Abstract
Background
In March 2020, the WHO declared the novel coronavirus outbreak a global pandemic. While great success in coronavirus disease 2019 (COVID-19) control has been achieved in China, imported cases have become a major challenge. This study aimed to describe the epidemiological and clinical characteristics of imported COVID-19 cases and to assess the effectiveness of screening strategies in Beijing, China.
Methods
This retrospective study included all imported cases transferred to Beijing Ditan Hospital from 29 February to 20 March 2020 who were screened by both chest computed tomography (CT) and reverse-transcriptase-polymerase chain reaction (RT-PCR) at the initial presentation. Demographic, clinical and laboratory data, in addition to chest CT imaging, were collected and analysed.
Results
In total, 2545 cases were included, among which 71 (2.8%) were finally diagnosed with laboratory-confirmed COVID-19. The majority 63 (88.7%) were from Europe. The most common initial symptoms were cough and fever, which accounted for 49.3% and 42.3%, respectively. Only four cases (5.6%) had lymphocytopenia, and thirteen cases (18.3%) demonstrated elevated levels of C-reactive protein (CRP). All cases had normal serum levels of procalcitonin (PCT). At initial presentation, among the 71 confirmed cases, 59 (83.1%) had a positive RT-PCR assay, and 35 (49.3%) had a positive chest CT. Twelve (16.9%) had a negative RT-PCR assay but a positive chest CT.
Conclusions
A combination of RT-PCR and chest CT is an effective strategy for the screening of imported COVID-19 cases. Our findings provide important information and clinical evidence about the infection control of imported COVID-19 cases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
In December 2019, a cluster of patients with pneumonia of unknown cause occurred in Wuhan, Hubei Province, China [1,2,3,4,5]. The novel coronavirus, identified as the causative agent, is now formally named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the disease caused by this novel coronavirus is called coronavirus disease 2019 (COVID-19) [6, 7]. Due to the lack of immunity against SARS-CoV-2 virus in humans, as well as its efficient transmission among humans, this virus spread rapidly across the world. Concerning COVID-19, the World Health Organization (WHO) raised the threat of the CoV epidemic to the "very high" level on February 28, 2020 [8].
Data provided by the WHO Health Emergency Dashboard (24 March 2020, 10:00 AM CET) report 332,930 confirmed cases of COVID-19 worldwide since the beginning of the epidemic [9]. Outside of China, the main endemic areas are Europe, the Americas and the Eastern Mediterranean region. Due to global economic integration, large numbers of Chinese people travel to endemic countries for trade, tourism, labour, study and other purposes. Subsequently, with the outbreak of COVID-19 abroad and the control of the epidemic in China, importation of COVID-19 from highly endemic areas into China is inevitable [10].
To address this new challenge, the rapid and accurate detection of imported cases is of great significance. In this study, we implemented border entry screening (BES) for overseas travellers and in-hospital screening for suspected cases. This provided us with a good opportunity to describe the characteristics of imported COVID-19 cases and to assess the effectiveness of the screening strategy in the first few months of the COVID-19 epidemic in Beijing, China.
Methods
Study design and subjects
A retrospective analysis was carried out on 71 overseas confirmed COVID-19 cases admitted to Beijing Ditan Hospital from 29 February to 20 March 2020, a designated hospital for the treatment of patients with COVID-19. All COVID-19 cases were diagnosed according to the Seventh Revised Trial Version of the Novel Coronavirus Pneumonia Diagnosis and Treatment Guidance [11]. A laboratory-confirmed COVID-19 case was defined as positive for SARS-CoV-2 nucleic acid on nasopharyngeal swabs and/or sputum specimens by reverse transcription polymerase chain reaction (RT-PCR) assays, which were performed by using TaqMan One-Step RT-PCR Kits from Da An Gene Co., Ltd. of Sun Yat-sen University (Da An; Guangzhou, China) and Shanghai BioGerm Medical Biotechnology Co., Ltd (BioGerm; Shanghai, China). The minimum detection limit of both kits was 500 copies/mL, and the specificity was 100% within the detection range.
Screening process and data collection
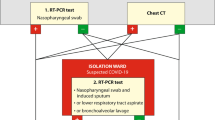
Imported COVID-19 cases admitted to our hospital were detected using two detection routes in the fever clinic (Fig. 1):
-
1.
