Abstract
Background
The spatiotemporal epidemiological evidence supporting joint endoscopic screening for esophageal cancer (EC) and gastric cancer (GC) remains limited. This study aims to identify combined high-risk regions for EC and GC and determine optimal areas for joint and separate endoscopic screening.
Methods
We analyzed the association of incidence trends between EC and GC in cancer registry areas across China from 2006 to 2016 using spatiotemporal statistical methods. Based on these analyses, we divided different combined risk regions for EC and GC to implement joint endoscopic screening.
Results
From 2006 to 2016, national incidence trends for both EC and GC showed a decline, with an average annual percentage change of -3.15 (95% confidence interval [CI]: -5.33 to -0.92) for EC and -3.78 (95% CI: -4.98 to -2.56) for GC. A grey comprehensive correlation analysis revealed a strong temporal association between the incidence trends of EC and GC, with correlations of 79.00% (95% CI: 77.85 to 80.14) in males and 77.62% (95% CI: 76.50 to 78.73) in females. Geographic patterns of EC and GC varied, demonstrating both homogeneity and heterogeneity across different regions. The cancer registry areas were classified into seven distinct combined risk regions, with 33 areas identified as high-risk for both EC and GC, highlighting these regions as priorities for joint endoscopic screening.
Conclusion
This study demonstrates a significant spatiotemporal association between EC and GC. The identified combined risk regions provide a valuable basis for optimizing joint endoscopic screening strategies for these cancers.
Similar content being viewed by others
Background
Upper gastrointestinal cancer (UGIC), including esophageal cancer (EC) and gastric cancer (GC), impose a significant global burden [1]. In 2022, there were 511,054 new cases of EC and 968,764 new cases of GC worldwide, with China contributing to 37.02% of EC cases and 43.83% of GC cases, respectively [2, 3]. Consequently, China bears the heaviest burden of UGIC globally, with the highest number of cases and deaths.
Higher incidences of both cancers are observed in the Taihang Mountains and Huaihe Basin, while the Kazakh-inhabited Xinjiang region has a higher incidence of EC. In contrast, Northwest China (e.g., Gansu and Qinghai provinces) and East Shandong show higher incidences of GC [4]. These findings reveal distinct geographical patterns of EC and GC, with notable overlaps and associations, suggesting shared risk factors and the potential for joint early prevention and screening.
The geographical distribution of EC and GC exhibits both similarities and differences worldwide. East Asia reports the highest incidence rates for both cancers, with elevated rates also observed in southeastern Africa and western South America [5]. The concentrating and deviating patterns of EC and GC vary by subsite and location across specific regions, demonstrating both positive and inverse correlations [6]. For example, Mongolia and China rank among the top five countries globally in terms of EC and GC incidence, with rates exceeding 10.8 per 100,000 persons. In contrast, Japan has a higher incidence of EC relative to GC, whereas South Korea exhibits the opposite trend. Similarly, South Africa reports a significantly higher incidence of EC compared to GC, with a difference exceeding tenfold [6, 7]. Our previous research identified common high-risk regions for EC and GC in China and quantified the associated risk levels. However, regional disparities and similarities in risk persist [4]. Higher incidences of both cancers are observed in the Taihang Mountains and Huaihe Basin, while the Kazakh-inhabited Xinjiang region has a higher incidence of EC. In contrast, Northwest China (eg, Gansu and Qinghai provinces) and East Shandong have higher incidences of GC [4]. These findings indicated distinct geographical patterns of EC and GC, with notable overlaps and associations, suggesting shared risk factors and the potential for joint early prevention and screening.
Since the 1970s, China has implemented early endoscopic screening programs for EC and GC and intensified research on UGIC in high-risk areas [8]. Prior studies have predominantly focused on high-risk regions for EC or GC separately, neglecting their geographical co-occurrence patterns [9, 10]. Despite being different cancer types, EC and GC share anatomical proximity and several analogous risk factors. Furthermore, early screening for both cancers can be conducted collectively using endoscopy. In 2015, the National Cancer Center of China (NCCC) launched a multi-center joint screening program for EC and GC to assess the cost-effectiveness of joint screening among residents aged 40–69 years in six regions of China, reporting 23% and 57% reductions in UGIC incidence and mortality rates, respectively [11]. Our previous studies have indicated that biennial joint endoscopic screening for EC and GC could be an optimal strategy in common high-risk areas, supporting its implementation in China [12, 13]. Nevertheless, limited research has explored the temporal and spatial associations between EC and GC, leaving unanswered questions regarding the necessity to expand screening regions and the identification of priority areas for joint endoscopic screening.
Therefore, this study aims to elucidate the temporal and spatial associations between EC and GC, and accurately divide different combined risk regions. The findings will provide robust evidence for targeting high-risk areas and inform the rational planning and implementation of joint endoscopic screening programs for these cancers in China.
