Abstract
Electronic waste (E-waste) generation is evaluated at 20–50 million tons, representing 1–3% of the general waste generated yearly worldwide. The large quantities of outdated and life-ended electrical and electronic equipment make it a fast-growing waste production all over the world. Printed circuit boards (PCBs) are the most highly valued precious components of E-waste. Apart from valuable metals, PCBs contain many dangerous and hazardous substances. The very unpredictable mix of such different important and hazardous materials combined in a small volume poses serious challenges for the recovery and recycling of these constituents. To prevent toxicity of these contaminants to humans and environment, it is inevitable to analyze the peculiarities and compositions of various materials in E-waste and determine how to manage their recycling via green ecofriendly processes. This paper will deal with the outline of E-waste problem, its diverse categories, composition, management, and various recycling processes especially the green ecofriendly ones with unique attention toward extraction of valuable metals. Unfortunately, despite the fact that many efforts to develop recycling technologies have been endeavored, these technologies are still rather exclusive and inadequate because of the intricacy of the E-waste system. Hence, the demerits of each process are debated and discussed from the viewpoint of technical advancement and environmental protection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental management of noxious wastes has attracted a main interest across the world due to the unbridled dumping into the ecosystem in a reckless way leading to possible risks. Currently, nearly 1.3 billion tons of wastes are generated yearly across the world [1], which will be expected to increase to 4.3 billion tons per annum by the year 2025. Electronic waste (E-waste) generation is growing very fast around the world [2], with the rate of generation being threefold higher than any other waste [3]. It is one of the principal contributors to environmental contamination, the reason why it is strongly required to develop effective recycling techniques for its mitigation [4, 5]. It is predicted that E-waste generation from old computers would rise by 500% and that from the discarded mobile phones would be nearly 18 times higher by the year 2020 compared with the year 2007 [6].
E-waste contains both valuable and hazardous metals which require special handling and recycling methods to diminish environmental contamination and hazardous effects on human health. Knowledge and understanding the compositions of E-waste components help to recycle the components effectively. Several existing recycling processes and techniques are convenient for management of E-waste, but the selection and adoption of an appropriate process need a cautious approach, which depends upon the material composition, and thus it becomes quite important to know about its components. Advanced electronic equipment contains nearly 60 different elements including valuable and hazardous materials. The most complex and valuable materials are found on printed circuit boards [7]. Printed circuit boards (PCBs) are the basic component whereupon electronic items are assembled. They are used to uphold and connect the electronic components together using conductive tracks etched from copper sheets laminated onto them [8]. PCBs are viewed as the most economically valuable component of E-waste. Yet, the fact that such a highly complex blend of various valuable and sometimes hazardous materials is constrained in such a small volume poses serious challenges for recycling the constituent substances and materials. The heterogeneous mix of organics, metals, fiber glass, toxic materials including heavy metals, and plastics makes the processing of PCBs a really challenging task. Recycling of waste PCBs (WPCBs) is a very crucial issue for treating waste and recovering valuable metals. In general, WPCBs are incorporated with around 30% metals such as copper, iron, tin, nickel, lead, zinc, aluminum, and precious metals [9, 10]. Beside metals, toxic substances, such as brominated flame retardants (BFR) and polyvinyl chloride (PVC) are also included [11, 12]. Their recycling is a complicated, costly process due to the variety of components, and thus, the associated difficulty in separating them. Marques et al.’s [8] processes based on pyrometallurgy and hydrometallurgy [13,14,15,16,17,18] are usually used for extracting and recovering metals from WPCBs. In industries, the recycling of E-waste is carried out basically through pretreatment and pyrometallurgical processes. One of the main international copper manufacturing companies, Aurubis Global, professionally processes electronic waste in addition to other copper materials and precious metals [19, 20]. The main pyrometallurgical procedures used are two reduction processes in a submerged furnace, followed by oxidation process in an anode furnace. The product which is 99% copper is cast into copper anodes, and electrowinning is then applied to purify copper [19, 20]. Other international companies, such as Umicore integrated smelting and refining facility, the Noranda process in Quebec, Rönnskär smelters in Sweden, and Kosaka’s plant in Japan, applied several industrial methods for recovering metals from E-waste [21].
Only partial extraction of metals can be attained by thermal processing, leading to a limited recovery of metals. Moreover, further leaching or electrochemical processing is required. Precious metals stay for an extended period during the process and are recovered later at the end of processing. Recent research done on energy recovery from personal computers waste offers an associated example for use of plastics in electronic waste. It indicates that thermal processing of E-waste provides a possible approach for recovery of energy from E-waste if an efficient emission control system is installed [11].
Recently, there have been many researches on hydrometallurgical treatment of E-waste for metal recycling because it entails lower cost, less environmental impact, and is easier to manage compared with pyrometallurgy [3]. Research on the hydrometallurgical method is directed to precious and common metals’ recycling [22]. The targeted metal for recycling in hydrometallurgy is mainly copper because of its high content in waste PCBs [23]. Mostly, the essential steps in hydrometallurgical treatment consist of leaching, purification, and recovery. Leaching treatments of waste printed circuit board can be sorted into four categories: acid, ammonia, ammonium salt, and chloride; other ways of leaching and some of these methods are industrially applied [24, 25]. Commonly, copper is dissolved in leachants, then the solution goes through solvent extraction to improve copper purity, and finally, pure copper is obtained by electrowinning [26,27,28,29].
Some of the E-waste recycling processes are related with certain disadvantages that limit their application on the industrial scale because of their slowness, time consuming nature, and adverse impacts on the environment. Some leachants used in hydrometallurgical processes are hazardous and pose serious health risks to the people, and should, therefore, be manufactured or used in accordance with high safety standards. Moreover, there are issues concerning the costs of hydrometallurgical processes compared with pyrometallurgical processes used for the extraction of metals from E-waste [11]. Pyrometallurgical processes have also some disadvantages such as generation of a large amount of slag, loss of precious metals, and difficulty in the recovery of Al and Fe, and other metals [11].
Hence, it is necessary to summarize and discuss in detail the recycling processes of E-waste, especially WPCBs, taking into consideration the concept of “Green process”. Based on environmental protection and resource recycling utilization for the metals, some green integrated recycling processes free from pollution and having high efficiency for E-waste treatment were presented and discussed.
Different Categories of E-Waste
E-waste is an expression referring to all spent electric and electronic devices that have been discarded by its users. Based on the European WEEE Directives 2002/96/EC and 2012/19/EU [30, 31], it is sorted into different types. These types include big and small household appliances, information technology and telecom equipment, consumer equipment, lighting devices, electrical and electronic nonindustrial tools, toys, relaxation and sports equipment, medical noninfected devices, monitors and control units, and automatic dispensers as shown in Fig. 1 [32, 33].
