Abstract
Background
Continuous theta burst stimulation (cTBS) is a form of repetitive transcranial magnetic stimulation which has been shown to alter cortical excitability in the upper limb representation of primary somatosensory cortex (SI). However, it is unknown whether cTBS modulates cortical excitability within the lower limb representation in SI. The present study investigates the effects of cTBS over the SI lower limb representation on cortical somatosensory evoked potentials (SEPs) and Hoffmann reflex (H-reflex) following tibial nerve stimulation at the knee. SEPs and H-reflex were recorded before and in four time blocks up to 30 minutes following cTBS targeting the lower limb representation within SI.
Results
Following cTBS, the P1-N1 first cortical potential was significantly decreased at 12–16 minutes. CTBS also suppressed the P2-N2 second cortical potential for up to 30 minutes following stimulation. The H-reflex remained statistically unchanged following cTBS although there was a modest suppression observed.
Conclusion
We conclude that cTBS decreases cortical excitability of the lower limb representation of SI as evidenced by suppressed SEP amplitude. The duration and magnitude of the cTBS after effects are similar to those observed in upper limb studies.
Similar content being viewed by others
Background
Decades of research in animal models have formulated principles that underlie plasticity within the primary somatosensory cortex (SI) [1]. In humans, plasticity-inducing transcranial magnetic stimulation (TMS) protocols may be applied over SI to promote rehabilitation following injury [2], investigate somatic influences in motor control [3] and to understand the neural basis of touch perception [4]. To date, studies have focused primarily on altering excitability of upper limb representations within SI. However, investigating the effects of these same protocols on lower limb representations would provide insight into both the generalizability of these plasticity- inducing techniques to other body representations, and potentially lead to the development of new strategies to alter cortical activity in patients with sensory and/or motor impairments affecting the lower limb.
Continuous theta burst stimulation (cTBS) is a plasticity-inducing protocol that alters activity within SI such that somatosensory evoked potentials (SEPs) are decreased [5, 6]. Specifically, cTBS applied over SI suppresses low frequency median nerve SEP components for up to 13 minutes [5] or leaves them unchanged [6]. High frequency oscillations are also modified by cTBS over SI such that early and late components are facilitated and suppressed, respectively, for up to 15 minutes following stimulation [6]. Of the plasticity-inducing protocols, cTBS offers the advantage of requiring short stimulation durations (i.e. 40 seconds) and relatively long-lasting effects [7]. Although the mechanisms of cTBS effects are not fully understood, cTBS appears to alter inhibitory and excitatory circuitry such that GABAergic [8, 9], glutamatergic [10, 11] and dopaminergic [12] systems are altered following stimulation. Studies in rat models reveal that TBS alters inhibitory neuronal circuitry within targeted cortex [13, 14]. In support of these findings, cTBS increases GABA concentration within human cortex as measured using magnetic resonance spectroscopy [9]. However, NMDA receptor blockade eliminates the effects of cTBS thereby suggesting that glutamatergic systems are also influenced by cTBS [10, 11]. Similarly, blockade of the dopamine D2 receptor eliminates the TBS effects [12].
To date, cTBS protocols have aimed to alter the excitability of upper limb representations within SI [5, 6] and M1 [5, 7, 15]. However, there exist distinct differences between hand and lower limb which may cause cTBS induced effects to differ between skin surfaces. Within SI of non-human primates, the hand representation exhibits a much larger cortical territory and higher cortical magnification factor than the lower limb [16]. The lower limb representation is intersected by the foot representation, resulting in two discrete foci dedicated to the lower limb, a trait not seen in the upper limb and hand [16, 17]. Last, the glabrous skin of the hand has a propensity for inducing experience-dependent plasticity within SI [18, 19] whereas hairy skin, as seen in the lower limb, does not [20]. Further evidence is also found in humans such that a greater number of cortical synapses are involved in the processing of upper limb versus lower limb afferent input [21] thereby increasing the opportunity to modify afferent processing originating from the upper limb. Last, plasticity induced by the lower limb is not identical to that induced by other non-upper limb areas, such as the face. Following rTMS over the leg area, the motor output of ischemic-nerve-blocked upper limb was unchanged, whereas rTMS over the face resulted in long lasting decreases [22]. The aforementioned findings lead to the suggestion that the hand and upper limb may be targeted and modulated via cTBS protocols to a greater extent and with greater ease compared to the lower limb.