Border entry screening: When an overseas flight arrived at Beijing International Airport, travellers were required to complete body temperature monitoring and self-health declarations during the customs check. Any traveller who was deemed to have symptoms of COVID-19 (including close contacts) was transferred to a COVID-19-designated hospital.
-
2.
In-hospital screening: First, suspected travellers after initial screening at the airport would have been placed under respiratory isolation conditions. Then, in addition to a medical history and laboratory tests, SARS-CoV-2 tests and chest computed tomography (CT) were performed. The confirmed cases with positive RT-PCR results were admitted to the COVID-19 confirmed ward for further treatment. For highly suspected cases with a positive CT but a negative RT-PCR, they were quarantined in a single room in the COVID-19 suspected ward, and repeated RT-PCR tests were performed with a time interval of two days for further confirmation during 14 days of quarantine, especially for clustered cases. The diagnosis of COVID-19 was confirmed if they became positive for SARS-CoV-2. Otherwise, they were excluded as COVID-19.
Data were collected from each of the confirmed cases, including demographic data (i.e., sex, age, cluster, country of departure), clinical and laboratory results and chest CT features at the initial presentation. The laboratory results included a complete blood count, C-reactive protein (CRP) and procalcitonin (PCT). The measurement of PCT in the serum was performed by a VIDAS B.R.A.H.M.S. PCT assay (bioMérieux, Durham, NC), a one-step immunoassay sandwich method with enzyme-linked fluorescent assay detection that has a detection limit of 0.05 ng/ml.
Statistical analysis
We described the categorical variables as frequencies and percentages (%) and continuous variables as mean and standard deviation (SD) for data with normal distribution (according to Kolmogorov–Smirnov test), otherwise as median and interquartile range (IQR) values. All statistical analyses were performed using SPSS (Statistical Package for the Social Sciences) version 22.0 software (SPSS Inc.).
Results
Demographic characteristics of the overseas imported COVID-19 cases
From 29 February to 20 March 2020, 71 (2.8%) imported COVID-19 cases were identified from among 2545 overseas travellers screened at the emergency department of infectious diseases, Beijing Ditan Hospital, Capital Medical University. The demographic characteristics of the cases are described in Table 1. There were 27 (38.0%) males and 44 (62.0%) females. The median age was 24 years (IQR 20–39; range, 6–55 years). The imported cases were mainly from Europe. Among these, 22 cases (40.0%) were from Spain, followed by 17 cases (23.9%) from the United Kingdom and 16 cases (22.5%) from Italy. A total of 11 clusters occurred, accounting for 39.4% of all COVID-19 cases, including four from Italy, three from the United Kingdom, three from Spain, and one from Austria (Table 1). The period from 29 February to 10 March 2020 was characterized by low numbers of imported cases. From 11 March onward, there was a gradual increase in the number of imported cases, among which the maximum was 14 per day (Fig. 2).
Clinical characteristics of the overseas imported COVID-19 cases
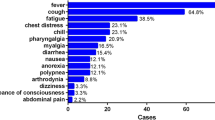
The median time from the illness onset to hospital admission was 4.0 days (IQR 2–7; range 11 h to 13 days). The most common symptoms at the onset of the illness were cough (35, 49.3%), followed by fever (30, 42.3%). Of 71 imported cases, 66 cases (93.0%) had more than one symptom. Among the other three symptomatic cases (4.2%), one only had fatigue and two only had low fever. Only two cases (2.8%) were asymptomatic but were members of family clusters (Table 2).
On admission, the leucocytes were above the normal range in two cases (2.8%) and below the normal range in seven cases (9.9%). Four cases (5.6%) had lymphocytopenia. Platelets were above the normal range in eight cases (11.3%). C-reactive protein (CRP) was above the normal range in 13 cases (18.3%). All cases had normal serum levels of procalcitonin (Table 3).
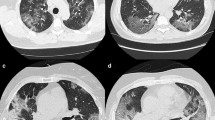
According to the chest imaging findings at the initial presentation, of 71 imported cases, 35 cases (49.3%) showed abnormal chest CT images, consisting of 19 cases (26.8%) of bilateral pneumonia and 16 cases (22.5%) of unilateral pneumonia (Table 2), with typical findings of patchy ground-glass opacity (GGO) in the lungs (Fig. 3A, B). There were six cases (8.5%) of unilateral patchy consolidation and five cases (7.0%) of bilateral consolidation in the lungs (Fig. 3C, D). Thirty-six cases (50.7%) had no abnormalities in the parenchyma of either lung.