E-Waste Composition and Characteristics
The structural composition of E-waste depends commonly on the type and the model of the electronic device, its manufacturer, date of manufacture, and the age of the scrap. Larger amounts of precious metals are included in scrap from IT and telecommunication systems than those in the scrap from household equipment [23]. For instance, a cellular phone contains more than 40 elements, base metals such as copper (Cu) and tin (Sn) and precious metals such as silver (Ag), gold (Au), and palladium (Pd) [34,35,36]. Circuit boards in the majority of the electronic equipment may contain toxic elements such as arsenic (As), chromium (Cr), lead (Pb), and mercury (Hg). Ended-life cathode ray tubes (CRTs) in televisions and computer monitors contain barium, copper, lead, zinc, and other rare earth metals. Therefore, most countries have prohibited cathode ray tubes disposal through landfilling. The changing composition of constituents due to the development of technology has led to a severe challenge in evolving policies to manage E-waste [37]. Various factors affect the composition of E-waste, including economic conditions, the reuse market, the recycling industry, waste separation programs, and control execution. Figure 2 adapted from [32] shows the characteristic material fractions of E-waste components.
Characteristic material fractions in e-waste [32] (color figure online)
E-Waste Management
Commonly, in waste management, waste materials are gathered, transported, disposed or processed, and recycled aiming to diminish their harmful influences on health or the environment. It is also conducted to regain resources from it. Some of the important processes utilized in dealing with E-waste incorporate the following:
Landfill Disposal
One of the used methods for disposing E-waste is to bury it. Mining voids or burrow depths can be used in land filling. E-wastes ending up as landfills may release pollutants to the environs after some years in natural ways. Leaching some wastes such as batteries may possibly release acids and heavy metals like mercury, nickel, and cadmium. Moreover, E-waste landfills may pollute groundwater [38, 39]. After diffusing into the land soil, polluted water will mix with other water sources such as rivers and streams, and thus cause a potential harm when used by animals and humans [40]. Organic and decayed materials in landfills decompose and penetrate through the ground as landfill leachate containing high amounts of polluting substances depending on the waste type and its decay stage [41].
Thermal Treatment
Thermal treatment of e-waste is carried out by either incineration or pyrolysis. Incineration is a method of throwing out waste by burning it [42]. It usually acts as an alternative to other disposal methods, especially landfilling. Incineration can lessen the volume of waste and the energy content of its combustible materials. When burning the waste materials, a reduction in its volume occurs, and the materials’ energy content can be utilized. Thermal treatment also includes pyrolysis (heating the substance in the absence of oxygen) wherein the substances are converted to fumes, oils, and charcoal.
When burning the plastic or PVC circuit board, the fumes consist of carcinogenic polycyclic aromatics, dioxins and polychlorinated dibenzofurans, and gases such as oxides of carbon, sulfur, and nitrogen are released and minor quantities of heavy metal oxides could be present in the smoke. Although the burning method is a simple, low-cost process, it has been prohibited because of its serious pollution of the environment [43,44,45]. E-waste incineration plants contribute significantly to the yearly emissions of cadmium and mercury [46, 47].
Re-use Method
Herein, the original hardware is second-hand or used after minor changes. This method has an advantage of reducing generated e-waste volume. However, inducements offered by the retailers to monetize the old appliances by exchanging against new ones are marketing gimmicks for accelerating sales volume. The actual benefits to the consumer in the new-for-old exchange practice are notional once seen commercially [48].
Recycling Method
Recycling is defined as the reworking of the wasted materials for performing the original function or for some other different purposes. Recycling of E-waste comprises disassembly and/or destruction of the wasted equipment to recover their substances. It is an important process in terms of waste treatment and recovering valuable materials. It is also a beneficial alternative relative to disposal. The US Environmental Protection Agency (EPA) has identified distinguished real advantages, such as energy savings and decrease in pollutions, when scrap materials are utilized rather than virgin materials.
Utilizing recycled materials in place of virgin materials results in noteworthy energy savings [49]. The prime targeted aim of recycling E-waste scrap is to diminish the harmful environmental impact caused by hazardous materials and ensure maximum material recovery. To accomplish these goals, detailed information of the E-waste components is required for choosing the right recycling method and facility [50].
Waste Printed Circuit Boards
While dealing with the growing volume of E-wastes, recycling of printed circuit boards (PCBs) is known as one of the most difficult tasks considering their complex structure and the consequent complicated blend of materials [51]. PCBs are fundamental constituent materials in an extensive variety of electrical and electronic hardware (televisions, personal computers, mobile phones, and laptops).
Printed circuit boards (PCBs) are composed of three types of materials: a nonconducting substrate or laminate, printed conducting tracks, and components mounted on the substrate. The substrate is typically composed of glass fiber reinforced with epoxy resin or paper reinforced with phenolic resin, both with brominated flame retardants [50]. Polymers and industrial plastics are other major constituents of PCBs that contain polyethylene, polypropylene, epoxies, and polyesters [21].
WPCBs have been paid careful attention by researchers and industrial personnel, due to their wealthy resource content and associated feasible risks on human health and environment when recycled informally and inappropriately. In this manner, factors that affect metal extraction are financial viability, recovery effectiveness, and environmental impact. WPCBs recycling process for the highest optimal recovery of metals generally includes three stages: pretreatment, size reduction, and metallurgical treatment. Pretreatment means compositional analysis and careful disassembly of the toxic parts by thermally or chemically desoldering [52, 53]. The materials are then shredded, crushed, and screened to reduce their size [3, 5, 11, 49, 54,55,56,57]. Metallurgical treatment involves thermal treatment [58, 59], leaching [60], electrolysis [61], and biological [22, 62] processes for recovery and purification of the metals [63,64,65,66].
E-waste Recycling and Treatments
Incineration, landfilling, and export to abroad are banned in recent years for E-waste management due to the strict laws that are enforced in developed countries such as the European Union (EU), the United States (US), Australia, and Japan [67]. Thus, environmental worries and the presence of reusable metals or components provoke the need to recover heavy and precious metals from E-waste, before disposing them off into the environment. However, environmental consequences and high-energy demand are the major limitations which hinder their use at large scale. Pyrometallurgical methods may be energy-intensive and high-cost processes [68]. They generate large amounts of slag, and may lead to the formation of mixed halogenated dioxins and furans which can be avoided by availing proper off-gas treatment [11, 21]. Hydrometallurgical treatment of E-waste frequently uses cyanide [69, 70], halide [71], thiourea, and thiosulfate [72, 73] as leaching agents to extract most valuable components [8, 74,75,76,77]. Bio-hydrometallurgy has also been applied, to leach valuable metals from waste printed circuit board (WPCBs) [60, 78]. Valuable metals can be further recovered from leaching solution through adsorption by means of biomass waste including microbial biomass [79].