Methods
Data source
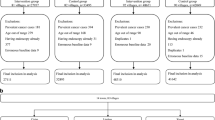
The data for this study were obtained from the China Cancer Annual Report, issued by the NCCC. This report provides comprehensive and standardized annual data on cancer incidence and mortality, stratified by sex and across various regions of China. Since 2005, population-based cancer statistics have been reported to the NCCC by local cancer registries, which collect data from hospitals, community health centers, basic medical insurance systems, the new rural cooperative medical system, and death surveillance centers [14]. The data collection process adheres to the Guidelines for China Cancer Registration, the standards of the International Agency for Research on Cancer (IARC), and the Cancer Incidence in Five Continents (CI5) series [15]. The NCCC ensures data quality through rigorous evaluation of comparability, completeness, and validity using multiple indicators, including the mortality-to-incidence ratio, the percentage of morphologically verified cases, the percentage of death certificate-only cases, and the stability of cancer trends over time. Although the cancer registration system has improved over time, regional disparities and discontinuities in data remain due to the varying number of cancer registries. In 2006, there were 34 cancer registration areas (CRAs), expanding to 487 by 2016, with 31 CRAs providing continuous data throughout the study period. Detailed information on the number and population coverage of these CRAs is provided in Additional file 1: Table S1 and S2.
We extracted data on EC and GC using the International Classification of Diseases, 10th Revision (ICD-10) codes C15 and C16, respectively. The dataset included annual case counts, crude incidence rates, and age-standardized incidence rates (ASIRs) adjusted using Segi’s world standard population, stratified by sex and urban–rural classification. According to China’s urban–rural administrative division guidelines, a CRA in a county-level city is classified as rural, while a CRA in a prefecture-level city or municipal district is classified as urban. To ensure consistency in administrative region names over time, we merged and matched all CRAs based on year and identical administrative division codes, enabling accurate integration and analysis across different regions in China.
To address potential deviations in geographical locations caused by administrative division changes, we calibrated these deviations by CRA location and year. First, we matched CRA locations with administrative divisions using identical codes and then extracted matched CRAs. Unmatched CRAs were calibrated by revising names or codes and re-matching them. For administrative division adjustments (e.g., separations or mergers) in specific years, we aligned CRA locations using corresponding codes. Name changes were addressed by updating codes based on the latest administrative names and matching them again. Ultimately, we integrated the matched CRA locations and administrative divisions to establish the Geographical Information Database of EC and GC in China from 2006 to 2016. More details on this process are provided in Additional file 2:Fig. S1 and S2.
Statistical analysis
Temporal association between EC and GC
To assess overall trends in ASIRs for EC and GC, we calculated the average annual percentage change (AAPC) using linear logarithmic regression, with ASIR as the dependent variable and year as the independent variable. Additionally, the annual percentage change (APC), a geometrically weighted average of the annual percentage changes, was computed to identify the year with the most significant changes during the study period. We used the Joinpoint regression model to analyze both overall and segmented trends in ASIRs for EC and GC, stratified by sex, at the national and CRA levels from 2006 to 2016 (Joinpoint Regression Program, Version 4.5.0.1, Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute, Rockville, MD, USA). A significant upward trend was defined as APC or AAPC > 0 with the lower bound of the 95% confidence interval (CI) > 0 (P < 0.05). Conversely, a significant downward trend was defined as APC or AAPC < 0 with the upper bound of the 95% CI < 0 (P < 0.05). If neither condition was met, the trend was considered non-significant. For instance, an APC or AAPC of 0.1 would indicate an annual or average annual increase of 0.1% over the period. The principles and interpretations of APC and AAPC are discussed in a previous study [16]. Subsequently, the temporal association between EC and GC from 2006 to 2016 was examined using the grey comprehensive correlation (GCC), a summary indicator measuring temporal associations over a fixed period. GCC is a weighted measure of grey absolute correlation (GAC) and grey relative correlation (GRC), indicating the concordance and fluctuation association between EC and GC, with values ranging from 0 to 100%. Due to non-continuous CRA data between 2006 and 2016, we selected data with at least three years for temporal association analysis by sex. Detailed GCC calculations are provided in Additional file 2 (p3-p4) and are described elsewhere [17].
Spatial association between EC and GC
We mapped the geographical distribution of ASIRs for EC and GC by sex from 2006 to 2016, using the ASIR in 2016 as a reference. Moran’s I index was used to analyze the spatial patterns of EC and GC in adjacent areas, determining whether the spatial distribution was clustered, dispersed, or random. A Moran’s I > 0 with P < 0.05 suggests a clustered pattern, while a Moran’s I < 0 with P < 0.05 indicates a dispersed pattern. A random pattern is indicated when neither condition is met. This analysis was conducted using ArcGIS 10.6, with further details provided in Additional file 2 (p5). Given the large population and extensive CRA coverage in 2016, we calculated the relative risk (RR) of EC and GC for that year to assess their spatial association. A concordant direction between RREC and RRGC suggests a homogeneous association (both high-risk or low-risk regions) between EC and GC, while an opposite direction suggests a heterogeneous association (separately high-risk or low-risk regions). Spatial scan statistics were employed using SatScanTM 9.6.1, with detailed methods described in Additional file 2 (p6-p7).
Combined risk regions of EC and GC
We classified combined risk regions for EC and GC by sex using the ratio of ASIR at each CRA to the national level, providing insights into prioritizing areas for endoscopic screening. The combined risk regions were classified as follows: double high-risk EC and GC, single high-risk EC, single high-risk GC, double middle-risk EC and GC, middle-risk EC and low-risk GC, middle-risk GC and low-risk EC, and double low-risk EC and GC. Classification methods and definitions are detailed in Additional file 2: Fig. S3, and Table S1.
