Abstract
Purpose of Review
Gut microbiota contribute to several physiological processes in the host. The composition of the gut microbiome is associated with different neurological and neurodevelopmental diseases. In psychiatric disease, stress may be a major factor leading to gut microbiota alterations. Depressive disorders are the most prevalent mental health issues worldwide and patients often report gastrointestinal symptoms. Accordingly, evidence of gut microbial alterations in depressive disorders has been growing. Here we review current literature revealing links between the gut microbiome and brain function in the context of depression.
Recent Findings
The gut-brain axis could impact the behavioral manifestation of depression and the underlying neuropathology via multiple routes: the HPA axis, immune function, the enteric nervous system, and the vagus nerve. Furthermore, we explore possible therapeutic interventions including fecal microbiota transplant or probiotic supplementation in alleviating depressive symptoms.
Summary
Understanding the mechanisms by which bidirectional communication along the gut-brain axis can be dysregulated in patients with depression could lead to the development of personalized, microbiome-targeted therapies for the treatment of this disorder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number of genes within the human gut microbiome outnumbers those in the human genome by 100-fold [1]. With such a vast collection of genes, the gut microbiome exerts a huge influence on the host in homeostasis and disease. Note, in this review we use the terms “gut microbiota” and “gut microbiome” interchangeably; however, the term "gut microbiota” refers to all microorganisms residing within the host gastrointestinal (GI) tract, including bacteria, fungi, viruses, and archaea, while the term “microbiome” refers to the genes expressed by microbiota. Furthermore, we primarily consider the contributions of bacteria because they are the most abundant and well-studied microbes in the mammalian gut.
Gut microbiota are involved in multiple physiological processes in both health and disease. For example, studies have shown that during development, gut microbiota are required for immune cell maturation at different developmental stages, ranging from in utero, with effects of maternal microbiota influencing prenatal development, to bacterial exposure in the early postnatal phase informing T cell differentiation [2]. Furthermore, during early-life development, the composition of organ-specific microbiota evolves in concert with host organ maturation [3]. Beyond development, gut microbiota also participate in nutrient metabolism, generating functional metabolites like short-chain fatty acids (SCFAs), tryptophan derivatives, carbohydrates, and amino acids. Gut microbiota are also associated with pathways that produce neurotransmitters like serotonin (5-HT), dopamine, GABA, acetylcholine and glutamate and have been shown make critical contributions to brain development and function [4]. Consequently, dysbiosis–i.e., disruption of the microbial ecosystem–of the gut microbiome is increasingly implicated in the etiology and symptomatology of metabolic, immunological, and neurological disorders.
Dysbiosis of the gut microbiome is associated with several host and environmental factors. Diet plays the most significant role in shaping the gut microbiota [5, 6]. For example, “Western diet”, a diet composed of highly processed food and elevated levels of saturated fat and sugar, increases the relative abundance pro-inflammatory bacteria and leads to increased risk for metabolic and inflammatory diseases [7, 8]. Antibiotic regimens can also lead to substantial changes in the gut microbiome, which could contribute to disease development [9, 10]. Moreover, host genetics has also been shown to play a role in regulating gut microbiota composition and its interplay with diet. For example, genes that are associated with lactose intolerance are dominantly inherited and have been shown to affect gut microbiota composition in the lactose-intolerant population [11]. Another study revealed a relationship between host genetics and microbiome using genome-wide association studies (GWAS) identifying a potential link between disease risk genes (for colorectal cancer and major depression disorders) and specific bacterial species [12]. Moreover, psychological stress has been implicated in changes in gut microbial ecology, with recent studies showing evidence of dysbiosis in mouse models for stress and highly stressed human subjects [13]. These environmental and genetic factors likely interact to drive dysbiosis of the gut microbiota and can be difficult to delineate from one another. Given the complex nature of gut microbiota interactions with different organ systems, this remains an intriguing field of study for the development of novel therapeutics targeting the gut microbiota to reduce risk for and treat symptoms across multiple disease states, including for neuropsychiatric disorders.
Here, we review literature revealing connections between gut microbiota and neuropsychiatric disorders, with an emphasis on major depressive disorder (MDD), as well as the therapeutic potential of targeting the gut microbiome to prevent the onset of disease and alleviate adverse symptoms.
Dysbiosis of the Gut Microbiome in Mental Health and Neuropsychiatric Disorders
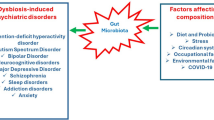
Emerging data are uncovering a significant role for gut microbiota in neuropsychiatric disorders [14]. Indeed, studies in both humans and preclinical animal models are beginning to implicate host-microbe interactions in the pathogenesis of neurodevelopmental-to-neurodegenerative disorders and even in traumatic brain injury. For example, changes in gut microbiota composition have been identified among some cohorts of patients with autism spectrum disorder (ASD) as well as in preclinical mouse models for ASD [15,16,17]. While not the focus of this review, the relationship between gut microbiota and ASD in hosts is the subject of multiple excellent reviews [13]. Interestingly, gut microbial diversity has been shown to be markedly altered in tobacco smokers [18], implicating a potential for gut microbiota involvement in tobacco addiction [19]. Taken together with data showing that microbiota can influence the function of the dopaminergic reward system [15,16,17] and therefore impact host behavior or disease phenotypes, dysbiosis of the gut microbiota is likely associated with several neuropsychiatric disorders.
Among neuropsychiatric diseases, depressive disorders are among the most prevalent and clinically best defined, with several scales available; however, broadly effective pharmacological treatments remain elusive. New data indicate that the gut microbiome could provide diagnostic, preventative, and therapeutic targets for major depressive disorder (MDD) [20, 21]. While the causal relationship between dysbiosis of the gut microbiome and risk for specific neuropsychiatric diseases remains an active area of investigation, it is valuable to evaluate the therapeutic potential of modulating the gut microbiome to promote neuropsychiatric health, identify novel biomarkers for early diagnosis, prevent disease, and manage symptoms.