Chelating agents have been used by many researchers for the effective and efficient removal of metals such as Zn, Cu, and Pb from E-waste [80,81,82]. Chelating agents could be used to extract metals, due to their ability to form soluble and stable metal complexes [83]. EDTA and Nitrilotriacetic acid (NTA) have been found capable of extracting up to 86% of Cu, Pb, and Zn. Di-Palma and Mecozzi [84] found that Cu and Pb can be more effectively extracted with EDTA than with citric acid, whereas Kolencík et al. [85] used Aspergillus niger fungi, along with citric acid and oxalic acid, and found it to be effective as a pretreatment or a final phase of E-waste metal recycling [86].
Unfortunately, even though several conventional methods are available, they sometimes just transfer the pollutants from one place to another. Researchers are therefore trying to develop more environmentally friendly processes that can efficiently solve this problem. Most of the used recycling practices aim to recover valuable metals from E-waste using physical and chemical methods.
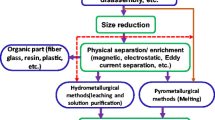
It is usual in waste management that wasted materials are collected, transported, disposed off, or processed and recycled to diminish their harmful effects on health or the environment. Several recycling processes are used and the proper process depends on type of the material, its metal content, and volume. However, to extract valuables from E-waste, it must endure a basic process including collection, breaking, and detaching, pretreatment, and final metal recovery as revealed in Fig. 3.
Several issues have to be considered in evolving a new treatment for recycling WPCBs driven by novelties, societal effects, and influence on the environment, a unified waste management plan, and the procedure economics. The WPCBs are various and complex in terms of type, size, configuration, components, and composition. Over time, the composition of PCBs is continuously changing, making it more challenging to acquire a constant material composition. In general, the recycling techniques of WPCBs can be abridged as physical recycling methods and chemical recycling methods.
Separation of metals and nonmetals from WPCBs as a rule completed by physical techniques relies upon various parameters, for example, separation by shape, density, electric conductivity, and electrostatic properties [11]. Then again, chemical recycling forms incorporate gasification, burning, and pyrolysis. Metallic fraction can be treated by pyrometallurgical, hydrometallurgical, or biotechnological process, and the recovery process becomes more complicated when the elements are available in minor concentrations. The recovery of metal values, which is nearly 30% of the full weight of WPCBs, is the primary motive for recycling, while the nonmetallic materials (approximately 70%) have rather less economic values. The main targeted aim of most recycling processes is to recover the most valuable metals from WPCBs using processes which occasionally are not very ecofriendly.
Physical Treatment Processes
Physical processes are commonly applied during the upgradation stage when various metals and nonmetals contained in E-waste are liberated and come apart by some means of shredding and crushing processes. The effort to recover the valuable metals in particular Au, Ag, Pd, and Cu has received enormous attention in recent years using extraction processes such as physical, chemical, and combined pyro/hydro leaching separation routes. Physical processes include dry crushing and pulverizing and then high-voltage electrostatic separation to get a variety of metal powders (Cu, Pb, Zn, Al, Sn, Au, Ag, etc.) which are conductive and nonmetallic resin powder materials which are nonconductive. The consequence of a recycling process can be measured from two aspects: the material recuperation proficiency and the effect on the environment. Physical separation processes gain low running expenses and experience from a high valuable metal loss (10–35%) due to insufficient metal liberation. To evade pollution with dust, a three-stage dust removal equipment (i.e., cyclone, bag, and air cleaner) are commonly practiced. This type of physical WPCBs recycling systems has a separation efficiency reaches 99%.
Mechanical method has been considered as the preferable recovery technology for E-waste since no secondary pollution was taken out during the process [87,88,89,90]. Magnetic separation has been generally used in mineral processing or solid waste industry to recover magnetic materials from other materials [91]. Conversely, to recover the nonferrous metals such as Cu, Al, Pb, and Zn from the solid E-wastes, eddy current separation has been used [92, 93]. Table 1 summarizes physical methods for recycling E-waste.
Physical recycling methods have many advantages. They are simple, appropriate and environmentally friendly processes. Their equipment and energy used are low cost and potential application of the products is distinguished. Significant dust generation and metal loss during shredding and grinding are some important weaknesses of the performance. Nowadays, some developed countries generally use physical processing of E-waste for the recovery of raw materials. Their process flowcharts include manual selective disassembly, shredding, magnetic and electrostatic Eddy current separations [100].
Chemical Treatment Processes
Various researchers studied the extraction methods of copper, lead, and zinc and precious metals from E-waste using chemical methods [88, 101,102,103]. These methods are based on the classic hydrometallurgical technology of metal extractions from their ores. Acids or alkalis are used as leachants for dissolution of precious metals from E-waste, and then they are separated and purified for the metal content enrichment and removing impurities. The wanted metal is separated through solvent extraction, adsorption, or ion exchange processes. Lastly, metals are recovered from solution via electrorefining or chemical reduction processes [26, 27, 29, 104, 105]. The processes utilized for recovering metals from E-waste were surveyed and described by Cui and Zhang [11]. Hydrometallurgical chemical processes were found to have extra benefits when contrasted with pyrometallurgical processes since they are more particular, expectable, and easily controllable [106]. A concise clarification of the green hydrometallurgical processes is given in Table 2.
Although chemical processes have been successfully used to recover metals from E-waste, they are accompanied with some disadvantages limiting their industrial-scale application [112, 113]. Chemical process operations are tedious, time consuming, and impact recycling economy. However, mechanically handling the E-waste to reduce the size required for efficient dissolution consumes long time. Approximately 20% of metals is mechanically lost during the liberation process that leads to a substantial reduction in the overall metal recovery. Moreover, halide leaching is difficult to enforce due to strong corrosive acids and oxidizing conditions; besides, special equipment made of stainless steel or rubbers is required for the leaching process. Also, there are risks of metal losses during subsequent steps affecting the overall recovery of metals.
