Abstract
Purpose of Review
Many of the diseases and dysfunctions described in the paradigm of the developmental origins of health and disease have been studied in relation to prenatal nutrition or environmental toxicant exposures. Here, we selectively review the current research on four exposures—two nutritional and two environmental—that have recently emerged as prenatal risk factors for long-term health outcomes.
Recent Findings
Recent studies have provided strong evidence that prenatal exposure to (1) excessive intake of sugar-sweetened beverages, (2) unhealthy dietary patterns, (3) perfluoroalkyl substances, and (4) fine particulate matter may increase risk of adverse health outcomes, such as obesity, cardiometabolic dysfunction, and allergy/asthma.
Summary
Emerging prenatal nutritional factors and environmental toxicants influence offspring long-term health. More work is needed to identify the role of paternal exposures and maternal exposures during the preconception period and to further elucidate causality through intervention studies. The ubiquity of these emerging nutritional and environmental exposures makes this area of inquiry of considerable public health importance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
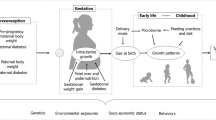
The developmental origins of health and disease (DOHaD) hypothesis posits that chronic diseases, in particular obesity, diabetes, and cardiovascular disease, have their origins during early development [1, 2]. This notion was most famously promoted by David Barker in the 1980s who through ecological correlations of infant mortality [3] and early life size [4] with heart disease mortality provided some of the earliest evidence that adverse early life conditions could have long-term effects on diseases previously thought to be caused by differences in genetics and adult behaviors. The DOHaD concept that exposure to an adverse early life environment has widespread consequences for later health now encompasses a broader scope of developmental cues extending beyond the in utero period [5].
Models to explain the DOHaD hypothesis have largely centered on two developmental pathways—the first is the evolutionary, adaptive programming of a disease phenotype resulting from a mismatch of in utero and postnatal environments (i.e., predictive adaptive response, as proposed by Gluckman and Hanson [6]). The second pathway is through direct exposure of the fetus to environmental factors that increase the risk of developing chronic diseases later in life [7]. These exposures are largely anthropogenic and “novel” (in evolutionary terms). Therefore, the physiological responses to these exposures have yet to be subjected to selection pressure [5, 7] and could disrupt normal signaling pathways during development and even lead to adverse effects evident at birth, such as birth defects and poor fetal growth. In many instances, however, the initial functional changes after exposure may be subtle, with no phenotype changes at birth, but increased risk of physiological dysfunction and/or disease later in life [5]. Two broad categories of these anthropogenic exposures are the subject of this review: nutritional factors and environmental toxicants. Evidence also suggests that social and built environments may influence many of the same outcomes, but those exposures are beyond the scope of the current review.
Extensive previous literature has demonstrated that nutritional factors in utero [either global undernutrition (e.g., caloric restriction in rodents [8], natural experiments of famine in humans [9]) or overnutrition (e.g., maternal obesity [10], excessive gestational weight gain [11], and gestational hyperglycemia [12])] influence health later in life, predisposing to conditions such as obesity, coronary heart disease, type 2 diabetes, and respiratory diseases. Similarly, several animal and epidemiological studies have shown in utero exposure to ubiquitous environmental chemicals present in everyday household items, such as plastics, clothing, furniture, and pesticides/herbicides, to be associated with a myriad of health consequences that manifest across an individual’s lifespan and are potentially transmitted to future generations [13].
In this review, we focus on four specific prenatal exposures—two nutritional and two environmental—as examples of emerging exposures within the DOHaD field. We discuss the literature surrounding maternal prenatal exposure to (1) excessive sugar-sweetened beverages, (2) unhealthy dietary pattern, (3) fine particulate matter, and (4) perfluoroalkyl substances. A large body of recent literature investigates associations between each of these exposures and later health, and the ubiquity of these exposures in humans makes them of considerable public health importance. In this selective review, we describe the recent literature surrounding these emerging exposures during pregnancy and their documented effects on later disease risk in the offspring.
Prenatal Nutritional Factors as Emerging Risk Factors for Later Disease
Maternal Sugar-Sweetened Beverage Intake During Pregnancy
Consumption of free sugars (either in the form of beverages or in foods) is high in many parts of the world and suggests a poor dietary quality [14]. The calories provided by consumption of free sugars have little nutritional value, which increases total energy intake leading to unhealthy weight gain [15]. Animal experiments have provided a proof-of-principle that free sugar consumption during pregnancy can lead to increased adipogenesis and cardiometabolic dysfunction in offspring [16]. These experiments, however, often study dietary sugar intake greater than what is typically consumed by humans and therefore may not directly translate to humans.
Previous population-based meta-analyses have shown that intake of sugar-sweetened beverages (SSB) during childhood or adulthood increases risks of obesity, type 2 diabetes, and metabolic syndrome [17, 18]. There is emerging evidence that prenatal intake of SSBs may influence intrauterine programming to increase obesity risk in childhood, after accounting for socio-demographics, overall dietary quality, and other obesity risk factors. Pre-birth cohorts Project Viva (USA) and Generation R (Netherlands) have reported prenatal SSB intake to be associated with higher offspring body mass index (BMI) z-scores, fat mass index, skinfold thicknesses, and waist circumference during mid-childhood (~ 6–7 years) [19••, 20]. For example, in Project Viva, for each additional maternal serving per week of SSB during pregnancy, children had 0.07 SD units (95% CI − 0.01, 0.15) higher BMI z-score. For comparison, in the same cohort, the effect estimates for traditional prenatal exposures of maternal pre-pregnancy BMI (per 5 kg/m2 increase) and gestational weight gain (per 5 kg increase) with mid-childhood BMI z-scores in the same cohort were 0.27 SD units (0.21, 0.32) and 0.11 SD units (0.06, 0.17) respectively [21].
