Abstract
Allergic rhinitis, as a medical condition, merits attention because of its prevalence in the population as well as the substantial economic impact of treating it. By virtue of their efficacy and low adverse effect profile, intranasal corticosteroids have gained recognition by healthcare providers as the first-line therapy for allergic rhinitis. For managed care decision makers, the use of intranasal corticosteroids as the gold standard of treatment in allergic rhinitis makes comparative economic and humanistic (patient preference or health-related quality of life [HR-QOL]) data between the various intranasal corticosteroids increasingly important for formulary decisions.
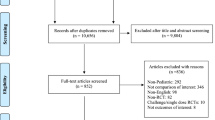
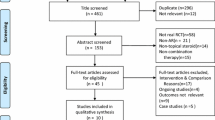
Although the equal efficacy and safety of intranasal corticosteroid products in the treatment of allergic rhinitis is well documented, research that compares the different economic and humanistic aspects of intranasal corticosteroid products is limited and less conclusive. In this article, we review published studies reporting pharmacoeconomic and humanistic analyses of intranasal corticosteroids in the treatment of allergic rhinitis and make recommendations for managed care decision makers in the selection of intranasal corticosteroids for allergic rhinitis. Based on inclusion/exclusion criteria, 15 pharmacoeconomic and 19 patient preference/HR-QOL studies were selected and reviewed.
The literature reviewed does not provide evidence of the superiority of a single intranasal corticosteroid product with respect to pharmacoeconomic, patient preference, or HR-QOL considerations. This finding is primarily owing to the lack of published head-to-head studies comparing pharmacoeconomic or humanistic outcomes between the different intranasal corticosteroids. Without further head-to-head studies on intranasal corticosteroids for the treatment of allergic rhinitis, cost minimization results may be the best decision strategy for managed care organizations (MCOs). Ideally, the results of cost-effectiveness or cost-utility studies comparing the different intranasal corticosteroids should guide the final formulary decision. In the absence of such studies, pharmacoeconomic and humanistic outcomes data from studies reported in the literature should be included into a pharmacoeconomic model, which considers the prevalence of allergic rhinitis in the MCOs to guide formulary inclusion decisions. Managed care decision makers will increasingly need to request this information from drug manufacturers if an informed, evidence-based decision is to be made.






Similar content being viewed by others
References
Law AW, Reed SD, Sundy JS, et al. Direct costs of allergic rhinitis in the United States: estimates from the 1996 medical expenditure panel survey. J Allergy Clin Immunol 2003; 111: 296–300
Crystal-Peters J, Crown WH, Goetzel RZ, et al. The cost of productivity losses associated with allergic rhinitis. Am J Manag Care 2000; 6: 373–8
Malone DC, Lawson KA, Smith DH, et al. A cost of illness study of allergic rhinitis in the United States. J Allergy Clin Immunol 1997; 99: 22–7
Trangsrud AJ, Whitaker AL, Small RE. Intranasal corticosteroids for allergic rhinitis. Pharmacotherapy 2002; 22: 1458–67
Corren J. Intranasal corticosteroids for allergic rhinitis: how do different agents compare? J Allergy Clin Immunol 1999; 104: S144–9
Blaiss MS. Efficacy, safety, and patient preference of inhaled nasal corticosteroids: a review of pertinent published data. Allergy Asthma Proc 2001; 22: S5–10
Dupclay Jr L, Doyle J. Assessment of intranasal corticosteroid use in allergic rhinitis: benefits, costs, and patient preferences. Am J Manag Care 2002; 8: S335–40