Results
Temporal trends between EC and GC
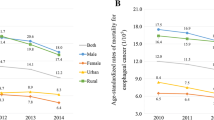
As shown in Fig. 1, the ASIR of EC and GC for both sexes combined increased nationally from 2006 to 2010 (EC: APC = 6.75, 95% CI: 1.20 to 12.61; GC: APC = 1.45, 95% CI: -1.55 to 4.54). Following this period, there was a significant decrease in EC (APC = -6.01, 95% CI: -7.41 to -4.57) and GC (APC = -5.45, 95% CI: -6.31 to -4.56). When stratified by sex, this pattern remained consistent. Notably, the incidence trends differed between urban and rural areas. In urban areas, the ASIR of EC and GC initially rose before declining, whereas in rural areas, a linear decrease was observed throughout the study period. This trend persisted after further stratification by sex in both urban and rural regions.
The annual percentage change (APC) in the ASIR of EC and GC by sex and urban–rural types from 2006 to 2016 in China (A, B and C denote whole population, males and females at national level, respectively; D, E and F denote whole population, males and females in urban area, respectively; G, H and I denote whole population, males and females in rural area, respectively)
As presented in Fig. 2, the ASIR for both EC and GC combined showed a significant decline from 2006 to 2016. The ASIR for EC decreased from 13.11 per 100,000 persons in 2006 to 11.46 per 100,000 persons in 2016, with an AAPC of -3.15 (95% confidence interval [CI]: -5.33 to -0.92). Similarly, the ASIR for GC declined from 24.14 per 100,000 persons in 2006 to 17.62 per 100,000 persons in 2016, with an AAPC of -3.78 (95% CI: -4.98 to -2.56). This consistent downward trend was observed in both males and females over the same period.
Table 1 presents the AAPC in ASIR for EC and GC across 31 CRAs with continuous data from 2006 to 2016. Most CRAs exhibited a decline in ASIR for both EC and GC (AAPC < 0), with the exception of Fusui. Specifically, significant reductions in ASIR for EC were observed in 25 CRAs for males and 23 CRAs for females, while GC rates significantly decreased in 23 CRAs for males and 20 CRAs for females. The most notable decrease in ASIR for EC among males was recorded in Jianhu, with an AAPC of -8.73 (95% CI: -11.38 to -6.00), while Nangang showed the largest decrease among females, with an AAPC of -14.35 (95% CI: -23.46 to -4.16). For GC, the largest declines were observed in Jianhu and Dafeng, with AAPCs of -7.85 (95% CI: -9.59 to -6.07) for males and -10.29 (95% CI: -13.94 to -6.48) for females, respectively. Detailed data can be found in Table 1.
Temporal association between EC and GC
As shown in Table 2, from 2006 to 2016, the average GAC, GRC, GCC between EC and GC for males were 73.63% (95CI: 72.12 to 75.14), 84.37% (95CI: 83.19 to 85.55) and 79.00% (95CI: 77.85 to 80.14). For females, these correlation values were 73.81% (95% CI: 72.33 to 75.29), 81.42% (95% CI: 80.07 to 82.78), and 77.62% (95% CI: 76.50 to 78.37), respectively. These findings indicate significant associations in the trends of concordance, fluctuation, and comprehensiveness between EC and GC.
Geographical patterns of between EC and GC
The geographical patterns of EC and GC over time are presented in Additional File 2 (Fig. S4-S7). Figure 3 exhibits the geographical patterns of the ASIR for EC and GC, both of which exhibit spatial clusters (Moran’s I > 0 and P < 0.01), indicating a significant spatial association of EC and GC with that in adjacent areas.Additionally, the temporal variations in Moran’s I for both cancers over the period suggest consistent spatial clustering trends, reflecting temporal concordance in their spatial associations.
Spatial association of EC and GC
Figure 4 shows substantial geographical variation in the spatial association between EC and GC, reflecting both homogeneous and heterogeneous risk patterns across CRAs. In regions with homogeneous high-risk for both EC and GC (RREC and RRGC > 1), the RREC and RRGC among males were 2.75 (95% CI: 2.73 to 2.77) and 2.44 (95% CI: 2.43 to 2.46), respectively, while among females they were 4.54 (95% CI: 4.41 to 4.67) and 2.28 (95% CI: 2.22 to 2.33), respectively (Additional File 3: Table S1). The differences in RREC between the highest- and lowest-risk areas for males and females were 10.60-fold and 62.33-fold, respectively, while the differences for RRGC were 6.71-fold and 9.82-fold, respectively (Additional File 3:Tables S2 and S3).
In heterogeneous risk regions, where RREC and RRGC exhibited opposite trends, there were significant differences in risk magnitudes and patterns (Fig. 4). In areas where RREC > 1 and RRGC < 1, the RREC and RRGC among males were 1.71 (95% CI: 1.68 to 1.74) and 0.66 (95% CI: 0.64 to 0.68), respectively, while among females they were 1.92 (95% CI: 1.80 to 2.05) and 0.64 (95% CI: 0.59 to 0.70), respectively, indicating a higher risk for EC and a lower risk for GC. Conversely, in areas where RREC < 1 and RRGC > 1, the RREC and RRGC among males were 0.57 (95% CI: 0.53 to 0.61) and 1.58 (95% CI: 1.56 to 1.60), respectively, while among females they were 0.36 (95% CI: 0.34 to 0.39) and 1.63 (95% CI: 1.59 to 1.69), respectively (Additional File 3: Table S1).
