Summary
Abstract
Liposomal doxorubicin (Myocet™) has been developed with the aim of improving the therapeutic index of the anthracycline doxorubicin by reducing the drug’s cardiotoxicity, but maintaining its antitumor efficacy. Liposomal doxorubicin plus cyclophosphamide is no less effective than conventional doxorubicin or epirubicin plus cyclophosphamide in the first-line treatment of women with metastatic breast cancer. In addition, the drug is significantly less cardiotoxic than conventional doxorubicin. The median cumulative dose to the first cardiac event for liposomal doxorubicin is higher than that for conventional doxorubicin; the potential to use doxorubicin for a longer period of time may benefit a number of patients. Liposomal doxorubicin has also shown potential as part of a chemotherapy regimen in patients with non-Hodgkin lymphoma (NHL). Thus, liposomal doxorubicin represents an important advance in terms of offering reduced cardiotoxicity compared with conventional doxorubicin, and can be used in preference to conventional doxorubicin in a combination regimen with cyclophosphamide in the first-line treatment of patients with metastatic breast cancer.
Pharmacologic Properties
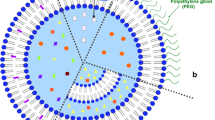
The rationale used as the basis for the design of intravenous liposomal doxorubicin is that the liposomes cannot exit the circulation in healthy tissues, but can exit through leaky tumor-associated vessels. In animal models, liposomal doxorubicin is less cardiotoxic than conventional doxorubicin, and reduces mammary tumor growth to a significantly greater extent than conventional doxorubicin.
Encapsulation of doxorubicin markedly alters the pharmacokinetic properties of the drug. In patients with metastatic breast cancer, the area under the plasma concentration-time curve and maximum plasma concentration values for total doxorubicin were higher in liposomal doxorubicin recipients than conventional doxorubicin recipients, while the volume of distribution and clearance of total doxorubicin were lower with liposomal doxorubicin.
In an animal model, liposomal doxorubicin was still delivered effectively to tumors; however, compared with conventional doxorubicin, the peak distribution to the heart and gastrointestinal mucosa was reduced.
Metabolism and elimination of doxorubicin occur via the hepatobiliary route. The main metabolite is doxorubicinol. The pharmacokinetic properties of this metabolite following the administration of liposomal doxorubicin suggest that the drug is metabolized in a similar manner to, but at a slower rate than, conventional doxorubicin.
Therapeutic Efficacy
The efficacy of liposomal doxorubicin, as monotherapy or in combination with cyclophosphamide, has been assessed as first-line therapy in three fully-published, randomized, multicenter trials (all n ≥160) in women with metastatic breast cancer.
Liposomal doxorubicin was no less effective than conventional doxorubicin as monotherapy in terms of objective tumor response rates (both 26%), median time to disease progression (TTP), or median time to treatment failure (TTF).
Likewise, in terms of objective response rates, liposomal doxorubicin plus cyclophosphamide was no less effective than comparator combination regimens of conventional doxorubicin plus cyclophosphamide (43% vs 43%) or epirubicin plus cyclophosphamide (46% vs 39%). The median TTP and TTF with liposomal doxorubicin plus cyclophosphamide were non-inferior to those with conventional doxorubicin plus cyclophosphamide and significantly longer than those for epirubicin plus cyclophosphamide. In a subgroup of patients who had received prior adjuvant doxorubicin, the response rate and median TTF, but not median TTP or overall survival duration, were significantly greater for patients who received liposomal doxorubicin than conventional doxorubicin (both as monotherapy or in combination with cyclophosphamide).
Additionally, in small phase I/II and II trials in patients with locally advanced or metastatic breast cancer, liposomal doxorubicin has shown anti-tumor activity in combination with trastuzumab, paclitaxel and trastuzumab, gemcitabine and docetaxel, and cyclophosphamide and fluorouracil.
Liposomal doxorubicin shows potential as a replacement for conventional doxorubicin as part of commonly used chemotherapy regimens in patients with NHL; in three phase I/II or II trials, the overall response rate was >80%.
Tolerability
Liposomal doxorubicin was generally at least as well tolerated as conventional doxorubicin (both as monotherapy or in combination with cyclophosphamide) or epirubicin (in combination with cyclophosphamide) in three trials in patients with metastatic breast cancer. Neutropenia (absolute neutrophil count of <500 cells/μL) was the most common hematologic toxicity in both treatment arms of all trials (50–87%). The incidence of neutropenia with liposomal doxorubicin plus cyclophosphamide was lower than that with conventional doxorubicin plus cyclophosphamide but greater than that with epirubicin plus cyclophosphamide. Anemia was also common (14–27% of patients) in all arms of all trials.