Depression is a Major Neuropsychiatric Disorder with Few Effective Therapeutics
Depression affects approximately 300 million people world-wide and ranks as the largest contributor to global disability among mental disorders, as reported by the World Health Organization [22, 23]. In 2021, during the COVID-19 pandemic, there was a significant 27% rise in cases of major depressive disorder (MDD) throughout the globe [24]. Furthermore, up to 31% of COVID-19 patients reported depression [25] and clinical trials have shown a potential link between long COVID’s effect on gut microbiota composition and depression disorders [26, 27]. According to the American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders (DSM-5), depression is characterized by persistent feelings of sadness and/or a loss of interest in previously enjoyable activities, leading to various behavioral symptoms. In the United States, approximately 10% of the population suffers MDD. However, due to the diverse symptoms and relatively unknown underlying causes of MDD, effective treatments have proven elusive [28]. For example, antidepressants like selective serotonin reuptake inhibitors (SSRIs) may be effective for some, but one-third of depression patients respond only partially or poorly to the medication [29]. Other treatment options like NMDA modulators, brain stimulation, anti-inflammatory agents, and psychedelics either have a short time window of effectiveness that requires the patient make frequent clinic visits, have undesirable side effects like gastrointestinal dysfunction, or are still in development (reviewed in Marwaha et al., 2023) [28]. Interestingly, a substantial percentage, ranging from 60 to 70% of individuals with MDD report gastrointestinal issues as comorbidities [30,31,32] and a significant proportion of irritable bowel disease (IBD) patients also reported depression [33]. This connection underscores the need to develop a deeper understanding of how alterations in the gut-brain axis can impact mental health and whether it could provide new therapeutic targets for MDD.
The gut-brain axis is comprised of the gut microbiota, immune system, vagus nerve, and enteric nervous system. It integrates hormonal, metabolic, immunological, and neural signals allowing gut microbes and their metabolites to influence host physiology. The majority of existing studies focus on how gut microbes influence brain function; however, given the bidirectionality of the axis, the brain could also modulate gut physiology by means of regulating motility or mucin production. Immune cytokine secretion is also regulated by both the brain and the gut microbiota.
Dysbiosis of the gut microbiome has been demonstrated in patients with major depression disorders (MDD) [34, 35]. Correlations between gut microbiota composition in early-stage depression and disease severity as well as remission post-treatment have been reported [36]. For instance, a study in Rotterdam with over 1,000 participants identified 13 bacterial genera and 1 family associated with depressive symptoms and verified the results with a second cohort with 1,539 participants [37]. Moreover, gut microbiota from MDD patients was shown to transfer depression-like behaviors to mice – fecal microbiota transplant (FMT) from depression patients’ sample successfully recapitulated the depressive-like phenotypes in microbiota-depleted antibiotic rats [38]. In preclinical research employing rodent models for depression, shifts in gut microbial ecology have been shown in parallel with the onset of depression-like behaviors [39]. These data illustrate that the gut microbiota could modulate host behavior via the gut-brain axis, and could therefore serve as a potential therapeutic target for treating psychiatric diseases.
Pathways in the Gut-Brain Axis Associated with Mental Health/Psychiatric Disorders
The HPA Axis
The hypothalamic–pituitary–adrenal (HPA) axis is a pathway activated in response to stress composed of the hypothalamus, pituitary gland, and the adrenal gland. It is activated when input from the parasympathetic, sympathetic, or limbic systems is received by the paraventricular nucleus of the hypothalamus (PVN) stimulating the release of corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) from the anterior pituitary gland, which then leads to the release of adrenocorticotrophic hormone (ACTH) into circulation thereby inducing the release of glucocorticoids like cortisol or corticosterone, the principal endogenous adrenal steroids in humans and rodents, respectively, from adrenal cortex into the system [40].
Dysfunction of the HPA axis has been shown to contribute to stress and depressive disorders [41]. As previously reviewed, early-life stress can lead to persistent changes in patterns of HPA axis activation in response to stress in adulthood [42]. Notably, HPA axis activation has been proposed as a biomarker for differentiating depression subtypes [43]. Interest in the interaction between the HPA axis and the gut microbiome in psychiatric disorders emerged within the past decade. In a landmark study, Wu et al. demonstrated that over-activation of the HPA axis associated with elevated levels of corticosterone in germ-free and antibiotics-treated mice contributed to social dysfunction in that animals that could be reversed through surgically (adrenalectomy) or pharmacologically-mediated reductions in corticosterone output [44]. Conversely, activation of corticosterone-producing neurons in the PVN was sufficient to induce social deficits in conventionally colonized mice. Similarly, Luo et al. found that germ-free (GF) mice that had received a fecal microbiota transplant from patients with depression (diagnosed with DSM-IV-TR and with high 17-item Hamilton rating scores) showed elevated HPA axis activity, as indicated by increased expression of glucocorticoid receptor pathway genes [45]. Moreover, O’Mahony et al. observed increased plasma corticosterone levels and changes in fecal microbiota in a rat model for maternal separation, drawing a correlation between activation of HPA axis and gut microbiota alterations [46]. In the same model, hyperactivation of the HPA axis – as measured by corticosterone levels in both stool and circulation and catecholamine in the hypothalamus –was corrected by administration of the probiotic strain Bifidobacterium CECT 7765 during early-life development [47]. Finally, Tian et al. identified increased levels of serum corticosterone in mice with depressive-like behavior that were corrected by ingestion of Bifidobacterium breve CCFM1025 during a 5-week stress-induction period in the chronic unpredictable mild stress (CUMS) mouse model for depression [48]. The two studies mentioned above were among the first to demonstrate the utility of probiotics in dampening stress induced HPA axis activation. Together, these data suggest that gut microbiota can modulate HPA axis activation in response to stress and further suggest that therapeutic targeting of the gut microbiome could be an innovative approach to treating stress-induced conditions like depressive disorders.