Bioleaching Treatment Processes
Bioleaching process is a well-thought-out one of the most auspicious technologies in metallurgical processing. It is considered a green technology [114, 115] having a lower operative cost and energy demands when comparing with chemical methods [116]. It is an excellent process for extracting metals from low-grade ores [117,118,119,120,121]. Bioleaching is technically practicable using bacteria-assisted reaction to extract base metals such as Cu, Ni, Zn, Cr, and precious metals such as Au, Ag, from E-waste. Recently, some studies for extracting metals from E-waste scrap have been done by many researchers [122,123,124]. Heavy metals were excellently leached when Acidophilic bacteria were used [125,126,127]. For example, Wang et al. [128] succeeded in mobilizing metals from WPCBs using Acidobacillus bacteria. The bacteria, Acidithiobacillus ferrooxidans (A. ferrooxidans) and Acidithiobacillus thiooxidans (A. thiooxidans), were grown and accustomed in WPCBs and then used as bioleaching bacteria to solubilize metals. Very few studies reported the use of fungus for recovery of metals from E-waste [81, 85, 122, 129]. Because of constant supply of nutrients for fungal growth, handling of fungi in turnover and long processing time restricts the use of fungus [130]. Besides these limitations, fungal bioleaching has several advantages over bacterial bioleaching: they can grow at high pH, which makes them efficient for alkaline materials bioleaching. They can leach metals rapidly and conceal organic acids that chelate metal ions, thereby, being useful in metal-leaching process [122, 131]. Even with such advantages, still, there is an information shortage about using fungi to leach metals from E-waste [132,133,134]. The E-waste has no source of energy that is required for growing the bacteria, and therefore, an external supply of nutrients is a must for leaching metals from E-waste. Table 3 shows the mostly used bacteria, and microorganisms for E-waste leaching are given in the table. Although bioleaching process has many advantages, commercial performance of the process is still in the nascent stage. This is imputable to the completely slow nature of this process. Many of the bioleaching processes require long time ranging from 48 to 245 h to recover metals without recovering all the metals present in E-waste [135]. Therefore, there is a necessity to develop a fast and economic bioleaching process that can be applied industrially.
Vacuum Recycling Processes
Metals can be recovered from E-waste utilizing vacuum processes which have no wastewater pollution. Here, the nonmetallic components from E-waste can be removed by vacuum pyrolysis so the metals can be easily recovered [14, 143]. Metals separated out and recovered from WPCBs depend on their vapor pressures at the same temperature. Zhou research group [97, 144] recovered metals from WPCBs by a two-step vacuum pyrolysis process. The chief process separated and recovered the solder alloy at temperature ranges from 400 to 600 °C. In the ensuing process, WPCBs were pyrolyzed and the residue heated under vacuum to recover the solder by centrifugal separation. Glass fiber and other inorganic metals and materials in the resulting residues still need additional treatment. Cadmium (Cd) and zinc (Zn) metals from WPCBs were recycled using vacuum process for their separation [145,146,147,148]. The research group used their self-made vacuum furnace, and they succeeded to favorably evaporate Cd and separate Zn because of their vapor pressure differences. The separation of lead found in the solders of WPCBs was more difficult because the Pb–Bi alloy was formed with a low vapor pressure.
He et al. effectively recovered metallic indium from a distinct sort of E-waste which is waste liquid crystal display (LCD) panels, using coke powder as a reducing agent [149]. Indium oxide (In2O3) was reduced to metallic indium under high-temperature condition by reducing atmosphere. The processing conditions were 1223 K and 1 Pa with 30 wt% carbon additions for 30 min. and the recovery rate of indium reached 90 wt%. Indium was also recovered from wasted LCD panels by chlorinated vacuum-separation method [150]. In this method, a chlorinating agent, ammonium chloride (NH4Cl), was used at temperature of 400 °C. The weight ratio of the used NH4Cl-to-glass powder was 1:2, and the optimal particle size was less than 0.13 mm. Purity of the recovered indium chloride reached 99.50%, and its recovery rate attained 98.02%.
The environmental and financial benefits of vacuum processes for the E-waste recycling have been validated. Gas flow can be efficiently controlled, and furthermore, no wastewater will be released or dust emission will occur. This process is a promising and environment-friendly method for metal recycling from E-waste, still, it needs to be improved for further advancement. On the one hand, vacuum processes have many benefits for separating metals having low boiling point and high saturation vapor pressure, such as Zn, Pb, and Cd. In contrast, separation of the valuable and rare metals having low saturated vapor pressure through vacuum condensation method is not so great. Still, pretty much hypothetical issues are waiting for additional clearance before its modern industrial application [151].
Conclusion
E-waste is not a common rubbish or an ordinary waste. Instead, it is a valuable scrap that contains considerable quantities of metal resources and should not be dumped wrongfully anywhere. Traditional processes may not meet the future industry requirements because of environmental contamination, high cost, and low efficiency. It would be greatly important to develop green processes for the recycling of WPCBs for both the economy and environment. The authors wish that this review will bring issues to light regarding E-waste management using cleaner economical recycling process. Even though various activities about development of recycling processes have been attempted, even now there is still a huge area left to achieve the environment-friendly recycling for metals. Furthermore, using a single technology still has some limitations and cannot solve all problems because of the complexity of the E-waste system. A combination of more than one process or technology should be applied to recover metals later on. We can also prefigure that future recycling technologies of metals from E-waste will turn out to be more effective, of low cost, and meeting the necessities of environmental safety.
References
