Abstract
Purpose of Review
This paper provides a reader who has little to none technical chemistry background with an overview of the working principles of lithium-ion batteries specifically for grid-scale applications. It also provides a comparison of the electrode chemistries that show better performance for each grid application.
Recent Findings
Two of the main causes driving the growth of stationary energy storage technologies are the increasing environmental regulations that promote a high penetration of non-dispatchable generation and policy changes in the electricity markets that benefit the profit of fast response energy resources such as a battery. The combination of these two factors is drawing the attention of investors toward lithium-ion grid-scale energy storage systems.
Summary
We review the relevant metrics of a battery for grid-scale energy storage. A simple yet detailed explanation of the functions and the necessary characteristics of each component in a lithium-ion battery is provided. We also discuss the chemistries currently used for cathode and anode materials. Discussed will be the trade-off of several materials with respect to cost, thermal stability, cyclability, environmental friendliness, and other important characteristics to be considered for grid applications. This paper also discusses the commercial availability of lithium-ion batteries for grid-scale storage and presents some of the containerized battery storage solutions available in the market.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the US Department of Energy (DOE) energy storage database [1], electrochemical energy storage capacity is growing exponentially as more projects are being built around the world. The total capacity in 2010 was of 0.2 GW and reached 1.2 GW in 2016. Lithium-ion batteries represented about 99% of electrochemical grid-tied storage installations during the first quarter of 2016 [2]. The International Renewable Energy Agency (IRENA) predicts a worldwide battery storage capacity of 150 GW by 2030 [3]. A report from Greentech Media research [2] estimates that the battery installed capacity in the USA will grow to 2.1 GW by 2021 and only the US market will be worth 2.9 billion USD. China is the second largest market for battery storage, after the USA, with a predicted market size of 8.5 billion USD by 2025 [4].
One of the main forces driving the growth for grid-scale energy storage systems in the market is the environmental regulations that has led to higher penetration of non-dispatchable generation (e.g., wind and solar power). For example, in California, electric utilities were required to procure 1.325 GW [5] of energy storage by 2020 in preparation for increasing the share of renewable electricity to 33% by year 2020 and 50% by 2030 [6]. The contributing factor to the growth of batteries in particular is that the Federal Energy Regulatory Commission (FERC) requires power market operators to distinguish high-quality reserve providers and compensate them accordingly [7, 8]. In particular, batteries are fast and efficient in following regulating reserve signals, which makes them an ideal choice for such services in electricity markets.
Lithium-ion batteries have been getting much attention among rechargeable batteries, given their high round trip efficiency close to 99%, no memory effects, long cycle life withstanding thousands of cycles [10•, 11], and large energy densities up to 200 Wh/kg [9], compared to 50 Wh/kg on lead-acid batteries [10•]. They also have lower degradation at partial state of charge compared with lead-acid batteries [12]. It has been the investments on research for non-stationary applications, mainly by the electric car industry, that has brought lithium-ion batteries to be technically and economically an attractive battery option, even for grid-scale applications. Lithium-ion batteries have the highest energy densities with respect to both weight and volume, of all commercially available secondary batteries. The high energy density of Li-ion batteries is due to the low molecular weight and low redox potential of lithium [13•]. The low weight of Li-ion batteries makes them especially attractive for portable devices such as cell phones and digital cameras. They are increasingly replacing lead-acid batteries and have been the choice of major electric vehicle manufacturers including Tesla Motors, Nissan, Chevrolet, BMW, Toyota, and Ford Motor [9].
The Electricity Storage Handbook published by Sandia National Laboratories [14] identifies and describes the services energy storage can supply to the power system, such as energy time shift, supply capacity, regulation reserves, voltage support, and transmission upgrade deferral. Batteries that can provide those services can be sorted into two categories, high energy and high power densities [15]. Batteries with high energy rate can discharge a moderate amount of power during a long period of time. These are useful for energy arbitrage, supply capacity, transmission and distribution congestion relief, and black start. Batteries with high power rate can discharge a large amount of power for a small period of time, which can be used for frequency regulation and voltage support.
For the batteries to be useful in the power system, they need to be paired with a Battery Management System (BMS) and a Power Conversion System, also referred to as Power Conditioning System (PCS) to form a complete electric energy storage system. To control and operate the energy storage system, the BMS and PCS must be in constant communication. In order to be able to supply the needed power levels for grid-scale applications, several battery cells need to be arranged into modules [15,16,17]. Generally, batteries suffer from capacity fade, which could be increased if the battery is exposed to extreme operation conditions that include overcharging, discharging beyond a safe level, applying a charge rate greater than the designed, and going beyond cut-off voltages [15, 16]. Furthermore, battery capacity could be wasted if the cells within a module are not charged evenly; i.e., whenever one cell of the module is fully charged, the module has to stop charging, leaving the rest of the cells in a partial state of charge. Likewise, when a cell reaches its discharge limit, the module must stop discharging, leaving unused energy in the rest of the cells [16]. A BMS is needed to monitor the safe operation of each module of batteries and to balance the charge within the cells of the modules.
The PCS is the intermediary device connecting the batteries with a direct current (DC) operation and the grid operating in alternating current (AC). In order to be able to use the power being delivered by the batteries, this needs to be converted and synchronized with the grid’s frequency, so it can act as a synchronous generator. Likewise, the power needed for charging the batteries needs to be converted from AC to DC [18]. The PCS regulates the power flow between the batteries and the grid [19]. The power rating of the PCS defines the maximum power the energy storage system can deliver [20]. The power electronics on the PCS are able to take signals from the grid and respond on sub-cycle time scales [19].