The Immune System
The immune system is a critical component of the microbiota-gut-brain axis. Microbe-immune interactions are mediated by bacterial components interacting with pattern recognition receptors and antigen-presenting cells as well as the release of microbially-derived metabolites such as short-chain fatty acids (SCFAs) which can then activate both the innate and adaptive immune systems and ultimately influence the activity of the nervous system [49]. Pathological activation of the immune response has been widely implicated in pathophysiology of stress-associated conditions like depressive disorders and is also associated with gut microbial changes [50, 51]. Bailey et al. showed that in mice, intruder-induced social stress resulted in increased levels of the pro-inflammatory cytokines TNF-α and IL-6 in circulation as well as changes in cecal microbiota, both of which were attenuated by prophylactic antibiotic administration prior to stressor presentation [52]. This study demonstrated a critical role for the gut microbiome in inducing inflammation in response to stress. Another study using the CUMS rat model for depression showed that stress could initiate or exacerbate inflammation in the colon, as demonstrated by increased CD-45 positive cells and elevated IFN-γ and IL-6 in the colon. The authors also reported differences in β-diversity of the gut microbiome and changes in phyla-level abundance with a decrease in the Firmicutes to Bacteroidetes ratio, a commonly reported metric of dysbiosis, as measured by 16S ribosomal RNA (rRNA) gene amplicon sequencing analysis of fecal microbiota [53]. In another study, Wong et al. used genetic and pharmacological approaches to negatively regulate caspase-1, which decreases inflammasome function, and showed that mice with reduced caspase-1 activation were more resistant to depressive-like phenotypes following stress exposures. Furthermore, caspase-1 KO mice and WT mice treated with caspase-1 inhibitors showed similar changes in microbiota composition: increases in the relative abundance of Akkermansia spp., Blautia spp., and Lanchnospiraceae. This study thus identified the inflammasome as a potential contributor to regulation of the gut microbiota-immune-brain axis in response stress which could ultimately lead to depression onset [54]. Intriguingly, multiple studies have shown that depressive phenotypes induced by dysbiosis-mediated activation of the immune response can be transferred to stress-naïve recipients via FMT. For instance, a study illustrated that transferring feces from stress-vulnerable rats to germ-free, stress naïve recipients not only recapitulated the depressive-like phenotypes in the recipient rats but also increased the inflammatory state, as evidence by an increase in Iba-1-positive cells (activated microglia), elevated levels of IL-1β in the ventral hippocampus, and increased plasma corticosterone levels [55]. This study demonstrates that dysbiosis of the gut microbiome can independently induce pro-inflammatory processes associated with depression-like phenotypes. While a consensus depression-linked microbial signature in psychiatric diseases remains to be found, the studies reviewed here reveal that gut microbiota can modulate immune activation in response to stress. Future research into functional compositions of the gut microbiome that yield stress resilience versus vulnerability will be key to further elucidating the mechanisms at play as well as identifying potential biomarkers that precede disease onset as well as the development of microbiome-targeted therapies to dampen pathological immune activation and the neuropathology underlying some cases of depression.
The Vagus Nerve
The vagus nerve – the 10th cranial nerve – provides parasympathetic innervation and plays a crucial role in modulating multiple physiological functions including mood, immune responses, digestion, and appetite [56]. Vagal dysfunction can result in obesity [57], inflammation [58], and mood disorders and it is thus a therapeutic target of interest for multiple disorders [56]. Evidence for vagal nerve involvement in neuropsychiatric diseases has largely been demonstrated in therapeutic applications like vagus nerve stimulation (VNS), an FDA-approved therapy in patients aged greater than 12 years with treatment-resistant depression (TRD). Remarkably, while VNS has been found to effectively alleviate depression scores in TRD patients [59, 60] and change the functional connectivity within default mode network in the brain using fMRI that’s associated with decreased depression scores (24-item Hamilton Depression Rating Scale) [61], the mechanism by which VNS exerts its effects on mental health remains unclear.
Anatomical studies of the vagus nerve show that it is composed of 20% efferent (motor) and 80% afferent (sensory) fibers, which together enable bidirectional communication between the brain and peripheral organs [62,63,64], including the gastrointestinal tract. Given that the majority of signaling in the GI tract is mediated by gut microbiota, the vagus nerve also plays a crucial role in communication along the microbiota-gut-brain axis in both health and in psychiatric disease [65]. In a pioneering study, Bravo et al. showed that bilateral subdiaphragmatic vagotomy – surgical means of obliterating vagal signaling beyond the diaphragm – attenuates the anxiolytic effect of Lactobacillus rhamnosus (JB-1) administration to mice exposed to stress via modulation of CNS GABA receptor expression [66]. A similar study that also used vagotomy to disrupt vagal transmission in mouse model for depression induced by exposure to the bacterial outer membrane molecule lipopolysaccharide (LPS), a bacterial toxin, demonstrated that vagotomized mice were protected from developing depressive-like behaviors, gut microbial changes, and systemic inflammation [67]. Furthermore, FMT from nicotinic acetylcholine receptor alpha 7 knockout mice (Chrna7 KO), which have depressive-like phenotypes shown by the forced swim and tail suspension tests, and to antibiotics-treated mice was sufficient to transfer the disease phenotype. An intact vagus nerve was required for the phenotype transfer of FMT, which resulted in systemic inflammation in the prefrontal cortex of the recipient mice [68]. Additionally, antibiotic-treated mice receiving FMT from donor mice exposed to chronic social defeat stress showed depressive-like phenotypes and increased IL-6 levels which was ameliorated in vagotomized recipient mice. Together, these data suggest that the vagus nerve is required for bidirectional communication along the gut-brain axis in depressive disorders [69•, 70]. In an effort to delineate the mechanisms by which targeting the vagal nerve can ameliorate stress phenotypes, a study employed VNS in rats with strong depressive-like behaviors. VNS effectively restored hippocampal 5HT1B receptor and BDNF levels and relieved depressive-like behaviors [71]. Overall, these findings demonstrated that the vagus nerve plays a crucial role in regulating the gut-microbiome-brain axis in psychiatric disorders. Consequently, it is likely that VNS stimulation acts via the gut-brain axis to exert some of its beneficial effects and, furthermore, that modulating the microbiome could serve as a potential therapeutic target for treatment-resistant depression.
The Enteric Nervous System
The enteric nervous system (ENS) is composed of enteric neurons and ganglia spread across the myenteric plexi (control of the gut muscle) and Meissner’s plexi (located beneath the mucus) in the intestinal tract [72]. It innervates the epithelial layer of the gut where the gut microbiome can interact directly with host cells. The ENS can control gut motility independent of central nervous system (CNS) input and can also be modulated by external factors like the immune system or gut microbiome. The ENS serves as a channel of communication along the microbiota-gut-brain axis, relaying signals from the gut lumen to the vagus nerve which ultimately reach the CNS [73•].
