Abstract
Objective
There is much evidence linking the involvement of oxidative stress in the pathogenesis of aging. Paraoxonase 1 (PON1) is an HDL-associated antioxidant enzyme that inhibits the oxidative modification of low-density lipoproteins (LDL). We have investigated the changes in plasma PON1 activity, LDL oxidation, radical scavenging activity and lipid peroxidation in d-galactose-induced aging rat model and also compared the results with 24-month naturally aged rats.
Method
Arylesterase activity of PON1, susceptibility of LDL for oxidation, plasma radical scavenging activity and plasma thiobarbituric acid reactive substances (TBARS) were measured in normal control rats (4-months-old control rats subjected to d-galactose-induced experimental aging, and 24-month-old naturally aged rats).
Results
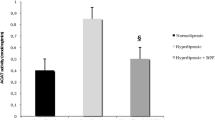
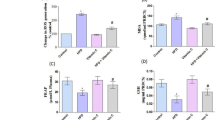
There was a significant decrease in plasma PON1 arylesterase activity in both subcutaneous d-galactose-treated groups and 24-month-old aged rats (P < 0.05, for each). TBARS, an oxidative stress marker, was seen to increase in the experimental groups (P < 0.01). In both subcutaneous galactose-treated and naturally aged rats, there was a significant rise in plasma LDL oxidation (P < 0.05, for each). However, radical scavenging activity was decreased significantly (P < 0.01) in both groups, as compared to control.
Conclusions
The d-galactose-induced rat model of aging mimics the naturally aged rat with reference to PON1 arylesterase activity and susceptibility to LDL oxidation. The results emphasize the importance of PON1 with respect to aging and its association with redox balance of the body.





Similar content being viewed by others
References
Kawakami K, Kadota J, Iida K, Shirai R, Abe K, Kohno S (1999) Reduced immune function and malnutrition in the elderly. Tohoku J Exp Med 187:157–171
Harman D (2006) Free radical theory of aging: an update: increasing the functional life span. Ann N Y Acad Sci 1067:10–21
Xu FB (1985) Sub-acute toxicity of d-galactose. In: Proceedings of the Second National Conference on Aging Research. Herbin China
Zhang X, Li WB, Zhang BL (1990) Biochemical changes in d-galactose induced subacute toxicity and mimetic aging in mice. Chin J Pharmacol Toxicol 4:309–310
Zheng JY, Fu YR, Li M, Gao KS, Zhang XG (2009) Effect of LTA isolated from bifidobacteria on d-galactose-induced aging. Exp Gerontol 44:760–765
Cui X, Zuo P, Zhang Q, Li X, Hu Y, Long J, Packer L, Liu J (2006) Chronic systemic d-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: protective effects of R-alpha-lipoic acid. J Neurosci Res 84:647–654
Zhang Q, Li XK, Cui X, Zuo PP (2009) d-galactose injured neurogenesis in the hippocampus of adult mice. Neurol Res 27:552–556
Yanar K, Aydın S, Cakatay U, Mengi M, Buyukpınarbas N, Atukeren P, Sitar ME, Sonmez A, Uslu E (2011) Protein and DNA oxidation in different anatomic regions of rat brain in a mimetic ageing model. Basic Clin Pharmacol Toxicol 109(6):423–433
Durrington PN, Mackness B, Mackness MI (2001) Paraoxonase and atherosclerosis. Arterioscler Thromb Vasc Biol 21:473–480
Goswami B, Tayal D, Gupta N, Mallika V (2009) Paraoxonase: a multifaceted biomolecule. Clin Chim Acta 410:1–12
Van Lenten BJ, Wagner AC, Nayak DP, Hama S, Navab M, Fogelman AM (2001) High-density lipoprotein loses its anti-inflammatory properties during acute influenza infection. Circulation 103:2283–2288
Cherki M, Berrougui H, Isabelle M, Cloutier M, Koumbadinga GA, Khalil A (2007) Effect of PON1 polymorphism on HDL antioxidant potential is blunted with aging. Exp Gerontol 42:815–824