Greater childhood adiposity has been observed not only in relation to prenatal intake of SSBs, but also to beverages with artificial sweeteners (ASB), which were presumably thought to be a healthier alternative to SSBs due to lower caloric content. Recent findings from the Canadian Healthy Infant Longitudinal Development (CHILD) Study demonstrate that daily consumption of ASBs during pregnancy (vs. no consumption) was associated with higher offspring BMI z-score and risk of overweight at 1 year of age [22••]. Similar observations were reported in the Danish National Birth Cohort, where daily ASB intake during pregnancy (vs. no consumption) was associated with higher risk of offspring large-for-gestational age at birth, higher BMI z-scores, and risk of overweight/obesity at 7 years [23]. It has been proposed that prenatal exposure to ASBs may exacerbate glucose intolerance through changes in the gut microbiome [24], which may in turn predispose offspring to obesity and metabolic disorders in later life.
Consumption of SSBs during pregnancy has also been related to other adverse outcomes during childhood, such as respiratory diseases. Maslova et al. reported that maternal intake of ≥ 1 soft drink per day during pregnancy was associated with increased odds of asthma in children [25]. More recently, Wright et al. reported that higher maternal pregnancy intake of SSBs was associated with greater odds of asthma during mid-childhood [26••]. Experimental models have suggested that these observations could be explained via adiposity-independent mechanisms, through reduced nitric oxide-related bronchodilation and increased oxo-nitrosative stress [27].
Prenatal Dietary Patterns
Much of the work surrounding the role of maternal diet during pregnancy on health outcomes in offspring has focused on individual nutrients [28], while a handful of studies have also examined foods or food groups such as fish (rich in n-3 fatty acids) [29] or dairy products (rich in calcium and vitamin D) [30]. While these studies may provide insights on causal mechanisms [28], the human diet does not consist solely of individual nutrients—rather, the meals we eat consist of a variety of nutrients that are likely to interact [31]. Over the past decade, research in nutritional epidemiology has begun to focus on dietary patterns as opposed to individual nutrients or foods, to account for interactions of nutrients within the diet. These patterns can be determined a priori using existing dietary guidelines such as the Healthy Eating Index (HEI) [32], or a posteriori using data-driven statistical techniques such as principal components or latent class analysis [31].
Emerging evidence suggests that prenatal dietary patterns may be associated with future disease risk, independent of confounders such as maternal socio-demographics, overall energy intake, and BMI. Findings from various birth cohorts such as the Healthy Start Study, Growing Up in Singapore Towards healthy Outcomes (GUSTO) Study, and Growing Up Today Study (GUTS) reported that greater adherence to a “healthy diet” during pregnancy (derived a priori using HEI) was associated with lower neonatal adiposity (BMI, skinfold thicknesses, percentage body fat) and lower obesity risk during mid-childhood (~ 9–14 years) [33, 34••, 35]. Healthy dietary patterns derived a posteriori have shown similar observations, for example, greater adherence to the “vegetables and fruits” dietary pattern during pregnancy was associated with lower BMI, fat mass index, risk of overweight [36•, 37, 38], and blood pressure during childhood [39]. More recently, birth cohorts have investigated maternal adherence to the Mediterranean diet, characterized by a high intake of fruits, vegetables, legumes, nuts, and whole grain products, already known to have a protective role against obesity, type 2 diabetes mellitus, and metabolic syndrome in adults [40, 41]. Birth cohorts in Spain [42], the USA [43•], Greece [43•], and Iran [44] demonstrated that greater adherence to the Mediterranean diet during pregnancy was associated with lower BMI z-score, waist circumference, waist-to-height ratio, skinfold thicknesses, and blood pressure in offspring during early and mid-childhood (~ 4–7 years). On the other hand, adherence to a “pro-inflammatory” dietary pattern during pregnancy was associated with lower fetal growth, higher BMI, waist circumference, and risk of overweight during childhood [45•, 46•].
Recent evidence also points to the role of prenatal dietary patterns on respiratory and allergic outcomes during childhood. Adherence to the Mediterranean diet during pregnancy has been associated with a lower risk of asthma, allergic sensitization, and allergic rhinitis during childhood [47], while a “fast food” dietary pattern during pregnancy has been associated with higher risk of asthma during early childhood (~ 3.5 years) [48]. Possible mechanisms explaining these observations include the transfer of dietary and environmental antigens from mother-to-child through human breast milk during breastfeeding [47].
Prenatal Exposure to Environmental Toxicants as Emerging Risk Factors for Later Disease
Perfluoroalkyl Substances
Perfluoroalkyl substances (PFASs) are synthetic fluorinated compounds used in manufacturing industrial and consumer products, such as stain-resistant and non-stick coatings for furniture, food packaging, insecticides, and firefighting foams [49]. PFASs can persist in the environment and in humans for extended periods (2–5 years or longer) due to stable carbon-fluorine bonds [50]. In humans, exposure to PFASs can occur through dietary intake of contaminated food or drinking water, and are widespread; detectable levels were found in 95% of Americans who participated in the US National Health and Nutrition Examination Survey between 1999 and 2008 [51].