The combined risk regions of EC and GC
Table 3 summarizes the classification of combined risk regions for EC and GC, highlighting areas that should be prioritized for joint endoscopic screening. The double high-risk regions, identified as top priorities for joint screening of both EC and GC, encompass 33 areas. In these regions, the average ASIR for EC and GC among males were 54.23 per 100,000 persons (ratio = 3.16) and 78.42 per 100,000 persons (ratio = 3.11), respectively. Among females, the corresponding rates were 27.21 per 100,000 persons (ratio = 4.66) and 31.05 per 100,000 persons (ratio = 3.02), respectively. For regions classified as single high-risk for EC, where targeted screening for EC is recommended, 38 areas were identified for males and 27 for females. In these regions, the average ASIR for EC and GC among males were 49.75 per 100,000 persons (ratio = 2.89) and 30.78 per 100,000 persons (ratio = 1.22), respectively, while among females, the rates were 20.10 per 100,000 persons (ratio = 3.44) and 12.66 per 100,000 persons (ratio = 1.23), respectively. For regions classified as single high-risk for GC, where screening efforts should focus on GC, 25 areas were identified for males and 15 areas for females. In these regions, the average ASIRs for EC and GC among males were 20.70 per 100,000 persons (ratio = 1.20) and 63.71 per 100,000 persons (ratio = 2.53), respectively, while among females, the rates were 5.97 per 100,000 persons (ratio = 1.02) and 27.96 per 100,000 persons (ratio = 2.72), respectively. Further details on regional classifications are depicted in Additional File 2: Fig. S9 and S10. Comprehensive data on ASIRs and risk levels across all categorized CRAs are available in Additional File 2: Table S3.
Discussion
This study is the first to explore the spatiotemporal associations between EC and GC in China and to identify their combined risk regions using population-reported data and a series of spatiotemporal analyses. We identified seven distinct combined risk regions for EC and GC, providing critical evidence for prioritizing joint endoscopic screening areas and understanding the overlapping and divergent risks associated with these cancers in China.
Our findings indicate that ASIR of EC and GC initially increased before showing a significant decline from 2006 to 2016. This trend is consistent with previous studies on the epidemiological profiles of EC and GC both in China and globally. For example, research has documented a decreasing annual trend in the ASIR of EC and GC of -4.2% (95% CI: -4.6 to -3.7) and -3.0% (95% CI: -3.5 to -2.4) between 2005 and 2015, respectively, with projections of continued declines through 2030 [18]. The Global Burden of Disease study also estimated that the global ASIR of EC and GC decreased by 22% and 28%, respectively, with China contributing to a significant proportion of these cases [19, 20].
The decline in ASIR for both EC and GC was more pronounced in rural areas compared to urban areas, regardless of sex. This difference is likely attributable to the implementation of early screening programs in rural areas since the 1970s. A case–control study in Linzhou, China, demonstrated that early endoscopic screening reduced GC mortality by 28% [21]. Additionally, a nine-year screening program in rural areas led to a 20% reduction in incidence and a 32% decrease in mortality risk for EC [22]. Between 2012 and 2018, over 2.16 million individuals in 194 rural areas participated in early screening programs, with a detection rate of UGIC at 2.05% compared to 0.20% in urban areas [23]. Nutritional interventions with beta-carotene, vitamin E, and selenium in rural areas have also been effective in reducing EC and GC incidence by 8% and 16%, respectively [24]. Despite limited access to treatment facilities in rural areas, China's central government has implemented early diagnosis and treatment projects for UGIC in high-incidence areas since 2005. With the enhancement of the medical insurance system and the improvement of primary healthcare services, the medical care level for residents in rural areas has significantly improved. Policies and guidelines have been issued, and medical resources have been allocated to facilitate early diagnosis and treatment [25,26,27]. For instance, in Cixian, a high-incidence area, early screening and treatment increased five-year survival rates by 14.11% for EC, 16.71% for cardia GC, and 14.92% for noncardia GC due to early screening and treatment from 2003 to 2013 [28]. These results highlight the importance of ongoing and targeted screening efforts, particularly in rural regions, to further mitigate the burden of EC and GC in China.
Our study found a significant temporal association between EC and GC, with concordance rates of 79.00% (95% CI: 77.85 to 80.14) among males and 77.62% (95% CI: 76.50 to 78.73) among females. This suggests that both cancers may share common risk factors and etiological pathways. Although the exact reasons for this association are not fully understood, several factors likely contribute, including the decreasing prevalence of smoking, alcohol use, and Helicobacter pylori (Hp) infection [5, 29, 30], as well as improved dietary habits and access to medical services have likely played a role [31]. The increased consumption of fruits and vegetables, which are rich in antioxidants, has been associated with a reduced risk of both cancers, potentially explaining the declining incidence [32]. Enhanced access to medical facilities through health insurance and public health initiatives has likely improved early detection and treatment, further contributing to the decrease in advanced cancers [33]. Although our study could not evaluate the specific effects of these factors due to data limitations, previous research supports their role in reducing cancer incidence [34].