La Du BN (1996) Structural and functional diversity of paraoxonases. Nat Med 2:1186–1187
Nguyen DS, Sok DE (2003) Oxidative inactivation of paraoxonase 1, an antioxidant protein and its effect on antioxidant action. Free Radic Res 37:1319–1330
Berliner JA, Heinecke JW (1996) The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med 20:707–727
Marathe GK, Zimmerman GA, McIntyre TM (2003) Platelet activating factor acetylhydrolase, and not paraoxonase-1, is the oxidized phospholipid hydrolase of high density lipoprotein particles. J Biol Chem 278:3937–3947
Mehdi MM, Rizvi SI (2012) Human plasma paraoxonase 1 (PON1) arylesterase activity during aging: correlation with susceptibility of LDL oxidation. Arch Med Res 43:438–443
Mehdi MM, Rizvi SI (2012) Plasma protein hydroperoxides during aging in humans: correlation with paraoxonase 1 (PON1) arylesterase activity and plasma total thiols. Arch Med Res 44:136–141
Sheng-lang J, Yong-guang Y (2012) In vivo antioxidant activity of total flavonoids from indocalamus leaves in aging mice caused by d-galactose. Food Chem Toxicol 50:3814–3818
Song X, Mingmin B, Diandong L, Yong LM (1999) Advanced glycation in d-galactose induced mouse aging model. Mech Ageing Dev 108:239–251
Ayub A, Mackness MI, Arrol S, Mackness B, Patel J, Durrington PN (1999) Serum paraoxonase after myocardial infarction. Arterioscler Thromb Vasc Biol 19:330–335
Schnitzer E, Pinchuk I, Bor A, Fainaru M, Samuni AM, Lichtenberg D (1998) Lipid oxidation in unfractionated serum and plasma. Chem Phys Lipids 92:151–170
Szabo MR, Iditoiu C, Chambre D, Lupea AX (2007) Improved DPPH determination for antioxidant activity spectrophotometric assay. Chem Paper 61:214–216
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malondialdehyde and 4-hydroxynonenal. Methods Enzymol 186:407–413
Wei H, Li L, Song Q, Ai H, Chu J, Li W (2005) Behavioural study of the d-galactose induced aging model in C57BL/6Jmice. Behav Brain Res 157:245–251
Moyer JR Jr, Furtak SC, McGann JP, Brown TH (2009) Aging-related changes in calcium-binding proteins in rat perirhinal cortex. Neurobiol Aging 32:1693–1706
Kong WJ, Wang Y, Wang Q, Hu YJ, Han YC, Liu J (2006) The relation between d-galactose injection and mitochondrial DNA 4834 bp deletion mutation. Exp Gerontol 41:628–634
Hua X, Lei M, Zhang Y, Ding J, Han Q, Hu G, Xiao M (2007) Long-term d-galactose injection combined with ovariectomy serves as a new rodent model for Alzheimer’ disease. Life Sci 80:1897–1905
Parameshwaran K, Irwin MH, Steliou K, Pinkert CA (2010) d-galactose effectiveness in modeling aging and therapeutic antioxidant treatment in mice. Rejuvenation Res 13:729–735
Lei M, Hua X, Xiao M, Han Q, Hu G (2008) Impairments of astrocytes are involved in the d-galactose induced brain aging. Biochem Biophys Res Commun 369:1082–1087
Santanam N, Parthasarathy S (2007) Aspirin is a substrate for paraoxonase-like activity: implications in atherosclerosis. Atherosclerosis 191:272–275
Tsuzura S, Ikeda Y, Suehiro T, Ota K, Osaki F, Arii K, Kumon Y, Hashimoto K (2004) Correlation of plasma oxidized low-density lipoprotein levels to vascular complications and human serum paraoxonase in patients with type 2 diabetes. Metabolism 53:297–302
Jaouad L, Milochevitch C, Khalil A (2003) PON1 paraoxonase activity is reduced during HDL oxidation and is an indicator of HDL antioxidant capacity. Free Radic Res 37:77–83
Li WF, Furlong CE, Costa LG (1995) Paraoxonase protects against chlorpyrifos toxicity in mice. Toxicol Lett 76:219–226