During pregnancy, certain PFASs can cross the placenta, thereby creating a potential for direct fetal exposure. As a result, there is growing interest in the relationship of PFAS exposure, especially during critical windows of susceptibility in early life, such as gestation, with later health effects. Animal studies demonstrate that PFASs can alter estrogen- and androgen-receptor function [52], activate peroxisome proliferator-activated receptors (PPAR) [53], and disrupt thyroid hormone homeostasis [54], all of which have known regulatory roles in metabolic function. Some studies have shown higher weight gain and body fat accumulation in rodents prenatally exposed to PFASs [55, 56]. Emerging evidence from human epidemiological studies, accounting for relevant socio-demographic covariates, also appears to support these observations; higher PFAS exposure in utero has been associated with more rapid increase in BMI during early to mid-childhood [57••], higher waist-to-height ratio [58], higher total cholesterol and low-density lipoprotein cholesterol [59], and greater risk of overweight/obesity in offspring [60]. Other human studies have reported inconsistent findings, however. In Project Viva, there was no evidence for an adverse effect of prenatal PFAS exposure on offspring adiposity in early childhood or on leptin, adiponectin, or insulin resistance in mid-childhood [61••]. Although maternal prenatal plasma PFAS concentration was associated with small increases in adiposity in girls in mid-childhood, the estimates had confidence intervals that crossed the null [62••]. Similarly, in the INMA birth cohort, there was no evidence of associations between prenatal maternal prenatal plasma PFAS concentration and cardiometabolic outcomes in mid-childhood [63].
PFASs may adversely affect respiratory outcomes through immunosuppression of cytokine production [64], but recent evidence demonstrates an inconsistent relationship between prenatal PFAS exposure and child respiratory and allergic outcomes. While some studies [65, 66] reported inverse associations between prenatal PFAS exposure and risk of allergic diseases in early childhood, others [67, 68] reported no clear evidence of associations between prenatal PFAS exposure and allergy or asthma-related outcomes.
Fine Particulate Matter
Particulate air pollution is a pro-oxidant environmental exposure that may promote programming of adiposity and cardiometabolic risk. Fine particulate matter with an aerodynamic diameter of less than 2.5 μm (PM2.5) can readily enter the lower airways, and prenatal exposure is hypothesized to adversely affect fetal development by inducing maternal oxidative stress, vascular dysfunction, and potentially inhibiting nutrient transfer from mother to fetus [69]. Air pollution exposure in early life may additionally induce adipose inflammation and hypertrophy [70].
Animal models have demonstrated that prenatal exposure to PM2.5 is linked to obesity and its related comorbidities in the offspring [71]. Recent evidence from human epidemiological studies accounting for sociodemographic covariates also support these observations; Fleisch et al. [72••] reported that prenatal exposure to PM2.5 was associated with faster weight gain during infancy and Schembari et al. [73••] reported associations with higher skinfold thicknesses, while Lavigne et al. [74•] and Madhloum et al. [75] showed that increased prenatal PM2.5 exposure was associated with changes in cord adiponectin and insulin, both of which regulate glucose and fatty acid breakdown in the fetus and may contribute to later childhood obesity. Furthermore, recent studies have reported that increased prenatal PM2.5 exposure was associated with increased BMI z-scores, fat mass, and risk of overweight or obesity during childhood [76, 77].
Prenatal PM2.5 exposure has also been related to other adverse outcomes during childhood, such as respiratory diseases. Findings from the Asthma Coalition on Community, Environment, and Social Stress (ACCESS) [78], Programming Research in Obesity, Growth, Environment and Social Stressors (PROGRESS) [79], and Kaiser Air Pollution and Pediatric Asthma [80] cohorts reported that an increased prenatal exposure to PM2.5 was associated with an increased risk of child asthma, which appeared to be stronger under conditions of maternal stress [78, 79].
Nutritional Factors and Environmental Toxicants: Two Sides of the Same Coin?
It is notable that the disease patterns linked to nutritional factors in utero are also often linked to prenatal exposure to environmental toxicants. Both exposures share similar features, such as functional changes in gene expression and cell metabolism that may lead to subtle morphological changes in the tissues and organs, resulting in altered susceptibility to future disease risk [5]. They may also exert their effects within common ranges of exposure (i.e., at a normal range of nutrition or at low doses of environmental toxicants) [5]. This similarity suggests a common pathway for influences of prenatal exposure to nutritional and toxicant stresses, the effects of which ultimately promote programming of future disease risk in the offspring [5].
These factors may compensate for each other’s effects, for example, prenatal consumption of seafood (such as fish and shellfish), a rich source of polyunsaturated fatty acids, could confer benefits to brain and visual system development in infants [81]. On the other hand, seafood is also a major source of methylmercury, a known neurotoxicant that is particularly harmful to fetal brain development [82]. In this case, it is important to balance the risks and benefits of prenatal seafood consumption for proper neurodevelopment in infants [82].
These nutritional and toxicant exposures may also act synergistically to generate a stronger impact, for example, emerging evidence from rodent models demonstrate that concurrent exposure to high-fat diet and bisphenol A during pregnancy increased the incidence of mammary tumor formation in the offspring through alterations in DNA methylation, with differential effects when compared with rodents who were exposed only to a high-fat diet in the absence of BPA [83••]. While there are currently no analogous epidemiological studies conducted in humans, Schaider et al. [84••] recently reported a high prevalence of PFASs and fluorinated chemicals in fast food packaging in the USA, which can leach into food and may significantly contribute to dietary PFAS exposure [85]. Given the link between fast food consumption and later obesity risk [86], the added contribution of dietary PFAS exposure from fast food packaging may serve to further exacerbate the impact of fast food consumption on future disease risk. These observations serve as an illustration of the need to study these exposures in tandem, as both are important and potentially synergistic drivers of future disease risk. It also highlights the necessity for an interdisciplinary approach to this research paradigm—a collaboration between nutritionists, toxicologists, endocrinologists, and epidemiologists to better understand the effects of these developmental exposures on long-term health outcomes in the offspring.