Our analysis revealed that high-risk areas for EC and GC are primarily concentrated in the adjacent regions of Henan, Hebei, Shanxi, Shandong, Jiangsu, and Anhui provinces, as well as in parts of Xinjiang and Sichuan provinces. Spatial scanning statistics identified homogeneous high-risk areas in the Taihang Mountains and Huaihe Basin, suggesting that environmental factors may significantly contribute to the high incidence of EC and GC in these regions. While excessive smoking and drinking have been highlighted as risk factors in past studies, case–control studies suggested their role in carcinogenesis may be limited [35, 36]. Therefore, further research is needed to clarify the influence of these factors across both high- and low-risk regions [37]. The elevated incidence risks of EC and GC in these areas may be associated with water pollution. Previous studies documented that approximately 80% of EC cases occurred within 10 km of rivers, and rural residents often used untreated surface water from rivers and lakes for drinking [38, 39]. Additionally, 76% and 88% of cancer villages in high-risk areas located within 3 km and 10 km of rivers, respectively, and the correlation coefficient between sewage discharge and high-risk villages was 0.94 (P < 0.05) [40]. However, the incidence risks significantly decreased with strict pollution control measures.
The heterogeneous risk patterns for EC and GC suggest that variations in geographical patterns are driven by different risk exposures. Although specific risk factors for this distribution are not fully identified, the higher prevalence of Hp infection in Northwest China (e.g., Ningxia, Qinghai, and Gansu provinces) is associated with increased GC rates in these regions [41, 42]. This indicates that Hp infection may play a significant role in the spatial distribution of EC and GC. The common low-risk areas in the southern Yangtze River regions, despite being surrounded by high-risk areas, have notably lower cancer rates compared to traditional high-risk areas. This discrepancy highlights the need for further research to uncover the protective factors and risk exposures contributing to lower incidence rates in these regions.
Our previous research demonstrated that joint screening could be a cost-effective strategy in high-risk regions for both EC and GC [12, 43]. This study further validates that areas with a high prevalence of EC often also have a high prevalence of GC, supporting the rationale for joint screening initiatives. Identifying different combined risk regions for EC and GC allows for prioritizing joint screening efforts and guiding cancer prevention and control strategies. These findings can inform cancer prevention and control plans by delineating various risk regions, including those without joint screening, and guide related research on cancer burden and risk factor assessment. The First National Death Survey (1973–1975) defined high-risk areas for EC as those with a crude mortality rate of ≥ 60 per 100,000 males and ≥ 30 per 100,000 females. Similarly, high-risk areas for GC were defined as ≥ 50 per 100,000 males and ≥ 25 per 100,000 females [44]. While this classification reflected the realities of EC and GC in China at that time, it did not account for population structure and lacks comparability at a global level. Another study classified high-risk, medium-risk, and low-risk areas of GC worldwide by ASIR > 20 per 100,000 persons, 11–19 per 100,000 persons, and < 10 per 100,000 persons based on 2005 cancer statistics, respectively [45]. However, this classification is outdated for China, where GC incidence has notably declined, though it remains higher than the global average. Our study, in contrast, divided classifies combined risk areas of EC and GC using the RR in CRAs compared to the national level, based on spatiotemporal associations in China. Areas with an RR ≥ 2 are prioritized for joint endoscopic screening for EC and GC, reflecting current realities in China and helping policymakers understand the similarities and differences between these cancers. We recommend implementing joint endoscopic screening in the following areas: Shexian, Cixian, Wu'an, Pingshun, Yangcheng, Jintan, Yandu, Jianhu, Danyang, Yangzhong, Feixi, Lujiang, Chaohu, Yi'an, Tianchang, Songxian, Luoning, Lushan, Linzhou, Neixiang, Jiyuan, Yanting, Shangzhou, Liangzhou, and Xinyuan. By focusing on high-risk regions, resources can be allocated more effectively to reduce the burden of EC and GC. The geographic and epidemiological data provided enhance the precision of identifying critical areas, improving upon previous methods that relied on broader regional data. Additionally, the effective joint screening in rural areas faces substantial challenges and barriers, including logistic and financial constraints. Our study acknowledges these challenges and suggests that future research should explore innovative solutions, such as mobile screening units and telemedicine, to overcome these barriers.
Public health implications
The classification of risk regions for EC and GC has significant public health implications. Understanding the spatiotemporal patterns of these cancers is crucial for developing national screening standards. First, many risk factors contributing to disparities in EC and GC remain poorly understood despite decades of research. It is essential to strengthen efforts to implement early screening strategies, improve compliance with screening programs, and expand cancer registries to reduce exposure to specific risks. Second, collaboration with local governments and research institutions is vital to establish screening programs in uncovered regions and to ensure effective diagnosis and treatment capacities. Finally, future research should focus on environmental and screening-related factors to explore potential etiological pathways between EC and GC. Expanding joint screening initiatives and conducting comprehensive cost-effectiveness analyses in identified high-risk areas will further optimize resource allocation and policy decisions. Addressing these key aspects will enhance early detection, improve prevention efforts, and ultimately reduce the burden of EC and GC in high-risk populations.