The goal of the present study was to investigate the effects of cTBS applied over the lower limb representation of SI on cortical excitability as measured with SEPs. The ability to modify cortical excitability within lower limb representations creates new opportunities for developing therapeutic strategies in clinical populations who demonstrate impairments in lower limb control. Further, it remains to be seen whether cTBS effects on upper limb SEPs may be generalized to lower limb representations. To address these questions SEPs were recorded before and for up to 30 minutes following cTBS over the lower limb representation within SI. Further, we obtained measurements of Hoffman reflexes (H-reflexes) in soleus muscle to identify whether cTBS induced effects occur at the cortical and/or spinal level. Based on the timeline of cTBS effects on early median nerve SEPs [5] it was hypothesized that a decrease in the first tibial nerve cortical potential would be seen up for up to 13 minutes following stimulation.
Methods
Participants
Nineteen healthy participants were tested (14 male, mean age = 25.1, SD = 5.24). Thirteen individuals participated in Experiment 1 (10 male, mean age = 25.2, SD = 5.0), six in control Experiment 2 (4 male, mean age = 20.5, SD = 1.22), and three in control Experiment 3 (3 male, mean age = 25.7, SD = 3.06). Three individuals participated in both Experiments 1 and 3 (3 male, mean age = 25.7, SD = 3.06). All participants were right handed, as determined by the Waterloo Handedness questionnaire, a derivative of the Edinburgh Handedness Inventory [23]. All participants wore 30 dB earplugs throughout all experimental procedures. All participants provided informed written consent prior to the experiment. This study conformed to the Declaration of Helsinki and was approved by the University of Waterloo.
Electromyography (EMG)
Muscle activity was recorded over the right soleus (SOL) and first dorsal interosseous (FDI) muscles using 9 mm Ag-AgCl surface electrodes. For SOL, two electrodes were positioned approximately 2 cm apart over the muscle belly. For FDI, the active electrode was placed over the muscle belly while the reference electrode was placed over the metacarpophalangeal joint of the index finger. EMG was amplified 1000x and filtered from 20–2500 Hz using an Intronix Model 2024F isolated preamplifier (Intronix Technologies Corporation, Bolton, Canada) and acquired using Signal Software and a Cambridge Electronic device (Power 1401, Cambridge Electronic Design, Cambridge, UK).
Somatosensory evoked potentials
The tibial nerve was stimulated (square wave pulse, 0.5 ms duration, 0.5 Hz) at the popliteal fossa (Grass SD 9, Grass Technologies, West Warwick, USA) with the anode of the stimulating electrode placed approximately 2 cm proximal to the popliteal crease, and the cathode proximal to the anode, to elicit an H-reflex in SOL [24]. Nerve stimulation intensity was maintained at an intensity to evoke an M wave of 10% of the maximal M wave. The 10% M wave intensity was monitored and maintained throughout the experiment.
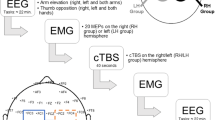
SEPs were recorded from CPz located over the somatosensory vertex and referenced to FPz [25] with a clavicle ground. In 8 participants, we were able to additionally obtain a MRI to confirm the location of CPz over SI using Brainsight Neuronavigation Software (Rogue Research, Montreal, Canada), as shown in Figure 1A. MRI was collected with a 3 Tesla GE scanner (172 images) with 3DFSPGR-IR sequences using a 20 cm FOV (256x256). EEG recordings were amplified 10000x and filtered from 2–500 Hz (Intronix 2024F Isolated Preamplifier, Intronix Technologies Corporation, Bolton, Canada). Electrode impedances were maintained at <5kΩ throughout the experiment (UFI Checktrode, Model 1089 Mk III, UFI, Morro Bay, California, USA). SEPs were collected in epochs of 150 ms including a 30 ms pre-stimulus period. SEPs were averaged from 120 nerve stimuli delivered every 2 seconds. Previous studies examining tibial nerve evoked SEPs used 50 to 80 [26], 100 [27] to 128 [28, 29] nerve stimuli. The frequency of stimulation was chosen to ensure H-reflexes were not suppressed.