Limitations—Bias and Measurement Error
Selection bias is often an issue in studies of prenatal nutrition and environmental toxicants, which are normally performed in observational cohorts. In cases where cohort recruitment is not representative of the general population, it would limit generalizability of the study findings. Participant retention and loss to follow-up may also be differentially related to exposure and outcome, which could bias the study findings causing either an overestimate or an underestimate of the observed associations.
Other issues include the potential for measurement error; in studies of prenatal nutrition, data are often obtained from food frequency questionnaires (FFQs) which rely on self-reported information. The possibility of recall bias therefore cannot be excluded. Misclassification is also a concern with any dietary reporting method. Methods to overcome these errors often include adjusting for total energy intake, validating and calibrating dietary questionnaires, or combining different dietary assessment methods (e.g., assessing levels of dietary biomarkers together with self-reported intakes). Novel technologies, such as mobile phone applications that provide digital images for food identification and portion-size estimation, could be integrated with traditional dietary assessment methods in future studies to reduce recall bias and improve accuracy.
Unlike PFASs which are measured objectively from blood samples, measurement error is more of a concern in studies of ambient pollutant exposure such as PM2.5. Cohort studies typically estimate long-term exposure to pollution particles using observed pollutant concentrations at the closest, or the average of the closest ambient monitors. However, the locations of these monitors may not match the locations where health outcomes are observed (e.g., at residential addresses). Other methods take advantage of satellites, whose coverage depends on weather conditions. Predicting the spatial distribution of local pollutant concentrations is crucial, as improper prediction can lead to biased estimates of health effects. Recent cohort studies [72••, 73••, 74•] have incorporated statistical models that allow for prediction of pollutant exposures outside of the participant’s residence, thereby accounting for spatial variations in ambient PM2.5 concentrations. State-of-the-art knowledge on atmospheric chemistry and meteorology has also been used to estimate spatially resolved exposure to PM constituents [87]. Instrument-related measurement errors, however, have not been extensively investigated in epidemiological studies of PM constituents and remain an ongoing area of research.
Future Research Directions
Role of Preconception Period
While much of the recent evidence supports a link between nutritional factors and/or exposure to environmental toxicants during pregnancy and offspring disease risk, there is a need for future studies to explore the role of preconception exposures. If women are provided with information regarding healthy habits prior to pregnancy, their ability to plan ahead and prepare for a healthier pregnancy may be greatly increased [88]. Recent findings from human epidemiological studies have provided evidence that preconception exposures may influence perinatal outcomes. Greater exposures to SSB intake, PFASs, and air pollution prior to pregnancy lowered fecundability [89] and increased risk of gestational diabetes [87, 90•], which may contribute towards future disease risk in the offspring. A challenge, however, will be to separate the effects of preconception versus prenatal exposures on offspring health outcomes, especially for persistent dietary habits or environmental toxicants with long biological half-lives, as the extent of these exposures would be expected to be relatively stable prior to conception and during pregnancy. Another challenge would be in recruiting representative preconception cohorts; ideally, the cohort should include couples of reproductive age who are at risk of pregnancy (i.e., having unprotected intercourse in the fertile window) and recruited from the first time they are at risk of pregnancy. As the reproductive process involves complex human behavior, this design could introduce left-truncation bias (e.g., women who became pregnant prior to the start of the study would not enter the study and their information would be truncated), as well as differential loss to follow-up, both of which could affect accuracy of study findings.
Role of Fathers
Another area of future study will be the role of paternal exposures to these factors on disease risk in offspring. Although not extensively studied, there are indications that paternal exposure to environmental chemicals can potentially impact the long-term health offspring, primarily through epigenetic modifications in the father’s sperm, which could consequently influence embryo and fetal development [91]. Furthermore, there is emerging evidence to suggest that paternal lifestyles, including diet and physical activity, can alter the sperm epigenome and subsequently affect the health of their children [92••]. To our knowledge, there are few prospective studies examining relationships between paternal environmental exposures (in a non-occupational setting) and subsequent offspring health. This area of investigation will be relevant to healthcare providers who are advising couples on lifestyle decisions prior to conception.
Intervention Studies
A key limitation of observational studies is the difficulty of inferring causality between prenatal exposure to nutritional imbalances and/or environmental toxicants and long-term offspring health outcomes. There is a need to address whether the observed associations represent true relationships or are merely artifacts of unmeasured confounding. This may be especially true for environmental and nutritional exposures, which often substantially vary with differences in socio-economic and demographic factors [93, 94].
Randomized controlled trials (RCTs) are typically considered the gold standard in health research to determine “cause and effect” [95], as the random allocation of subjects to intervention and control groups minimizes systematic differences and biases between groups. While observational research has made substantive contributions to the current understanding of prenatal exposure to nutritional imbalances and/or environmental toxicants with long-term offspring health outcomes, RCTs can provide more definitive evidence of causality. Intervention studies on reducing SSB consumption have been conducted only in children and non-pregnant adults [96, 97], but not in pregnant women. Recent studies of lifestyle interventions to improve prenatal dietary habits have not included outcomes after birth [98]. RCTs also have not been widely embraced in the field of environmental health [99]. More RCTs are therefore needed to complement the substantial findings of observational research, by providing more definitive evidence of causality and identifying potentially efficacious interventions. It is, however, important to acknowledge the challenges of conducting RCTs, including generally high costs, complex nature of certain interventions (e.g., behavioral interventions), and ability to examine only a limited number of exposures per study. Conducting intervention studies from preconception may be more challenging due to a limited population base from which to recruit, but are arguably more important than interventions during pregnancy, given the profound implications for preconception health on the growth, development, and long-term health of the offspring [100••].