The role of the ENS in mediating the stress response has been widely described [73•]. It has been shown in BALB/c mice, which have an intrinsic anxiogenic phenotype, that an intact epithelium and ENS are required for therapeutic efficacy of SSRIs, which significantly increase vagal activity, as evidenced by ex vivo recordings [74]. For in vivo evidence, a study using a chronic unpredictable stress mouse model showed decreased intestinal motility in stressed mice. This study further demonstrated that stress induces transcriptional changes in genes encoding neurotransmitters (dopamine and noradrenaline) as well as ENS markers involved in plasticity and motility (Gdnf and Bdnf) in the proximal colon. In addition, the authors report a significant negative correlation between serotonin (5-HT) and catecholamine levels with neurotrophic factors in the colon, indicating a correlation between stress-induced intestinal motility deficits and ENS activity-related transcriptional changes [75]. Furthermore, a recent study in a chronic restraint stress rodent model for depression combining colonic single-cell and single-nucleus transcriptomics showed that ENS glia promote intestinal inflammation upon exposure to glucocorticoids during chronic stress, which results in an elevated inflammatory response to colitis-inducing insults. The study also demonstrated that in humans (subjects with IBD and undergoing stress vs. no stress), stress leads to decreased gut motility. Finally, transcriptomic studies performed on stressed mice showed a shift in the profile of ENS cell types, many of which reverted to a more immature state, which not only reduced gut motility but also made the host more susceptible to gut inflammation. Thus, the ENS relays stress signals from the brain to the gut, both priming inflammation as well as affecting gut motility [76••].
Interaction between the ENS and microbiota was shown by GF mice studies, in which the ENS morphology deficits in GF mice were rescued by colonization of complex gut microbiota flora [77]. Since the ENS is at the interface between the gut lumen and the CNS, it can be stimulated by bacterial metabolites and then relay the signals to the sympathetic and parasympathetic and autonomic nervous systems [78], which transmit information to the brain. SCFAs, including but not limited to acetate, propionate, and butyrate, are metabolites generated upon microbial fermentation of dietary fibers [79]. Decreased SCFA production correlates with changes in gut microbiome composition in the chronic restraint stress mouse model for depression [80] and also in human patients (recently reviewed in Liu et al., 2023) [81]. Changes in SCFA abundance can reduce gut motility [82], increase gut permeability/disrupt the gut barrier, and increase inflammation via modulation of the ENS [83]. Administration of SCFA in the chronic social defeat mouse model for depression ameliorated anhedonia – the perception of pain in response to normally innocuous stimuli – and normalized intestinal permeability [84]. In addition to SCFAs, synaptic neurotransmission via direct ENS signaling is crucial for GI motility and mood. The activity of 5-HT within the gut-brain axis is largely implicated in psychiatric diseases [85]. Indeed, it has been shown that mice with a mutation in tryptophan hydroxylase 2 (TPH2), the rate-limiting enzyme in 5-HT production, have abnormal ENS development, decreased gut motility, and reduced epithelial growth, phenotypes which can be rescued by administration of slow release 5-HT in adulthood [86]. The inhibitory neurotransmitter GABA also plays a critical role in ENS signaling that modulates mood. In the chronic restraint stress mouse model for depression, application of a modified nano GABA molecule was shown to alleviate the depressive phenotype by targeting the enteric nervous system [87]. Furthermore, a study applying GABA-producing microbes in mice fed with high-fat diet showed that the intervention reduced the depressive phenotypes, corrected corticosterone and increased GABA levels in the intestine [88]. The data from these associative studies warrants further investigation into the impact of host-microbe interactions regulating ENS function and its potential role in the pathophysiology of depression.
Toward Realizing the Therapeutic Potential of the Gut Microbiome in Mental Health Management
The etiology of neuropsychiatric disorders like major depressive disorder is complex, with genetic and environmental factors, as well as their interactions, contributing to disease risk and onset, making it challenging to address clinically. Current therapeutic strategies targeting synaptic transmission have shown limited effects, with one third of depression patients remaining resistant to current pharmacological treatments. Emerging research demonstrating contributions of the gut-microbiome-brain axis to depressive disorders is beginning to reveal the diagnostic and therapeutic potential of the gut microbiome for patients with depression.
Probiotics are a common mode of intervention for targeting gut microbiome in psychiatric diseases [89]. In the rat maternal separation model for depression, administration of Bifidobacterium infantis was shown to alleviate depressive-like phenotypes [90]. Another study in the CUMS mouse model showed that treatment with Limosilactobacillus reuteri was sufficient to improve despair behavior and metabolic alterations via inhibiting the kynurenine pathway [91]. A meta-analysis of probiotics and prebiotics in clinical trials with depression patients showed that there is a positive effect of probiotics but not prebiotics – nutrients that promote the expansion of specific microbial species – in mitigating depressive symptoms [89]. Certain probiotic strains have also shown beneficial effects as add-on treatment in patients with depression. In a study, patients received multi-strain probiotics (Vivomixx, which is a commercially available probiotic cocktail comprised of 8 bacterial strains) while maintaining their normal treatment regimen for 4 weeks, after which the severity of depression was measured using the HAM-D score. The probiotic intervention increased abundance of genus Lactobacillus in the gut, which was negatively correlated with HAM-D score immediately post-intervention and at a 4-week follow-up [92]. This study suggests that regardless of the polypharmacy of patient’s medication, add-on treatment of probiotics has the potential to further ameliorate depression symptoms. While the heterogeneity of patient compliance to the probiotic treatments can make it difficult to assess the long-term effects of probiotics, preclinical and clinical evidence shows potential for the use of probiotics as treatment in relieving depression.
Beyond probiotics, an alternative treatment strategy for targeting the gut microbiome is via FMT, the transfer of fecal microbiota from healthy donor subjects to patients. The first study to apply FMT as a possible antidepressant treatment was reported in 2019. A 79-year-old woman who suffered from depression and GI symptoms, and for whom none of the prescribed medicine alleviated her symptoms, received an FMT from her healthy grandson. The patient’s depression score decreased from 21 to 4, as measured by the PHQ–9 scale. The patient had also suffered from chronic constipation, which was likewise decreased at 6 months post-FMT [93]. Following the previous case reported in 2019, Doll et al. conducted a randomized clinical trial using FMT as adjuvant therapy for two female treatment-resistant Caucasian depression patients who also suffered from constipation. The patients were administered frozen FMT-capsules daily and were followed up after 4 weeks. Consistent with the previous study, the patients in this small clinical trial showed decreased HAM-D scores and improved GI symptoms after 4 weeks of treatment. Furthermore, 16S rRNA gene amplicon sequencing revealed a significant increase in species evenness and a concomitant decrease in species diversity following the FMT [94]. Despite the low number of cases reporting use of FMT for treating depressive disorders and the absence of robust controls, the existing clinical results are consistent with preclinical studies in rodent models of depression, including work in humanized mice in which patient microbiomes were transplanted into germ-free mice. Notably, the humanized mice showed promising results in successfully recapitulating patient phenotypes in laboratory assays for depressive-like phenotypes [34, 38, 95]. Conversely, FMT from healthy donor animals or human subjects has been shown to alleviate depressive-like phenotypes rodents after stress induction [96,97,98]. Therefore, FMT could serve as a great platform to develop more precise microbial treatments for patients with depression.
