Abstract
Purpose of Review
Importation of schistosomiasis by migrant populations is increasingly being recognized as a global health issue in non-endemic countries, with consequences for the infected individuals and for public health. The purpose of this review is to assess the extent of the problem and the possible ways to mitigate its impacts.
Recent Findings
Published studies on schistosomiasis in migrants to the main refugee-hosting countries were identified and reviewed. The use of sensitive tests for screening indicated that the prevalence of schistosomiasis among migrants to non-endemic countries was higher than previously recognized. The establishment of schistosomiasis transmission in southern Europe had also demonstrated the ease with which the disease could be spread to new areas by moving populations.
Summary
The high prevalence of schistosomiasis among refugees and migrant populations is documented by several reports from Europe, North America, Australia, and New Zealand. It is also clear that there are no uniform international protocols for screening and treatment of migrants with schistosomiasis. Moreover, the existing protocols are not being consistently implemented and may not be inclusive of all vulnerable migrants. There is a need for more research on the implementation, feasibility, cost-effectiveness, and efficacy of different protocols for screening and treatment of refugees and migrant populations from high-risk areas. There is also a need for development and evaluation of newer, more accurate diagnostic screening tests for this purpose.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
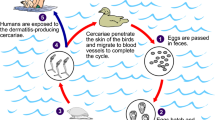
Schistosomiasis is a helminthic infection caused by flukes of the genus Schistosoma. The disease occurs in two major forms—intestinal and urogenital—caused by five species of schistosomes (Table 1) [1]. Transmission of schistosomiasis requires a freshwater snail intermediate host. The worm life cycle is completed when people suffering from schistosomiasis contaminate freshwater sources with their excreta containing parasite eggs, which hatch in water and develop in the snail host to the infective cercarial form that penetrates the skin of other individuals that come in contact with infested water. In the infected individual, the larvae develop into adult worms that live in the blood vessels where the females release eggs. Some of the eggs are passed in stools and urine and complete the life cycle, but some eggs are trapped in the tissues and may persist for more than 25 years and cause progressive damage to organs and long-term chronic complications. Long-term sequelae of Schistosoma haematobium infection include obstructive disease of the urinary tract and squamous cell carcinoma of the bladder. Complications of intestinal schistosomiasis caused by Schistosoma mansoni include intestinal polyposis, liver periportal fibrosis, and esophageal varices. There are also general systemic effects like anemia, stunting, and impaired cognition.
Schistosomiasis is endemic in 78 countries in the tropics and subtropics in areas with limited access to safe water supply and poor sanitation (Fig. 1). WHO estimated that in 2015, at least 218 million people in 52 countries with moderate to high transmission required preventive treatment [2]. People become exposed to infested water as a result of occupational and domestic activities. School-aged children are particularly vulnerable to infection due to swimming or fishing in contaminated water. An estimated 85% of the world cases of schistosomiasis are in Africa [2]. Distribution of the disease tends to be focal, depending on the presence of specific snail intermediate host and human activity. Schistosomiasis control is mainly based on mass treatment with praziquantel in countries with moderate to high transmission. Other measures include provision of potable water, improvement of sanitation, and snail control.
Schistosomiasis, countries or areas at risk (Reprinted with permission from WHO. “Schistomiasis, Countries or Areas at Risk, 2014.” Available at: http://gamapserver.who.int/mapLibrary/Files/Maps/Global_ShistoPrevalence_ITHRiskMap.png?ua=1 Accessed September 8, 2017)
The transmission of schistosomiasis in many endemic areas is greatly influenced by population movements. In Africa, the persistence and spread of schistosomiasis are mainly influenced by absence of control activities, low access to safe water, and human migration [3]. As an example, in Congo, the significant population movements in recent conflicts have introduced the disease in new areas [3]. Another example has been described in China, in a study comparing the epidemiology and risk factors for schistosomiasis among immigrants, emigrants, and permanent resident agricultural workers in three villages of Hunan province [4]. Although all participants had similar water contact risk, the prevalence rate of schistosomiasis in the immigrants was twice as high as in the permanent residents. There was also significant lack of awareness about the disease and its prevention among migrants compared to the permanent residents. Consequently, migrant workers took no personal protective measures. Moreover, resident workers had better access to antischistosomal chemotherapy. The discrepancy between the migrant population and permanent residents showed the impact of inadequate coverage of migrant workers by the schistosomiasis control program. In addition, it has been reported that in some cases, migrating people moved to areas where the snails were still present even after the transmission was controlled or interrupted [5]. Such issues may cause a new challenge to the control operations in the areas progressing towards the stage of transmission control or interruption, even leading to a risk of re-bouncing or re-emerging transmission.
By 2016 worldwide, an estimated 65.6 million people have been forcibly displaced from their homes because of war, violence, or oppression [6]. Nearly 22.5 million of these were refugees, and 40.3 million were internally displaced. Of the 58 million international migrants added in the north between 1990 and 2015, 44 million, or 76%, were born in the south [7]. Given these figures, the risk of importation of schistosomiasis by migrants is evident. In individuals who visited GeoSentinel clinics from March 1997 to November 2009, schistosomiasis was diagnosed in 370 of 2804 migrants from Africa (13%); although 48% were diagnosed in the first year, cases continued to be diagnosed up to 10 years after arrival [8].
The present paper reviews the published epidemiological reports on importation of schistosomiasis by migrant populations in the main countries receiving migrants from endemic areas. The main objectives are to investigate the prevalence of schistosomiasis among migrant populations and to explore the long-term impact of schistosomiasis on the migrants and on public health in the host countries.