Conclusions
The emerging prenatal nutritional factors and environmental exposures described in this selective review—intake of sugar-sweetened beverages, dietary patterns, perfluoroalkyl substances, and fine particulate matter—provide examples of prenatal exposures emerging as risk factors for adverse long-term health. These emerging risk factors are attractive targets for prevention of future disease risk, as they are amenable to behavior change or environmental interventions during pregnancy. Prevention strategies at the earliest stages of developmental plasticity, including before and during pregnancy, hold much promise for prevention of non-communicable diseases across the life course. Future research and disease prevention strategies should continue to focus on these and other emerging exposures.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Barker DJ. The developmental origins of chronic adult disease. Acta Paediatr Suppl. 2004;93(446):26–33.
Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73. https://doi.org/10.1056/NEJMra0708473.
Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1(8489):1077–81.
Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2(8663):577–80.
Barouki R, Gluckman PD, Grandjean P, Hanson M, Heindel JJ. Developmental origins of non-communicable disease: implications for research and public health. Environ Health. 2012;11:42. https://doi.org/10.1186/476-069X-11-42.
Gluckman PD, Hanson MA. Developmental origins of disease paradigm: a mechanistic and evolutionary perspective. Pediatr Res. 2004;56(3):311–7. https://doi.org/10.1203/01.PDR.0000135998.08025.FB.
Hanson MA, Gluckman PD. Developmental origins of health and disease—global public health implications. Best Pract Res Clin Obstet Gynaecol. 2015;29(1):24–31. https://doi.org/10.1016/j.bpobgyn.2014.06.007.
Roseboom TJ, Watson ED. The next generation of disease risk: are the effects of prenatal nutrition transmitted across generations? Evidence from animal and human studies. Placenta. 2012;33(Suppl 2):e40–4. https://doi.org/10.1016/j.placenta.2012.07.018.
Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Mol Cell Endocrinol. 2001;185(1–2):93–8.
Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VW, Eriksson JG, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017;5(1):53–64. https://doi.org/10.1016/S2213-8587(16)30107-3.
Poston L. Gestational weight gain: influences on the long-term health of the child. Curr Opin Clin Nutr Metab Care. 2012;15(3):252–7. https://doi.org/10.1097/MCO.0b013e3283527cf2.
Hiersch L, Yogev Y. Impact of gestational hyperglycemia on maternal and child health. Curr Opin Clin Nutr Metab Care. 2014;17(3):255–60. https://doi.org/10.1097/MCO.0000000000000030.
Padmanabhan V, Cardoso RC, Puttabyatappa M. Developmental programming, a pathway to disease. Endocrinology. 2016;157(4):1328–40.
Ambrosini GL. Sugar: what are the current facts and where to now? Curr Nutr Rep. 2014;3(4):299–301. https://doi.org/10.1007/s13668-014-0097-z.
Choo VL, Ha V, Sievenpiper JL. Sugars and obesity: is it the sugars or the calories? Nutr Bull. 2015;40(2):88–96. https://doi.org/10.1111/nbu.12137.
Zheng J, Feng Q, Zhang Q, Wang T, Xiao X. Early life fructose exposure and its implications for long-term cardiometabolic health in offspring. Nutrients. 2016;8(11).
Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98(4):1084–102. https://doi.org/10.3945/ajcn.113.058362.
Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010;33(11):2477–83.
•• Gillman MW, Rifas-Shiman SL, Fernandez-Barres S, Kleinman K, Taveras EM, Oken E. Beverage intake during pregnancy and childhood adiposity. Pediatrics 2017;140(2).(pii):peds.2017–0031. doi: https://doi.org/10.1542/peds.2017-0031. The most recent study from a US pre-birth cohort linking prenatal intake of sugar-sweetened beverages with increased child adiposity. Findings suggested that the associations were due primarily to maternal, not child, sugar-sweetened beverage intake and to sugary soda rather than fruit drinks or juice.
Jen V, Erler NS, Tielemans MJ, Braun KV, Jaddoe VW, Franco OH, et al. Mothers’ intake of sugar-containing beverages during pregnancy and body composition of their children during childhood: the generation R study. Am J Clin Nutr. 2017;105(4):834–41. https://doi.org/10.3945/ajcn.116.147934.
Perng W, Gillman MW, Mantzoros CS, Oken E. A prospective study of maternal prenatal weight and offspring cardiometabolic health in midchildhood. Ann Epidemiol 2014;24(11):793–800.e1. doi: https://doi.org/10.1016/j.annepidem.2014.08.002.
•• Azad MB, Sharma AK, de Souza RJ, Dolinsky VW, Becker AB, Mandhane PJ, et al. Association between artificially sweetened beverage consumption during pregnancy and infant body mass index. JAMA Pediatr. 2016;170(7):662–70. https://doi.org/10.1001/jamapediatrics.2016.0301. This paper describes the first human evidence that prenatal consumption of artificial sweeteners during pregnancy may influence infant body mass index and risk of overweight.
Zhu Y, Olsen SF, Mendola P, Halldorsson TI, Rawal S, Hinkle SN, et al. Maternal consumption of artificially sweetened beverages during pregnancy, and offspring growth through 7 years of age: a prospective cohort study. Int J Epidemiol. 2017;46(5):1499–508.
Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514(7521):181–6. https://doi.org/10.1038/nature13793.
Maslova E, Strom M, Olsen SF, Halldorsson TI. Consumption of artificially-sweetened soft drinks in pregnancy and risk of child asthma and allergic rhinitis. PLoS One. 2013;8(2):e57261. https://doi.org/10.1371/journal.pone.0057261.