There are different batteries suitable and commercially available for grid-scale energy storage, including advanced lead-acid batteries [21], flow batteries [22], and sodium-sulfur batteries [23]. This paper focuses on the lithium-ion battery component of an energy storage system. This paper does not discuss BMS nor PCS. We will be focusing on aspects that matter the most in stationary, large-scale, grid applications. Observe that different services require different attributes; for example, if a battery is meant to be the power source of a car, weight and volume are important features to consider. However, if the battery will be sitting on a substation, weight and volume may not always be that important. In the section ‘Working Principles of Lithium-Ion Batteries’ we review the working principles of a Li-ion battery. The chemistries used for its elements and their attributes are analyzed in the section ‘Elements of a Li-Ion Battery’. The section ‘Discussion’ encloses the discussion where we rank the commonly used chemistries and present their availabilities in the market. Finally, the “Conclusions” section has the conclusion of the paper.
Working Principles of Lithium-Ion Batteries
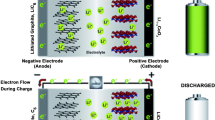
A lithium-ion battery constitutes an anode, a cathode, an electrolyte, a separator, and current collectors. Schematics of a lithium-ion battery is shown in Fig. 1.
Electrochemical cells convert electrical energy into chemical energy and vice-versa. In Li-ion batteries, when the cell is being charged, the lithium ions flow from the cathode to the anode where they are stored. When the cell is being discharged, lithium ions disassociate from the anode move to the cathode through the electrolyte and electrons are carried by the external circuit to do useful work.
The anode is the negative electrode, and since electrons leave the battery through the anode, it must be a material with high electronic conductivity and large cycling capacity. Graphite is typically used; the most common kind of graphite used in Li-ion batteries is mesocarbon microbeds, also known as MSMB [24].
The cathode is the positive electrode; it must be able to accept and release lithium ions and electrons. Cathodes are usually made of a lithium metal oxide that can undergo oxidation when lithium is removed. A common composition is LiMO2, (M = cobalt (Co), nickel (Ni), or manganese (Mn)).
The electrolyte is the means of transportation for the Li-ions between the electrodes during the charging and discharging cycles; therefore, it has to be a good ionic conductor and electronic insulator. Typically, electrolytes consist of a lithium salt solution in an organic solvent; the most popular one is lithium hexafluorophosphate (LiPF6) with ethylene carbonate-dimethyl carbonate (EC-DMC) [25].
The separator is a membrane separating anode and cathode and it is the host of the electrolyte. Its function is to prevent a short circuit between anode and cathode but allowing the lithium ions to flow during the charge and discharge cycles [26].
The current collectors as the name suggests collect the electrons flowing to and from the battery through an external circuit. They must be made of a material that is stable at the operating voltages of the electrodes. They are usually made of aluminum and copper for the cathode and anode, respectively [27].
For the typical lithium-ion battery chemical configuration, the chemical reaction is expressed as follows [26].
The energy and power rating of a battery are delimited by the composition and characteristics of its electrodes and electrolyte materials [28]. The energy storage capacity of a battery depends on the number of active components the electrodes can stock, and the power capacity is a function of the surface area of the electrodes and the internal resistance of the battery. However, energy and power ratings are dependent on each other [29]. This energy-power relation is expressed with the C-rate, which represents the rate at which a battery is charged or discharged relative to its maximum capacity. Furthermore, energy density depends on the chemistry of the battery while power density depends on the kinetics and design of the cell [30]. Thin electrodes can provide high power densities, and thick electrodes yield high energy densities [10•, 30]; thus, batteries can be designed to deliver high power or high energy, but not both [31].
The improvement in energy density over the past 10 years is due to progress in battery construction technologies. However, this is expected to saturate within a few years, and thus, other innovations are going to be needed [32••]. Using cathodes that exhibit higher voltages is one approach; however, operating at higher voltages (above 4V vs Li+) increases the likelihood of unwanted chemical reactions that lead to the degradation of the electrolyte and a reduction in the number of charging cycles.
Other approaches to improve performance of lithium-ion batteries are doping, nano-structuring, and coating of electrodes. Doping is the introduction of other particles to the main composition of an electrode material. Depending on the attribute that wants to be enhanced and the original composition of the electrode, different particles can be doped to obtain the desired operating voltage, capacity, and cycling performances.
Nano-structured electrode materials have shown to improve battery performance due to short ion diffusion length, better mechanical robustness, and high surface areas with lots of active sites, since a large surface area increases the contact between electrolyte and electrode for the reaction [28, 33•, 34, 35].
Coating is covering the electrode with a different element to take advantage of the attributes that element provides to the battery. It can be used to increase safety acting as a physical barrier between cathode and electrolyte, preventing the oxygen release and therefore reducing fire hazard [33•, 36]. Carbon is a common particle to coat electrodes with since it increases electrical conductivity [28].
Elements of a Li-Ion Battery
In this section, we provide a more detail overview of the four main components of a Li-ion battery. There are several alternative chemistries for electrodes. Those alternatives are meant to improve the performance of Li-ion batteries one way or another. Thus, we first discuss performance attributes for a battery and then review the most commonly used chemistries on Li-ion batteries for grid-scale applications.
Electrochemical Performance Attributes
There are a number of attributes that need to be understood in order to explain alternative battery chemistries. Those attributes include theoretical capacity, cycling stability, thermal stability, capacity fade, electronic conductivity, lithium storage capacity, and wettability. Even though improving these attributes would enhance the performance of any battery, they are especially important for grid-scale applications, since large amounts of power and energy need to be stored.
Theoretical capacity is the amount of energy that ideally can be withdrawn from an electrode if using all of its active material. It defines the maximum limit of a battery’s energy capacity; it is usually measured in milliampere hours per gram [37]. A large theoretical capacity increases the energy density of the battery.
Cycling stability is the capacity a material has to accept and release lithium ions repeatedly without major changes in its physical structure, over-heating or losing other electrochemical properties. This attribute becomes more critical as the size of the battery increases.