Diagnostic and Screening Tests for Schistosomiasis
The following brief update of schistosomiasis diagnostic tests could be helpful for understanding the main epidemiological data reviewed in this paper:
Diagnosis of schistosomiasis is mainly based on parasitological tests to detect eggs in urine or fecal samples. These parasitological techniques are specific, relatively simple, and cheap. They remain as the gold standard for diagnosis of schistosomiasis in endemic areas. However, their main limitations are that the techniques are slow and labor-intensive and they are not highly sensitive. For intestinal schistosomiasis, the most widely used parasitological methods are the Kato thick smear [9, 10] and the concentration of formalin-preserved stool [11]. Parasitological diagnosis of urinary schistosomiasis is done by filtration techniques for urine samples [12].
Immunodiagnosis of schistosomiasis relies mainly on detection of specific antibodies or antigens. Antibody detection tests provide only indirect proof of exposure because they detect antibodies produced by the host’s immune response to the parasite [13••]. Positive antibody tests indicate past or present exposure to infection. However, these tests are less sensitive in the identification of species other than S. mansoni [14]. For identification of other species, antibody detection tests are commonly combined with antigen-specific immunoblot test [15]. Another disadvantage of antibody detection tests is that they may miss prepatent and early infections, resulting in false negative tests. False positive tests could occur due to cross reactions with other parasitic infections or due to previous schistosome infection. Yet, due to their high sensitivity, antibody detection tests are recommended for screening of travelers and in populations with low prevalence of schistosomiasis [16]. Many antibody-detecting tests are being used by different laboratories, but they need to be standardized [13••].
Antigen tests therefore offer direct proof of the presence of parasites as detection of eggs. Recently developed antigen detection tests based on monoclonal antibody to detect circulating antigens have been demonstrated to have high diagnostic accuracy [17••]. These assays detect parasite-excreted circulating anodic antigen (CAA) or circulating cathodic antigen (CCA) in serum or urine samples at very low levels and have been demonstrated to have high sensitivity in field studies in China [18] and Tanzania [19]. A commercially available lateral flow-immune chromatographic reagent strip test which detects CCA in urine has been developed as a point-of-care (POC) test. This test accurately detected infections with S. mansoni, Schistosoma japonicum, and Schistosoma mekongi [17••]. A meta-analysis of 15 studies comparing the POC-CCA test and microscopy for S. mansoni demonstrated that POC-CCA detected a very large proportion of infections identified by microscopy, but it misclassified a large proportion of microscopy negatives as positives in endemic areas with a moderate to high prevalence of infection, possibly because the test is potentially more sensitive than microscopy [20].
A variety of molecular techniques and a range of DNA targets for detection of schistosomes have been described [21]. PCR-based technology is highly specific and sensitive and has the potential for high throughput, but DNA detection tests are hardly used for clinical diagnosis within Schistosoma-endemic countries because they require expensive laboratory equipment and highly skilled personnel [22].
In endemic areas, visible or microscopic hematuria is a sensitive proxy marker for urinary schistosomiasis. Persistent eosinophilia in a person with history of exposure to schistosomiasis should also be cause for suspicion of infection. Eosinophilia has been recommended as an indication for investigations for parasitic infections in individuals newly arriving from endemic areas [23]. Clinical examination could also provide indirect clues for the diagnosis of schistosomiasis. S. haematobium infection is associated with symptoms and signs in the urogenital tract; S. mansoni and S. japonicum may cause diarrhea and bloody diarrhea, hepatomegaly, and splenomegaly. Late complications could be detectable by radiological imaging, particularly ultrasound examination.
Schistosomiasis and Global Migration
To explore the prevalence of schistosomiasis in migrant populations, we searched Medline to identify studies on schistosomiasis in migrants or refugees in North America, Europe, Australia, and New Zealand published between 2000 and 2017. Boolean operators (not, and, or) were also used in succession to narrow and widen the searches. Other articles were identified by reviewing the reference list of articles.
The terms “refugee” and “migrant” are frequently used interchangeably although there is a significant difference [24]. The basic difference is that a refugee is defined by the 1951 Geneva Convention [25] as a person fleeing armed conflict or persecution and thus became internationally recognized as “refugee” with access to assistance from states and international organizations. On the other hand, “migrants” choose to move not because of a direct threat of persecution or death, but mainly to improve their lives by finding work or, in some cases, for education, family reunion, or other reasons. There is also a distinction between “migrant” and “immigrant.” The term “migrant“ is a general designation defined by the Webster dictionary as “a person who goes from one place to another specially to find work,” whereas an “immigrant” is a person who comes to a country to take up permanent residence. The present review recognizes that the use of these terms has not been uniform in the different published reports and that individuals moving from schistosomiasis-endemic countries to non-endemic countries do share similar risks and vulnerabilities and face similar conditions as the refugees. We therefore included in the review groups reported as refugees, immigrants, or migrants.
Different countries have different policies and practices regarding the screening of refugees for diseases [26•]. Thus, information about prevalence of schistosomiasis in migrant populations is found in various sources: health screening reports for refugees, clinical audits in primary care facilities, or infectious disease units that deal with migrants and refugees. In total, we have identified 38 published studies on schistosomiasis in migrant populations, including 11 studies in the USA and Canada, 13 studies in Europe, and 14 studies in Australia and New Zealand.