Hoornweg D, Bhada-Tata P (2012) What a waste: a global review of solid waste management, vol 15. World Bank, Urban Development & Local Government Unit, Washington, DC, p 98
Hadi P, Ning C, Ouyang W, Xu M, Lin CSK, McKay G (2015) Toward environmentally-benign utilization of nonmetallic fraction of waste printed circuit boards as modifier and precursor. Waste Manag 35:236–246
Tuncuk A, Stazi V, Akcil A, Yazici E, Deveci H (2012) Aqueous metal recovery techniques from e-scrap: hydrometallurgy in recycling. Miner Eng 25:28–37
Guan J, Wang J, Min X, Wu W (2012) The products characteristics of calcium-basic compounds pyrolysis with waste printed circuit boards (PCB), the 7th international conference on waste management and technology. Proc Environ Sci 16:461–468
Veit HM, Diehl TR, Salami AP, Rodrigues JS, Bernardes AM, Tenorio JAS (2005) Utilization of magnetic and electrostatic separation in the recycling of printed circuit boards scrap. Waste Manag 25:67–74
United Nations Environment Programmed (UNEP) Report (2010) Urgent need to prepare developing countries for surge in E-wastes. http://www.unep.org/Documents.Multilingual
Rankin WJ (2011) Minerals, metals and sustainability: meeting future material needs. CSIRO Publishing, Collingwood
Marques A, Cabrera J, Malfatti C (2013) Printed circuit boards: a review on the perspective of sustainability. J Environ Manag 131:298–306
Duan H, Hou KL, Zhu X (2011) Examining the technology acceptance for dismantling of waste printed circuit boards in light of recycling and environmental concerns. J Environ Manag 92:392–399
He W, Li G, Ma X, Wang H, Huang J, Xu M, Huang C (2006) WEEE recovery strategies and the WEEE treatment status in China. J Hazard Mater 136:502–512
Cui J, Zhang L (2008) Metallurgical recovery of metals from electronic waste: a review. J Hazard Mater 158:228–256
Sohaili J, Muniyandi S, Suhaila SM (2012) A review on printed circuit boards waste recycling technologies and reuse of recovered nonmetallic materials. J Emerging Trends Eng Appl Sci 3:138–144
Bernardes AM, Bohlinger I, Milbrandt H, Rodriguez D, Wuth W (1997) Recycling of printed wire boards by melting with oxidizing/reducing top blowing process. In: TMS annual meeting, Orlando, Florida 9–13 February. The Minerals, Metals & Materials Society, Warrendale, pp 363–375
Hall WJ, Williams PT (2006) Fast pyrolysis of halogenated plastics recovered from waste computers. Energy Fuels 20:1536–1549
Jha M, Kumari A, Choubey P, Lee J, Kumar V, Jeong J (2012) Leaching of lead from solder material of waste printed circuit boards (PCBs). Hydrometallurgy 124:28–34
Pozzo R, Malicsi A, Iwasaki I (1991) Removal of lead from printed wire board scrap by an electrodissolution-delamination method. Resour Conserv Recycl 5:21–34
Xiu F, Qi Y, Zhang F (2013) Recovery of metals from waste printed circuit boards by supercritical water pretreatment combined with acid leaching process. Waste Manag 33:1251–1257
Yazici E, Deveci H (2013) Extraction of metals from waste printed circuit boards (WPCBs) in H2SO4-CuSO4-NaCl solutions. Hydrometallurgy 139:30–38
Aurubis Global (2015) Metal recycling. https://www.aurubis.com/en/en/corp/products/recycling/metal-recycling. Accessed Feb 2015
Aurubis Global (2015) Recycling technology. https://www.aurubis.com/en/en/. Accessed Feb 2018
Khaliq A, Rhamdhami MA, Brooks G, Masood S (2014) Metal extraction process for electronic waste and existing industrial routes: a review and Australian perspective. Resources 3:152–179
Bryan CG, Watkin E, McCredden TJ et al (2015) The use of pyrite as a source of lixiviant in the bioleaching of electronic waste. Hydrometallurgy 152:33–43
Chancerel P, Meskers CE, Hagelüken C, Potter VS (2009) Assessment of precious metal flows during preprocessing of waste electrical and electronic equipment. J Ind Ecol 13(5):791–810
Dhawan N, Kumar M, Kumar V, Wadhwa M (2008) Recovery of metals from electronic scrap by hydrometallurgical route. In: Mishra B, Ludwig C, Das SK (eds) Global symposium on recycling, waste treatment and clean technology (REWAS 2008). The Minerals, Metals & Materials Society, Warrendale, PA, pp 693–698
Zeng XL, Zheng LX, Xie HH et al (2012) Current status and future perspective of waste printed circuit boards recycling. Procedia Environ Sci 16:590–597
Ritcey GM (2006) Solvent extraction in hydrometallurgy: present and future. Tsinghua Sci Technol 11(2):137–152
Safarzadeh SM, Bafghi MS, Moradkhani D, Ilkhchi OM (2007) A review on hydrometallurgical extraction and recovery of cadmium from various resources. Miner Eng 20(3):211–220
Sum EYL (1991) The recovery of metals from electronic scrap. JOM 43(4):53–61
Yang B (1994) Ion exchange in organic extractant system. Ion Exch Adsorpt 10(2):168–179
European Parliament (2003) Directive 2002/96/EC of the European Parliament and of the Council of 27 January 2003 on waste electrical and electronic equipment (WEEE). Off J Eur Union L37:24–38
European Parliament (2012) Directive 2012/19/EU of the European Parliament and of the Council of 4 July 2012 on waste electrical and electronic equipment (WEEE). Off J Eur Union L197:38–71
Widmer R, Oswald-Krapf H, Sinha-Khetriwal D, Böni H, Schnellmann M (2005) Global perspectives on e-waste. Environ Impact Assess Rev 25(5):436–458. www.ChemistryWorld.org, June 2007, The gadget scrap heap: electronic waste, pp 44–48
Sharma P, Fulekar MH, Pathak B (2012) E-waste—a Challenge for Tomorrow. Res. J. of Recent Sci. 1(3):86–93
Liu Q, Li KQ, Zhao H, Li G, Fan FY (2009) The global challenge of electronic waste management. Environ Sci Pollut Res 16(3):L248–249
Shao G, Li Y, Xu X, Liu J, Wu K, Gu C (2008) The hazard of chromium exposure to neonates in Guiyu of China. Sci Total Environ 403(1–3):99–104
Blass VD, Favret L, Fuji M, Mahdavi S, Miller R, Neira, J (2006) End-of-life management of cell phones in the United States. MS Thesis, Donald Bren School of Environmental Science and Management University of California, Santa Barbara, CA
Robinson B (2009) E-waste: an assessment of global production and environmental impacts. Sci Total Environ 408:183–191
Schmidt CW (2002) E-junk explosion. Environ, Health Perspectives, p 110
Yang GCC (1993) Environmental threats of discarded picture tubes and printed circuit boards. J Hazard Mater 34:235–243
Kasassi A, Rakimbei P, Karagiannidis A, Zabaniotou A, Tsiouvaras K, Nastis A, Tzafeiropoulou K (2008) Soil contamination by heavy metals: measurements from a closed unlined landfill. Bioresour Technol 99:8578–8584
Qasim SR, Chiang W (1994) Sanitary landfill leachate: generation, control and treatment. CRC Press, New York