Thermal stability is the temperature range a material can hold before decomposing, corroding, or starting to experience uncontrolled chemical reactions; for electrode materials, this could lead to fire and explosions. Regarding separators, thermal stability is the ability to maintain its original shape and porosity at high temperatures. The safety of the battery is highly dependent on the thermal stability of its elements.
Capacity fade is a rate of how fast the capacity decreases after a certain number of cycles. Capacity retention is the opposite rate, meaning how much of its initial capacity is held after a certain number of cycles. Capacity fade on the electrodes is directly related to the cycle life of a battery.
Electrical conductivity is the ability of a material to effectively transport electrons. Likewise, ionic conductivity is the ability of a material to effectively transport ions, lithium ions for the purpose of lithium batteries.
Lithium storage capacity shows how many lithium ions can be accommodated within the anode’s structure [37]. A large lithium storage capacity yields high energy density batteries.
Wettability is the amount of electrolyte the separator and the electrodes can absorb. Higher wettability is better for battery’s kinetics, since electrolyte is the transportation means of lithium ions.
Cathode Materials
Most of the research on Li-ion batteries is being conducted to find a material that is low in cost, shows improved cycling performance, has good thermal stability, and leads to high energy and power densities [38]. Particularly, thermal stability is a big concern when selecting cathode materials given that heat generation on the cathode is three to four times greater than that of the anode [36].
There are three kinds of materials commonly used as cathodes on lithium-ion batteries, namely layered lithium transition metal oxides, lithium manganese oxide spinels, and lithium transition metal phosphates [36].
Layered lithium transition metal oxides are the preferred choice for the cathode in lithium-ion batteries, since they have high energy densities when cycled under high voltages and show high cycling efficiency given their crystal structure [33•]. However, this kind of materials show a high capacity fade when used or stored at high temperatures, due to chemical reactions with the electrolyte [36].
Lithium manganese oxide spinels have low cost, are environmentally friendly, and show excellent safety characteristics and high power capability. However, they can be corroded in acidic electrolytes. When paired with a graphite electrode, the manganese (Mn) migrates from cathode to anode, especially at high temperatures [36].
Lithium transition metal phosphate have a stable discharge platform, high thermal stability, and moderate discharge capacity [33•]; however, most of them have low electronic conductivity.
The first commercial Li-ion battery was introduced by Sony in 1990, using a LiCoO2 cathode [39, 40]. Since then, LiCoO2 has been widely used in batteries for many applications. Although LiCoO2 presents good characteristic for a cathode material, there has been extensive research into the optimum chemistry for cathode materials that can supply all the characteristics needed for each of the applications given to batteries [41]. To date, the three most common layered lithium transition metals used for lithium-ion batteries cathodes are cobalt, nickel, and manganese. However, the main interest of today’s research is on lithium manganese oxide spinels and lithium transition metal phosphates [32••]. Some of the commonly used cathode chemistries for grid-scale batteries are discussed below.
Lithium Nickel Oxide
Lithium nickel oxide (LiNiO2) is a commonly used cathode; it has a practical capacity of about 156 mAh/g while its theoretical capacity is 275 mAh/g [42] and a voltage of 4.2 V [43]. It shows 20% higher energy density than LiCoO2 [41]. It has a good cycling stability [43]. It is also fairly friendly with environment and not a costly material compared to LiCoO2 [44]. However, it is less stable than LiCoO2, is difficult to manufacture, and has low thermal stability. Also, its structure is not ordered, which leads nickel ions to occupy places in the lithium sites, what impedes the insertion and extraction of lithium ions [43, 44]. A way to overcome this issue is by adding cobalt to LiNiO2 to increase the order in the structure [41]; however, this also increases the cost. Coating LiNiO2 with a cobalt-manganese mix improves its capacity retention [41].
Lithium Nickel Manganese Cobalt Oxide
Combining Ni, Mn, and Co at equal ratios forms lithium nickel manganese cobalt oxide (Li[Ni,Mn,Co]O2) which has a high practical capacity of 170 mAh/g and can operate at a voltage around 3.8 V vs Li+ [41]. It has good cycling stability; however, capacity decreases as the discharge rate increases. Aluminum can be doped into Li(Ni,Mn,Co)O2 to increase its rate capability [41]. This material is well suited for high voltage batteries [33•] and it is a safe cathode for large-scale batteries [42]. A variation of lithium nickel manganese cobalt is LiNi0.6Co0.2Mn0.2O2 which has attracted attention given its high capacity of 170 mAh/g due to large Ni content and low cobalt content which makes it less harmful to environment [42]. Liu et al. [45] coated LiNi0.6Co0.2Mn0.2O2 with lithium disilicate (Li2Si2O5) to increase its rate capability and cycling performance.
Lithium Iron Phosphate
Lithium iron phosphate (LiFePO4) has been widely used as a cathode because of its environmental friendliness and its long cycle life. Also, the decrease in capacity after cycling is lower than that of LiCoO2 and Li(Ni,Mn,Co)O2 [41, 46]. It has a high capacity of 170 mAh/g, has a flat voltage profile around 3.5 V, and does not release oxygen, which makes it a safe material to use with better thermal stability [26, 47]. Batteries using LiFePO4 have shown to retain 95% capacity after 1000 cycles [12]. Tests conducted by Kim et al. [48] yield the results shown in Fig. 2.
LiFePO4 battery voltage profile before and after cycling [48]
On the other hand, its electronic conductivity is low [41], but it has been proven that this can be undermined by carbon coating the cathode [28]. Carbon-coated LiFePO4 has the right qualities to be used in batteries for high-power applications, but it is not as appropriate for high energy applications [26, 41]. Another good coating option for LiFePO4 is tin (Sn), which prevents the dissolution of iron (Fe) in LiPF6-based electrolyte [49].
Another way to improve electrochemical performance is to nano-structure LiFePO4 particles [49]. Nano-structured LiFePO4 demonstrated improved safety and higher stability during long-term cycling [50] and, especially when carbon coated, showed super capacitor-like discharge rates [51].