Schistosomiasis in Migrant Populations in North America
The USA is one of the main western countries that receive refugees. In 2015, the USA received 69,920 refugees, including 22,492 refugees from Africa [27]. Most of these come from sub-Saharan Africa, where schistosomiasis is highly prevalent. The Center for Disease Control (CDC) publishes guidelines for medical screening and treatment of USA-bound refugees including guidelines for domestic screening for intestinal parasites [28]. CDC recommends the use of serological tests as the most sensitive tools for screening for schistosomiasis besides stool and urine tests. Stool testing for schistosomiasis is recommended to be done on three specimens on three separate days. In 2005, CDC issued recommendations for predeparture treatment of schistosomiasis with praziquantel in refugees from sub-Saharan Africa who do not have contraindications [29]. An updated table of countries that are currently implementing predeparture presumptive treatment can be found at the CDC website [30••].
Table 2 shows the published reports of screening for schistosomiasis in the USA [31,32,33,34,35,36,37,38,39,40] and Canada [42]. The reported prevalence of schistosomiasis in refugees varied widely depending on many factors, including the county of origin of the refugees and the laboratory tests used for screening. The highest prevalence rates were reported from sub-Saharan Africa, with rates reaching 73% in refugees from Somalia [35] and 64% in a cohort from Sudan [36]. Screening based on stool examination showed very low sensitivity compared to serology. In the epidemiological data reviewed by Chang et al [39], serology identified 18 refugees as Schistosoma-positive (7.6%) but none of these was identified by stool microscopy. The results are also affected by the exact technique used in testing. In the data reported by Geltman et al [31], stool screening was based on examination of a single stool sample instead of the CDC-recommended three samples, resulting in prevalence rate of 1% in African refugees. However, methodology recommended by CDC [43] was used by most screening studies in the USA [35,36,37,38,39]. This approach uses a combination of tests with purified adult worm antigens for antibody detection. Serum specimens are tested by the Falcon assay screening test enzyme-linked immunosorbent assay (FAST-ELISA) using S. mansoni adult microsomal antigen (MAMA). A positive reaction indicates infection with Schistosoma species. Sensitivity for S. mansoni infection is 99%, 95% for S. haematobium infection, and < 50% for S. japonicum infection. Specificity of this assay for detecting schistosome infection is 99%. Because the test sensitivity with the FAST-ELISA is low for species other than S. mansoni, immunoblots of the species appropriate to the patient’s travel history are also tested to ensure detection of S. haematobium and S. japonicum infections. Immunoblots with adult worm microsomal antigens are species-specific, so a positive reaction indicates the infecting species [15].
There is evidence that the CDC policy of presumptive treatment with albendazole has markedly reduced the prevalence of intestinal helminths in refugees from Middle East and south Asia [39]. However, the institution of the CDC recommendations on schistosomiasis has been variable due to funding restrictions and logistical challenges [38]. As an example, the domestic screening and presumptive treatment guidelines were not fully instituted in Illinois in 2015 due to funding restrictions and screening results for intestinal parasite in 2015 were based on stool ova and parasite examinations [44]. Review of reports from the USA shows a need for additional data on the prevalence, screening methods, and presumptive treatment of schistosomiasis among refugees.
During the period 2010−2014, Canada admitted about 49,516 refugees, excluding Quebec [45•]. Newly arrived refugees undergo health assessment and screening for diseases. For schistosomiasis, the official policy is to screen newly arriving refugees from Africa with serology and treat if positive with praziquantel [41]. Based on serological screening in 2011–2014, a prevalence of 15% was reported in African refugees in Toronto, Canada [42].
Schistosomiasis in the International Migrant Population in Europe
Europe hosts 6% of the world’s displaced people [6]. One significant route of migration to Europe is the recent influx of African immigrants though the Mediterranean which has contributed to the expanding threat of importation of schistosomiasis from the highly endemic countries in sub-Saharan Africa into Europe. Of 13 published studies on schistosomiasis in refugees and migrants in Europe, six studies were from Spain [46,47,48,49,52,51], three were from Germany [52••, 53, 54], two were from France [55•, 56, 57], and one of each was from Switzerland [58] and Italy [59•].
There are no unified guidelines for screening and treatment of migrants in Europe for schistosomiasis [60]. Table 3 shows details of the screening studies for schistosomiasis done in Europe. Most of the published screening reports were not systematic screening studies but retrospective analyses of data on screening tests done in refugee or migrant patients seen in primary care clinics or referred to infectious disease hospitals. Each country and each health facilities has its own policy for the management of immigrants with schistosomiasis.
The prevalence of schistosomiasis among the refugees in Europe varied widely according to method of testing and the geographical origin of the refugees. Screening was done by serological methods in eleven studies. Four of these did not specify the type of serologic test used [50••, 52••, 53, 56], four used ELISA only [47,48,49, 51], one used IHA only [46], and two used a combination of serologic tests [58, 59•]. These two studies used combinations of serologic tests and also used antigen detection tests to evaluate the accuracy of using multiple tests for screening refugees. One study in Germany that did not use serology used PCR to detect schistosomal antigens in stools for screening, besides microscopy [54]. Ten studies reported using stool microscopy and urine examinations. Microscopy was mainly used to confirm results of serologic tests, but results based on microscopy alone gave very low positive rates. Stool examination was not always done according to standard techniques for schistosomiasis; only two studies confirmed using three samples for stool examination. When regions of origin of refugees were compared, the prevalence rates were the highest in sub-Saharan African refugees, ranging between 2.4% [47] and 22% [51]. For individual countries of origin, refugees from Eritrea had the highest prevalence rate reaching 56% by stool microscopy with PCR testing in Germany [54] and 50.5% by serology in Switzerland [58].