Lee JC, Song HT, Yoo JM (2007) Present status of the recycling of waste electrical and electronic equipment in Korea. Resour Conserv Recycl 50:380–397
Bi X, Simoneit BRT, Wang Z, Wang X, Sheng G, Fu J (2010) The major components of particles emitted during recycling of waste printed circuit boards in a typical e-waste workshop of South China. Atmos Environ 44(35):4440–4445
Luo C, Liu C, Wang Y, Liu X, Li F, Zhang G, Li X (2011) Heavy metal contamination in soils and vegetables near an e-waste processing site, south China. J Hazard Mater 186(1):481–490
Owens CJ, Lambright C, Bobseine K, Ryan B, Gray LJ, Gullett BK, Wilson VS (2007) Identification of estrogenic compounds emitted from the combustion of computer printed circuit boards in electronic waste. Environ Sci Technol 41(24):8506–8511
Funcke W, Hemminghaus HJ (1997) PXDF/D in flue gas from an incinerator charging wastes containing Cl and Br and a statistical description of the resulting PXDF/D combustion profiles. Organohalogen Compds 31:93–98
Stewart ES, Lemieux PM (2003) Emissions from the incineration of electronics industry waste. In: IEEE international symposium on proceedings of electronics and the environment, pp 271–275
Ari V (2016) A review of technology of metal recovery from electronic waste, Ch. 6. In: Florin-Constantin Mihai (ed) E-Waste in Transition - From Pollution to Resource. https://doi.org/10.5772/61569
Cui J, Forssberg E (2003) Mechanical recycling of waste electric and electronic equipment: a review. J Hazard Mater 99(3):243–263
Li J, Shrivastava P, Gao Z, Zhang HC (2004) Printed circuit board recycling: a state of the art survey. IEEE Trans Electron Pack Manuf 27(1):33–42
Taberman SO, Carlsson B, Erichson H, Brobech J, Gregersen JC (1995) Environmental consequences of incineration and landfilling of waste from electronic equipment. The Nordic Council of Ministers, Copenhagen
Jianzhi L, Shrivastava P, Gao Z, Zhang HC (2004) Printed circuit board recycling: a state-of-the-art survey. IEEE Trans Electron Pack 27:33–42
Yang T, Zhu P, Liu W, Chen L, Zhang D (2017) Recovery of tin from metal powders of waste printed circuit boards. Waste Manag 68:449–457
Guo JY, Guo J, Xu ZM (2009) Recycling of non-metallic fractions from waste printed circuit boards: a review. J Hazard Mater 168(2–3):567–590
Kasper AC, Berselli GBT, Freitas BD, Tenório JAS, Bernardes AM, Veit HM (2011) Printed wiring boards for mobile phones: characterization and recycling of copper. Waste Manag 31:2536–2545
Tan Z, He Y, Xie W, Zhou E, Zheng Y (2011) Size distribution of wet crushed waste printed circuit boards. Min Sci Technol (China) 21:359–363
Tilmatine A, Medles K, Bendimerad SE, Boukholda F, Dascalescu L (2009) Electrostatic separators of particles: application to plastic/metal, metal/metal and plastic/plastic mixtures. Waste Manag 29:228–232
Ortiifio N, Conesa JA, Molto J et al (2014) Pollutant emissions during pyrolysis and combustion of waste printed circuit boards, before and after metal removal. Sci Total Environ 499:27–35
Yang XN, Sun LS, Xiang J et al (2013) Pyrolysis and dehalogenation of plastics from waste electrical and electronic equipment (WEEE): a review. Waste Manag 33(2):462–473
Ghosh B, Ghosh MK, Parhi P, Mukherjee PS, Mishra BK (2015) Waste printed circuit boards recycling: an extensive assessment of current status. J Clean Prod 94:5–19
Cocchiaraa C, Piazza S, Sunseri C, Inguanta R (2017) Study of a novel electrochemical method for copper recovery from waste printed circuit boards. Chem Eng Trans 57:2017
Zhou PG, Zheng Z, Peng XC et al (2007) Leaching of copper from printed circuit board by Thiobacillus ferrooxidans. Environ Pollut Control 29(2):119–122 (in Chinese)
Dorella G, Mansur MB (2007) A study of the separation of cobalt from spent Li-ion battery residues. J Power Sources 170:210–215
Lister TE, Wang P, Anderko A (2014) Recovery of critical and value metals from mobile electronics enabled by electrochemical processing. Hydrometallurgy 149:228–237
Pranolo Y, Zhang W, Cheng CY (2010) Recovery of metals from spent lithium-ion battery leach solutions with a mixed solvent extractant system. Hydrometallurgy 102:37–42
Provazi Campos BA, Espinosa DCR, Tenório JAS (2011) Metal separation from mixed types of batteries using selective precipitation and liquid–liquid extraction techniques. Waste Manag 31:59–64
Herat S, Agamuthu P (2012) E-waste: a problem or an opportunity Review of issues, challenges and solutions in Asian countries. Waste Manag Res (11):1113–1129. https://doi.org/10.1177/0734242X1245337
Kaya M (2016) Recovery of metals and nonmetals from electronic waste by physical and chemical recycling processes. Waste Manag 57:64–90
Raphulu MC, Scurrell MS (2015) Cyanide leaching of gold catalysts. Catal Commun 67:87–89
Wang WD, Feng YL, Li HR, Yang ZC, Zhang X, Yi AF (2015) Recovering gold from cyanide residue by alkaline predesilication-cyanide leaching technique. Chin J Nonferrous Met 25(1):233–240
Behnamfard A, Salarirad MM, Veglio F (2013) Process development for recovery of copper and precious metals from waste printed circuit boards with emphasize on palladium and gold leaching and precipitation. Waste Manage 33(11):2354–2363
Ghasem A, Khoramnejadian S (2015) The extraction of gold from e-waste by hydrometallurgy. Orient J Chem 31(1):113–120
Kannan G, Prabhakaran D, Sivakumar P, Thirumarimurugan M (2014) Recovery of metals from electronic waste using electrowinning process. J Chem Pharm Sci 4:136–137
Chen M, Huang J, Ogunseitan OA, Zhu N, Wang Y (2015) Comparative study on copper leaching from waste printed circuit boards by typical ionic liquid acids. Waste Manag 41:142–147
Gonçalves MCA, Garcia EM, Taroco HA, Gorgulho HF, Melo JOF, Silva RRA, Souza AG (2015) Chemical recycling of cell phone Li-ion batteries: application in environmental remediation. Waste Manag 40:144–150
Navarro M, May PM, Hefter G, Konigsberger E (2014) Solubility of CuO(s) in highly alkaline solutions. Hydrometallurgy 147:68–72
Pant D, Singh P, Upreti MK (2014) Metal leaching from cathode ray tube waste using combination of Serratia plymuthica and EDTA. Hydrometallurgy 146:89–95
Sahni A, Kumar A, Kumar S (2016) Chemo-biohydrometallurgy—a hybrid technology to recover metals from obsolete mobile SIM cards. Environ Nanotechnol Monit Manag 6:130–133
Gurung M, Adhikari BB, Kawakita H, Ohto K, Inoue K, Alam S (2013) Recovery of gold and silver from spent mobile phones by means of acidothiourea leaching followed by adsorption using biosorbent prepared from persimmon tannin. Hydrometallurgy 133:84–93
Jadhao P, Chauhan G, Pant KK, Nigam KDP (2016) Greener approach for the extraction of copper metal from electronic waste. Waste Manag 57:102–112