Nausea/vomiting was the most common severe non-hematologic adverse event in all trials (13–24%); however, the incidence of this event did not differ significantly between treatment arms. In one trial, grade 3 stomatitis/mucositis was more frequent with liposomal doxorubicin plus cyclophosphamide than with epirubicin plus cyclophosphamide, while injection site toxicity was less common in liposomal doxorubicin plus cyclophosphamide recipients. Severe palmar-plantar erythrodysesthesia, which is a dose-limiting toxicity with pegylated liposomal doxorubicin, was not observed in any liposomal doxorubicin recipients.
Liposomal doxorubicin was significantly less cardiotoxic than conventional doxorubicin (both as monotherapy or in combination with cyclophosphamide). The incidence of congestive heart failure or protocol-defined cardiotoxicity compared with basal resting left ventricular ejection fraction was reduced >2-fold with liposomal doxorubicin monotherapy and >3-fold with liposomal doxorubicin plus cyclophosphamide.





Similar content being viewed by others
References
Assersohn L, Johnston SRD. Postmenopausal metastatic breast cancer: a review of first-line treatment options. Am J Cancer 2003; 2(2): 95–109
Marty M. Liposomal doxorubicin (Myocet) and conventional anthracyclines: a comparison. Breast 2001; 10 Suppl. 2: 28–33
Safra T. Cardiac safety of liposomal anthracyclines. Oncologist 2003, 8 Suppl. 2: 17–24
Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin. Cancer 2003Jun 1; 97(11): 2869–79
Batist G, Barton J, Chaikin P, et al. Myocet (liposome-encapsulated doxorubicin citrate): a new approach in breast cancer therapy. Expert Opin Pharmacother 2002Dec; 3(12): 1739–51
Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf 2000Apr; 22(4): 263–302
Elan. Myocet: summary of product characteristics [online]. Available from URL: http://http://www.emea.eu.it [Accessed 2004 Nov 3]
Batist G. Improving the therapeutic index when using Myocet in the treatment of metastatic breast cancer. Breast 2001; 10Suppl. 2: 16–21
Swenson CE, Perkins WR, Roberts P, et al. Liposome technology and the development of Myocet™ (liposomal doxorubicin citrate). Breast 2001; 10Suppl. 2:1–7
Mayer LD, Bally MB, Cullis PR, et al. Comparison of free and liposome encapsulated doxorubicin tumor drug uptake antitumor efficacy in the SC115 murine mammary tumor. Cancer Lett 1990; 53: 183–90
Swenson CE, Bolcsak LE, Batist G, et al. Pharmacokinetics of doxorubicin administered i.v. as Myocet (TLC D-99; liposome-encapsulated doxorubicin citrate) compared with conventional doxorubicin when given in combination with cyclophosphamide in patients with metastatic breast cancer. Anticancer Drugs 2003Mar; 14: 239–46
Mross K, Niemann B, Massing U, et al. Pharmacokinetics of liposomal doxorubicin (TLC-D99; Myocet) in patients with solid tumors: an open-label, single-dose study. Cancer Chemother Pharmacol 2004Aug; 54: 514–24
Batist G, Ramakrishnan G, Rao CS, et al. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J Clin Oncol 2001Mar 1; 19: 1444–54
EMEA. Myocet: scientific discussion [online]. Available from URL: http://http://www.emea.eu.it [Accessed 2005 Feb 14]
Roberts P, Janoff AS, Mayhew E. Doxorubicin fluorescence in a human breast carcinoma (MX-1), after treatment with liposome-associated or free doxorubicin [abstract no. 3327]. Proc Am Assoc Cancer Res 2000; 41: 522
Hamilton A, Biganzoli L, Coleman R, et al. EORTC 10968: a phase I clinical and pharmacokinetic study of polyethylene glycol liposomal doxorubicin (Caelyx®, Doxil®) at a 6-week interval in patients with metastatic breast cancer. Ann Oncol 2002; 13: 910–8
A phase I multicentre clinical and pharmacokinetic study of Myocet™ (liposomal doxorubicin) in combination with cyclophosphamide in patients with metastatic breast cancer who have normal or abnormal liver biochemistry. Oxford: Zeneus Pharma Ltd, 2005. (Data on file)
Iannitti D, Rathore R, Safran H, et al. TLC D-99 can be safely administered to patients with advanced hepatobiliary carcinomas and severe hepatobiliary dysfunction [abstract no. 1214]. Proc Am Soc Clin Oncol 2000May 20; 19
Harris L, Batist G, Belt R, et al. Liposome-encapsulated doxorubicin compared with conventional doxorubicin a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer 2002Jan 1; 94: 25–36
Chan S, Davidson N, Juozaityte E, et al. Phase III trial of liposomal doxorubicin and cyclophosphamide compared with epirubicin and cyclophosphamide as first-line therapy for metastatic breast cancer. Ann Oncol 2004Oct; 15(10): 1527–34