An increasing number of cases of schistosomiasis with complications have been reported among African immigrants in Europe [60, 61]. These cases were typically misdiagnosed and presented later with disease complications because they were initially seen by clinicians who were not aware of the clinical picture of schistosomiasis and lack of standard guidelines for screening of immigrants from endemic areas. In France, a study reported that in a clinic caring for vulnerable populations in Paris, no screening was done for intestinal or urinary parasitic infections in three out of five migrants from endemic areas, with a possible risk of future disease complications for missed infections [55•]. The need to educate primary care workers and physicians in Europe about schistosomiasis has been advocated [60]. Some of the published papers indicate that schistosomiasis in refugees was not considered as a public health concern as the diseases that are directly infectious. One study reporting infectious diseases in refugees in Spain did not include schistosomiasis and stated that “Eight diseases with a potential risk of transmission in our environment were studied” [62].
The potentially serious consequences of imported schistosomiasis in Europe have been realized after recent reports showing evidence of transmission of schistosomiasis in Corsica [63, 64••, 65]. These reports also stressed the potential of an even wider spread of disease transmission in Europe because Bulinus truncatus, the snail intermediate host for S. hematobium, is endemic in southern Europe, including Spain, Italy, France, and Greece. Climatic change, the establishment of an intermediate host, and the presence of non-treated schistosomiasis patients are the main factors posing a threat of establishing schistosomiasis transmission in Europe [63].
Schistosomiasis in the Immigrant Population in Australia and New Zealand
The 2015–2016 humanitarian program of Australia granted 15,552 offshore refugee and humanitarian visas, of which 58.9% were granted to persons born in Middle East,
29.3% to persons born in Asia, and 11.8% to persons born in Africa [66].
Many centers in Australia currently screen and treat people from refugee-like backgrounds for schistosomiasis. In general, the health authorities in the different centers follow the policy recommended by the Australasian Society for Infectious Diseases (ASID) which is as follows [67, 68•]:
Offer blood testing for schistosomiasis serology if people have lived in/travelled through endemic countries.
-
If serology is negative, no follow-up is required.
-
If serology is positive or equivocal
-
Treat with praziquantel in two doses of 20/mg/kg, 4 hours apart, orally. (40 mg/kg total, no upper limit)
-
Perform stool microscopy for ova.
-
Perform urine dipstick for hematuria and end-urine microscopy for ova if hematuria.
-
-
If positive for ova on urine or stool, evaluate further for end-organ disease with ultrasound and LFTs. See flowchart for further details.
-
Seek advice from a pediatric specialist on treatment of children < 5 years.
Table 4 shows details of screening studies in Australia [69,70,71,72,73,74,75,76,77,78,79,80] and New Zealand [81, 82].
Most reports of screening data on schistosomiasis are about refugees from Africa. Positive schistosomiasis serology in African refugees ranged between 2% [70] and 40.8% [75]. Asian refugees from Myanmar tested positive in 5.4% [79] and 7% [80] Most of the reports were based on patients seen in refugee clinics, but this may not cover all the refugees with schistosomiasis. Raman et al [72] reported that while New South Wales state of Australia received 1,557 refugee children (< 14 years) in 2005, only about one in five (n = 331) was seen in a refugee-specific clinic. Of those assessed, 27% were serology-positive for schistosomiasis.
New Zealand accepts 750 “quota” refugees annually. On arrival in New Zealand, refugees are received into the Mangere Refugee Resettlement Centre (MRRC) where they undergo medical screening for communicable disease control [83]. Retrospective studies on screening of refugees in New Zealand reported prevalence of rates of 21.9% [81] and 4% [82] in groups of refugees from different origins.
Conclusions
The need for better detection and treatment of schistosomiasis in migrant population is increasingly being recognized as a global health issue that needs to be addressed. After using sensitive serologic tests for screening, it was realized that prevalence of this infection was greater than had been previously detected by conventional parasitological tests. The increase in global migration from endemic areas has increased the risk of exporting cases of the disease to non-endemic areas. Early screening and treatment of schistosomiasis could forestall development of serious disease complications downstream. At the public health level, the recent evidence of the transmission of schistosomiasis in southern Europe showed how easily the disease could be introduced into new territories. This calls for reviewing the screening and treatment policies in countries where the disease is not endemic. There are also implications for the global strategies for schistosomiasis elimination. The published reports on screening for schistosomiasis show a need for further evaluation of the feasibility and cost-effectiveness of the different options for screening refugees and migrants, including domestic and predeparture screening and treatment protocols. The value of laboratory tests needs to be reviewed in different settings. Such a review should consider targeted testing, the diagnostic accuracy of serology, stool antigen testing, and combinations of laboratory tests. Screening for schistosomiasis should not be limited to those officially defined as refugees but should also include other vulnerable migrants who could benefit from the refugee screening strategies.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
WHO Media center, Schistosomiasis fact sheet, updated January 2017. Available from: http://www.who.int/mediacentre/factsheets/fs115/en/
WHO. Status of schistosomiasis endemic countries, 2015. Available from: http://apps.who.int/neglected_diseases/ntddata/sch/sch.html
Madinga J, Linsuke S, Mpabanzi L, Meurs L, Kanobana K, Speybroeck N, et al. Schistosomiasis in the Democratic Republic of Congo: a literature review. Parasites & Vectors. 2015;8:601. https://doi.org/10.1186/s13071-015-1206-6.
Cao C, Baoa Z, Lia S, Weib W, Yib P, Yua Q, et al. Schistosomiasis in a migrating population in the lake region of China and its potential impact on control operations. Acta Tropica. 2015;145:88–92.