Madrigal AJ, Argumedo DR, Alarcón A, Mendoza-L R, Barradas O, Cruz SJ, Ferrera-C R, Jiménez-F M (2015) Bioleaching of gold, copper and nickel from waste cellular phone PCBs and computer gold finger motherboards by two Aspergillus niger strains. Braz J Microbiol 46:707–713
Pociecha M, Lestan D (2012) Recycling of EDTA solution after soil washing of Pb, Zn, Cd and As contaminated soil. Chemosphere 86(8):843–846
Li L, Dunn JB, Zhang XX, Gaines L, Chen RJ, Wu F, Amine K (2013) Recovery of metals from spent lithium-ion batteries with organic acids as leaching reagents and environmental assessment. J Power Sources 233:180–189
Di-Palma L, Mecozzi R (2007) Heavy metals mobilization from harbour sediments using EDTA and citric acid as chelating agents. J Hazard Mater 147(3):768–775
Kolenčík M, Urík M, Čerňanský S, Molnárová M, Matúš P (2013) Leaching of zinc, cadmium, lead and copper from electronic scrap using organic acids and the Aspergillus niger strain. Fresenius Environ Bull 22:3673–3679
Gadd GM (1999) Fungal production of citric and oxalic acid: importance in metal speciation: physiology and biogeochemical processes. Adv Microb Physiol 41:47–92
Lu Y, Xu Z (2016) Precious metals recovery from waste printed circuit boards: a review for current status and perspective. Resour Conserv Recycl 113:28–39
Park Y, Fray D (2009) Recovery of high purity precious metals from printed circuit boards. J Hazard Mater 164:1152–1158
Li, J., Dong, Q., Liu, L., Song, Q., 2016. Measuring treatment costs of typical waste electrical and electronic equipment: a pre-research for Chinese policy making. Waste Manage 57:36–45
Li J, Lu H, Guo J, Xu Z, Zhou Y (2007) Recycle technology for recovering resources and products from waste printed circuit boards. Environ Sci Technol 41:1995–2000
Waton J, Younas I (1998) Superconducting discs as permanent magnets for magnetic separation. Mater Sci Eng B 53:220–224
Lungu M, Schlett Z (2001) Vertical drum eddy-current separator with permanent magnets. Int J Miner Process 63:207–216
Zhang S, Rem P (1999) Particle trajectory simulation of two-drum eddy current separators. Resour Conserv Recycl 26:71–90
Meng L, Gao J, Zhong Y, Wang Z, Chen K, Guo Z (2018) Supergravity separation for recovering Pb and Sn from electronic waste. Sep Purif Technol 191:375–383
Lee J, Kim Y, Lee JC (2012) Disassembly and physical separation of electric/electronic components layered in printed circuit boards (PCB). J Hazard Mater 241–242:387–394
Habib M, Miles NJ, Hall P (2013) Recovering metallic fractions from waste electrical and electronic equipment by a novel vibration system. Waste Manag 33:722–729
Zhou YH, Qiu KQ (2010) A new technology for recycling materials from waste printed circuit boards. J Hazard Mater 175:823–828
Estrada-Ruiz RH, Flores-Campos R, Gamez-Altamirano HA, Velarde-Sanchez EJ (2016) Separation of the metallic and non-metallic fraction from printed circuit boards employing green technology. J Hazard Mater 311:91–99
Zhang ZY, Zhang FS, Yao TQ (2017) An environmentally friendly ball milling process for recovery of valuable metals from E-waste scraps. Waste Manag 68:490–497
Tsydenova O, Bengtsson M (2011) Chemical hazards associated with treatment of waste electrical and electronic equipment. Waste Manag 31(1):45–58
Chehade Y, Siddique A, Alayan H, Sadasivam N, Nusri S, Ibrahim T (2012) Recovery of gold, silver, palladium, and copper from waste printed circuit boards. In: Proceedings of the international conference on chemical, civil and environment engineering (ICCEE), Dubai, United Arab Emirates, pp 24–25
Delfini M, Ferrini M, Manni A, Massacci P, Piga L (2011) Antonio Scoppettuolo optimization of precious metal recovery from waste electrical and electronic equipment boards. J Environ Prot 2:675–682
Dhawan N, Kumar V, Kumar M (2009) Recovery of metals from electronic scrap by hydrometallurgical route. In: Howard SM (ed.) EPD Congress 2009. TMS, Warrendale, PA, pp 1107–1109
Shamsuddin M (1986) Metal recovery from scrap and waste. J Met 38(2):24–31
Tavlarides LL, Bae JH, Lee CK (1985) Solvent extraction, membranes, and ion exchange in hydrometallurgical dilute metals separation. Sep Sci Technol 22(2–3):581–617
Paretsky VM, Antipov NI, Tarasov AV (2004) Hydrometallurgical method for treating special alloys, jewelry, electronic and electrotechnical scrap. In: Proceedings of the Minerals, Metals & Materials Society (TMS) annual meeting, Charlotte, NC, 14–18 March, pp 713–721
Neto IFF, Sousa CA, Brito MSCA, Futuro AM, Soares HMVM (2016) A simple and nearly-closed cycle process for recycling copper with high purity from end life printed circuit boards. Sep Purif Technol 164:19–27
Kumari A, Jha MK, Lee JC, Singh RP (2016) Clean process for recovery of metals and recycling of acid from the leach liquor of PCBs. J Clean Prod 112:4826–4834
Jiang P, Harney M, Song Y, Chen B, Chen B, Chen Q, Chen T, Lazarus G, Lawrence HD, Korzenski MB (2012) Improving the end-of-life for electronic materials via sustainable recycling methods. Proc Environ Sci 16:485–490
Zhang ZY, Zhang FS (2014) A green process for copper recovery from waste printed circuit boards. Adv Mater Res 878:374–379
Zhang ZY, Zhang FS (2014) Selective recovery of palladium from waste printed circuit boards by a novel non-acid process. J Hazard Mater 279:46–51
Hilson G, Monhemius AJ (2006) Alternatives to cyanide in the gold mining industry: what prospects for the future? J Clean Prod 14:1158–1167
La Brooy SR, Linge HG, Walker GS (1994) Review of gold extraction from ores. Miner Eng 7:1213–1241
Brombacher CH, Bachofen R, Brandl H (1998) Development of a laboratory-scale leaching plant for metal extraction from fly ash by Thiobacillus strains. Appl Environ Microbiol 64(4):1237
Mishra D, Dong JK, Ralph DE, Jong-Hwan AHN, Young HAR (2008) Bioleaching of spent hydro-processing catalyst using acidophilic bacteria and its kinetics aspect. J Hazard Mater 152(3):1082–1091
Ilyas S, Anwar MA, Niazi SB, Ghauri MA (2007) Bioleaching of metals from electronic scrap by moderately thermophilic acidophilic bacteria. Hydrometallurgy 88(1–4):180
Kinnunen PHM, Heimala S, Vanhanen ML, Puhakka J (2006) Chalcopyrite concentrate leaching with biologically produced ferric sulphate. Biores Technol 97:1727–1734
Liu YG, Zhou M, Zeng GM, Wang LX, Fan T, Xu WH (2008) Bioleaching of heavy metals from mine tailings by indigenous sulfur-oxidizing bacteria: effects of substrate concentration. Bioresour Technol 99:4124–4129
Nakade DB (2013) Bioleaching of copper from low grade ore bornite using halophilic Thiobacillus ferrooxidans, N-11. Res J Recent Sci 2:162–166