Importantly, gut microbiota form unique, defined communities in each GI subregion: the small intestine (subdivided into duodenum, ileum and jejunum), large intestine, and colon. Each region is different in terms of acidity, villi length, intestinal immunity, and lumen nutritional metabolite content [99,100,101]. Therefore, gut microbiota distribute differently along the GI tract and perform specialized functions in their established niche. While fecal microbiota transplants are the gold standard for demonstrating causality of microbial actors in host phenotypes and show promising results for treatment-resistant depression, it is possible that whole intestinal microbiota transplant (WIMT) – which could be made possible by negative pressure capsules designed to sample discrete gut compartments in a pH-dependent manner, analogous to sampling made possible by “smart capsules” [102] – comprised of the diverse array of microbes present within each GI sub-region would be a better approach. Thus, it is crucial to investigate differences in efficacy between FMT versus WIMT and to determine whether WIMT could potentially elicit a better therapeutic outcome in treating psychiatric disorders in the future. Overall, modulation of the gut microbiome holds great promise for relieving depressive and GI symptoms in patients with depression (Fig. 1). However, further research into the microbes, metabolites, and means of treatment is warranted to improve treatment safety and effectiveness.
Bidirectional microbiota-gut-brain pathways could contribute to susceptibility and disease onset in depression, they also represent a promising therapeutic target. Major communication pathways along the microbiota-gut-brain axis include: 1) Hypothalamic–pituitary–adrenal axis (HPA axis), a neuroendrocrine pathway along which the hypothalamus releases corticotropin-releasing factor that stimulates the pituitary gland to produce adrenocorticotropic hormone and leading to cortisol secretion from the adrenal gland into circulation. Activation of the HPA axis results in local and systemic inflammation that could alter the gut microbiota, while the gut microbiota can reciprocally influence the activity of the HPA axis. 2) Microbiome-immune interactions can likewise affect host brain function and physiology. 3) The enteric nervous system (ENS) can respond to SCFAs or neurotransmitters (NTMR) and their precursors produced by gut bacteria to modulate gut physiology and relay signals to the vagus nerve that are then transmitted to the brain. 4) The vagus nerve can transduce sensory signals from the gut to the brain via direct synaptic connections with neuropod cells, neuroendocrine cells located within the gut epithelium, and can also be activated by neurotransmitters produced by bacteria, including GABA and serotonin. Conversely, the vagus nerve can also modulate gut physiology via afferent signals from the brain. Therapeutic targeting of the gut microbiota could be achieved by precision probiotic administration or via microbial transplant, which has been shown to alleviate depressive-like phenotypes in preclinical studies and in limited clinical interventions, reviewed here. HPA axis, hypothalamus–pituitary–adrenal axis; ENS: enteric nervous system; SCFA: short-chain fatty acid; NTMR: neurotransmitter; FMT, fecal microbiota transplant
Considering findings supporting a role for dysbiosis of the gut microbiome in depression, antibiotics have also been explored as a possible treatment strategy for depression. Minocycline is one of the most common antibiotics used in patient treatments [103, 104]; however, it remains in limited use, being reserved for specific populations of depression patients like those with baseline low-grade inflammation [105]. That said, prolonged administration of antibiotics could decrease diversity of the gut microbiome, which could unintentionally increase risk for disease progression. Thus, antibiotic administration is not a standard treatment for patients with depression and is unlikely to emerge as a new treatment frontier.
Conclusion and Future Directions
Effective treatments for patients with major depressive disorder (MDD) is a global unmet need. Unfortunately, the complex interplay of genetics and environmental factors in MDD makes diagnosis, treatment, and prevention across the heterogeneous patient population difficult. While previous research has focused on brain-specific manipulations, commonly by using drugs that modulate synaptic transmission, this “one size fits all” approach has not proven particularly successful. In recent decades, research has revealed sizable contributions of the microbiome-gut-brain axis to mental health and the potential for microbiome-targeted therapies to treat neuropsychiatric disorders. In this review, we examined recent literature highlighting the relationship between the gut microbiome and neuropsychiatric disorders, with an emphasis on depressive disorders. We also explored the different pathways that mediate bidirectional communication along the microbiome-gut-brain axis: the HPA axis, immune system, vagus nerve, and enteric nervous system. Signals initiated in either the brain or the gut can induce downstream response pathways that result in physiological changes, enteroendocrine secretion, gut motility changes, and inflammation responses that can ultimately influence brain function and behavior. While investigation into the mechanisms by which gut microbes influence risk for neuropsychiatric disorders, their onset, and the therapeutic utility of targeting the gut microbiome to relieve GI and behavioral symptoms in depression remains in its infancy, a growing body of literature clearly indicates that host-microbe interactions exert profound effects on host physiology, including on brain function, and identify the gut-brain axis as a clear target for therapeutic intervention. Now, more research is needed to determine whether the gut microbiome could serve as a biomarker for developing personalized medicine for treatment of depression and other psychiatric disorders.
Data Availability
No datasets were generated or analysed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474(11):1823–36.
Ganal-Vonarburg SC, Hornef MW, Macpherson AJ. Microbial-host molecular exchange and its functional consequences in early mammalian life. Science (New York, NY). 2020;368(6491):604–7.
Kundu P, Blacher E, Elinav E, Pettersson S. Our gut microbiome: the evolving inner self. Cell. 2017;171(7):1481–93.
Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693(Pt B):128–33.
Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–84.
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63.
Zinocker MK, Lindseth IA. The Western diet-microbiome-host interaction and its role in metabolic disease. Nutrients. 2018;10(3).
Perler BK, Friedman ES, Wu GD. The role of the gut microbiota in the relationship between diet and human health. Annu Rev Physiol. 2023;85:449–68.
