Abstract
Shear strength of saturated, remolded clay mineral samples was measured in an attempt to relate strength to forces acting between clay particles. The fine fractions of the clays, purified when necessary, were consolidated to different void ratios and subjected to quick, translatory shear in a direct shear device. Relative values for shear strength were obtained in this way. Interparticle forces in the clays were altered and the effect on shear strength was observed.
For a high-swelling, Na-saturated montmorillonite, strength at constant void ratio decreased with increasing salt concentration. To explain the decrease in strength with decrease in force of repulsion between particles a model is suggested where strength results from the force of repulsion resisting displacement of particles in the shear plane. Addition of small amounts of dispersing and of soil-conditioning chemicals had little effect on shear strength.
Calcium montmorillonite had a lower strength than sodium montmorillonite when compared at the same void ratios. This is related to the lower surface area of interaction and lower repulsion found with divalent ions.
Water content at the liquid limit showed the same dependence upon interparticle forces in the montmorillonite clays as did shear strength.
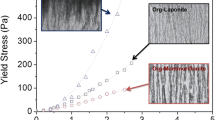
In kaolinite, interparticle forces of attraction result in a structure or particle arrangement which has the major influence on shear strength. A clay at low pH which was flocculated in a network, edge-to-face structure owing to presence of positive edge-charge had higher strength than a dispersed clay, but a clay flocculated by electrolyte which produced a lamellar, face-to-face structure had a lower strength.
Similar content being viewed by others
References
Aylmore, L. A. G. and Quirk, J. P. (1959) Swelling of clay-water systems: Nature, v. 183, pp. 1752–1753.
Blackmore, A. V. and Warkentin, B. P. (1960) Swelling of calcium montmorillonite: Nature, v. 186, pp. 823–824.
Lambe, T. W. (1951) Soil Testing for Engineers: John Wiley & Sons, New York, 165 pp.
Lambe, T. W. (1958) The engineering behavior of compacted clay: J. Soil Mech. Found. Div., Proc. Amer. Soc. Civil Engineers, v. 84, no. SM2, 35 pp.
Rosenqvist, I. Th. (1959) Physico-chemical properties of soils: Soil-water systems: J. Soil Mech. Found. Div., Proc. Amer. Soc. Civil Engineers, v. 85, no. SM2, pp. 31–35.
Schofield, R. K. and Samson, H. R. (1955) Flocculation of kaolinite due to the attraction of oppositely charged crystal faces: Disc. Faraday Soc, no. 18, pp. 135–145.
Warkentin, B. P., Bolt, G. H. and Miller, R. D. (1957) Swelling pressure of montmorillonite: Soil Sci. Soc. Amer. Proc., v. 21, pp. 495–497.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Warkentin, B.P., Yong, R.N. Shear Strength of Montmorillonite and Kaolinite Related to Interparticle Forces. Clays Clay Miner. 9, 210–218 (1960). https://doi.org/10.1346/CCMN.1960.0090111
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.1960.0090111