A. Depiction of localization of cTBS target over CPz. CTBS was applied over the CPz location which was also the site for recording SEPs following tibial nerve stimulation. CS (central sulcus), PCG (postcentral gyrus). B. Experiment timeline. SEPs and H-reflexes were recorded before (T 0 ) and at 5 to 9 (T 1 ), 12 to 16 (T 2 ), 19 to 23 (T 3 ) and 26 to 30 (T 4 ) minutes following SI cTBS.
Transcranial magnetic stimulation (TMS)
TMS was applied using a MagPro stimulator (MCF-B65, Medtronic, Minneapolis, Minnesota, USA) using a 90mm diameter figure-of-eight coil. The coil was positioned over left M1 at a 45° angle to the mid-sagittal line to evoke motor evoked potentials (MEPs) in the right FDI. Once an optimal location for FDI MEPs was located, the resting motor threshold (RMT), defined as the lowest intensity required to evoke MEPs of at least 500 μV in 5 of 10 consecutive trials while the muscle is at rest, was found. RMT was determined at the beginning of the experiment prior to nerve stimulation set-up. Continuous theta burst stimulation (cTBS) using 600 pulses [7] was applied over the location of the sensory vertex electrode (CPz). We chose to use a cTBS intensity determined from RMT obtained from FDI based on a pilot study in which we noted that MEPs from the tibialis anterior and soleus muscles were inconsistent, frequently evoked activity in gluteal, hamstrings and quadriceps muscles, and required high intensities (i.e. ~ 85-95% of the MSO) using our figure-of-eight coil which created participant discomfort. Further, cTBS applied at these intensities may result in current spread, making inferences about results due to changes in SI difficult. To improve the opportunity to modify the excitability within the lower limb representation, we subsequently chose to increase the intensity required for RMT and increase the cTBS intensity to 80% RMT, as opposed to the 80% active motor threshold value used in most upper limb studies. Further, we chose to use RMT rather than AMT to avoid metaplasticity effects of cTBS [30] which may contribute to the variable effects of cTBS observed elsewhere [31].
For cTBS, the coil handle was positioned to induce an initial current in the anterior to posterior direction. Prior to application of cTBS, the CPz electrode was removed from the scalp to ensure accurate application of the cTBS and following the completion of cTBS the CPz electrode was replaced at the original location on the scalp and impedances were checked to ensure proper re-application similar to that performed elsewhere [4, 32]. This procedure was performed to allow the cTBS coil to be placed directly upon the cranium though we recognize that it may also introduce some variability in the data.
Experiment 1: Real cTBS over SI
Thirteen subjects participated in Experiment 1. Measures of SEPs and H-reflexes were obtained immediately before cTBS (T 0 ) and at four time blocks following stimulation: 5 to 9 (T 1 ), 12 to 16 (T 2 ), 19 to 23 (T 3 ), and 26 to 30 (T 4 ) minutes. The experiment timeline is shown in Figure 1B.
Experiment 2: Sham cTBS over SI
Six subjects participated. Experiment 2 was identical to Experiment 1 with the exception that Sham cTBS was applied over SI. For sham stimulation, the real cTBS protocol was delivered with the coil tilted perpendicular to the cranium and positioned over the CPz electrode position. In this orientation, the current flow is mainly directly towards outside of the cranium.
Experiment 3: Extended duration real cTBS over SI
Three subjects participated. Experiment 3 was identical to Experiment 1 with the exception that three additional time blocks were included to extend the recording window to 60 minutes following cessation of cTBS. These time blocks were 36 to 40 (T 5 ), 46 to 50 (T 6 ), and 56 to 60 (T 7 ) minutes following cTBS. Based on the findings of Experiment 1, this control experiment investigated whether P2-N2 changes returned to baseline by 60 minutes following cTBS.
Data analysis
Two one-way repeated measures ANOVA were performed, one analyzing peak-to-peak amplitude of SEP components and the H-reflex, and a second analyzing onset latency of SEP components and the H-reflex, both with within-subject factor TIME (5 levels: T 0 ; T 1 ; T 2 ; T 3 ; T 4 ). Peak-to-peak SEP amplitudes were measured for the P1-N1 and P2-N2 tibial nerve cortical potentials by averaging 120 epochs during each time block. Trials displaying movement, blink, or noise artifacts were identified and rejected during off-line analysis. A priori hypotheses tested that P1-N1 would be suppressed at T 1 (5 to 9 minutes following cTBS) and T 2 (12 to 16 minutes following cTBS) as compared to T 0 . Post-hoc analysis was performed using Tukey’s tests.