Leclercq S, Mian FM, Stanisz AM, Bindels LB, Cambier E, Ben-Amram H, et al. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat Commun. 2017;8:15062.
Desbonnet L, Clarke G, Traplin A, O’Sullivan O, Crispie F, Moloney RD, et al. Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav Immun. 2015;48:165–73.
Lopera-Maya EA, Kurilshikov A, van der Graaf A, Hu S, Andreu-Sanchez S, Chen L, et al. Effect of host genetics on the gut microbiome in 7,738 participants of the Dutch Microbiome Project. Nat Genet. 2022;54(2):143–51.
Qin Y, Havulinna AS, Liu Y, Jousilahti P, Ritchie SC, Tokolyi A, et al. Combined effects of host genetics and diet on human gut microbiota and incident disease in a single population cohort. Nat Genet. 2022;54(2):134–42.
Vuong HE, Yano JM, Fung TC, Hsiao EY. The microbiome and host behavior. Annu Rev Neurosci. 2017;40(1):21–49.
Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877–2013.
Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa-Mattioli M. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell. 2016;165(7):1762–75.
Sgritta M, Dooling SW, Buffington SA, Momin EN, Francis MB, Britton RA, et al. Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron. 2019;101(2):246-59.e6.
Buffington SA, Dooling SW, Sgritta M, Noecker C, Murillo OD, Felice DF, et al. Dissecting the contribution of host genetics and the microbiome in complex behaviors. Cell. 2021;184(7):1740-56 e16.
Curtis K, Stewart CJ, Robinson M, Molfese DL, Gosnell SN, Kosten TR, et al. Insular resting state functional connectivity is associated with gut microbiota diversity. Eur J Neurosci. 2019;50(3):2446–52.
Lakosa A, Rahimian A, Tomasi F, Marti F, Reynolds LM, Tochon L, et al. Impact of the gut microbiome on nicotine’s motivational effects and glial cells in the ventral tegmental area in male mice. Neuropsychopharmacology. 2023;48(6):963–74.
Nikolova VL, Smith MRB, Hall LJ, Cleare AJ, Stone JM, Young AH. Perturbations in gut microbiota composition in psychiatric disorders: a review and meta-analysis. JAMA Psychiat. 2021;78(12):1343–54.
Sanada K, Nakajima S, Kurokawa S, Barcelo-Soler A, Ikuse D, Hirata A, et al. Gut microbiota and major depressive disorder: a systematic review and meta-analysis. J Affect Disord. 2020;266:1–13.
Moreno-Agostino D, Wu YT, Daskalopoulou C, Hasan MT, Huisman M, Prina M. Global trends in the prevalence and incidence of depression:a systematic review and meta-analysis. J Affect Disord. 2021;281:235–43.
WHO. Depression and other common mental disorders: global health estimates. 2017. Retrieved from https://policycommons.net/artifacts/546082/depression-and-other-common-mental-disorders/
Santomauro DF, Mantilla Herrera AM, Shadid J, Zheng P, Ashbaugh C, Pigott DM, et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398(10312):1700–12.
Mazza MG, Palladini M, Villa G, Agnoletto E, Harrington Y, Vai B, et al. Prevalence of depression in SARS-CoV-2 infected patients: an umbrella review of meta-analyses. Gen Hosp Psychiatry. 2023;80:17–25.
Blackett JW, Sun Y, Purpura L, Margolis KG, Elkind MSV, O’Byrne S, et al. Decreased gut microbiome tryptophan metabolism and serotonergic signaling in patients with persistent mental health and gastrointestinal symptoms after COVID-19. Clin Transl Gastroenterol. 2022;13(10):e00524.
Ramakrishnan RK, Kashour T, Hamid Q, Halwani R, Tleyjeh IM. Unraveling the mystery surrounding post-acute sequelae of COVID-19. Front Immunol. 2021;12:686029.
Marwaha S, Palmer E, Suppes T, Cons E, Young AH, Upthegrove R. Novel and emerging treatments for major depression. Lancet. 2023;401(10371):141–53.
Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Focus (Am Psychiatr Publ). 2018;16(4):420–9.
Aguado A, García Del Álamo M. Gastrointestinal comorbidity and symptoms associated with depression in patients aged over 60 years. Semergen. 2020;46(1):27–32.
Huang J, Cai Y, Su Y, Zhang M, Shi Y, Zhu N, et al. Gastrointestinal symptoms during depressive episodes in 3256 patients with major depressive disorders: findings from the NSSD. J Affect Disord. 2021;286:27–32.
Haj Kheder S, Heller J, Bär JK, Wutzler A, Menge BA, Juckel G. Autonomic dysfunction of gastric motility in major depression. J Affect Disord. 2018;226:196–202.
Zhang B, Wang HE, Bai YM, Tsai SJ, Su TP, Chen TJ, et al. Bidirectional association between inflammatory bowel disease and depression among patients and their unaffected siblings. J Gastroenterol Hepatol. 2022;37(7):1307–15.
Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry. 2016;21(6):786–96.
Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–94.
Madan A, Thompson D, Fowler JC, Ajami NJ, Salas R, Frueh BC, et al. The gut microbiota is associated with psychiatric symptom severity and treatment outcome among individuals with serious mental illness. J Affect Disord. 2020;264:98–106.
Radjabzadeh D, Bosch JA, Uitterlinden AG, Zwinderman AH, Ikram MA, van Meurs JBJ, et al. Gut microbiome-wide association study of depressive symptoms. Nat Commun. 2022;13(1):7128.
Kelly JR, Borre Y, O’ Brien C, Patterson E, El Aidy S, Deane J, et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;2016(82):109–18.
Jianguo L, Xueyang J, Cui W, Changxin W, Xuemei Q. Altered gut metabolome contributes to depression-like behaviors in rats exposed to chronic unpredictable mild stress. Transl Psychiatry. 2019;9(1):40-.
Bellavance MA, Rivest S. The HPA - immune axis and the immunomodulatory actions of glucocorticoids in the brain. Front Immunol. 2014;5:136.
Malhi GS, Mann JJ. Depression. Lancet. 2018;392(10161):2299–312.
Juruena MF. Early-life stress and HPA axis trigger recurrent adulthood depression. Epilepsy Behav. 2014;38:148–59.
Juruena MF, Bocharova M, Agustini B, Young AH. Atypical depression and non-atypical depression: is HPA axis function a biomarker? A systematic review. J Affect Disord. 2018;233:45–67.
