Ca2+-ATPase. J Neurochem 68:1124–1134
Parsons JT, Churn SB, Kochan LD, DeLorenzo RJ (2000) Pilocarbin-induced status epilepticus causes N-methyl-D-aspartate receptor-dependent inhibition of microsomal Mg2+/Ca2+-ATPase- mediated increases in Ca2+ uptake. J Neurochem 75:1209–1218
Kumar R, Azam S, Sullivan JM, Owen C, Cavener DRC, Zhang P, Ron D, Harding HP, Chen J-J, Han A, White BC, Krause GS, DeGracia DJ (2001) Brain ischemia and reperfusion activates the eukaryotic initiation factor 2a kinase, PERK. J Neurochem 77:1418–1421
Calfon M, Zeng H, Urano F, Till JH, Hubbart SR, Harding HP, Clark SG, Ron D (2002) IREI couples endoplasmic reticulum load to secretory capacity by processing XBP-1 mRNA. Nature 415:92–96
Paschen W, Aufenberg C, Hotop S, Mengesdorf T (2003) Transient cerebral ischemia activates processing of xbpl mRNA indicative of endoplasmic reticulum stress. J Cereb Blood Flow Metabol, in press
Althausen S, Mengesdorf T, Mies G, Oláh L, Nairn AC, Proud CG, Paschen W (2001) Changes in phosphorylation of initiation factor eIF2a, elongation factor eEF-2 and p70 S6 kinase after transient focal cerebral ischemia in mice. J Neurochem 78:779–787
Chung KKK, Dawson VL, Dawson TM (2001) The role of the ubiquitin-proteasomal pathway in Parkinson’s disease and other neurodegenerative disorders. Trends Neurosci 24 (11, Suppl. 1):S7–S14
Mattson MP (2002) Oxidative stress, perturbed calcium homeostasis and immune dysfunction in Alzheimer’s disease. J Neurovirol 8:539–550
Katayama T, Imaizumi K, Sato N, Miyoshi K, Kudo T, Hitomi J, Morihara T, Yoneda T, Gomi F, Mori Y, Nakano J, Takeda J, Tsuda T, Itoyama Y, Murayama O, Takashima A, St George-Hyslop P, Takeda M, Tohyama M (1999) Presenilin-1 mutations downregulate the signalling pathway of the unfolded protein response. Nature Cell Biol 1:479–485
Roy J, Minotti S, Dong LC, Figlewicz DA, Durham HD (1998) Glutamate potentiates the tocixity of mutant Cu/Zn-superoxide dismutase in motor neurons by postsynaptic calcium-dependent mechanisms. J Neurosci 18:9673–9684
Kourroku Y, Fujita E, Jimbo A, Kikuchi T, Yamagata T, Momoi, MY, Kominami E, Kuida K, Sakamaki K, Yonehara S, Momoi T (2002) Polyglutamine aggregates stimulate ER stress signals and caspase-12 activation. Hum Mol Gen 11:1505–1515
Bence NF, Sampat RM, Kopito RR (2001) Impairment of the ubiquitin-proteasomal system by protein aggregation. Science 292:1552–1555
Mengesdorf T, Jensen PH, Mies G, Aufenberg C, Paschen W (2002) Down-regulation of parkin protein in transient focal cerebral ischemia: A link between stroke and degenerative disease? Proc Natl Acad Sei USA 99:15042–15047
Imai Y, Soda M, Takahashi R (2000) Parkin suppress unfolded protein stress-induced cell death through its E3 ubiquitin-ligase activity. J Biol Chem 275:35661–35664
Ledesma MD, Galvan C, Hellias B, Dotti C, Jensen PH (2002) Astrocytic but not neuronal increased expression and redistribution of parkin during unfolded protein stress. J Neurochem 83:1431–1440
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2004 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Paschen, W. (2004). The Organelles II: Endoplasmic Reticulum and its Overload. In: Herdegen, T., Delgado-García, J. (eds) Brain Damage and Repair. Springer, Dordrecht. https://doi.org/10.1007/1-4020-2541-6_8
Download citation
DOI: https://doi.org/10.1007/1-4020-2541-6_8
Publisher Name: Springer, Dordrecht
Print ISBN: 978-90-481-6538-4
Online ISBN: 978-1-4020-2541-9
eBook Packages: Springer Book Archive