Chest CT images of COVID-19 cases. GGO, patchy ground-glass opacity; RR, respiratory rate; BP, blood pressure. A Chest CT image of a patient from The United Kingdom on the 1st day of hospitalization showing patchy ground-glass opacity (GGO) in the subpleural area of the left lower lobe (dry cough, no dyspnoea, no supplementary O2 required, SpO2 98%, RR 18 times/min, BP 151/66 mmHg). B Chest CT image of a patient from America on the 1st day of hospitalization showing multiple patchy and spherical GGOs in the lower lobe of the bilateral lungs with interlobular septal thickening (fever, sore throat, no dyspnoea, no supplementary O2 required, SpO2 96%, RR 20 times/min, BP 110/70 mmHg). C Chest CT image of a patient from The United Kingdom on the 1st day of hospitalization showing multiple patchy consolidations and GGOs in the left lung (fever, dry cough, no dyspnoea, no supplementary O2 required, SpO2 97%, RR 18 times/min, BP 124/67 mmHg). D Chest CT image of a patient from Spain on the 1st day of hospitalization showing multiple patchy GGOs and early consolidation in the bilateral lungs (fever, dyspnoea, supplementary O2 required 2 L/min by nasal cannula, SpO2 97%, RR 21 times/min, BP 127/86 mmHg)
During the diagnostic procedure, the positive rates of the initial RT-PCR assay and chest CT imaging in our cohort were 83.1% (59/71) and 49.3% (35/71) for the diagnosis of COVID-19 cases, respectively. However, among the remaining twelve cases with initial nonpositive results, five of whom were eventually confirmed to have COVID-19 by two repeated RT-PCR tests, four were confirmed by three tests, one was confirmed by four tests, and two were confirmed by five tests.
The effectiveness of the screening strategy
From 29 February to 20 March 2020, with the combination of RT-PCR and CT, 2.8% (71/2545) of entry screening cases were detected and quarantined in time. However, those who were excluded as COVID-19 were repeatedly tested for SARS-CoV-2 during 14 days of quarantine and all SARS-CoV-2 results were negative as confirmed by telephone follow-up with doctors at designated places. At the same time, the local Centers for Disease Control and Prevention (CDC) had not reported any new confirmed cases in this population.
Discussion
The COVID-19 epidemic was dominated by overseas imported cases between March and June 2020 [12], which became a new challenge for the control of COVID-19 in Beijing, China. In this retrospective study, 2.8% (71/2545) of entry screening cases were diagnosed with COVID-19 by a combination of RT-PCR and chest CT. The RT-PCR assay of the respiratory specimens diagnosed most COVID-19 cases (83.1%). A positive chest CT was found in 49.3% of cases; however, among these, 34.3% were also diagnosed by repeated RT-PCR.
Focusing on the period from 29 February to 20 March 2020, there was a consistent increase in the number of imported COVID-19 cases from overseas into Beijing, China. The countries of origin of the imported cases reflected the patterns of SARS-CoV-2 activity in that country at that time. Screening should focus on travellers coming from countries with high COVID-19 activity. The first imported case seen in Beijing was from Iran on 29 February 2020, corresponding to the severity of the COVID-19 outbreak that occurred in Iran at the same time. However, over the course of the next few days, the case exporting regions expanded, and cases from around the world were identified. This demonstrated the global spread of the disease during the development of the pandemic. This also suggests that a detailed epidemiological history is of paramount importance for the early detection of COVID-19 patients.
All imported cases were screened first at customs via temperature monitoring and self-health declarations and then transferred to the emergency department of infectious diseases in Beijing Ditan Hospital. In this study, the most common symptoms were cough and fever, similar to the cohorts reported in the currently available literature [13,14,15]. However, compared with those with SARS-CoV (99%) and MERS-CoV (98%) [16], fever was less frequent in those with SARS-CoV-2. Notably, two cases in our study were asymptomatic but were members of family clusters, but they were positive for SARS-CoV-2 nucleic acids, indicating that contacts of the confirmed cases within their clusters cannot be ignored during screening [17]. It is vital to strengthen the surveillance and tracing of this population.