Strengths and limitations
In this study, our primary contributions are being the first to examine the spatiotemporal associations between EC and GC and to divide different combined risk areas for both cancers. This provides crucial evidence to support joint endoscopic screening and identifies high-risk areas for EC and GC in China. Second, this study applies interdisciplinary and integrated approaches, utilizing spatiotemporal techniques to analyze the association between EC and GC. The combined application of these methods and models allows us to explore potential etiological associations between EC and GC, offering important references for specific cancers with previously unexplored relationships. Third, our findings establish a theoretical framework and explicit analytical solutions for tailoring standards of high- and low-risk areas for UGIC at the national level. However, there are some limitations to this study. First, EC and GC have different subsites that were not analyzed due to a lack of data, so the results predominantly reflect the dominant subsites (esophageal squamous cell carcinoma and gastric cardia cancer) in China. Second, we acknowledge the potential discrepancy between the locations where cancer cases are identified and where patients receive treatment, particularly in rural areas with limited healthcare access. Although our study focuses on incidence locations using data from the NCCC—which includes diverse sources like hospitals, community health centers, and insurance systems—these challenges highlight the need for further research. Future studies should assess how access barriers influence screening outcomes and explore solutions such as mobile screening units and telemedicine initiatives [46]. Third, due to disparities in population density and economic conditions across CRAs, and the concentration of CRAs in eastern regions, potential biases may exist in the findings for western regions. However, the existing CRAs in Western China still reflect real epidemiological process because the CRA settings are based on population sampling data. Fourth, this study was conducted during the COVID-19 pandemic, which caused a significant lag in the latest data. Data spanning from 2006 to 2016 only present profiles up to that time. However, given the typically stable geographical patterns of EC and GC in China, these findings still offer valuable guidance for joint screening efforts. Lastly, the unavailability of combined data for both sexes within each CRA limits our ability to examine sex-specific associations comprehensively. Despite this, the consistency in results across sexes strengthens the robustness and validity of our conclusions.
Conclusions
This study utilized spatiotemporal statistical approaches to investigate the associations between EC and GC, identifying various combined risk regions and supporting the implementation of joint endoscopic screening efforts. From 2006 to 2016, the ASIRs of EC and GC initially increased slightly but then experienced a significant decline, with a notable reduction observed particularly among females and in rural areas. Identifying high-risk regions provides a foundation for targeted screening and prevention strategies, which are crucial for refining public health policies and enhancing cancer control efforts in China. The findings underscore the importance of sustained, targeted screening programs to further mitigate the burden of EC and GC, offering valuable insights for developing effective prevention and control measures.
Availability of data and materials
The source of esophageal and gastric cancers in this study can be obtained from China Cancer Registry Annual Report, respectively. The important data (e.g., the divided regions in Fig. 5) of this study were listed in Additional file 1,2 and 3. The whole analysis process can be obtained on reasonable request.
Abbreviations
- AAPC:
-
Average annual percentage change
- APC:
-
Annual percentage change
- ASIR:
-
Age-standardized incidence rate
- CI:
-
Confidence interval
- CRAs:
-
Cancer registry areas
- EC:
-
Esophageal cancer
- GAC:
-
Grey absolute correlation
- GC:
-
Gastric cancer
- GCC:
-
Grey comprehensive correlation
- GRC:
-
Grey relative correlation
- Hp:
-
Helicobacter pylori
- NCCC:
-
National Cancer Center of China
- RR:
-
Relative risk
- UGIC:
-
Upper gastrointestinal cancer
References
Wang S, Zheng R, Li J, Zeng H, Li L, Chen R, et al. Global, regional, and national lifetime risks of developing and dying from gastrointestinal cancers in 185 countries: a population-based systematic analysis of GLOBOCAN. The Lancet Gastroenterology & Hepatology. 2024;9:229–37.
World Health Organization. Cancer Today. 2022. https://gco.iarc.who.int/today/.
Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond). 2021;41:1037–48.
Li P, Jing J, Li R, Ge M, Jia P, Hu W, et al. Upper Gastrointestinal Cancer in China: Spatial Epidemiologic Evidence from Screening Areas. Cancer Prev Res (Phila). 2020;13:935–46.
Arnold M, Ferlay J, van Berge Henegouwen MI, Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020;69:1564–71.
Li M, Park JY, Sheikh M, Kayamba V, Rumgay H, Jenab M, et al. Population-based investigation of common and deviating patterns of gastric cancer and oesophageal cancer incidence across populations and time. Gut. 2022;72:1–9.
Li Y, Xu J, Gu Y, Sun X, Dong H, Chen C. The Disease and Economic Burdens of Esophageal Cancer in China from 2013 to 2030: Dynamic Cohort Modeling Study. JMIR Public Health Surveill. 2022;8:e33191.
Chen W, Zeng H, Chen R, Xia R, Yang Z, Xia C, et al. Evaluating efficacy of screening for upper gastrointestinal cancer in China: a study protocol for a randomized controlled trial. Chin J Cancer Res. 2017;29:294–302.
Thrift AP, Wenker TN, El-Serag HB. Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention. Nat Rev Clin Oncol. 2023;20:338–49.
Lander S, Lander E, Gibson MK. Esophageal Cancer: Overview, Risk Factors, and Reasons for the Rise. Curr Gastroenterol Rep. 2023;25:275–9.
Chen R, Liu Y, Song G, Li B, Zhao D, Hua Z, et al. Effectiveness of one-time endoscopic screening programme in prevention of upper gastrointestinal cancer in China: a multicentre population-based cohort study. Gut. 2021;70:251–60.
Xia R, Zeng H, Liu W, Xie L, Shen M, Li P, et al. Estimated Cost-effectiveness of Endoscopic Screening for Upper Gastrointestinal Tract Cancer in High-Risk Areas in China. JAMA Netw Open. 2021;4:e2121403.