Anode Materials
The anode composition and electrochemical properties are of fundamental importance for the performance of a battery; the features needed for an anode material to be effective are high electronic conductivity, low working potential, high cycling stability, and low volume change during lithium insertion and extraction [52].
Something to take into consideration when choosing the anode material is that when voltages are lower than the reduction potential of the electrolyte; a thin layer of electrolyte reduction products is formed on the anode’s surface. This film is called solid electrolyte interface (SEI) [53]. Even though SEI works as a Li-ion conductor and a protective coating to prevent anode corrosion, uncontrolled SEI formation represents a safety risk, especially in large batteries [28, 52, 53]. Some of the commonly used anode materials are described below.
Graphite (C)
Regarding conductivity, cycling stability, and energy density, graphite is the first choice for anode material on lithium-ion batteries [48]. However, its thermal stability when lithiated is a safety concern [36]; also at high discharge rate, crystallized lithium may accumulate on the surface, which could break through the separator and cause a short circuit [13•]. Also, many materials, such as transition metals, have larger lithium storage capacities than that of graphite, which is 372 mAh/g [50].
An approach to improve electrochemical performance of a graphite anode is to treat it with molybdenum oxide to get graphite with pores and channels that facilitate the diffusion of lithium ions. These pores can also accommodate the volume changes during charging and discharging processes. Deng and Zhou [54] proved that this modified graphite anode has longer cycle life than regular graphite anodes, even at rates of 5 C.
Given their low voltages, carbonaceous anodes tend to form SEI [28]; capacity fade is attributed to the loss of active lithium during the formation of SEI [48]. The latest work on graphite anodes focuses on the addition of additives to stimulate the formation of an artificial and stable SEI; the trade-off of these additives is the increase of the impedance, making the cells less suitable for high-power applications [36].
Using transition metal oxides with a carbon coating on the anode is a common practice since they exhibit higher capacity and higher discharge rate than graphite, and the carbon coating increases the electric conductivity [55]. Also, silicon (Si)- or tin (Sn)-based anodes are an excellent choice for high energy density batteries [36].
Silicon Compounds
Currently, silicon (Si) is one of the most promising anode materials. Researchers have focused on lithium alloy anodes such as SiO2, mainly because its theoretical capacity of 4000 mAh/g is much larger than that of graphite and comparable to that of elemental Li [26]. The downside of using silicon is its poor electronic conductivity [56] and its volume change up to 300% during charge-discharge process [57], which leads to the disintegration of the anode and therefore, the failure of the battery [26, 53]. This problem can be solved by modifying the morphology of the anode. Song et al. [28] proposed that pores in silicon particles act as a buffer to reduce the volume changes during lithium insertion and extraction. It has been proven that carbon coating silicon helps improve the cycling stability and the formation of stable SEI layers [57]. However, gas generation is increased with higher surface area due to amplified reactions with the electrolyte [36].
Forming composites with carbon, metals, or conductive polymers and nano-structuring are common practices to improve electrochemical performance of silicon [56].
Fukata et al. [58] synthesized a silicon iron (Si-Fe) nano-composite, which showed higher capacity than graphite anodes and better cycling stability than pure Si electrodes; the higher capacity was given by the silicon and the longer cycle life by the iron component of the compound. Polat and Keles [52] mixed copper (Cu) with silicon (Si) to create an anode material with increased electron pathways, and longer cycle life due to copper absorbing the volume change of silicon during cycling. Furthermore, the presence of copper on the anode increases the adhesion to the current collector, usually made of copper, leading to longer life. Huang et al. [56] used a cobalt nano-sheet to enhance the electron conductivity and stabilize the silicon anode, getting a discharge capacity of approximately 2400 mAh/g after 100 cycles.
Lithium Titanate
Lithium titanate (Li4Ti5O12) is considered a high safety anode material; even though when compared to graphite, it has a lower capacity of 170 mAh/g and higher voltage level of 1.5 V. Li4Ti5O12 is still considered a viable anode material given its low volume change of less than 1%. The fact that it does not react with organic electrolytes, thus, no SEI is formed, and that it has a high thermal stability making Li4Ti5O12 an excellent option for large-scale battery anode material [26, 28, 59, 60]. However, it has poor electronic conductivity and low lithium-ion diffusion coefficient [13•]. To overcome these issues, many strategies have been researched, one of them is nano-structuring, which has proved to increase rate performance; however, it might affect the cycling stability by increasing reactivity with electrolyte [13•]. When Li4Ti5O12 particles are nano-structured, it shows a high discharge capability of 227 mAh/g at 1 C and 120 mAh/g at 20 C after 50 cycles [61].
Mu et al. [62] incorporated sucrose, which is a type of sugar, as a carbon source to enhance Li4Ti5O12 performance, coming up with a composite with higher electronic conductivity and smaller particle size. This compound showed remarkable performance at high charging rates as high as 60 and 80 C with capacities of 98 and 82 mAh/g respectively after 200 cycles. Liu et al. [60] made a composite with graphene, which on top of the regular advantages of Li4Ti5O12 showed an excellent rate capability of 108 mAh/g after 1000 cycles at a rate of 10 C. Results are shown on Fig. 3.
Li4Ti5O12 battery voltage profile on 1st and 100th cycles [60]
Electrolyte
Electrolytes have a direct impact on the electrochemical performance of a lithium-ion battery, as they serve as lithium-ion transportation means. Also, the safety of a battery is related to the interaction between electrolyte and electrode materials [63]. The electrolyte selection for a battery depends on the chemistry of its electrodes, because if the cathode’s potential is not compatible with the electrolytes’ electrochemical window, the electrolyte would be decomposed; by using more stable electrolytes, decomposition can be avoided [32••].