Results
Experiment 1: Real cTBS over SI
All participants successfully completed the experiment. Data from one individual was removed due to movement related noise in the SEP recordings. Analyses were subsequently performed on data from the remaining 12 participants (10 male, mean age = 25.3, SD = 5.45). The group-averaged RMT and cTBS intensity (with standard deviation) were 55 ± 8.4 % and 44 ± 6.8 % of the maximum stimulator output (MSO), respectively. The latencies of the P1, N1, P2, N2 SEP components and H-reflex (with standard deviation) for each time block are shown in Table 1. With the exception of P2, the latencies of all components remained unchanged following cTBS (P1: F(4,44)=0.71, p=0.59, N1: F(4,44)=0.68, p=0.61, P2: F(4,44)=3.36, p=0.017, N2: F(4,44)=0.99, p=0.42, H-reflex: F(4,44)=0.0, p=1.0). Post hoc Tukey’s test revealed that the latency of the P2 component was significantly altered at T2, T3, and T4 compared to T0. Therefore, the P2-N2 amplitude was analyzed two ways. Method one used the P2-N2 amplitude derived by the latency values obtained from the pre-cTBS block. Method two used the P2-N2 amplitude defined by the P2 latency from each time block separately (i.e. from T0, T1….).
Somatosensory Evoked Potentials
Figure 2A displays the P1-N1 group-averaged peak-to-peak amplitude with standard error bars. One-way ANOVA revealed no effect of TIME (F(4,44)=1.53, p=0.21). However, in support of the hypothesis, a priori comparison of pre-cTBS amplitude versus T2 revealed that P1-N1 is significantly suppressed from 12 to 16 minutes following cTBS (paired t-test, p=0.023) and this effect was observed in 9 of 12 participants. P1-N1 suppression was also nearly significant at T1 from 5 to 9 minutes following cTBS (paired t-test, p=0.03).
SEPs before and after cTBS. A. Group-averaged P1-N1 (with standard error) recorded before and at each time block following cTBS. B. Group-averaged P2-N2 (with standard error) recorded before and at each time block following cTBS. C. SEP trace from an individual participant depicting location of the P1, N1, P2 and N2 components at T 0 (before) and at time block T 2 (12 to 16 minutes) following cTBS. Asterisks indicate p < 0.05.
Figure 2B displays the group-averaged P2-N2 amplitude with standard error bars. One-way ANOVA revealed a significant main effect of TIME for both analyses (Method one: F(4,44)=9.81, p=<0.0001, Method two: F(4,44)=3.72, p=0.01). Post-hoc Tukey’s tests revealed that P2-N2 was significantly suppressed at each time block compared to pre-cTBS amplitude using Method one and at T 2 , T 3 and T 4 using Method two.
H-reflex
Figure 3 displays group-averaged H-reflex and M wave amplitudes with standard error for each time block. For the H-reflex, one-way ANOVA revealed no effect of TIME (F(4,44)=1.77, p=0.15) suggesting that cTBS over SI did not significantly alter spinal motor neuron excitability. Similarly, for the M wave, one-way ANOVA revealed no effect of TIME (F(4,44)=0.56, p=0.69) indicating that nerve stimulation intensity was maintained across time blocks.
Experiment 2: Sham cTBS over SI
All participants successfully completed the experiment. Data from one individual was removed due to excessively large potentials deemed to be statistical outliers in the pre-cTBS condition. Analyses were subsequently performed on data from the remaining 5 participants (3 male, mean age = 20.4, SD = 1.34). The group-averaged RMT and cTBS intensity (with standard deviation) were 51 ± 8.9 % and 41 ± 7.3 % of the MSO respectively. Figure 4 displays the group-averaged M wave, H-reflex, P1-N1, and P2-N2 peak-to-peak amplitudes (with standard error) for each time block. One-way ANOVA revealed no effect of TIME for M wave (F(4 16)=0.48, p=0.75), H-reflex (F(4 16)=0.36, p=0.83), P1-N1 (F(4 16)=1.52, p=0.24), or P2-N2 (F(4 16)=1.97, p=0.15) suggesting that sham cTBS had no significant effect on either cortical or spinal excitability.