Costa LG, Vitalone A, Cole TB, Furlong CE (2005) Modulation of paraoxonase (PON1) activity. Biochem Pharmacol 69:541–550
Van Himbergen TM, van der Schouw YT, Voorbij HA, van Tits LJ, Stalenhoef AF, Peeters PH, Roest M (2008) Paraoxonase (PON1) and the risk for coronary heart disease and myocardial infarction in a general population of Dutch women. Atherosclerosis 199:408–414
Ozturk H, Gungor M, Yigit S, Tacyildiz H, Basinoglu F, Koldas M (2009) Effect of exercise on the paraoxonase activity. Clin Biochem 42:343–346
La Du BN, Aviram M, Billecke S, Navab M, Primo-Parmo S, Sorenson RC, Standiford TJ (1999) On the physiological role(s) of the paraoxonases. Chem Biol Interact 14(119–120):379–388
Costa LG, Giordano G, Cole TB, Marsillach J, Furlong CE (2013) Paraoxonase 1 (PON1) as a genetic determinant of susceptibility to organophosphate toxicity. Toxicol 10(307):115–122
Tavori H, Aviram M, Khatib S, Musa R, Nitecki S, Hoffman A, Vaya J (2009) Human carotid atherosclerotic plaque increases oxidative state of macrophages and low-density lipoproteins, whereas paraoxonase 1 (PON1) decreases such atherogenic effects. Free Radic Biol Med 46:607–615
Mackness B, Durrington PN, Boulton AJM, Hine D, Mackness MI (2002) Serum paraoxonase activity in patients with type 1 diabetes compared to healthy controls. Eur J Clin Invest 32:259–264
Unal E, Uzun H, Kusaslan R, Dogan M, Genc H, Gunes P, Titiz I (2005) Serum paraoxonase (a high-density lipoprotein-associated lipophilic antioxidant) activity and lipid profile in experimental acute pancreatitis. Pancreas 31:84–87
Marchegiani F, Mara M, Oliveri F, Cardelli M, James RW, Boemi M, Franceschi C (2008) Paraoxonase 1: genetics and activities during aging. Rejuvenation Res 11:113–127
Polat D, Ezgi D, Ahmet B, Hakan Y (2006) Decreased serum paraoxonase 1 (PON1) activity: an additional risk factor for atherosclerotic heart disease in patients with PCOS? Hum Reprod 21:104–108
Caroline AA, Michael IM, Sudhesh K, Andrew JB, Paul ND (1995) Serum paraoxonase activity, concentration, and phenotype distribution in diabetes mellitus and its relationship to serum lipids and lipoproteins. Arterioscler Thromb Vasc Biol 15:1812–1818
Jaouad L, de Guise C, Berrougui H, Cloutier M, Isabelle M, Fulop T, Payette H, Khalil A (2006) Age-related decrease in high-density lipoproteins antioxidant activity is due to an alteration in the PON1’s free sulfhydryl groups. Atherosclerosis 185:191–200
Aviram M, Rosenblat M, Bisgaier CL, Newton RS, Primo-Parmo SL, La Du BN (1998) Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J Clin Invest 101:1581–1590
Mackness B, Durrington PN, Mackness MI (1998) Review human serum paraoxonase. Gen Pharmacol 31:329–336
Rozenberg O, Aviram M (2006) S-Glutathionylation regulates HDL associated paraoxonase 1 (PON1) activity. Biochem Biophys Res Commun 351:492–498
Ates O, Azizi S, Alp HH, Kiziltunc A, Kocer I, Baykal O (2009) Decreased serum paraoxonase 1 activity and increased serum homocysteine and malondialdehyde levels in age-related macular degeneration. Tohoku J Exp Med 217:17–22
Acknowledgments
The authors are grateful to University Grants Commission, New Delhi for financial support in the form of grant F 37-392/2009 to SIR.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, D., Rizvi, S.I. Plasma paraoxonase 1 arylesterase activity in d-galactose-induced aged rat model: correlation with LDL oxidation and redox status. Aging Clin Exp Res 26, 261–267 (2014). https://doi.org/10.1007/s40520-013-0170-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-013-0170-2