Bian C-R, Lu D-B, Su J, Zhou X, Zhuge HX, PHL L. Serological prevalence of Schistosoma japonicum in mobile populations in previously endemic but now non-endemic regions of china : a systematic review and meta-analysis. PLoSONE. 2015;10(6):e0128896. https://doi.org/10.1371/journal. pone.0128896.
UNHCR, Global trends, forced displacement in 2016, trends at a glance, http://www.unhcr.org/globaltrends2016/
United Nations Department of Economic and Social Affairs, Population Division. Trends in international migration, 2015. December 2015. http://www.un.org/en/development/desa/population/migration/publications/migrationreport/docs/MigrationReport2015.pdf
McCarthy AE, Weld LH, Barnett ED, So H, Coyle C, Greenaway C, et al. Spectrum of illness in international migrants seen at GeoSentinel clinics in 1997-2009, part 2: migrants resettled internationally and evaluated for specific health concerns. Clin Infect Dis. 2013 Apr;56(7):925–33. https://doi.org/10.1093/cid/cis1016.
Montresor A, Crompton DWT, Hall A, Bundy DAP, Savioli L. Guidelines for the evaluation of soil-transmitted helminthiasis and schistosomiasis at community level. Geneva: World Health Organization; 1998. p. 1–49.
Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop São Paulo. 1972;14:397–400.
WHO. Bench aids for the diagnosis of intestinal parasites. Geneva: World Health Organization; 1994. p. 1–20.
Peters PA, Mahmoud AA, Warren KS, Ouma JH, Siongok TK. Field studies of a rapid, accurate means of quantifying Schistosoma haematobium eggs in urine samples. Bull World Health Organ. 1976;54:159–62.
•• Hinz R, Schwarz NG, Hahn A, Frickmann H. Serological approaches for the diagnosis of schistosomiasis—a review. Mol Cell Probes. 2017;31:2–21. This review presents results of published studies on serological assays detecting antibodies against different Schistosoma species. It provides insights in the diagnostic performance and an overview on the availability of described assays and their suitability for case management and large-scale screening. It is helpful for understanding the value of the assays in screening for schistosomiasis
Kinkel H-F, Dittrich S, Bäumer B, Weitzel T. Evaluation of eight serological tests for diagnosis of imported schistosomiasis. Clin Vaccine Immunol. 2012;19:948–53.
Tsang VC, Wilkins PP. Immunodiagnosis of schistosomiasis. Immunol Invest. 1997;26:175–88.
Bierman WF, Wetsteyn JC, van Gool T. Presentation and diagnosis of imported schistosomiasis: relevance of eosinophilia, microscopy for ova, and serology. J Travel Med. 2005;12:9–13.
•• Utzinger J, Becker SL, van Lieshout L, van Dam GJ, Knopp S. New diagnostic tools in schistosomiasis. Clin Microbiol Infect. 2015;21(6):529–42. This is a review on the latest developments in schistosomiasis diagnosis, including microscopic techniques, rapid diagnostic tests for antigen detection, polymerase chain reaction (PCR) assays, and proxy markers for morbidity assessments. This is relevant to understanding interpretation of screening tests used in migrants
van Dam GJ, Xu J, Bergquist R, de Dood CJ, Utzinger J, Qin ZQ, et al. An ultra-sensitive assay targeting the circulating anodic antigen for the diagnosis of Schistosoma japonicum in a low-endemic area, People’s Republic of China. Acta Trop. 2015;141:190–7.
Knopp S, Corstjens PLAM, Koukounari A, Cercamondi CI, Ame SM, de Dood CJ, et al. Sensitivity and specificity of a urine circulating anodic antigen test for the diagnosis of Schistosoma haematobium in low endemic settings. PLoS Negl Trop Dis. 2015;9:e0003752.
Ochodo EA, Gopalakrishna G, Spek B, et al. Circulating antigen tests and urine reagent strips for diagnosis of active schistosomiasis in endemic areas. Cochrane Database Syst Rev. 2015;3:CD009579.
Verweij JJ, Stensvold CR. Molecular testing for clinical diagnosis and epidemiological investigations of intestinal parasitic infections. Clin Microbiol Rev. 2014;27:371–418.
van Lieshout L, Roestenberg M. Clinical consequences of new diagnostic tools for intestinal parasites. Clin Microbiol Infect. 2015 Jun;21(6):520–8. https://doi.org/10.1016/j.cmi.2015.03.015.
Seybolt LM, Christiansen D, Barnett ED. Diagnostic evaluation of newly arrived asymptomatic refugees with eosinophilia. Clin Infect Dis. 2006;42:363–7.
Edwards A. 2015. UNHCR viewpoint: ‘refugee’ or ‘migrant’. Available from: http://www.unhcr.org/en-us/news/latest/2016/7/55df0e556/unhcr-viewpoint-refugee-migrant-right.html
UNHCR Convention and protocol relating to the status of refugees http://www.unhcr.org/3b66c2aa10.html
• Hvass AMF, Wejse C. Systematic health screening of refugees after resettlement in recipient countries: a scoping review. Annals of Human Biology. 2017;44(5):475–83. https://doi.org/10.1080/03014460.2017.1330897. This is a review of studies on health screening of refugees in North America, Australia/New Zealand, and Europe. Because of differences in country policies, the screenings were conducted differently in the various locations. The studies demonstrated great variation in who was targeted for screening and how screening was conducted.
Department of Homeland Security. Yearbook of immigration statistics 2015. Available from: https://www.dhs.gov/immigration-statistics/yearbook/2015
Domestic intestinal parasite guidelines. Available from: https://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/intestinal-parasites-domestic.html.