Batist G, Harris L, Azarnia N, et al. Improved therapeutic index of TLC D-99 (liposome-encapsulated doxorubicin) compared to free doxorubicin in first-line treatment of metastatic breast cancer in patients who had received prior adjuvant doxorubicin [abstract no. 405]. Proc Am Soc Clin Oncol 2000; 19: 105a
Theodoulou M, Campos SM, Batist G, et al. TLC D99 (D, Myocet) and herceptin (H) is safe in advanced breast cancer (ABC): final cardiac safety and efficacy analysis [abstract no. 216]. Proc Am Soc Clin Oncol 2002; 21: 55a
Cortes J, Climent M, Lluch A, et al. Updated results of a phase II study (M77035) of Myocet® combined with weekly Herceptin ®and paclitaxel in patients (pts) with HER2-positive locally advanced or metastatic breast cancer (LABC/MBC) [abstract no. 3041]. Breast Cancer Res Treat 2004; 88Suppl. 1: 125–126. Plus poster presented at the 27th Annual San Antonio Breast Cancer Symposium; 2004 Dec 8–11; San Antonio (TX)
Schmid P, Krocker J, Dieing A, et al. Gemcitabine, myocet and docetaxel for primary treatment of breast cancer [abstract no. v269]. Onkologie 2003; 26 Suppl. 5: 22
Valero V, Buzdar AU, Theriault RL, et al. Phase II trial of liposome-encapsulated doxorubicin, cyclophosphamide, and fluorouracil as first-line therapy in patients with metastatic breast cancer. J Clin Oncol 1999May; 17: 1425–34
National Cancer Institute. Adult non-Hodgkin’s lymphoma (PDQ®): treatment - health professional version [online]. Available from URL: http://http://www.cancer.gov [Accessed 2004 Sep 29]
Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large B-cell lymphoma. N Engl J Med 2002Jan 24; 346(4): 235–42
Levine AM, Tulpule A, Espina B, et al. Liposome-encapsulated doxorubicin in combination with standard agents (cyclophosphamide, vincristine, prednisone) in patients with newly diagnosed AIDS-related non-Hodgkin’s lymphoma: results of therapy and correlates of response. J Clin Oncol 2004Jul 1; 22(13): 2662–70
Tulpule A, Espina BM, Berman N, et al. A phase I/II trial of liposomal doxorubicin (TLC-D99, myocet) in combination with cyclophosphamide, vincristine, and prednisone (COMP) for newly diagnosed intermediate and high grade non-Hodgkin’s lymphoma (NHL) [abstract no. 329]. Eur J Cancer 2001Oct; 37 Suppl. 6: S91. Plus poster presented at the European Cancer Conference; 2001 Oct 21–25; Lisbon
Federico M, Dyer MJS, Caballero MD, et al. The MYOCAN study: a phase II study of cyclophosphamide, oncovin, Myocet™, and prednisone plus rituximab (R-COMP) in the treatment of elderly patients with diffuse large B-cell non-Hodgkin lymphoma (DLBCL) [abstractno. 4586]. Blood 2004Nov 16; 104(11Pt 2): 230b
Cancer Care Ontario Guidelines Initiative. Epirubicin, as a single agent or in combination, for metastatic breast cancer: practice guideline report #1-6 (update) [online]. Available from URL: http://http://www.cancercare.on.ca [Accessed 2005 May 12]
Barcelon: Zeneus Pharma Ltd, 2005. (Data on file)
American Cancer Society. Cancer facts & figures 2005 [online]. Available from URL: http://http://www.cancer.org [Accessed 2005 Jan 21]
National Comprehensive Cancer Network. Clinical practice guidelines in oncology - version 1.2005: breast cancer [online]. Available from URL: http://http://www.nccn.org [Accessed 2005 Jan 21]
Chung CT, Carlson RW. Goals and objectives in the management of metastatic breast cancer. Oncologist 2003; 8: 514–20
European Society for Medical Oncology. Minimal clinical recommendations for diagnosis, treatment and follow-up of locally recurrent or metastatic breast cancer (MBC) [online]. Available from URL: http://http://www.esmo.org [Accessed 2005 Jan 21]
National Cancer Institute. Breast cancer (PDQ®): treatment: health professional version [online]. Available from URL: http://http://www.cancer.gov [Accessed 2004 Sep 29]
Crown J, Dieras V, Kaufmann M, et al. Chemotherapy for metastatic breast cancer: report of a European expert panel. Lancet Oncol 2002Dec; 3: 719–26
Rivera E. Liposomal anthracyclines in metastatic breast cancer: clinical update. Oncologist 2003; 8Suppl. 2: 3–9
O’Brien MER, Wigler N, Inbar M, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HC1 (CAELYX™/Doxil®) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol 2004; 15: 440–9
Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344(11): 783–92
Shapiro CL, Ervin T, Welles L, et al. Phase II trial of high-dose liposome-encapsulated doxorubicin with granulocyte colony-stimulating factor in metastatic breast cancer. J Clin Oncol 1999May; 17: 1435–41
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dando, T.M., Keating, G.M. Liposomal Doxorubicin. Am J Cancer 4, 193–206 (2005). https://doi.org/10.2165/00024669-200504030-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00024669-200504030-00006