Tipre DR, Dave SR (2004) Bioleaching process for Cu-Pb-Zn bulk concentrate at high pulp density. Hydrometallurgy 75:37–43
Zhou HB, Zeng WM, Yang ZF, Xie YJ, Qiu GZ (2009) Bioleaching of chalcopyrite concentrate by a moderately thermophilic culture in a stirred tank reactor. Bioresour Technol 100:515–520
Brandl H, Bosshard R, Wegmann M (2001) Computer-munching microbes: metal leaching from electronic scrap by bacteria and fungi. Hydrometallurgy 59:319–326
Choi MS, Cho KS, Kim DS, Kim DJ (2004) Microbial recovery of copper from printed circuit boards of waste computer by Acidithiobacillus ferrooxidans. J Environ Sci Health A 39(11–12):2973–2982
Faramarzi MA, Stagars M, Pensini E, Krebs W, Brandl H (2004) Metal solubilization from metal-containing solid materials by cyanogenic Chromobacterium violaceum. J Biotechnol 113:321–326
Debaraj M, Young HR (2010) Current research trends of microbiological leaching for metal recovery from industrial wastes. Curr Res Technol Educ Topics Appl Microbiol Microb Biotechnol 4:1289–1296
Liang G, Mo Y, Zhou Q (2010) Novel strategies of bioleaching metals from printed circuit boards (PCBs) in mixed cultivation of two acidophiles. Enzyme Microb Technol 47:322–326
Panda S, Akcil A, Pradhan N, Deveci H (2015) Current scenario of chalcopyrite bioleaching: a review on the recent advances to its heap-leach technology. Bioresour Technol 196:694–706
Wang J, Bai J, Xu J, Liang B (2009) Bioleaching of metals from printed wire boards by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans and their mixture. J Hazard Mater 172:1100–1105
Saidan M, Brown B, Valix M (2012) Leaching of electronic waste using biometabolised acids. Chin J Chem Eng 20:530–534
Kim MJ, Seo JY, Choi YS, Kim GH (2016) Bioleaching of spent Zn-Mn or Ni-Cd batteries by Aspergillus species. Waste Manag 51:168–173
Xu TJ, Ramanathan T, Ting YP (2014) Bioleaching of incineration fly ash by Aspergillus niger—precipitation of metallic salt crystals and morphological alteration of the fungus. Biotechnol Rep 3:8–14
Amiri F, Yaghmaei S, Mousavi SM, Sheibani S (2011) Recovery of metals from spent refinery hydrocracking catalyst using adapted Aspergillus niger. Hydrometallurgy 109:65–71
Biswas S, Bhattacharjee K (2014) Fungal assisted bioleaching process optimization and kinetics: scenario for Ni and Co recovery from a lateritic chromite overburden. Sep Purif Technol 135:100–109
Rasoulnia P, Mousavi SM (2016) V and Ni recovery from a vanadium-rich power plant residual ash using acid producing fungi: Aspergillus niger and Penicillium simplicissimum. RSC Adv 6:9139–9151
Jadhav U, Hocheng H (2015) Waste solder and printed circuit board: the emerging secondary sources for recovery of metals. Arch Mater Sci Eng 72:5–15
Yang T, Xu Z, Wen J, Yang L (2009) Factors influencing bioleaching copper from waste printed circuit boards by Acidithiobacillus ferrooxidans. Hydrometallurgy 97(1, 2):29
Mražíková A, Marcinčáková R, Kaduková J, Velgosová O, Willner J, Fornalczyk A, Saternus M (2013) The effect of specific conditions on Cu, Ni, Zn and Al recovery from PCBS waste using acidophilic bacterial strains. J Polish Miner Eng Soc 59–62
Brandle H, Lelmann S, Faramarzi MA, Martinelli D (2008) Biomobilization of silver, gold, and platinum from solid waste materials by HCN-forming microorganisms. Hydrometallurgy 94(1–4):14
Ilyas S, Ruan CH, Bhatti HN, Ghauri MA, Anwar MA (2010) Column bioleaching of metals from electronic scrap. Hydrometallurgy 101(3, 4):135
Velgosová O, Kaduková J, Marcinčáková R, Mražíková A, Fröhlich L (2014) The role of main leaching agents responsible for Ni bioleaching from spent Ni-Cd batteries. Sep Sci Technol 49:438–444
Velgosová O, Kaduková J, Marcinčáková R, Palfy P, Trpcevská J (2013) Influence of H2SO4 and ferric iron on Cd bioleaching from spent Ni–Cd batteries. Waste Manag 33:456–461
Sheel A, Pant D (2017) Recovery of gold from electronic waste using chemical assisted microbial biosorption (hybrid) technique. Bioresour Technol 247:1189–1192
Alston SM, Clark AD, Arnold JC, Stein BK (2011) Environmental impact of pyrolysis of mixed WEEE plastics Part 1: Experimental pyrolysis data. Environ Sci Technol 45:9380–9385
Zhou YH, Wu WB, Qiu KQ (2011) Recycling of organic materials and solder from waste printed circuit boards by vacuum pyrolysis-centrifugation coupling technology. Waste Manag 31:2569–2576
Zhan L, Xu Z (2009) Separating and recycling metals from mixed metallic particles of crushed electronic wastes by vacuum metallurgy. Environ Sci Technol 43:7074–7078
Zhan L, Xu Z (2011) Separating and recovering Pb from copper-rich particles of crushed waste printed circuit boards by evaporation and condensation. Environ Sci Technol 45(12):5359–5365
Zhan L, Xu Z (2012) Separating criterion of Pb, Cd, Bi and Zn from metallic particles of crushed electronic wastes by vacuum evaporation. Sep Sci Technol 47:913–919
Zhan L, Xu ZM (2008) Application of vacuum metallurgy to separate pure metal from mixed metallic particles of crushed waste printed circuit board scraps. Environ Sci Technol 42:7676–7681
He Y, Ma E, Xu Z (2014) Recycling indium from waste liquid crystal display panel by vacuum carbon-reduction. J Hazard Mater 268:185–190
Ma E, Lu R, Xu Z (2012) An efficient rough vacuum-chlorinated separation method for the recovery of indium from waste liquid crystal display panels. Green Chem 14:3395–3401
Zhan L, Xu ZM (2014) State-of-the-art of recycling e-wastes by vacuum metallurgy. Sep Environ Sci Technol 48:14092–14102
Xiu FR, Weng H, Qi Y, Yu G, Zhang Z, Chen M (2017) A novel recovery method of copper from waste printed circuit boards by supercritical methanol process: preparation of ultrafine copper materials. Waste Manag 60:643–651
Xue M, Yan G, Li J, Xu Z (2012) Electrostatic separation for recycling conductors, semiconductors, and nonconductors from electronic waste. Environ Sci Technol 46(19):10556–10563
Acknowledgements
The authors are grateful to the Central Metallurgical R&D Institute for providing financial support to this work under Grant No. ID 72/2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No competing financial interest is declared.
Additional information
The contributing editor for this article was Bernd Friedrich.
Rights and permissions
About this article
Cite this article
Abdelbasir, S.M., El-Sheltawy, C.T. & Abdo, D.M. Green Processes for Electronic Waste Recycling: A Review. J. Sustain. Metall. 4, 295–311 (2018). https://doi.org/10.1007/s40831-018-0175-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-018-0175-3