The most common electrolyte material today is LiFP6 organic carbonate solution. Some concerns about this material’s performance are the narrow electrochemical stability domain, which keeps cathode voltages low; its high flammability, which affects safety since when overheated, the LiFP6 electrolyte could easily catch fire; and the fact that it is a toxic material. Researchers are trying to overcome these issues by enhancing thermal stability through additives, such as flame retardant, and the use of redox shuttles to avoid overcharge and lithium salts to decrease the toxicity of the electrolyte [26]. All these hazardous conditions increase with the size of the battery, which makes it unsuitable for grid-scale energy storage systems [64]. Constructing non-flammable electrolytes with high anodic stability, good lithium-ion conductivity, and that can form stable SEI is key to being able to produce powerful large size lithium-ion batteries [33•, 63, 65].
Electrolyte composition also has a direct influence on SEI formation. When LiFP6 is used, the SEI is primarily made of LiF, which reduces the capacity of the battery by absorbing lithium [64]. Some additives have been studied to form stable SEI on the electrodes to prevent further decomposition; these additives include phosphides, sulfonate esters, monomers, carboxyl anhydrides, fluorine-containing compounds, and some special ether carbonates [33•].
An emerging class of electrolyte is that of molten salts, called ionic liquids. These are non-toxic, non-flammable, non-volatile, highly conductive and exhibit a wide temperature stability [26]. Ionic liquids could be made of organosilicon compounds, hydroflouroethers, or phosphates. The latter seem to be an excellent fire retardant because of their low viscosity and high solubility [65].
Another kind of electrolyte are the solid electrolytes, which have many advantages over liquid ones, such as higher energy density and flexible geometry, which can also solve problems such as dissolution of transition metals, electrolyte decomposition, and lithium dendrite growth [66]. Solid electrolytes also do the function of the separator; however, because of their low ionic conductivity, they are limited to high-energy batteries that charge-discharge slowly [36, 67]. Solid electrolytes are the most promising candidates for high-temperature lithium-ion batteries [68, 69].
Separator
Even though the separator is not directly involved in the battery’s chemical reactions, it plays a major role in its performance, especially in the security of lithium-ion batteries; since it keeps the cathode and anode from short-circuiting. The separator must be chemically compatible with the electrolyte and electrode materials [70]. The separator also accommodates the electrolyte and must provide appropriate channels for Li-ion transportation [57, 70]. Porosity and pore size are important in electrolyte uptake by the separator [40]. Wettability is an important feature given that most of the separator materials are not ionically conductive [70]. Another desired feature of separators is high temperature withstand capacity, so deformation does not occur when operated under extreme conditions [70].
Batteries with thin separators tend to have lower resistance, therefore higher densities. On the other hand, thick separators have better mechanical strength, thus are safer [70].
Discussion
Given the price decrease on supplies and manufacturing processes for lithium-ion batteries, there is a large number of companies offering Li-ion-based solutions. All companies are betting on different chemistries trying to find the balance between performance and cost. Some companies are adopting the strategy of specialization on certain chemistry for a specific application in order to dominate a market niche (Table 1).
A comparison of the products being offered by some of the active companies in grid-scale energy storage systems is listed in Table 2. It is worth noting that all the companies’ products offer scalability as well as custom designs for specific needs a client may have.
It can be noted on Table 2 that Li(NiMnCo)O2 is the most popular cathode material, and graphite is the more widely used anode material. It is well known that batteries are designed to serve a specific purpose; thus, the chemistry and morphology of the electrodes should be selected accordingly. In this section, we compare and rank the electrode materials used on the available products, with respect to their performance on grid-scale applications.
The relevant attributes that are particularly important for grid-scale applications are cost, cycle stability, theoretical capacity, and operating voltage, for both electrodes. In addition to those, for the cathode, thermal stability and environmental friendliness are considered and for the anode, electrical conductivity and volume change are important. To be able to compare the chemistries, all the values have been normalized on a scale from one to five, being five better for battery performance.
The cost comparison was made reviewing the prices for battery grade materials on the market [79]; in order to be consistent with the used scale, the materials with high costs get a one and the cheaper ones get a five.
Cycling stability of the electrodes is the attribute that has the most influence in defining the battery’s cycle life; since large initial investments are needed for grid-scale systems, replacement of the batteries must be delayed as long as possible.
Theoretical capacity sets the upper bound on the amount of energy the material is able to store.
A battery with a high open-circuit voltage is able to provide more power. Therefore, it is desired for the cathode to have a high voltage vs Li+ and for the anode to have a low voltage vs Li+. The difference in voltages between cathode and anode defines the voltage of the battery.
For the cathode materials, thermal stability is especially important, since uncontrolled reactions could lead to fire and explosions, especially in large batteries. Thermal stability on the cathode is related to the safety of the battery.
Environmental friendliness is also related to safety, as how hazardous to the environment each material is and how easy it is to dispose each material after the battery’s end of life.
On the anode side, electrical conductivity is important given that electrons leave the battery trough the anode. It is related to the charge-discharge rate the battery is rated at.
When the battery is being charged, the anode must accept lithium ions into its structure. If the anode’s material does not have a buffer to store these ions, it will naturally increase in volume, leading to reduced cycling life and safety of the battery.
Figure 4 shows a comparison of the relevant attributes of a cathode for grid-scale applications. LiNiO2 is the cheapest among the compared chemistries and it has the highest voltage, but it falls short on the rest of the attributes compared. Given its high voltage, it is a good option for high-power applications. Li(NiMnCo)O2 has a high theoretical capacity and moderate voltage level, it is on the middle range regarding cost, and it has good thermal stability and cycle life. This is a well-balanced material when a cost-benefit analysis is made, since it provides a really good performance at a moderate cost. LiFePO4 is a well suited chemistry for grid-scale applications, given its good thermal and cycling stability and environmental friendliness, which make LiFePO4 a safe material for large-scale batteries. It also has a high energy capacity and moderate voltage level. However, it is more expensive than the other materials.