Li W-Q, Qin X-X, Li Z-X, Wang L-H, Liu Z-C, Fan X-H, et al. Beneficial effects of endoscopic screening on gastric cancer and optimal screening interval: a population-based study. Endoscopy. 2022;54:848–58.
Wei W, Zeng H, Zheng R, Zhang S, An L, Chen R, et al. Cancer registration in China and its role in cancer prevention and control. Lancet Oncol. 2020;21:e342–9.
Chen R, Zheng R, Zhang S, Wang S, Sun K, Zeng H, et al. Patterns and trends in esophageal cancer incidence and mortality in China: an analysis based on cancer registry data. Journal of the National Cancer Center. 2023;3:21–7.
Zhang J, Ma B, Han X, Ding S, Li Y. Global, regional, and national burdens of HIV and other sexually transmitted infections in adolescents and young adults aged 10–24 years from 1990 to 2019: a trend analysis based on the Global Burden of Disease Study 2019. The Lancet Child & Adolescent Health. 2022;6:763–76.
Jiao Y, Gong C, Wang S, Duan Y, Zhang Y. The Influence of Air Pollution on Pulmonary Disease Incidence Analyzed Based on Grey Correlation Analysis. Contrast Media Mol Imaging. 2022;2022:1–12.
Zhou J, Zheng R, Zhang S, Chen R, Wang S, Sun K, et al. Gastric and esophageal cancer in China 2000 to 2030: Recent trends and short-term predictions of the future burden. Cancer Med. 2022;11:1902–12.
GBD 2017 Oesophageal Cancer Collaborators*. The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:582–97.
GBD 2017 Stomach Cancer Collaborators. The global, regional, and national burden of stomach cancer in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol. 2020;5:42–54.
Chen Q, Yu L, Hao CQ, Wang JW, Liu SZ, Zhang M, et al. Effectiveness of endoscopic gastric cancer screening in a rural area of Linzhou, China: results from a case-control study. Cancer Med. 2016;5:2615–22.
Liu M, Yang W, Guo C, Liu Z, Li F, Liu A, et al. Effectiveness of Endoscopic Screening on Esophageal Cancer Incidence and Mortality: A 9-Year Report of the Endoscopic Screening for Esophageal Cancer in China (ESECC) Randomized Trial. J Clin Oncol. 2024;42:1655–64.
Fan X, Qin X, Zhang Y, Li Z, Zhou T, Zhang J, et al. Screening for gastric cancer in China: Advances, challenges and visions. Chin J Cancer Res. 2021;33:168–80.
Sun W, Yang H, Wang X, Fan J, Qiao Y, Taylor PR. The Association Between Family History of Upper Gastrointestinal Cancer and the Risk of Death from Upper Gastrointestinal Cancer–based on Linxian Dysplasia Nutrition Intervention Trial (NIT) Cohort. Front Oncol. 2022;12: 897534.
National Cancer Center of China. National Cancer Prevention and Control Network. http://www.chinancpcn.org.cn/. Accessed 15 Jun 2024.
Wang H, Liu Z, Guo C, Liu M, He Y, Tian H, et al. Health-seeking behavior and barriers to treatment of patients with upper gastrointestinal cancer detected by screening in rural China: real-world evidence from the ESECC trial. Lancet Reg Health West Pac. 2021;12: 100181.
Central Government of People's Republic China. Technical plan for population screening, early diagnosis and early treatment of upper gastrointestinal cancer. 2019. https://www.gov.cn/zhengce/zhengceku/2019-11/19/content_5453477.htm.
Li D, Li D, Song G, Liang D, Chen C, Zhang Y, et al. Cancer survival in Cixian of China, 2003–2013: a population-based study. Cancer Med. 2018;7:1537–45.
Yang S, Hao S, Ye H, Zhang X. Cross-talk between Helicobacter pylori and gastric cancer: a scientometric analysis. Front Cell Infect Microbiol. 2024;14:1353094.
Jamal Eddin TM, Nasr SMO, Gupta I, Zayed H, Al Moustafa A-E. Helicobacter pylori and epithelial mesenchymal transition in human gastric cancers: An update of the literature. Heliyon. 2023;9:e18945.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49.
He K-J, Gong G. Global trends and projections of colorectal, esophageal and stomach cancer burden among youth associated with diet: A analysis of 204 countries and territories from 1990 to 2019 and until 2040. Transl Oncol. 2024;46:101984.
Jiang Q, Shu Y, Jiang Z, Zhang Y, Pan S, Jiang W, et al. Burdens of stomach and esophageal cancer from 1990 to 2019 and projection to 2030 in China: Findings from the 2019 Global Burden of Disease Study. J Glob Health. 2024;14:04025.
Li W-Q, Park Y, Wu JW, Ren J-S, Goldstein AM, Taylor PR, et al. Index-based dietary patterns and risk of esophageal and gastric cancer in a large cohort study. Clin Gastroenterol Hepatol. 2013;11:1130-1136.e2.
Steevens J, Schouten LJ, Goldbohm RA, van den Brandt PA. Alcohol consumption, cigarette smoking and risk of subtypes of oesophageal and gastric cancer: a prospective cohort study. Gut. 2010;59:39–48.
Gao Y, Hu N, Han XY, Ding T, Giffen C, Goldstein AM, et al. Risk factors for esophageal and gastric cancers in Shanxi Province, China: A case–control study. Cancer Epidemiol. 2011;35:e91–9.