Sham cTBS over SI. Group averaged peak-to-peak amplitudes (with standard error) of SEPs and spinal reflexes before and at each time block following cTBS. No significance was found for any measure at any time point. The left ordinate reflects the M wave and H reflex and the right ordinate scale reflects the SEPs.
Experiment 3: Extended duration real cTBS over SI
All participants successfully completed the experiment. The group-averaged RMT and cTBS intensity (with standard deviation) were 56 ± 15.1 % and 45 ± 12.1 % of the MSO respectively. Figure 5 displays the group-averaged M wave, H-reflex, P1-N1, and P2-N2 peak-to-peak amplitudes (with standard error) for each time block. The data suggest that the P2-N2 amplitude returns to baseline by 60 minutes following stimulation.
Extended duration real cTBS over SI. Group averaged peak-to-peak amplitudes (with standard error) of SEPs and spinal reflexes before and at each time block following cTBS. Three additional time blocks were added to extend the recording window to 60 minutes following cTBS. The left ordinate reflects the M wave and H reflex and the right ordinate scale reflects the SEPs.
Discussion
In the present study we investigated the effects of cTBS over the SI representation of the lower limb on somatosensory evoked potentials and spinal reflexes originating from tibial nerve stimulation. Novel findings include suppression of the P1-N1 component from 12–16 minutes and reduced P2-N2 for up to 30 minutes following cTBS. CTBS did not significantly alter spinal H-reflexes. These results indicate that cTBS exerts similar effects on lower and upper limb SEPs and that cTBS can be applied over SI to modulate the excitability of the lower limb representation.
Early cortical SEP potentials following median nerve stimulation are thought to originate from the arrival of the thalamo-cortical volley from the thalamus into area 3b of SI [33] and a similar path is postulated for early cortical potentials following stimulation of the tibial nerve [34, 35]. The P1 is generated by depolarization of the apical dendritic membrane of pyramidal neurons in the cortex, resulting in a relative electronegativity in reference to the cell soma and basal dendrites [36]. As this current propagates towards the soma and basal dendrites a dipole is formed with the negative pole being located at the apical dendrites (the “sink”) and the positive pole being located at the basal dendrites (the “source”) [36]. This dipole is recorded via EEG as a positivity as the electrode only records from the “visible” side of the dipole, with the negative dipole being occluded [36]. Conversely, the N1 results from the dipole generated by the same population of pyramidal cells with the “sink” being located at the basal dendrites, and the “source” being located at the apical dendrites [36]. The amplitude of these potentials reflects the magnitude of the summation of extracellular postsynaptic potentials created by the depolarization of the pyramidal neurons located at the generator site and the resultant dipole [36]. A suppression of the P1-N1 therefore reflects decreased depolarization of cortical pyramidal neurons and therefore decreased activity within this neuronal population.
The finding that tibial nerve P1-N1 SEPs are suppressed following cTBS closely matches results obtained from a study examining median nerve SEPs. Tibial nerve P1-N1 is suppressed for up to 16 minutes following cTBS but is not suppressed by 19 minutes. Median nerve P25-N33 potentials are suppressed for up to 13 minutes but are unchanged when tested at 20 minutes [5]. Further, the magnitude of cTBS induced suppression is similar for the lower and upper limb. CTBS decreases tibial nerve P1-N1 by ~28% while median nerve P25-N33 is reduced by ~25% [5] or remains unchanged [6]. Comparison of the upper and lower limb data suggests that the time course and magnitude of changes induced by cTBS are similar for the first cortical potential for nerves of the upper and lower limb.