Figure 5 shows a comparison of the relevant attributes of an anode for grid-scale applications. Li4Ti5O12 has a good cycle stability and low volume change; however, its voltage is high, its electrical conductivity is low, and its theoretical capacity is low compared to the other materials. Despite this, for applications where lifetime and safety are more valuable than energy density, using a Li4Ti5O12 anode is a good option. SiO2 has an excellent theoretical capacity and good voltage level and is very cheap. However, it suffers a tremendous volume change, which also affects its cycling stability and has low electrical conductivity. This anode material is good for high energy applications with low cycling frequency. Graphite seems to be the most appropriate material for grid-scale batteries; it has good electrical conductivity, low volume change and voltage level, and a moderate theoretical capacity and cycle life. Even though graphite is the most expensive material, the rest of its attributes make up for it to be the most used anode among commercially available grid-scale energy storage systems.
Even though all materials described above can be used for power or energy applications, Table 1 shows a classification in terms of better material performance given an application.
Conclusions
Every day, it becomes more evident the growing need for a flexible and sustainable but still reliable electric power system. These characteristics can be achieved by including grid-scale energy storage in the system. Among electrochemical storage technologies, lithium ion is getting the most of the attention given its superior performance and the huge research effort by the electric vehicle industry. Grid applications can be categorized in high energy or high power. Depending on the chemistry of its components, Li-ion batteries can show a better performance for different applications on the grid. The most common uses given to batteries are frequency regulation, time shift, and reserve capacity; these uses can be staked together to get more economic value from the battery and thus make grid-scale battery storage more attractive to investors.
The anode, cathode, and electrolyte account for most of the electrochemical characteristics of a cell, having that the energy storage capacity depends on the features of the electrodes and electrolyte, and the power rating depends mainly on the electrodes’ morphology and their chemistry. Three common methods for improving electrode performance are coating the electrode with a different material, doping other particles into the electrode and nano-structuring the electrode. Depending on the desired outcome, the selection of method and element to use varies.
For grid-scale applications, a battery with a LiFePO4 cathode, improved either by doping or carbon coating paired with an enhanced graphite anode, seems like the most promising combination of electrodes that could yield a strong but yet flexible battery.
As the market for containerized grid-scale energy storage systems continues to grow, more companies will be willing to compete in it; thus, more options will become available and prices for lithium-ion batteries will become more competitive.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Department of Energy. DOE Energy Storage Database. 2016. http://www.energystorageexchange.org/.
G. research and ESA US Energy Storage Monitor. U. S. Energy Storage Monitor: Q2 2016 Executive Summary. Tech. Rep. June, 2016.
IRENA. REmap, Roadmap for a renewable energy future. Tech. Rep., 2016.
Blume S. Global Energy Storage Market Overview & Regional Summary Report. Energy Storage Council, Tech. Rep., 2015.
C. P. U. Commission. Energy storage goals for utilities. 2014;1–2.
S. O. F. California, E. L. Material. Senate Bill No. 350. no. 350, 2015.
Federal Energy Regulatory Commission. FERC order 755. no. 755, 2011.
Federal Energy Regulatory Commission. FERC order 784. no. 784, 2013.
Zhang SS. Status, opportunities, and challenges of electrochemical energy storage. Front Energy Res. 2013;1:1–6.
• Diouf B, Pode R. Potential of lithium-ion batteries in renewable energy. Renew Energy. 2015;76:375–80. Makes comparison of other batteries to lithium ion. It presents states and explains the electric vehicle industry as the driving factor of lithium-ion technological development. It points out the challenges that need to be overcome in order to have a better lithium-ion battery for stationary use.
Omar J, Posada G, Rennie AJR, Martins VL, Marinaccio J, Barnes A, et al. Aqueous batteries as grid scale energy storage solutions. Renew Sust Energ Rev. 2017;68(Part 2):1174–82.
Krieger EM, Cannarella J, Arnold CB. A comparison of lead-acid and lithium-based battery behavior and capacity fade in off-grid renewable charging applications. Energy. 2013;60:492–500.
• Zhao B, Ran R, Liu M, Shao Z. A comprehensive review of Li4Ti5O12-based electrodes for lithium-ion batteries: the latest advancements and future perspectives. Materials Science and Engineering R: Reports. 2015;98:1–71. A detailed review on anodes, their characteristics, and methods to improve them, with a focus on Li4Ti5O12. It offers a heavily chemistry-loaded explanation of Li4Ti5O12 anodes, their latest improvements, and challenges.
Akhil AA, Huff G, Currier AB, Kaun BC, Rastler DM, Chen SB, Cotter AL, Bradshaw DT, Gauntlett WD. SANDIA REPORT DOE / EPRI electricity storage handbook in collaboration with NRECA. 2015; February.
Rahimi-Eichi H, Ojha U, Baronti F, Chow M. Battery management system: an overview of its application in the smart grid and electric vehicles. Industrial Electronics Magazine, IEEE. 2013;7(2):4–16.
Lawder BMT, Suthar B, Northrop PWC, De S, Hoff CM, Leitermann O, et al. System (BESS) and battery management system (BMS) for grid-scale applications. Proc IEEE. 2014;102(6):1014–30.
Piao C, Wang Z, Cao J, Zhang W, Lu S. Lithium-ion battery cell-balancing algorithm for battery management system based on real-time outlier detection. Mathematical problems in engineering, Hindawi Publishing Corporation, 2015;2015. http://dx.doi.org/10.1155/2015/168529.
Ko Y-S, Na K-S, Park W-H, Seong H-J, Won C-Y. Dual battery pack charge/discharge system for ESS using 3-level NPC inverter. June 2016;497–502.