Guidelines for overseas presumptive treatment of strongyloidiasis, schistosomiasis, and soil-transmitted helminth infections. Available from: https://www.cdc.gov/immigrantrefugeehealth/guidelines/overseas/intestinal-parasites-overseas.html
•• Centers for Disease Control. Treatment schedules for presumptive parasitic infections. Available from: http://www.cdc.gov/immigrantrefugeehealth/guidelines/overseas/interventions/interventions.html .This site shows the US CDC intestinal parasite guidelines and an updated table for predeparture treatment. The guidelines address the parasitic infections most commonly encountered in refugees but focus on soil-transmitted helminthic infections ( Ascaris , Trichuris , hookworm), Strongyloides , and Schistosoma spp.
Geltman PL, Cochran J, Hedgecock C. Intestinal parasites among African refugees resettled in Massachusetts and the impact of an overseas pre-departure treatment program. Am J Trop Med Hyg. 2003;69(6):657–62.
Lifson AR, Thai D, O'Fallon A, Mills WA, Hang K. Prevalence of tuberculosis, hepatitis B virus, and intestinal parasitic infections among refugees to Minnesota. Public Health Reports. 2002;117(1):69–77.
Lowther SA, Johnson G, Hendel-Paterson B, Nelson K, Mamo B, Krohn K, et al. HIV/AIDS and associated conditions among hiv-infected refugees in Minnesota, 2000–2007. Int J Environ Res Public Health. 2012;9:4197–209. https://doi.org/10.3390/ijerph9114197.
Garg PK, Perry S, Dorn M, Hardcastle L, Parsonnet J. Risk of intestinal helminth and protozoan infection in a refugee population. Am J Trop Med Hyg. 2005;73(2):38691.
Posey D, Blackburn B, Weinberg M, Flagg E, Ortega L, Wilson M, et al. High prevalence and presumptive treatment of schistosomiasis and strongyloidiasis among African refugees. Clin Infect Dis. 2007;45:1310–5.
Franco-Paredes C, Dismukes R, Nicolls D, Hidron A, Workowski K, Rodriguez-Morales A, et al. Persistent and untreated tropical infectious diseases among Sudanese refugees in the United States. Am J Trop Med Hyg. 2007;77:633–5.
Quandelacy TM, Riefkohl A, Franco PC. Prevalence of untreated schistosomiasis among Sudanese refugees: “the lost boys of Sudan” in the United States. Bol Med Hosp Infant Mex. 503Vol. 67, Noviembre-Diciembre 2010. Boletin medico del Hospital Infantil de Mexico
Brodine SK, Thomas A, Huang R, et al. Community based parasitic screening and treatment of Sudanese refugees: application and assessment of Centers for Disease Control guidelines. Am J Trop Med Hyg. 2009;80:425–30.
Chang AH, Perry S, Du JN, Agunbiade A, Polesky A, Parsonnet J. Decreasing intestinal parasites in recent northern California refugees. Am. J. Trop. Med. Hyg. 2013;88(1):191–7. https://doi.org/10.4269/ajtmh.2012.12-0349.
Rapoport AB, McCormick D, Cohen PA. Screening for Schistosoma mansoni and Strongyloides stercoralis infection among Brazilian immigrants in the United States. Open Forum Infect Dis. 2015;2(1):ofv003. Published online 2015 Jan 8. https://doi.org/10.1093/ofid/ofv003. PMCID: PMC4438884.
Khan K, Heidebrecht C, Sears J, Chan A, Rashid M, Greenaway C, Stauffer W, Narasiah L, Pottie K; Canadian Collaboration for Immigrant and Refugee Health, guidelines for immigrant health, Appendix 8: intestinal parasites—Strongyloides and Schistosoma: evidence review for newly arriving immigrants and refugees http://www.cmaj.ca/content/suppl/2010/06/07/cmaj.090313.DC1/imm-para-8-at.pdf. This document reviews evidence on parasitic infection in refugee populations, the value of serologic testing and treatment of strongyloidiasis and schistosomiasis, and the use of ivermectin and praziquantel for treating strongyloidiasis and schistosomiasis.
Redditt VJ, Janakiram P, Graziano D, Rashid M. Health status of newly arrived refugees in Toronto, Ont: part 1: infectious diseases. Can Fam Physician. 2015;61(7):e303–9. PMCID: PMC4501620
CDC-DPDx-schistosomiasis infection—laboratory diagnosis. Available from: https://www.cdc.gov/dpdx/schistosomiasis/dx.html
Illinois Refugee Health Program. 2015 Annual screening summary report. Available from: http://www.dph.illinois.gov/sites/default/files/publications/publicationscmh2015-refugee-program-ar.pdf
• Citizenship and Immigration Canada. Statistics and open data. Available from: www.cic.gc.ca/english/resources/statistics/
López-Vélez R, Huerga H, Turrientes MC. Infectious diseases in immigrants from the perspective of a tropical medicine referral unit. Am J Trop Med Hyg. 2003;69(1):115–21.
Monge-Maillo B, Jiménez BC, Pérez-Molina JA, Norman F, Navarro M, Pérez-Ayala A, et al. Imported infectious diseases in mobile populations, Spain. Emerg Infect Dis. 2009;15:1745–52.
Monge-Maillo B, López-Vélez R, Norman FF, Ferrere-González F, Martínez-Pérez Á, Pérez-Molina JA. Screening of imported infectious diseases among asymptomatic sub-Saharan African and Latin American immigrants: a public health challenge. Am J Trop Med Hyg. 2015;92:848–56.