Currently, RT-PCR tests are the gold standard diagnostic tool for COVID-19 [18, 19]. In this study, all patients received the SARS-CoV-2 test at initial presentation. The positive rate of the initial RT-PCR assay for respiratory samples was 83.1%, which is consistent with a previous report [20]. In fact, from the results of this study, 16.9% (12/71) of the patients with negative RT-PCR results but a positive chest CT were diagnosed with COVID-19 through repeated RT-PCR. These negatives could result from improper sampling techniques or a low viral load in the area sampled [21, 22]. Therefore, for patients with a high clinical suspicion, specimens should be continuously collected for multiple tests to avoid a missed diagnosis.
Previous studies have shown that chest CT scans are of great significance to screen suspected cases of COVID-19 [23]. In the early stage, there was ground-glass opacification with or without consolidative abnormalities, especially with a peripheral distribution. In severe cases, lung consolidation may occur, but pleural effusion is rare [24]. In our study, nearly half of the imported cases showed typical CT features consistent with the study by Huang et al. [25]. For these cases, chest CT may be used for clinical staging of the diseases. In addition, 16.9% (12/71) had initial positive chest CT scans prior to the initial negative RT-PCR results, indicating that chest CT, where it is available, may identify cases that have progressed to pneumonia but are no longer shedding virus from the upper respiratory tract. Notably, normal chest CT imaging was found in 36 (50.7%) cases compared to 17% in a recent study by Pan et al. [26]. The imaging features of COVID-19 are diverse and depend on the stage of infection after the onset of symptoms. A retrospective analysis of chest CT in 121 patients with COVID-19 by Bernheim et al. [27] showed more frequent normal CT findings (56%) in the early stages of the disease (0–2 days). Therefore, a normal result from the initial CT scan does not completely rule out COVID-19.
There are several limitations to our study. First, due to the limited number of patients, our conclusions need to be further verified in large samples and multicentre data. Second, due to time constraints, those who were excluded as having COVID-19 at the initial presentation were not followed up for longer periods of time. Therefore, continued attention needs to be paid to the reports of the local CDC about COVID-19 outbreaks for further verification.
Conclusions
Currently, SARS-CoV-2 continues to spread globally. To accurately detect imported COVID-19 cases, the following aspects should be focused: (1) Strengthen the surveillance of overseas COVID-19 outbreaks. Airport customs personnel and doctors in hospitals should update the epidemic situation abroad synchronously. (2) Strengthen the understanding of the clinical characteristics of the imported cases and use combined screening.
Availability of data and materials
Data of the study can be available upon request from Ling-hang Wang.
Abbreviations
- COVID-19:
-
Coronavirus disease 2019
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- CT:
-
Computed tomography
- RT-PCR:
-
Reverse-transcriptase-polymerase chain reaction
- CRP:
-
C-reactive protein
- PCT:
-
Procalcitonin
- WHO:
-
World Health Organization
- BES:
-
Border entry screening
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- GGO:
-
Ground-glass opacity
- CDC:
-
Centers for Disease Control and Prevention
References
Hui DS, Madani TA, Ntoumi F, Kock R, Dar O, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan. China Int J Infect Dis. 2020;91:264–6. https://doi.org/10.1016/j.ijid.2020.01.009.
Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J Med Virol. 2020;92(4):401–2. https://doi.org/10.1002/jmv.25678.
Paules CI, Marston HD, Fauci AS. Coronavirus infections-more than just the common cold. JAMA. 2020. https://doi.org/10.1001/jama.2020.0757.
Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–3. https://doi.org/10.1016/S0140-6736(20)30185-9.
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33. https://doi.org/10.1056/NEJMoa2001017.
Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–15. https://doi.org/10.1016/S0140-6736(20)30360-3.
Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3): 105924. https://doi.org/10.1016/j.ijantimicag.2020.105924.
World Health Organization. WHO Director-General's opening remarks at the media briefing on COVID-19 - 28 February 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---28-february-2020.
World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report–63. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200323-sitrep-63-covid-19.pdf?sfvrsn=d97cb6dd_2.