Such MC, Hill C. Battery energy storage and wind energy integrated into the Smart Grid. 2012 I.E. PES Innovative Smart Grid Technologies (ISGT), 2012;1–4.
Schoenung S, Hassenzahl W. Long- vs. short-term energy storage technologies analysis: a life-cycle cost study: a study for the DOE energy storage systems program. Power Quality. 2003;SAND2011-2:84.
McKeon BB, Furukawa J, Fenstermacher S. Advanced lead- acid batteries and the development of grid-scale energy storage systems. Proc IEEE. 2014;102(6):951–63.
Zeng YK, Zhao TS, An L, Zhou XL, Wei L. A comparative study of all-vanadium and iron-chromium redox flow batteries for large-scale energy storage. J Power Sources. 2015;300:438–43.
Liao Q, Sun B, Liu Y, Sun J, Zhou G. A techno-economic analysis on NaS battery energy storage system supporting peak shaving. Int J Energy Res. 2016;40:241–7.
Nazri GA, Pistoia G. Lithium batteries science and technology. New York: Kluwer Academic Publishers; 2004.
Schaber C, Mazza P, Hammerschlag R. Utility-scale storage of renewable energy. Electricity Journal. 2004;17(6):21–9.
Scrosati B, Garche J. Lithium batteries: status, prospects and future. J Power Sources. 2010;195(9):2419–30.
Kang SW, Xie HM, Zhang W, Zhang JP, Ma Z, Wang RS, et al. Improve the overall performances of lithium ion batteries by a facile method of modifying the surface of cu current collector with carbon. Electrochim Acta. 2015;176:604–9.
Song MK, Park S, Alamgir FM, Cho J, Liu M. Nanostructured electrodes for lithium-ion and lithium-air batteries: the latest developments, challenges, and perspectives. Materials Science and Engineering R: Reports. 2011;72(11):203–52.
SBC Energy Institute. Electricity storage factbook. IEA workshop on energy storage Sep, 2013, 2013;1–13.
Spotnitz R. Lithium-ion batteries: the basics. Chem Eng Prog 2013;29.
M. E. V. Team. A guide to understanding battery specifications. Curr 2008;1–3.
•• Scrosati B, Abraham KM, van Schalkwijk W, Hassoum J. Lithium batteries: advanced technologies and applications. Pennington: Wiley; 2013. This book offers a detail explanation of how the batteries works; it opens the door to electrochemistry with a focus on lithium-ion batteries. It offers a good review of the chemistry, history, developments, and emergent technologies of lithium-ion batteries.
• Hu M, Pang X, Zhou Z. Recent progress in high-voltage lithium-ion batteries. J Power Sources. 2013;237:229–42. Shows a comprehensive review of the cathodes for lithium-ion batteries, digging really deep on their flaws and advantages for different applications.
Wu MS, Lee RH. Nanostructured manganese oxide electrodes for lithium-ion storage in aqueous lithium sulfate electrolyte. J Power Sources. 2008;176(1):363–8.
Deng Y, Fang C, Chen G. The developments of SnO2/graphene nanocomposites as anode materials for high performance lithium ion batteries: a review. J Power Sources. 2016;304:81–101.
Choi NS, Chen Z, Freunberger SA, Ji X, Sun YK, Amine K, et al. Challenges facing lithium batteries and electrical double-layer capacitors. Angewandte Chemie - International Edition. 2012;51(40):9994–10024.
Glaize C, Genies S. Lithium batteries and other electrochemical storage systems. Hoboken: John Wiley & Sons; 2013.
Bryner M. Lithium-ion batteries. Linden’s handbook of batteries 2013;36.
Chiang Y-M. Electrochemical energy storage for the grid. World 2010.
Nunes-Pereira J, Costa CM, Lanceros-Mendez S. Polymer composites and blends for battery separators: state of the art, challenges and future trends. J Power Sources. 2015;281:378–98.
Fergus JW. Recent developments in cathode materials for lithium ion batteries. J Power Sources. 2010;195(4):939–54.
Lee S-W, Kim H, Kim M-S, Youn H-C, Kang K, Cho B-W, et al. Improved electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode material synthesized by citric acid assisted sol-gel method for lithium ion batteries. J Power Sources. 2016;315:261–8.
Ohzuku T, Ueda A, Nagayama M. Electrochemistry and structural chemistry of LiNiO2 (R(3)over-bar-M) for 4 volt secondary lithium cells. J Electrochem Soc. 1993;140(7):1862–70.
Kraytsberg A, Ein-Eli Y, Kraytsberg A, Ein-Eli Y. Higher, stronger, better ... a review of 5 volt cathode materials for advanced lithium-ion batteries. Adv Energy Mater. 2012;2(8):922–39.
Liu S, Wu H, Huang L, Xiang M, Liu H, Zhang Y. Synthesis of Li2Si2O5-coated LiNi0.6Co0.2Mn0.2O2 cathode materials with enhanced high-voltage electrochemical properties for lithium-ion batteries. J Alloys Compd. 2016;674:447–54.
Shi W, Wang J, Zheng J, Jiang J, Viswanathan V, Zhang J-G. Influence of memory effect on the state-of-charge estimation of large- format Li-ion batteries based on LiFePO4 cathode. J Power Sources. 2016;312:55–9.
Gong C, Xue Z, Wen S, Ye Y, Xie X. Advanced carbon materials/olivine LiFePO4 composites cathode for lithium ion batteries. J Power Sources. 2016;318:93–112.
Kim JH, Woo SC, Park MS, Kim KJ, Yim T, Kim JS, et al. Capacity fading mechanism of LiFePO4-based lithium secondary batteries for stationary energy storage. J Power Sources. 2013;229:190–7.
Satyavani TVSL, Kumar AS, Subba PSV. Methods of synthesis and performance improvement of lithium iron. Engineering Science and Technology, an International Journal. 2015;19(1):178–88.