Bocanegra C, Salvador F, Sulleiro E, Sánchez-Montalvá A, Pahissa A, Molina I. Screening for imported diseases in an immigrant population: experience from a teaching hospital in Barcelona, Spain. Am J Trop Med Hyg. 2014;91:1277–81.
•• Delcor N, Maruri B, Arandes A, Guiu I, Essadik H, Soley M, et al. Infectious diseases in sub-Saharan immigrants to Spain. Am. J. Trop. Med. Hyg. 2016;94(4):750–6. https://doi.org/10.4269/ajtmh.15-0583. This paper describes infectious diseases in newly arrived sub-Saharan immigrants after standardized health visits at an international health center in Barcelona, Spain.
Salvador F, Molina I, Sulleiro E, Burgos J, Curran A, Van den Eynde E, et al. Tropical diseases screening in immigrant patients with human immunodeficiency virus infection in Spain. Am. J. Trop. Med. Hyg. 2013;88(6):1196–202. https://doi.org/10.4269/ajtmh.12-0714.
•• Herbinger K, Alberer M, Berens-Riha N, Schunk M, Bretzel G, von Sonnenburg F, et al. Spectrum of imported infectious diseases: a comparative prevalence study of 16,817 German travelers and 977 immigrants from the tropics and subtropics. Am. J. Trop. Med. Hyg. 2016;94(4):757–66. https://doi.org/10.4269/ajtmh.15-0731. This retrospective analysis shows a large spectrum of imported IDs among returning German travelers and immigrants, which varies greatly based not only on travel destination and origin of immigrants, but also on type of travel
Mockenhaupt F, Barbre K, Jensenius M, Larsen C, Barnett E, Stauffer W, Rothe C, Asgeirsson H, Hamer D, Esposito D, Gautret P, Schlagenhauf P. Profile of illness in Syrian refugees: a GeoSentinel analysis, 2013 to 2015. Euro Surveill. 2016;21(10). doi: https://doi.org/10.2807/1560-7917.ES.2016.21.10.30160
Maaßen W, Wiemer D, Frey 1C, Kreuzberg C, Tannich E, Hinz R, et al. Microbiological screenings for infection control in unaccompanied minor refugees: the German Armed Forces Medical Service’s experience. Military Medical Research. 2017;4:13. https://doi.org/10.1186/s40779-017-0123-8. PMCID: PMC5402321
• Deniaud F, Rouessé C, Collignon A, Domingo A, Rigal L. Failure to offer parasitology screening to vulnerable migrants in France: epidemiology and consequences. Sante. 2010;20(4):201–8.
Monpierre O, Baudino P, Rio-Rene P, Maurice S, Malvy D, Receveur MC. Global health of unaccompanied refugee minors in Gironde (France) between 2011 and 2013. Bull Soc Pathol Exot. 2016;109:99–106. https://doi.org/10.1007/s13149-016-0476-3. PMID: 26860845
Pierre Baudino. Etat de sant´e des mineurs isol´es ´etrangers accueillis en Gironde entre 2011 et ´ 2013. Masters Thesis, Université de Bordeaux, Medecine humaine et pathologie. 2015. HAL Id: dumas-01157256 https://dumas.ccsd.cnrs.fr/dumas-01157256
Chernet A, Kling K, Sydow V, Kuenzli E, Hatz C, Utzinger J, et al. Accuracy of diagnostic tests for Schistosoma mansoni infection in asymptomatic Eritrean refugees: serology and POC-CCA against stool microscopy. Clin Infect Dis. 2017; https://doi.org/10.1093/cid/cix366. This study was to evaluate the accuracy of a combination of microscopy, different serological tests, and POC-CCA tests for the screening of Eritrean refugees in Switzerland for schistosomiasis
• Beltrame A, Guerriero M, AnghebenA GF, Requena-Mendez A, Zammarchi L, et al. Accuracy of parasitological and immunological tests for the screening of human schistosomiasis in immigrants and refugees from African countries: an approach with latent class analysis. PLoS Negl Trop Dis. 2017;11(6):e0005593. https://doi.org/10.1371/journal.pntd.0005593. This study was to evaluate the accuracy of different tests for the screening of schistosomiasis in African migrants in Italy
Riccardi N, Nosenzo F, Peraldo F, Sarocchi F, Taramasso L, Traverso P, et al. Increasing prevalence of genitourinary schistosomiasis in Europe in the migrant era: neglected no more? PLOS Neglected Tropical Diseases. 2017; https://doi.org/10.1371/journal.pntd.0005237.
Poddighe D, Castelli L, Pulcrano G, Grosini A, Balzaretti M, Spadaro S, et al. Urinary schistosomiasis in an adolescent refugee from Africa: an uncommon cause of hematuria and an emerging infectious disease in Europe. J Immigr Minor Health. 2016;18:1237–40. https://doi.org/10.1007/s10903-015-0272-3. PMID: 26335551
Manzardo C, Treviño B, Gómez i Prat J, Cabezos J, Monguí E, Clavería I, et al. Communicable diseases in the immigrant population attended to in a tropical medicine unit: epidemiological aspects and public health issues. Travel Med Infect Dis. 2008 Jan-Mar;6(1-2):4–11. https://doi.org/10.1016/j.tmaid.2007.11.002.