Wu WL, Adame MD, Liou CW, Barlow JT, Lai TT, Sharon G, et al. Microbiota regulate social behaviour via stress response neurons in the brain. Nature. 2021;595(7867):409–14.
Luo Y, Zeng B, Zeng L, Du X, Li B, Huo R, et al. Gut microbiota regulates mouse behaviors through glucocorticoid receptor pathway genes in the hippocampus. Transl Psychiatry. 2018;8(1):187.
O’Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho A-M, Quigley EMM, et al. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiat. 2009;65(3):263–7.
Moya-Perez A, Perez-Villalba A, Benitez-Paez A, Campillo I, Sanz Y. Bifidobacterium CECT 7765 modulates early stress-induced immune, neuroendocrine and behavioral alterations in mice. Brain Behav Immun. 2017;65:43–56.
Tian P, O’Riordan KJ, Lee YK, Wang G, Zhao J, Zhang H, et al. Towards a psychobiotic therapy for depression: bifidobacterium breve CCFM1025 reverses chronic stress-induced depressive symptoms and gut microbial abnormalities in mice. Neurobiol Stress. 2020;12:100216.
Jacobson A, Yang D, Vella M, Chiu IM. The intestinal neuro-immune axis: crosstalk between neurons, immune cells, and microbes. Mucosal Immunol. 2021;14(3):555–65.
Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16(1):22–34.
Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20(2):145–55.
Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun. 2011;25(3):397–407.
Wei L, Li Y, Tang W, Sun Q, Chen L, Wang X, et al. Chronic unpredictable mild stress in rats induces colonic inflammation. Front Physiol. 2019;10:1228.
Wong ML, Inserra A, Lewis MD, Mastronardi CA, Leong L, Choo J, et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol Psychiatry. 2016;21(6):797–805.
Pearson-Leary J, Zhao C, Bittinger K, Eacret D, Luz S, Vigderman AS, et al. The gut microbiome regulates the increases in depressive-type behaviors and in inflammatory processes in the ventral hippocampus of stress vulnerable rats. Mol Psychiatry. 2019;1068–1079.
Browning KN, Verheijden S, Boeckxstaens GE. The vagus nerve in appetite regulation, mood, and intestinal inflammation. Gastroenterology. 2017;152(4):730–44.
de Lartigue G. Role of the vagus nerve in the development and treatment of diet-induced obesity. J Physiol. 2016;594(20):5791–815.
Bonaz B, Sinniger V, Pellissier S. Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. J Physiol. 2016;594(20):5781–90.
Rong P, Liu J, Wang L, Liu R, Fang J, Zhao J, et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: a nonrandomized controlled pilot study. J Affect Disord. 2016;195:172–9.
Carreno FR, Frazer A. Vagal nerve stimulation for treatment-resistant depression. Neurotherapeutics. 2017;14(3):716–27.
Fang J, Rong P, Hong Y, Fan Y, Liu J, Wang H, et al. Transcutaneous vagus nerve stimulation modulates default mode network in major depressive disorder. Biol Psychiatry. 2016;79(4):266–73.
Prechtl JC, Powley TL. The fiber composition of the abdominal vagus of the rat. Anat Embryol. 1990;181(2):101–15.
Paintal AS. Vagal sensory receptors and their reflex effects. Physiol Rev. 1973;53(1):159–227.
Breit S, Kupferberg A, Rogler G, Hasler G. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front Psychiatry. 2018;9:44.
Bonaz B, Bazin T, Pellissier S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci. 2018;12(FEB):1–9.
Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci. 2011;108(38):16050–5.
Zhang J, Ma L, Chang L, Pu Y, Qu Y, Hashimoto K. A key role of the subdiaphragmatic vagus nerve in the depression-like phenotype and abnormal composition of gut microbiota in mice after lipopolysaccharide administration. Transl Psychiatry. 2020;10(1):186.
Pu Y, Tan Y, Qu Y, Chang L, Wang S, Wei Y, et al. A role of the subdiaphragmatic vagus nerve in depression-like phenotypes in mice after fecal microbiota transplantation from Chrna7 knock-out mice with depression-like phenotypes. Brain Behav Immun. 2021;94:318–26.
• Wang S, Ishima T, Qu Y, Shan J, Chang L, Wei Y, et al. Ingestion of Faecalibaculum rodentium causes depression-like phenotypes in resilient Ephx2 knock-out mice: a role of brain-gut-microbiota axis via the subdiaphragmatic vagus nerve. J Affect Disord. 2021;292:565–73. This study demonstrated that probiotics study showing modulation of gut microbiota can affect depessive-like phenotypes.
Wang S, Ishima T, Zhang J, Qu Y, Chang L, Pu Y, et al. Ingestion of Lactobacillus intestinalis and Lactobacillus reuteri causes depression- and anhedonia-like phenotypes in antibiotic-treated mice via the vagus nerve. J Neuroinflammation. 2020;17(1):241.
Shin HC, Jo BG, Lee C-Y, Lee K-W, Namgung U. Hippocampal activation of 5-HT1B receptors and BDNF production by vagus nerve stimulation in rats under chronic restraint stress. Eur J Neurosci. 2019;50(1):1820–30.
Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9(5):286–94.
• Margolis KG, Cryan JF, Mayer EA. The microbiota-gut-brain axis: from motility to mood. Gastroenterology. 2021;160(5):1486–501. This review elaborated on how enteric nervous system modulate the gut microbiota in mood disorders.
McVey Neufeld KA, Bienenstock J, Bharwani A, Champagne-Jorgensen K, Mao Y, West C, et al. Oral selective serotonin reuptake inhibitors activate vagus nerve dependent gut-brain signalling. Sci Rep. 2019;9(1):14290.
Lobo B, Tramullas M, Finger BC, Lomasney KW, Beltran C, Clarke G, et al. The stressed gut: region-specific immune and neuroplasticity changes in response to chronic psychosocial stress. J Neurogastroenterol Motil. 2023;29(1):72–84.
•• Schneider KM, Blank N, Alvarez Y, Thum K, Lundgren P, Litichevskiy L, et al. The enteric nervous system relays psychological stress to intestinal inflammation. Cell. 2023;186(13):2823-38.e20. This study provides evidence of how ENS communicates with CNS via the immune system and glia activation.