Weinstein SF. Combination therapy in the treatment of allergic rhinitis. Allergy Asthma Proc 2002; 23: 1–3
Keith PK, Haddon J, Birch S. A cost-benefit analysis using a willingness-to-pay questionnaire of intranasal budesonide for seasonal allergic rhinitis. Rhinocort Study Group. Ann Allergy Asthma Immunol 2000; 84: 55–62
Kozma CM, Schulz RM, Sclar DA, et al. A comparison of costs and efficacy of intranasal fluticasone propionate and terfenadine tablets for seasonal allergic rhinitis. Clin Ther 1996; 18: 334–46
Trotter JP. The treatment of seasonal allergic rhinitis: cost implications of pharmacotherapy for managed care. Manag Care Interface 2000; 13: 60–2
Stahl E, van Rompay W, Wang EC, et al. Cost-effectiveness analysis of budesonide aqueous nasal spray and fluticasone propionate nasal spray in the treatment of perennial allergic rhinitis. Ann Allergy Asthma Immunol 2000; 84: 397–402
Nash DB, Sullivan SD, Mackowiak J. Optimizing quality of care and cost effectiveness in treating allergic rhinitis in a managed care setting. Am J Manag Care 2000; 6: S3–15
Schramm B, Ehlken B, Smala A, et al. Cost of illness of atopic asthma and seasonal allergic rhinitis in Germany: 1-yr retrospective study. Eur Respir J 2003; 21: 116–22
Santos R, Cifaldi M, Gregory C, et al. Economic outcomes of a targeted intervention program: the costs of treating allergic rhinitis patients. Am J Manag Care 1999; 5: S225–34
McMenamin P. Costs of hay fever in the United States in 1990. Ann Allergy 1994; 73: 35–9
Ross RN. Hay fever: an expensive disease for American business. Am J Manag Care 1996; 2: 285–90
Roundtable discussion. The health and economic impact of rhinitis. Am J Manag Care 1997; 3: S8–S18
Storms WW, Meltzer E, Nathan RA, et al. The economic impact of allergic rhinitis. J Allergy Clin Immunol 1997; 99: S820–4
Liao E, Griffis D, Huse DM. Cost of treating allergic rhinitis and sinusitis with intranasal corticosteroids in a managed care population. J Man Care Med 2002; 6: 6–12
Bachert C, El-Akkad T. Patient preferences and sensory comparisons of three intranasal corticosteroids for the treatment of allergic rhinitis. Ann Allergy Asthma Immunol 2002; 89: 292–7
Kaliner MA. Patient preferences and satisfaction with prescribed nasal steroids for allergic rhinitis. Allergy Asthma Proc 2001; 22: S11–5
Gerson I, Green L, Fishken D. Patient preference and sensory comparisons of nasal spray allergy medications. J Sens Stud 1999; 14: 491–6
Ciprandi G, Canonica WG, Grosclaude M, et al. Effects of budesonide and fluticasone propionate in a placebo-controlled study on symptoms and quality of life in seasonal allergic rhinitis. Allergy 2002; 57: 586–91
Naclerio RM, Baroody FM, Bidani N, et al. A comparison of nasal clearance after treatment of perennial allergic rhinitis with budesonide and mometasone. Otolaryngol Head Neck Surg 2003; 128: 220–7
Shah SR, Miller C, Pethick N, et al. Two multicenter, randomized, single-blind, single-dose, crossover studies of specific sensory attributes of budesonide aqueous nasal spray and fluticasone propionate nasal spray. Clin Ther 2003; 25: 2198–214
Hughes K, Glass C, Ripchinski M, et al. Efficacy of the topical nasal steroid budesonide on improving sleep and daytime somnolence in patients with perennial allergic rhinitis. Allergy 2003; 58: 380–5
Lumry W, Hampel F, LaForce C, et al. A comparison of once-daily triamcinolone acetonide aqueous and twice-daily beclomethasone dipropionate aqueous nasal sprays in the treatment of seasonal allergic rhinitis. Allergy Asthma Proc 2003; 24: 203–10