The generator of the tibial nerve derived P2-N2 is still largely unknown. However, somatosensory evoked fields following tibial nerve stimulation suggest that potentials occurring at approximately 50 ms arise from a generator located in area 1 of SI [35]. Further, the P2-N2 appears to originate from a similar large-diameter afferent source that may traverse a polysynaptic pathway before terminating within SI [37]. Similar to the P1-N1, P2-N2 decreased ~ 36% with the maximal suppression occurring from 12 to 16 minutes following cTBS. However, unlike the earlier potential, P2-N2 was significantly decreased for up to 30 minutes with the suppression maintained at ~30%. The robust effects of cTBS on P2-N2 compared to P1-N1 may relate to the spatial proximity between their respective generators and the position of cTBS delivery within SI. Area 1, where later occurring SEPs are thought to be generated, is located on the crown of the postcentral gyrus in close proximity to the focus of cTBS stimulation [38]. In comparison, area 3b, where early cortical potentials are generated, is located in the depths of the posterior bank of the central sulcus [38]. We also observed that the P2 latency increased at 12–16 minutes following cTBS which may also relate to the proximity of the cTBS delivery to the P2 generator. An alternative explanation is that cTBS alters activity in remote loci involved in the polysynaptic pathway through which the P2 travels before arriving in SI [37]. The latter explanation is plausible as cTBS is known to alter activity in remote loci [39].
The H-reflex remained statically unchanged both in latency and amplitude following cTBS. This agrees with previous work showing that cTBS over M1 did not alter H-reflexes [7] or F-waves following Brodmann area 5 stimulation [40]. However, we note that the H-reflex did show modest suppression beginning at ~ 12–16 minutes following cTBS. Specifically, the H-reflex was suppressed by ~ 15% from the pre-cTBS amplitude. This suggests that effects induced by cTBS over SI are mediated primarily via changes in the excitability of cortical neurons but may also include modest changes in the spinal motor neuron output. Intermittent TBS delivered for five consecutive days over M1 alters H-reflexes in patients with multiple sclerosis [41]. It may be that repeat sessions of cTBS over SI and/or M1 may exhibit changes in spinal motor neuron excitability.
Conclusion
The results of the present study indicate that cTBS may be used over SI to modify the excitability of the lower limb representation. These findings create new opportunities to investigate the utility of cTBS to modulate SI activity in clinical populations. Understanding the modulation of SI lower limb representations has importance in designing rehabilitation strategies for individuals with impaired gait or balance and/or altered somatosensory processing of the lower limb such as in stroke populations.
References
Nudo RJ: Neural bases of recovery after brain injury. J Commun Disord. 2011, 44: 515-520. 10.1016/j.jcomdis.2011.04.004.
Bashir S, Mizrahi I, Weaver K, Fregni F, Pascual-Leone A: Assessment and modulation of neural plasticity in rehabilitation with transcranial magnetic stimulation. PM R. 2010, 2: S253-S268. 10.1016/j.pmrj.2010.10.015.
Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, et al: Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol. 2000, 523 (Pt 2): 503-513.
Ragert P, Franzkowiak S, Schwenkreis P, Tegenthoff M, Dinse HR: Improvement of tactile perception and enhancement of cortical excitability through intermittent theta burst rTMS over human primary somatosensory cortex. Exp Brain Res. 2008, 184: 1-11.
Ishikawa S, Matsunaga K, Nakanishi R, Kawahira K, Murayama N, Tsuji S, et al: Effect of theta burst stimulation over the human sensorimotor cortex on motor and somatosensory evoked potentials. Clin Neurophysiol. 2007, 118: 1033-1043. 10.1016/j.clinph.2007.02.003.
Katayama T, Suppa A, Rothwell JC: Somatosensory evoked potentials and high frequency oscillations are differently modulated by theta burst stimulation over primary somatosensory cortex in humans. Clin Neurophysiol. 2010, 121: 2097-2103. 10.1016/j.clinph.2010.05.014.
Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC: Theta burst stimulation of the human motor cortex. Neuron. 2005, 45: 201-206. 10.1016/j.neuron.2004.12.033.
Cardenas-Morales L, Nowak DA, Kammer T, Wolf RC, Schonfeldt-Lecuona C: Mechanisms and applications of theta-burst rTMS on the human motor cortex. Brain Topogr. 2010, 22: 294-306. 10.1007/s10548-009-0084-7.
Stagg CJ, Wylezinska M, Matthews PM, Johansen-Berg H, Jezzard P, Rothwell JC, et al: Neurochemical effects of theta burst stimulation as assessed by magnetic resonance spectroscopy. J Neurophysiol. 2009, 101: 2872-2877. 10.1152/jn.91060.2008.
Huang YZ, Chen RS, Rothwell JC, Wen HY: The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol. 2007, 118: 1028-1032. 10.1016/j.clinph.2007.01.021.