Collins J, Borojevic R, Verdu EF, Huizinga JD, Ratcliffe EM. Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterol Motil. 2014;26(1):98–107.
Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28(2):203–9.
Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332–45.
Wu M, Tian T, Mao Q, Zou T, Zhou C-j, Xie J, et al. Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Transl Psychiatry. 2020;10(1):350.
Liu L, Wang H, Chen X, Zhang Y, Zhang H, Xie P. Gut microbiota and its metabolites in depression: from pathogenesis to treatment. EBioMedicine. 2023;90:104527.
Soret R, Chevalier J, De Coppet P, Poupeau G, Derkinderen P, Segain JP, et al. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology. 2010;138(5):1772–82.
O’Riordan KJ, Collins MK, Moloney GM, Knox EG, Aburto MR, Fulling C, et al. Short chain fatty acids: microbial metabolites for gut-brain axis signalling. Mol Cell Endocrinol. 2022;546:111572.
van de Wouw M, Boehme M, Lyte JM, Wiley N, Strain C, O’Sullivan O, et al. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations. J Physiol. 2018;596(20):4923–44.
Del Colle A, Israelyan N, Gross MK. Novel aspects of enteric serotonergic signaling in health and brain-gut disease. Am J Physiol Gastrointest Liver Physiol. 2020;318(1):G130–43.
Israelyan N, Del Colle A, Li Z, Park Y, Xing A, Jacobsen JPR, et al. Effects of serotonin and slow-release 5-hydroxytryptophan on gastrointestinal motility in a mouse model of depression. Gastroenterology. 2019;157(2):507-21.e4.
Tri BD, Shashni B, Matsui H, Nagasaki Y. Designing poly(gamma-aminobutyric acid)-based nanoparticles for the treatment of major depressive disorders. J Control Release. 2023;360:110–21.
Patterson E, Ryan PM, Wiley N, Carafa I, Sherwin E, Moloney G, et al. Gamma-aminobutyric acid-producing lactobacilli positively affect metabolism and depressive-like behaviour in a mouse model of metabolic syndrome. Sci Rep. 2019;9(1):16323.
Liu RT, Walsh RFL, Sheehan AE. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev. 2019;102:13–23.
Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170(4):1179–88.
Marin IA, Goertz JE, Ren T, Rich SS, Onengut-Gumuscu S, Farber E, et al. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci Rep. 2017;7:43859.
Schaub AC, Schneider E, Vazquez-Castellanos JF, Schweinfurth N, Kettelhack C, Doll JPK, et al. Clinical, gut microbial and neural effects of a probiotic add-on therapy in depressed patients: a randomized controlled trial. Transl Psychiatry. 2022;12(1):227.
Cai T, Shi X, Yuan LZ, Tang D, Wang F. Fecal microbiota transplantation in an elderly patient with mental depression. Int Psychogeriatr. 2019;31(10):1525–6.
Doll JPK, Vazquez-Castellanos JF, Schaub AC, Schweinfurth N, Kettelhack C, Schneider E, et al. Fecal Microbiota Transplantation (FMT) as an adjunctive therapy for depression-case report. Front Psychiatry. 2022;13:815422.
Sharon G, Cruz NJ, Kang D-W, Gandal MJ, Wang B, Kim Y-M, et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell. 2019;177(6):1600-18.e17.
Hu B, Das P, Lv X, Shi M, Aa J, Wang K, et al. Effects of “healthy” fecal microbiota transplantation against the deterioration of depression in fawn-hooded rats. mSystems. 2022;7(3):e0021822.
Rao J, Xie R, Lin L, Jiang J, Du L, Zeng X, et al. Fecal microbiota transplantation ameliorates gut microbiota imbalance and intestinal barrier damage in rats with stress-induced depressive-like behavior. Eur J Neurosci. 2021;53(11):3598–611.
Settanni CR, Ianiro G, Bibbò S, Cammarota G, Gasbarrini A. Gut microbiota alteration and modulation in psychiatric disorders: current evidence on fecal microbiota transplantation. Prog Neuropsychopharmacol Biol Psychiatry. 2021;109:110258.
Martinez-Guryn K, Leone V, Chang EB. Regional diversity of the gastrointestinal microbiome. Cell Host Microbe. 2019;26(3):314–24.
Hollister EB, Gao C, Versalovic J. Compositional and functional features of the gastrointestinal microbiome and their effects on human health. Gastroenterology. 2014;146(6):1449–58.
Gu S, Chen D, Zhang JN, Lv X, Wang K, Duan LP, et al. Bacterial community mapping of the mouse gastrointestinal tract. PLoS ONE. 2013;8(10):e74957.
Rehan M, Al-Bahadly I, Thomas DG, Young W, Cheng LK, Avci E. Smart capsules for sensing and sampling the gut: status, challenges and prospects. Gut. 2023;73(1):186–202.
Dinan K, Dinan T. Antibiotics and mental health: the good, the bad and the ugly. J Intern Med. 2022;292(6):858–69.
Hao W-Z, Li X-J, Zhang P-W, Chen J-X. A review of antibiotics, depression, and the gut microbiome. Psychiatry Res. 2020;284:112691.
Nettis MA, Lombardo G, Hastings C, Zajkowska Z, Mariani N, Nikkheslat N, et al. Augmentation therapy with minocycline in treatment-resistant depression patients with low-grade peripheral inflammation: results from a double-blind randomised clinical trial. Neuropsychopharmacology. 2021;46(5):939–48.
Funding
This study was supported the Veterans Health Administration (grant VHA I01CX001937 to RS) and The Robert & Janice McNair Foundation, NIH NICHD R01 HD109095, NIH NICHD R01 HD109780, and The Brain & Behavior Research Foundation, NARSAD Young Investigator Award 28918 to SAB. This study is in part the result of work supported with resources and the use of facilities at the Michael E. DeBakey Veterans Affairs Medical Center.
Author information
Authors and Affiliations
Contributions
I-C W, SAB, RS wrote the review.
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
The authors do not report new studies/experiments with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Genetic Epidemiology
Rights and permissions
About this article
Cite this article
Wang, IC., Buffington, S.A. & Salas, R. Microbiota-Gut-Brain Axis in Psychiatry: Focus on Depressive Disorders. Curr Epidemiol Rep 11, 222–232 (2024). https://doi.org/10.1007/s40471-024-00349-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40471-024-00349-z