•• Wright LS, Rifas-Shiman SL, Oken E, Litonjua AA, Gold DR. Prenatal and early life fructose, fructose-containing beverages, and midchildhood asthma. Ann Am Thorac Soc. 2018;15(2):217–24. https://doi.org/10.1513/AnnalsATS.201707-530OC. The most recent study conducted in a US pre-birth cohort that details the relationship between higher prenatal sugar-sweetened beverage intake and risk of asthma during mid-childhood. The observed associations were independent of child adiposity.
Singh VP, Aggarwal R, Singh S, Banik A, Ahmad T, Patnaik BR, et al. Metabolic syndrome is associated with increased oxo-nitrative stress and asthma-like changes in lungs. PLoS One. 2015;10(6):e0129850. https://doi.org/10.1371/journal.pone. eCollection 2015.
Perng W, Oken E. Chapter 15—programming long-term health: maternal and fetal nutrition and diet needs A2 - Saavedra, Jose M. In: Dattilo AM, editor. Early nutrition and long-term health. Woodhead Publishing; 2017. p. 375–411.
Starling P, Charlton K, McMahon AT, Lucas C. Fish intake during pregnancy and foetal neurodevelopment—a systematic review of the evidence. Nutrients. 2015;7(3):2001–14. https://doi.org/10.3390/nu7032001.
Brantsaeter AL, Olafsdottir AS, Forsum E, Olsen SF, Thorsdottir I. Does milk and dairy consumption during pregnancy influence fetal growth and infant birthweight? A systematic literature review. Food Nutr Res. 2012;56(pii):–20050. https://doi.org/10.3402/fnr.v56i0.
Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9.
Kant AK. Indexes of overall diet quality: a review. J Am Diet Assoc. 1996;96(8):785–91. https://doi.org/10.1016/S0002-8223(96)00217-9.
Shapiro AL, Kaar JL, Crume TL, Starling AP, Siega-Riz AM, Ringham BM, et al. Maternal diet quality in pregnancy and neonatal adiposity: the Healthy Start Study. Int J Obes. 2016;40(7):1056–62.
•• Chia AR, Tint MT, Han CY, Chen LW, Colega M, Aris IM, et al. Adherence to a healthy eating index for pregnant women is associated with lower neonatal adiposity in a multiethnic Asian cohort: the Growing Up in Singapore Towards healthy Outcomes (GUSTO) Study. Am J Clin Nutr. 2018;107(1):71–9. https://doi.org/10.1093/ajcn/nqx003. A recent paper that observed significant associations between higher maternal diet quality lower neonatal adiposity. One of the few studies in the world to detail this relationship in a multi-ethnic Asian birth cohort.
Dhana K, Zong G, Yuan C, Schernhammer E, Zhang C, Wang X, et al. Lifestyle of women before pregnancy and the risk of offspring obesity during childhood through early adulthood. Int J Obes. 2018;3(10):018–0052.
• Chen LW, Aris IM, Bernard JY, Tint MT, Chia A, Colega M, et al. Associations of maternal dietary patterns during pregnancy with offspring adiposity from birth until 54 months of age. Nutrients. 2016;9(1) Another paper from a multi-ethnic Asian birth cohort that describes the associations of a “healthy dietary pattern” with lower offspring adiposity. These associations were observed only at ages 18 months and older, until 54 months of age.
Martin CL, Siega-Riz AM, Sotres-Alvarez D, Robinson WR, Daniels JL, Perrin EM, et al. Maternal dietary patterns during pregnancy are associated with child growth in the first 3 years of life. J Nutr. 2016;146(11):2281–8. https://doi.org/10.3945/jn.116.234336.
van den Broek M, Leermakers ET, Jaddoe VW, Steegers EA, Rivadeneira F, Raat H, et al. Maternal dietary patterns during pregnancy and body composition of the child at age 6 y: the generation R study. Am J Clin Nutr. 2015;102(4):873–80. https://doi.org/10.3945/ajcn.114.102905.
Leermakers ETM, Tielemans MJ, van den Broek M, Jaddoe VWV, Franco OH, Kiefte-de Jong JC. Maternal dietary patterns during pregnancy and offspring cardiometabolic health at age 6 years: the generation R study. Clin Nutr. 2017;36(2):477–84. https://doi.org/10.1016/j.clnu.2015.12.017.
Huo R, Du T, Xu Y, Xu W, Chen X, Sun K, et al. Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: a meta-analysis. Eur J Clin Nutr. 2015;69(11):1200–8.
Kastorini CM, Milionis HJ, Esposito K, Giugliano D, Goudevenos JA, Panagiotakos DB. The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J Am Coll Cardiol. 2011;57(11):1299–313.
Fernandez-Barres S, Romaguera D, Valvi D, Martinez D, Vioque J, Navarrete-Munoz EM, et al. Mediterranean dietary pattern in pregnant women and offspring risk of overweight and abdominal obesity in early childhood: the INMA birth cohort study. Pediatr Obes. 2016;11(6):491–9. https://doi.org/10.1111/ijpo.12092.
•• Chatzi L, Rifas-Shiman SL, Georgiou V, Joung KE, Koinaki S, Chalkiadaki G, et al. Adherence to the Mediterranean diet during pregnancy and offspring adiposity and cardiometabolic traits in childhood. Pediatr Obes. 2017;12(Suppl 1):47–56. https://doi.org/10.1111/ijpo.12191. This study pooled data from two longitudinal birth cohorts in the USA and Greece to illustrate that greater adherence to Mediterranean diet during pregnancy protected against excess offspring cardiometabolic risk.