Gross G, Jacobs RL, Woodworth TH, et al. Comparative efficacy, safety, and effect on quality of life of triamcinolone acetonide and fluticasone propionate aqueous nasal sprays in patients with fall seasonal allergic rhinitis. Ann Allergy Asthma Immunol 2002; 89: 56–62
Grubbe R, Adelglass JM, Casale TB, et al. Intransal therapy with once daily triamcinolone acetonide aerosol versus twice daily beclomethasone dipropionate aqueous spray in patients with perennial allergic rhinitis. Curr Ther Res 1996; 57: 825–38
Adamopoulos G, Manolopoulos L, Giotakis I. A comparison of the efficacy and patient acceptability of budesonide and beclomethasone dipropionate aqueous nasal sprays in patients with perennial rhinitis. Clin Otolaryngol 1995; 20: 340–4
Saengpanich S, de Tineo M, Naclerio RM, et al. Fluticasone nasal spray and the combination of loratadine and montelukast in seasonal allergic rhinitis. Arch Otolaryngol Head Neck Surg 2003; 129: 557–62
Kaszuba SM, Baroody FM, de Tineo M, et al. Superiority of an intranasal cortico-steroid compared with an oral antihistamine in the as-needed treatment of seasonal allergic rhinitis. Arch Intern Med 2001; 161: 2581–7
Condemi J, Schulz R, Lim J. Triamcinolone acetonide aqueous nasal spray versus loratadine in seasonal allergic rhinitis: efficacy and quality of life. Ann Allergy Asthma Immunol 2000; 84: 533–8
Ratner PH, van Bavel JH, Martin BG, et al. A comparison of the efficacy of fluticasone propionate aqueous nasal spray and loratadine, alone and in combination, for the treatment of seasonal allergic rhinitis. J Fam Pract 1998; 47: 118–25
Craig TJ, Mende C, Hughes K, et al. The effect of topical nasal fluticasone on objective sleep testing and the symptoms of rhinitis, sleep, and daytime somnolence in perennial allergic rhinitis. Allergy Asthma Proc 2003; 24: 53–8
Jen A, Baroody F, de Tineo M, et al. As-needed use of fluticasone propionate nasal spray reduces symptoms of seasonal allergic rhinitis. J Allergy Clin Immunol 2000; 105: 732–8
Craig TJ, Teets S, Lehman EB, et al. Nasal congestion secondary to allergic rhinitis as a cause of sleep disturbance and daytime fatigue and the response to topical nasal corticosteroids. J Allergy Clin Immunol 1998; 101: 633–7
Potter PC, Van Niekerk CH, Schoeman HS. Effects of triamcinolone on quality of life in patients with persistent allergic rhinitis. Ann Allergy Asthma Immunol 2003; 91: 368–74
Juniper EF. Rhinitis management: the patient’s perspective. Clin Exp Allergy 1998; 28 Suppl. 6: 34–8
Sullivan SD, Lyles A, Luce B, et al. AMCP guidance for submission of clinical and economic evaluation data to support formulary listing in US health plans and pharmacy benefit management organizations. J Manag Care Pharm 2001; 7: 272–82
Mather DB, Sullivan SD, Augenstein D, et al. Incorporating clinical outcomes and economic consequences into drug formulary decisions: a practical approach. Am J Manag Care 1999; 5: 277–85
Langley PC. Formulary submission guidelines for Blue Cross and Blue Shield of Colorado and Nevada. Structure, application and manufacturer responsibilities. Pharmacoeconomics 1999; 16: 211–24
Acknowledgments
We would like to thank Chris Brunder, Sarah Herbert, Hui Li, and Xiaomin Ruan of the Economics department at the University of New Mexico for their assistance in locating and copying the articles used in this review. No sources of funding were used to assist in the preparation of this manuscript. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gupchup, G.V., Borrego, M.E., Santos, R. et al. Economic, Patient Preference, and Health-Related Quality of Life Considerations for Intranasal Corticosteroids in Allergic Rhinitis. Dis-Manage-Health-Outcomes 13, 169–184 (2005). https://doi.org/10.2165/00115677-200513030-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00115677-200513030-00003