Teo JT, Swayne OB, Rothwell JC: Further evidence for NMDA-dependence of the after-effects of human theta burst stimulation. Clin Neurophysiol. 2007, 118: 1649-1651.
Monte-Silva K, Ruge D, Teo JT, Paulus W, Rothwell JC, Nitsche MA: D2 receptor block abolishes theta burst stimulation-induced neuroplasticity in the human motor cortex. Neuropsychopharmacology. 2011, 36: 2097-2102. 10.1038/npp.2011.100.
Benali A, Trippe J, Weiler E, Mix A, Petrasch-Parwez E, Girzalsky W, et al: Theta-burst transcranial magnetic stimulation alters cortical inhibition. J Neurosci. 2011, 31: 1193-1203. 10.1523/JNEUROSCI.1379-10.2011.
Trippe J, Mix A, Aydin-Abidin S, Funke K, Benali A: Theta burst and conventional low-frequency rTMS differentially affect GABAergic neurotransmission in the rat cortex. Exp Brain Res. 2009, 199: 411-421. 10.1007/s00221-009-1961-8.
Suppa A, Ortu E, Zafar N, Deriu F, Paulus W, Berardelli A, et al: Theta burst stimulation induces after-effects on contralateral primary motor cortex excitability in humans. J Physiol. 2008, 586: 4489-4500. 10.1113/jphysiol.2008.156596.
Sur M, Nelson RJ, Kaas JH: Representations of the body surface in cortical areas 3b and 1 of squirrel monkeys: comparisons with other primates. J Comp Neurol. 1982, 211: 177-192. 10.1002/cne.902110207.
Nelson RJ, Sur M, Felleman DJ, Kaas JH: Representations of the body surface in postcentral parietal cortex of Macaca fascicularis. J Comp Neurol. 1980, 192: 611-643. 10.1002/cne.901920402.
Xerri C, Merzenich MM, Jenkins W, Santucci S: Representational plasticity in cortical area 3b paralleling tactual-motor skill acquisition in adult monkeys. Cereb Cortex. 1999, 9: 264-276. 10.1093/cercor/9.3.264.
Xerri C, Coq JO, Merzenich MM, Jenkins WM: Experience-induced plasticity of cutaneous maps in the primary somatosensory cortex of adult monkeys and rats. J Physiol Paris. 1996, 90: 277-287. 10.1016/S0928-4257(97)81438-6.
Coq JO, Xerri C: Environmental enrichment alters organizational features of the forepaw representation in the primary somatosensory cortex of adult rats. Exp Brain Res. 1998, 121: 191-204. 10.1007/s002210050452.
Kurusu K, Kitamura J: Long-latency reflexes in contracted hand and foot muscles and their relations to somatosensory evoked potentials and transcranial magnetic stimulation of the motor cortex. Clin Neurophysiol. 1999, 110: 2014-2019. 10.1016/S1388-2457(99)00166-2.
Ziemann U, Wittenberg GF, Cohen LG: Stimulation-induced within-representation and across-representation plasticity in human motor cortex. J Neurosci. 2002, 22: 5563-5571.
Oldfield RC: The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971, 9: 97-113. 10.1016/0028-3932(71)90067-4.
DeLisa J, Lee H, Baran E, Lai K, Spielholz N: Somatosensory Evoked Potentials. Manual of Nerve Conduction Velocity and Clinical Neurophysiology. 1994, Raven, New York, 195-274. 3
Seyal M, Emerson RG, Pedley TA: Spinal and early scalp-recorded components of the somatosensory evoked potential following stimulation of the posterior tibial nerve. Electroencephalogr Clin Neurophysiol. 1983, 55: 320-330. 10.1016/0013-4694(83)90210-9.
McIlroy WE, Bishop DC, Staines WR, Nelson AJ, Maki BE, Brooke JD: Modulation of afferent inflow during the control of balancing tasks using the lower limbs. Brain Res. 2003, 961: 73-80. 10.1016/S0006-8993(02)03845-3.
Terada K, Umeoka S, Baba K, Sakura Y, Usui N, Matsuda K, et al: Generators of tibial nerve somatosensory evoked potential: recorded from the mesial surface of the human brain using subdural electrodes. J Clin Neurophysiol. 2009, 26: 13-16. 10.1097/WNP.0b013e3181969043.