National Health Commission of the People's Republic of China website. Epidemic notification. 2020. http://www.nhc.gov.cn/xcs/yqtb/list_gzbd.shtml.
National Health Commission of the People's Republic of China website. Diagnosis and treatment of novel coronavirus pneumonia (trial version seventh). http://www.nhc.gov.cn/yzygj/s7652m/202003/a31191442e29474b98bfed5579d5af95.shtm.
The People's Government of Beijing Municipality website. Epidemic notification. 2020. http://www.beijing.gov.cn/ywdt/zwzt/yqfk/yqbb/index_31.html.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. https://doi.org/10.1016/S0140-6736(20)30183-5.
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–9. https://doi.org/10.1001/jama.2020.1585.
Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368: m606. https://doi.org/10.1136/bmj.m606.
Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet. 2015;386:995–1007. https://doi.org/10.1016/S0140-6736(15)60454-8.
Tan SS, Yan B, Saw S, Lee CK, Chong AT, Jureen R, et al. Practical laboratory considerations amidst the COVID-19 outbreak: early experience from Singapore. J Clin Pathol. 2021;74(4):257–60. https://doi.org/10.1136/jclinpath-2020-206563.
Liu R, Han H, Liu F, Lv Z, Wu K, Liu Y, et al. Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin Chim Acta. 2020;505:172–5. https://doi.org/10.1016/j.cca.2020.03.009.
Wang J, Cai K, Zhang R, He X, Shen X, Liu J, et al. Novel one-step single-tube nested quantitative real-time PCR assay for highly sensitive detection of SARS-CoV-2. Anal Chem. 2020;92(13):9399–404. https://doi.org/10.1021/acs.analchem.0c01884.
Xu M, Wang D, Wang H, Zhang X, Liang T, Dai J, et al. COVID-19 diagnostic testing: technology perspective. Clin Transl Med. 2020;10(4): e158. https://doi.org/10.1002/ctm2.158.
Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2019;2020: 200642. https://doi.org/10.1148/radiol.2020200642.
Winichakoon P, Chaiwarith R, Liwsrisakun C, Salee P, Goonna A, Limsukon A, et al. Negative nasopharyngeal and oropharyngeal swab does not rule out COVID-19. J Clin Microbiol. 2020;58(5):e00297-e320. https://doi.org/10.1128/JCM.00297-20.
Song F, Shi N, Shan F, Zhang Z, Shen J, Lu H, et al. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295:210–7. https://doi.org/10.1148/radiol.2020200274.
Wu J, Liu J, Zhao X, Liu C, Wang W, Wang D, et al. Clinical characteristics of imported cases of COVID-19 in Jiangsu Province: a multicenter descriptive study. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciaa199.
Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, et al. CT Imaging features of 2019 novel coronavirus (2019-nCoV). Radiology. 2020;295:202–7. https://doi.org/10.1148/radiol.2020200230.
Pan F, Ye T, Sun P, Gui S, Liang B, Li L, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020;295(3):715–21. https://doi.org/10.1148/radiol.2020200370.
Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295(3): 200463. https://doi.org/10.1148/radiol.2020200463.
Acknowledgements
We thank all cases included in this study. We are really grateful to all the health workers around the world. Their expertise is fundamental to stop SARS-CoV-2 from spreading further.
Funding
This work was supported by the National Key Research and Development Project of China (Grant Number: 2020YFA 0707600). The funder had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
CJM and LHW designed the study; LL, YFC, SYY and YXT collected data; LL, CJM and YFC performed data analyses; LL and CJM drafted and revised the manuscript. All of the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was approved by the Clinical Ethics Committees of Beijing Ditan Hospital, Capital Medical University (Record number 2020014–01). All methods were performed in accordance with the relevant guidelines and regulations. The need of informed consent was waived by ethics committee of Beijing Ditan Hospital due to the retrospective nature of the study. We confirmed that the identification information of all participants (including patient names, ID numbers, home addresses and telephone numbers) would not be included in recordings, written descriptions or publications.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, L., Ma, CJ., Chang, YF. et al. The characteristics of overseas imported COVID-19 cases and the effectiveness of screening strategies in Beijing, China. BMC Infect Dis 22, 59 (2022). https://doi.org/10.1186/s12879-021-06998-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-021-06998-5