Xu T, Wang W, Gordin ML, Wang D, Choi D. Lithium-ion batteries for stationary energy storage. JOM. 2010;62(9):24–30.
Gong H, Xue H, Wang T, He J. In-situ synthesis of monodisperse micro-nanospherical LiFePO4/carbon cathode composites for lithium-ion batteries. J Power Sources. 2016;318:220–7.
Polat BD, Keles O. Designing self-standing silicon-copper composite helices as anodes for lithium ion batteries. J Alloys Compd. 2016;677:228–36.
Voelker P, Scientific TF. Trace degradation analysis of lithium-ion battery components. April, 2014;1–9.
Deng T, Zhou X. Porous graphite prepared by molybdenum oxide catalyzed gasification as anode material for lithium ion batteries. Mater Lett. 2016;176:151–4.
Zhang W, Zhang Y, Yang Z, Chen G, Ma G, Wang Q. In-situ design and construction of lithium-ion battery electrodes on metal substrates with enhanced performances: a brief review. Chin J Chem Eng. 2014;24(1):48–52.
Huang X, Wu J, Cao Y, Zhang P, Lin Y, Guo R. Cobalt nanosheet arrays supported silicon film as anode materials for lithium ion batteries. Electrochim Acta. 2016;203:213–20.
Zhang M, Zhang T, Ma Y, Chen Y. Latest development of nanostructured Si/C materials for lithium anode studies and applications. Energy Storage Materials. 2016;4:1–14.
Fukata N, Mitome M, Bando Y, Wu W, Wang ZL. Lithium ion battery anodes using Si-Fe based nanocomposite structures. Nano Energy. 2016;26:37–42.
Liu C, Massé R, Nan X, Cao G. A promising cathode for Li-ion batteries: Li3V2(PO4)3. Energy Storage Materials. 2016;4:15–58.
Liu H-P, Wen G-W, Bi S-F, Wang C-Y, Hao J-M, Gao P. High rate cycling performance of nanosized Li4Ti5O12/graphene composites for lithium ion batteries. Electrochim Acta. 2016;192:38–44. http://linkinghub.elsevier.com/retrieve/pii/S0013468616301797
Liu G, Zhang R, Bao K, Xie H, Zheng S, Guo J, et al. Synthesis of nano-Li4Ti5O12 anode material for lithium ion batteries by a biphasic interfacial reaction route. Ceram Int. 2016;42(9):11468–72.
Mu D, Chen Y, Wu B, Huang R, Jiang Y, Li L, et al. Nano-sized Li4Ti5O12/C anode material with ultrafast charge/discharge capability for lithium ion batteries. J Alloys Compd. 2016;671:157–63.
Swiderska-Mocek A, Naparstek D. Physical and electrochemical properties of lithium bis(oxalate)borateorganic mixed electrolytes in Li-ion batteries. Electrochim Acta. 2016;204:69–77.
Suo L, Borodin O, Sun W, Fan X, Yang C, Wang F, et al. Advanced high-voltage aqueous lithium-ion battery enabled by “water-in-bisalt” electrolyte. Angew Chem Int Ed. 2016:1–7.
Zeng Z, Wu B, Xiao L, Jiang X, Chen Y, Ai X, et al. Safer lithium ion batteries based on nonflammable electrolyte. J Power Sources. 2015;279:6–12.
Ma J, Hu P, Cui G, Chen L. Surface and interface issues in spinel LiNi0.5Mn1.5O4: insights into a potential cathode material for high energy density lithium-ion batteries: Chemistry of Materials; 2016. https://doi.org/10.1021/acs.chemmater.6b00948.
Saikia D, Ho SY, Chang YJ, Fang J, Tsai LD, Kao HM. Blending of hard and soft organic-inorganic hybrids for use as an effective electrolyte membrane in lithium-ion batteries. J Membr Sci. 2016;503:59–68.
Zhang J, Ma C, Liu J, Chen L, Pan A, Wei W. Solid polymer electrolyte membranes based on organic/inorganic nanocomposites with star-shaped structure for high performance lithium ion battery. J Membr Sci. 2016;509:138–48.
Thangadurai V, Narayanan S, Pinzaru D. Garnet-type solid-state fast Li ion conductors for Li batteries: critical review. Chem Soc Rev. 2014;7(12):3857–86
Lee H, Yanilmaz M, Toprakci O, Fu K, Zhang X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ Sci. 2014;7(12):3857–86.
BYD. Byd web site. http://byd.com/energy/ess.html.
NEC. Nec energy solutions web site. https://www.neces.com/assets/NECES-Grid-Poster116142.pdf.
Kokam. Kokam web site. http://kokam.com/wp-content/uploads/2016/06/2016-Kokam-ESS-Brochure.pdf?PHPSESSID=67430cfb8734b8f2df678d7def11c3ed.
S. SDI. Samsung sdi web site. http://www.samsungsdi.co.kr/upload/essbrochure/Samsung%20SDI%20ESS%20brochure.pdf.
L. Chem. Lg chem web site. http://www.lgchem.com/upload/file/product/ESSLGChemENG[0].pdf.
SAFT. Saft web site. http://www.saftbatteries.com/battery-search/intensium%C2%AE-max.
Enerdel. Enerdel web site. http://www.enerdel.com/se210-600-z-secure.
Tesla. Tesla energy web site. https://www.tesla.com/enCA/powerpack.
SigmaAldrich. Sigma-aldrich website. http://www.sigmaaldrich.com/catalog.situ. Design and construction of lithium-ion battery electrodes on metal.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Energy Storage
Rights and permissions
About this article
Cite this article
Arteaga, J., Zareipour, H. & Thangadurai, V. Overview of Lithium-Ion Grid-Scale Energy Storage Systems. Curr Sustainable Renewable Energy Rep 4, 197–208 (2017). https://doi.org/10.1007/s40518-017-0086-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40518-017-0086-0