Beric A, Prevec TS: Distribution of scalp somatosensory potentials evoked by stimulation of the tibial nerve in man. J Neurol Sci. 1983, 59: 205-214. 10.1016/0022-510X(83)90038-2.
Vera CL, Perot PL, Fountain EL: Scalp recorded somatosensory evoked potentials to posterior tibial nerve stimulation in humans. Electroencephalogr Clin Neurophysiol. 1983, 56: 159-168. 10.1016/0013-4694(83)90070-6.
Gentner R, Wankerl K, Reinsberger C, Zeller D, Classen J: Depression of human corticospinal excitability induced by magnetic theta-burst stimulation: evidence of rapid polarity-reversing metaplasticity. Cereb Cortex. 2008, 18: 2046-2053.
Hamada M, Murase N, Hasan A, Balaratnam M, Rothwell JC: The Role of Interneuron Networks in Driving Human Motor Cortical Plasticity. Cereb Cortex. 2012, Epub ahead of print
Premji A, Ziluk A, Nelson AJ: Bilateral somatosensory evoked potentials following intermittent theta-burst repetitive transcranial magnetic stimulation. BMC Neurosci. 2010, 11: 91-10.1186/1471-2202-11-91.
Allison T, McCarthy G, Jones SJ, Jones SJ: Potentials evoked in human and monkey cerebral cortex by stimulation of the median nerve. A review of scalp and intracranial recordings. Brain. 1991, 114 (Pt 6): 2465-2503.
Kakigi R, Jones SJ: Influence of concurrent tactile stimulation on somatosensory evoked potentials following posterior tibial nerve stimulation in man. Electroencephalogr Clin Neurophysiol. 1986, 65: 118-129. 10.1016/0168-5597(86)90044-4.
Kakigi R, Koyama S, Hoshiyama M, Shimojo M, Kitamura Y, Watanabe S: Topography of somatosensory evoked magnetic fields following posterior tibial nerve stimulation. Electroencephalogr Clin Neurophysiol. 1995, 95: 127-134. 10.1016/0013-4694(95)00053-2.
Gloor P: Neuronal generators and the problem of localization in electroencephalography: application of volume conductor theory to electroencephalography. J Clin Neurophysiol. 1985, 2: 327-354. 10.1097/00004691-198510000-00002.
Nelson AJ, Brooke JD, McIlroy WE, Linklater CM, Staines WR: The afferent origin of the secondary somatosensory evoked potential from the lower limb in humans. Brain Res. 2000, 887: 432-435. 10.1016/S0006-8993(00)03024-9.
Nelson AJ, Chen R: Digit Somatotopy within Cortical Areas of the Postcentral Gyrus in Humans. Cereb Cortex. 2008, 18: 2341-2351. 10.1093/cercor/bhm257.
Stefan K, Gentner R, Zeller D, Dang S, Classen J: Theta-burst stimulation: remote physiological and local behavioral after-effects. Neuroimage. 2008, 40: 265-274. 10.1016/j.neuroimage.2007.11.037.
Premji A, Rai N, Nelson A: Area 5 influences excitability within the primary motor cortex in humans. PLoS One. 2011, 6: e20023-10.1371/journal.pone.0020023.
Mori F, Codeca C, Kusayanagi H, Monteleone F, Boffa L, Rimano A, et al: Effects of intermittent theta burst stimulation on spasticity in patients with multiple sclerosis. Eur J Neurol. 2010, 17: 295-300. 10.1111/j.1468-1331.2009.02806.x.
Acknowledgments
We thank Natural Sciences and Engineering Research Council to AJN.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CMZ conceived of the study, carried out the data collection, analyzed all data, and drafted the manuscript. DAEB assisted with the study design, data collection and editing of the manuscript. MJA, KGHL, and MFJ assisted with data collection, and aided in editing of the manuscript. AJN conceived of the study, assisted with data analysis and assisted with manuscript preparation. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Zapallow, C.M., Asmussen, M.J., Bolton, D.A.E. et al. Theta burst repetitive transcranial magnetic stimulation attenuates somatosensory evoked potentials from the lower limb. BMC Neurosci 13, 133 (2012). https://doi.org/10.1186/1471-2202-13-133
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2202-13-133