Li P, Jing J, Liu W, Wang J, Qi X, Zhang 6th G. The spatiotemporal patterns of esophageal cancer burden attributable to behavioral, metabolic, and dietary risk factors from 1990 to 2019: a population-based study. JMIR Public Health and Surveillance. 2023. https://doi.org/10.2196/46051.
Xu C, Xing D, Wang J, Xiao G. The lag effect of water pollution on the mortality rate for esophageal cancer in a rapidly industrialized region in China. Environ Sci Pollut Res. 2019. https://doi.org/10.1007/s11356-019-06408-z.
Lu Y, Song S, Wang R, Liu Z, Meng J, Sweetman AJ, et al. Impacts of soil and water pollution on food safety and health risks in China. Environ Int. 2015;77:5–15.
Cheng Y, Nathanail CP. A study of “cancer villages” in Jiangsu Province of China. Environ Sci Pollut Res. 2018;26:1932–46.
Zhou X-Z, Lyu N-H, Zhu H-Y, Cai Q-C, Kong X-Y, Xie P, et al. Large-scale, national, family-based epidemiological study on Helicobacter pylori infection in China: the time to change practice for related disease prevention. Gut. 2023;72:1–15.
Feng R, Su Q, Huang X, Basnet T, Xu X, Ye W. Cancer situation in China: what does the China cancer map indicate from the first national death survey to the latest cancer registration? Cancer Commun. 2022;43:1–12.
Xia R, Li H, Shi J, Liu W, Cao M, Sun D, et al. Cost-effectiveness of risk-stratified endoscopic screening for esophageal cancer in high-risk areas of China: a modeling study. Gastrointest Endosc. 2022;95:225-235.e20.
Li H, Ding C, Zeng H, Zheng R, Cao M, Ren J, et al. Improved esophageal squamous cell carcinoma screening effectiveness by risk-stratified endoscopic screening: evidence from high-risk areas in China. Cancer communications (London, England). 2021;41:715–25.
Fock KM, Talley N, Moayyedi P, Hunt R, Azuma T, Sugano K, et al. Asia-Pacific consensus guidelines on gastric cancer prevention. J Gastroenterol Hepatol. 2008;23:351–65.
Greenwald ZR, El-Zein M, Bouten S, Ensha H, Vazquez FL, Franco EL. Mobile Screening Units for the Early Detection of Cancer: A Systematic Review. Cancer Epidemiol Biomarkers Prev. 2017;26:1679–94.
Acknowledgements
The authors thank National Cancer Center of China for providing all involved data.
Funding
This work was supported provided by National funding program for postdoctoral researchers (Grant No. GZC20232085), National Key Research Development Program of China (Grant No. 2018YFC1313100), Shaanxi Provincial Administration of Traditional Chinese Medicine (Grant NO.2022-SLRH-LJ-009), and National Youth Science Foundation (Grant NO.82102976), Social Science Foundation Project of Shaanxi Province (Grant NO.2020F009), and Basic Research Program for Young Scientists of Shaanxi Province of Natural Science: (Grant NO.2021JQ-804).
Author information
Authors and Affiliations
Contributions
PL, XQ, and GHZ conceptualized and designed the study. PL and XQ contributed to statistical analysis, interpretation of data, and PL drafted the manuscript. JJ, RUB, RYX revised critically the manuscript for important intellectual content. MY, RYX, and JZW did administrative, technical, or material support. This work was completed under the supervision of GHZ and GJZ. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study approved from the First Affiliated Hospital of Xi'an Jiaotong University Research Ethics Committee (No. LLSBPJ-2024–304).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
12916_2024_3594_MOESM1_ESM.xlsx
Additional file 1: Table S1. The general information of China Cancer Registration between 2006 and 2016. Table S2. The number of cancerregistration areas in provicial regions from 2006 to 2016.
12916_2024_3594_MOESM2_ESM.pdf
Additional file 2: Table S1. The division method of the combined risk areas in EC and GC. Table S2. The division results of combined risk regions between EC and GC by sexes in 487 cancer registration areas of China. Fig. S1. Flow chart of geographical information database. Fig. S2. The geographical distribution of CRAs in 2006–2016. Fig. S3. The division diagram of combined risk regions of EC and GC. Fig. S4. The geographical patterns of EC ASIR among males in from 2006 to 2016. Fig. S5. The geographical patterns of EC ASIR among females in from 2006 to 2016. Fig. S6. The geographical patterns of GC ASIR among males in from 2006 to 2016. Fig. S7. The geographical patterns of GC ASIR among females in from 2006 to 2016. Fig. S8. The geographic patterns of the combined risk regions of EC and GC by sex in China. Fig. S9. The different combination risk regions between EC and GC among males in China. Fig. S10. The different combination risk regions between EC and GC among females in’ China.
12916_2024_3594_MOESM3_ESM.xlsx
Additional file 3: Table S1. The spatial association magnitude of EC and GC in China. Table S2. The relative risk of EC and GC among males in each cancer registration area. Table S3. The relative risk of EC and GC among females in each cancer registration areas.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, P., Qi, X., Bai, R. et al. The spatiotemporal associations between esophageal and gastric cancers provide evidence for its joint endoscopic screening in China: a population-based study. BMC Med 22, 364 (2024). https://doi.org/10.1186/s12916-024-03594-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-024-03594-7