European Centre for Disease Prevention and Control. Rapid risk assessment: local transmission of Schistosoma haematobium in Corsica, France. Stockholm: The Centre; 2015. http://ecdc.europa.eu/en/publications/Publications/risk-assessment-Schistosoma%20haematobium-Corsica-update_TOR1N6.pdf
•• Boissier J, Grech-Angelini S, Webster BL, Allienne JF, Huyse T, Mas-Coma S, et al. Outbreak of urogenital schistosomiasis in Corsica (France): an epidemiological case study. Lancet Infect Dis. 2016;16:971–9. https://doi.org/10.1016/S1473-3099(16)00175-4. This paper presents an updated account of the epidemiological studies investigating the outbreak of urogenital schistosomiasis in Corsica (France)
Berry A, Fillaux J, Martin-Blondel G, Boissier J, Iriart X, Marchou B, et al. Evidence for a permanent presence of schistosomiasis in Corsica, France, 2015. Euro Surveill. 2016; 21(1).
Australian Government, Department of Immigration and Border Protection. Australia’s offshore humanitarian programme: 2015–16 http://www.border.gov.au/ReportsandPublications/Documents/statistics/australia-offshore-humanitarian-programme-2015-16.pdf
The Royal Australasian College of Physicians policy on refugee and asylum seeker health, Royal Australasian College of Physicians, May 2015 https://www.racp.edu.au/docs/default-source/advocacy-library/policy-on-refugee-and-asylum-seeker-health.pdf
• Chaves NJ, Paxton G, Biggs BA, Thambiran A, Smith M, Williams J, Gardiner J, Davis JS; On behalf of the Australasian Society for Infectious Diseases and Refugee Health Network of Australia Guidelines writing group. Recommendations for comprehensive post-arrival health assessment for people from refugee-like backgrounds. https://www.asid.net.au/documents/item/1225. These are the Australian guidelines for post-arrival health assessment in refugee-like populations. It provides detail on 39 studies including more than 30,000 people from refugee-like backgrounds from primary care, primary, and tertiary care refugee and asylum seeker health services and detention centers around Australia, presented by age and region of origin.
Caruana SR, Kelly HA, Ngeow JY, Ryan NJ, Bennett CM, Chea L, et al. Undiagnosed and potentially lethal parasite infections among immigrants and refugees in Australia. J Travel Med. 2006;13(4):233–9.
Rice JE, Skull SA, Pearce C, Mulholland N, Davie G, Carapetis JR. Screening for intestinal parasites in recently arrived children from East Africa. J Paediatr Child Health. 2003;39(6):456–9.
Martin JA, Mak DB. Changing faces: a review of infectious disease screening of refugees by the Migrant Health Unit, Western Australia in 2003 and 2004. Med J Aust. 2006;185(11–12):607–10.
Raman S, Wood N, Webber M, Taylor K, Isaacs D. Matching health needs of refugee children with services: how big is the gap? Aust N Z J Public Health. 2009;33(5):466–70.
Davis J, Weber M. A prospective audit of 215 newly arrived African refugees. J Paediatr Child Health. 2007;42(A13).
Tiong ACD, Patel MS, Gardiner J, Ryan R, Linton KS, Walker K a, et al. Health issues in newly arrived African refugees attending general practice clinics in Melbourne. Med J Aust. 2006;185(11):602–6.
Gibney KB, Mihrshahi S, Torresi J, Marshall C, Leder K, Biggs BA. The profile of health problems in African immigrants attending an infectious disease unit in Melbourne, Australia. Am J Trop Med Hyg. 2009;80(5):805–11.
Sheikh M, Pal A, Wang S, MacIntyre CR, Wood NJ, Isaacs D, et al. The epidemiology of health conditions of newly arrived refugee children: a review of patients attending a specialist health clinic in Sydney. J Paediatr Child Health. 2009 Sep;45(9):509–13.
Mutch RC, Cherian S, Nemba K, Geddes JS, Rutherford DM, Chaney GM, et al. Tertiary paediatric refugee health clinic in Western Australia: analysis of the first 1026 children. J Paediatr Child Health. 2012;48(7):582–7.
Johnston V, Smith L, Roydhouse H. The health of newly arrived refugees to the top end of Australia: results of a clinical audit at the Darwin Refugee Health Service. Aust J Prim Health. 2012 Jan;18(3):242–7.
Chaves NJ, Gibney KB, Leder, O’Brien DP, Marshall C, Biggs BA. Screening practices for infectious diseases among Burmese refugees in Australia. Emerg Infect Dis. 2009;15:1769–72. https://doi.org/10.3201/eid1511.090777.
Paxton GA, Sangster KJ, Maxwell EL, McBride CRJ, Drewe RH. Post-arrival health screening in Karen refugees in Australia. PLoS ONE. 2012;7(5):e38194. https://doi.org/10.1371/journal.pone.0038194.
McLeod A, Reeve M. The health status of quota refugees screened by New Zealand’s Auckland Public Health Service between 1995 and 2000. N Z Med J. 2005;118(1224):U1702.
Rungan S, Reeve AM, Reed PW, Voss L. Health needs of refugee children younger than 5 years arriving in New Zealand. Pediatr Infect Dis J. 2013 Dec;32(12):e432–6.
The Auckland Regional Public Health Service. http://www.arphs.govt.nz/health-information/promoting-health-wellbeing/refugee-health/service
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ahmed A. Adeel declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Additional information
This article is part of the Topical Collection on Tropical Diseases in Conflict Regions
Rights and permissions
About this article
Cite this article
Adeel, A.A. Schistosomiasis in International Refugees and Migrant Populations. Curr Trop Med Rep 4, 256–267 (2017). https://doi.org/10.1007/s40475-017-0128-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40475-017-0128-0