Abstract
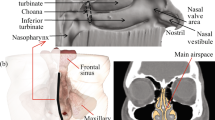
This article reviews the importance of topical nasal drug delivery in the management of chronic rhinosinusitis, in particular the delivery of topical drugs to the key area of the middle meatus. Administration methods of topical nasal drugs include powered nasal sprays, pumped nasal sprays and nasal drops — each method has perceived advantages and disadvantages. The ideal nasal delivery system would allow a large proportion of the drug to penetrate the nasal valve; would have significant drug delivery to the middle meatus and minimal delivery to the pharynx and lower respiratory tract; be consistent; and be user friendly.
Methods of assessment of intranasal drug distribution can be classified as quantitative or qualitative. Quantitative techniques include: imaging of radiolabels with gamma cameras; endoscopic photography of the anterior end of the middle turbinate; absorption of a dyed/labeled drug on a patty inserted in the middle meatus; and positron emission tomography. Each technique has limitations that must be borne in mind. Qualitative techniques for gathering information can also be used; however, the information obtained tends to be difficult to utilize to draw firm conclusions from.
There are a number of factors that have been studied to determine their effect on nasal drug distribution: the nasal valve; inferior turbinate hypertrophy and rhinitis; sniffing; administration devices; head position; and, importantly, inter- and intra-individual variability.
Future research needs to be directed toward the deposition and distribution characteristics of nasal drugs to the middle meatus, the carrying forward of preclinical research and hypotheses to prospective clinical trials (including trials investigating patient compliance with different devices), and the interaction between drugs and the nasal mucosa and mucus.




Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.References
Van Cauwenberge P, Watelet JB. Epidemiology of chronic rhinosinusitis. Thorax 2000; 55 Suppl. 2: S20–1
Drake-Lee AB. The physiology of the nose and paranasal sinuses. In: Kerr AG, editor. Scott-Brown’s otolaryngology. Vol. 1. Oxford: Butterworth-Heinemann, 1997: 3–5
Messerklinger W. Background and evolution of endoscopic sinus surgery. Ear Nose Throat J 1994; 73(7): 449–50
Stammberger H. Endoscopic endonasal surgery: concepts in treatment of recurring rhinosinusitis. Part I: anatomic and pathophysiologic considerations. Otolaryngol Head Neck Surg 1986; 94(2): 143–7
Drake-Lee AB. Nasal polyps. In: Kerr AG, editor. Scott-Brown’s otolaryngology. Vol. 4. Oxford: Butterworth-Heinemann, 1997: 1–16
Chalton R, Mackay I, Wilson R, et al. Double blind, placebo controlled trial of betamethasone nasal drops for nasal polyposis. BMJ 1985; 291: 788
Keith P, Nieminen J, Hollingworth K, et al. Efficacy and tolerability of fluticasone propionate nasal drops 400mg once daily compared with placebo for the treatment of bilateral polyposis in adults. Clin Exp Allergy 2000; 30(10): 1460–8
Dowley AC, Homer JJ. The effect of inferior turbinate hypertrophy on nasal spray distribution to the middle meatus. Clin Otolaryngol 2001; 26: 488–90
Newman SP, Moren F, Clarke SW. Deposition pattern from a nasal pump spray. Rhinology 1987; 25: 77–82
Homer JJ, Raine CH. An endoscopic photographic comparison of nasal drug delivery by aqueous spray. Clin Otolaryngol 1998; 23: 560–3
Bateman ND, Whymark AD, Clifton NJ, et al. A study of intranasal distribution of azelastine hydrochloride aqueous nasal spray with different spray techniques. Clin Otolaryngol 2002; 27: 327–30
Kayarkar R, Clifton NJ, Woolford TJ. An evaluation of the best head position for instillation of steroid nose drops. Clin Otolaryngol 2002; 27: 18–21
Tsikoudas A, Homer JJ. The delivery of topical nasal sprays and drops to the middle meatus: a semiquantitative analysis. Clin Otolaryngol 2001; 26: 294–7
Homer JJ, Maughan J, Burniston M. A quantitative analysis of the intranasal delivery of topical nasal drugs to the middle meatus: spray versus drop administration. J Laryngol Otol 2002; 116: 10–3
Berridge MS, Heald DL, Muswick GJ, et al. Biodistribution and kinetics of nasal carbon-11-triamcinolone acetonide. J Nucl Med 1998; 39: 1972–7
Bergstrom M, Cass LM, Valind S, et al. Deposition and disposition of [11C]zanamivir following administration as an intranasal spray: evaluation with positron emission tomography. Clin Pharmacokinet 1999; 36 Suppl. 1: 33–9
Hallworth GW, Padfield JM. A comparison of the regional deposition in a model nose of a drug discharged from metered aerosol and metered-pump nasal delivery systems. J Allergy Clin Immunol 1986; 77: 348–53
Kubba H, Spinou E, Robertson A. The effect of head position on the distribution of drops within the nose. Am J Rhinol 2000; 14: 83–6
Weber R, Keerl R, Radziwill R, et al. Videoendoscopic analysis of nasal steroid distribution. Rhinology 1999; 37: 69–73
Newman SP, Moren F, Clarke SW. Deposition pattern of nasal sprays in man. Rhinology 1988; 26: 111–20
Newman SP, Steed KP, Hardy JG, et al. The distribution of an intranasal insulin formulation in healthy volunteers: effect of different administration techniques. J Pharm Pharmacol 1994; 46: 657–60
Newman SP, Moren PF, Clarke SW. The nasal distribution of metered dose inhalers. J Laryngol Otol 1987; 101: 127–32
Hardy JG, Lee SW, Wilson CG. Intranasal drug delivery by spray and drops. J Pharm Pharmacol 1985; 37: 294–7
Aoki FY, Crowley JC. Distribution and removal of human serum albumin-technetium 99m instilled intranasally. Br J Clin Pharmacol 1976; 3: 869–78
Moren F, Bjornek K, Klint T, et al. A comparative distribution study of two procedures for administration of nose drops. Acta Otolaryngol 1988; 106: 286–90
Wilson R, Sykes DA, Chan KL, et al. Effect of head position on the efficacy of topical treatment of chronic mucopurulent rhinosinusitis. Thorax 1987; 42: 631–2
Gazis AG, Homer JJ, Henson DB, et al. The effect of six weeks topical nasal betamethasone drops on the hypothalamo-pituitary-adrenal axis and bone turnover in patients with nasal polyposis. Clin Otolaryngol 1999; 24: 495–8
Karagama YG, Lancaster JL, Karkanevatos A, et al. Delivery of nasal drops to the middle meatus: which is the best head position. Rhinology 2001; 39: 226–9
Bryant ML, Brown P, Gurevich N, et al. Comparison of the clearance of radiolabelled nose drops and nasal spray as mucosally delivered vaccine. Nucl Med Commun 1999; 20: 171–4
Acknowledgements
No sources of funding were used to assist in the preparation of this manuscript. The authors have no conflicts of interest that are directly relevant to the content of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Homer, J.J., Aggarwal, R. & Cordoza, A. Delivery of topical nasal drugs. Am J Drug Deliv 1, 125–131 (2003). https://doi.org/10.2165/00137696-200301020-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00137696-200301020-00004