Sedaghat F, Naja F, Darand M, Beyzai B, Rashidkhani B. Adherence to a Mediterranean dietary pattern and overweight and obesity among female adolescents in Iran. Int J Adolesc Med Health. 2017;23(10):2016–0160.
• Sen S, Rifas-Shiman SL, Shivappa N, Wirth MD, Hebert JR, Gold DR, et al. Dietary inflammatory potential during pregnancy is associated with lower fetal growth and breastfeeding failure: results from Project Viva. J Nutr. 2016;146(4):728–36. https://doi.org/10.3945/jn.115.225581. Most recent study from a US pre-birth cohort that describes associations of a pro-inflammatory diet during pregnancy with impaired fetal growth.
• Sen S, Rifas-Shiman SL, Shivappa N, Wirth MD, Hebert JR, Gold DR, et al. Associations of prenatal and early life dietary inflammatory potential with childhood adiposity and cardiometabolic risk in Project Viva. Pediatr Obes. 2017;10(10):12221. Most recent study from a US pre-birth cohort that describes associations of a pro-inflammatory diet during pregnancy may promote development of adiposity in the offspring.
Julia V, Macia L, Dombrowicz D. The impact of diet on asthma and allergic diseases. Nat Rev Immunol. 2015;15(5):308–22. https://doi.org/10.1038/nri3830.
von Ehrenstein OS, Aralis H, Flores ME, Ritz B. Fast food consumption in pregnancy and subsequent asthma symptoms in young children. Pediatr Allergy Immunol. 2015;26(6):571–7. https://doi.org/10.1111/pai.12433.
Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci. 2007;99(2):366–94. https://doi.org/10.1093/toxsci/kfm128.
Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115(9):1298–305.
Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. Trends in exposure to polyfluoroalkyl chemicals in the U.S. population: 1999–2008. Environ Sci Technol. 2011;45(19):8037–45. https://doi.org/10.1021/es1043613.
Kjeldsen LS, Bonefeld-Jorgensen EC. Perfluorinated compounds affect the function of sex hormone receptors. Environ Sci Pollut Res Int. 2013;20(11):8031–44. https://doi.org/10.1007/s11356-013-1753-3.
Zhang L, Ren XM, Wan B, Guo LH. Structure-dependent binding and activation of perfluorinated compounds on human peroxisome proliferator-activated receptor gamma. Toxicol Appl Pharmacol. 2014;279(3):275–83. https://doi.org/10.1016/j.taap.2014.06.020.
Long M, Ghisari M, Bonefeld-Jorgensen EC. Effects of perfluoroalkyl acids on the function of the thyroid hormone and the aryl hydrocarbon receptor. Environ Sci Pollut Res Int. 2013;20(11):8045–56. https://doi.org/10.1007/s11356-013-1628-7.
Taxvig C, Dreisig K, Boberg J, Nellemann C, Schelde AB, Pedersen D, et al. Differential effects of environmental chemicals and food contaminants on adipogenesis, biomarker release and PPARgamma activation. Mol Cell Endocrinol. 2012;361(1–2):106–15. https://doi.org/10.1016/j.mce.2012.03.021.
Hines EP, White SS, Stanko JP, Gibbs-Flournoy EA, Lau C, Fenton SE. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: low doses induce elevated serum leptin and insulin, and overweight in mid-life. Mol Cell Endocrinol. 2009;304(1–2):97–105. https://doi.org/10.1016/j.mce.2009.02.021.
•• Braun JM, Chen A, Romano ME, Calafat AM, Webster GM, Yolton K, et al. Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: the HOME study. Obesity (Silver Spring). 2016;24(1):231–7. https://doi.org/10.1002/oby.21258. One of the more recent studies in humans that have detailed associations between exposure to higher prenatal PFAS and greater adiposity in mid-childhood.
Hoyer BB, Ramlau-Hansen CH, Vrijheid M, Valvi D, Pedersen HS, Zviezdai V, et al. Anthropometry in 5- to 9-year-old Greenlandic and Ukrainian children in relation to prenatal exposure to perfluorinated alkyl substances. Environ Health Perspect. 2015;123(8):841–6. https://doi.org/10.1289/ehp.1408881.
Mora AM, Fleisch AF, Rifas-Shiman SL, Woo Baidal JA, Pardo L, Webster TF, et al. Early life exposure to per- and polyfluoroalkyl substances and mid-childhood lipid and alanine aminotransferase levels. Environ Int. 2018;111:1–13. https://doi.org/10.1016/j.envint.2017.11.008.
Lauritzen HB, Larose TL, Oien T, Sandanger TM, Odland JO, van de Bor M, et al. Prenatal exposure to persistent organic pollutants and child overweight/obesity at 5-year follow-up: a prospective cohort study. Environ Health. 2018;17(1):9. https://doi.org/10.1186/s12940-017-0338-x.
•• Fleisch AF, Rifas-Shiman SL, Mora AM, Calafat AM, Ye X, Luttmann-Gibson H, et al. Early-life exposure to perfluoroalkyl substances and childhood metabolic function. Environ Health Perspect. 2017;125(3):481–7. https://doi.org/10.1289/EHP303. A recent study form a US pre-birth cohort that showed prenatal PFAS plasma concentrations were not associated with markers of cardiometabolic risk (leptin, adiponectin, and insulin resistance) in children.
•• Mora AM, Oken E, Rifas-Shiman SL, Webster TF, Gillman MW, Calafat AM, et al. Prenatal exposure to perfluoroalkyl substances and adiposity in early and mid-childhood. Environ Health Perspect. 2017;125(3):467–73. https://doi.org/10.1289/EHP246. In this recent study, although prenatal PFAS exposure was associated with small increases in adiposity measurements in mid-childhood, the confidence intervals were wide and crossed the null.
Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, Ballester F, Iniguez C, Martinez D, et al. Prenatal exposure to perfluoroalkyl substances and cardiometabolic risk in children from the Spanish INMA birth cohort study. Environ Health Perspect. 2017;125(9):097018. https://doi.org/10.1289/EHP1330.
Qazi MR, Abedi MR, Nelson BD, DePierre JW, Abedi-Valugerdi M. Dietary exposure to perfluorooctanoate or perfluorooctane sulfonate induces hypertrophy in centrilobular hepatocytes and alters the hepatic immune status in mice. Int Immunopharmacol. 2010;10(11):1420–7.
Goudarzi H, Miyashita C, Okada E, Kashino I, Kobayashi S, Chen CJ, et al. Effects of prenatal exposure to perfluoroalkyl acids on prevalence of allergic diseases among 4-year-old children. Environ Int. 2016;94:124–32. https://doi.org/10.1016/j.envint.2016.05.020.
Okada E, Sasaki S, Kashino I, Matsuura H, Miyashita C, Kobayashi S, et al. Prenatal exposure to perfluoroalkyl acids and allergic diseases in early childhood. Environ Int. 2014;65:127–34. https://doi.org/10.1016/j.envint.2014.01.007.
Impinen A, Nygaard UC, Lodrup Carlsen KC, Mowinckel P, Carlsen KH, Haug LS, et al. Prenatal exposure to perfluoralkyl substances (PFASs) associated with respiratory tract infections but not allergy- and asthma-related health outcomes in childhood. Environ Res. 2018;160:518–23. https://doi.org/10.1016/j.envres.2017.10.012.
Timmermann CA, Budtz-Jorgensen E, Jensen TK, Osuna CE, Petersen MS, Steuerwald U, et al. Association between perfluoroalkyl substance exposure and asthma and allergic disease in children as modified by MMR vaccination. J Immunotoxicol. 2017;14(1):39–49. https://doi.org/10.1080/1547691X.2016.254306.
Kannan S, Misra DP, Dvonch JT, Krishnakumar A. Exposures to airborne particulate matter and adverse perinatal outcomes: a biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ Health Perspect. 2006;114(11):1636–42.
Xu X, Yavar Z, Verdin M, Ying Z, Mihai G, Kampfrath T, et al. Effect of early particulate air pollution exposure on obesity in mice: role of p47phox. Arterioscler Thromb Vasc Biol. 2010;30(12):2518–27. https://doi.org/10.1161/ATVBAHA.110.215350.
Bolton JL, Auten RL, Bilbo SD. Prenatal air pollution exposure induces sexually dimorphic fetal programming of metabolic and neuroinflammatory outcomes in adult offspring. Brain Behav Immun. 2014;37:30–44. https://doi.org/10.1016/j.bbi.2013.10.029.
•• Fleisch AF, Rifas-Shiman SL, Koutrakis P, Schwartz JD, Kloog I, Melly S, et al. Prenatal exposure to traffic pollution: associations with reduced fetal growth and rapid infant weight gain. Epidemiology. 2015;26(1):43–50. https://doi.org/10.1097/EDE.0000000000000203. One of the few studies that have shown associations between increased prenatal exposure to traffic-related pollution and rapid weight gain in infancy.
•• Schembari A, de Hoogh K, Pedersen M, Dadvand P, Martinez D, Hoek G, et al. Ambient air pollution and newborn size and adiposity at birth: differences by maternal ethnicity (the born in Bradford study cohort). Environ Health Perspect. 2015;123(11):1208–15. One of the few recent studies that have described associations of fine particulate matter exposure during pregnancy with newborn size and adiposity.
• Lavigne E, Ashley-Martin J, Dodds L, Arbuckle TE, Hystad P, Johnson M, et al. Air pollution exposure during pregnancy and fetal markers of metabolic function: the MIREC study. Am J Epidemiol. 2016;183(9):842–51. https://doi.org/10.1093/aje/kwv256. One of the few epidemiological studies that have assessed the association between prenatal exposure to air pollution and indicators of fetal metabolic function, in a relative large cohort ( n = 1,257).
Madhloum N, Janssen BG, Martens DS, Saenen ND, Bijnens E, Gyselaers W, et al. Cord plasma insulin and in utero exposure to ambient air pollution. Environ Int. 2017;105:126–32. https://doi.org/10.1016/j.envint.2017.05.012.
Chiu YH, Hsu HH, Wilson A, Coull BA, Pendo MP, Baccarelli A, et al. Prenatal particulate air pollution exposure and body composition in urban preschool children: examining sensitive windows and sex-specific associations. Environ Res. 2017;158:798–805. https://doi.org/10.1016/j.envres.2017.07.026.
Mao G, Nachman RM, Sun Q, Zhang X, Koehler K, Chen Z, et al. Individual and joint effects of early-life ambient exposure and maternal prepregnancy obesity on childhood overweight or obesity. Environ Health Perspect. 2017;125(6):067005. https://doi.org/10.1289/EHP261.
Lee A, Leon Hsu HH, Mathilda Chiu YH, Bose S, Rosa MJ, Kloog I, et al. Prenatal fine particulate exposure and early childhood asthma: effect of maternal stress and fetal sex. J Allergy Clin Immunol. 2017;8(17):31273–3.
Rosa MJ, Just AC, Kloog I, Pantic I, Schnaas L, Lee A, et al. Prenatal particulate matter exposure and wheeze in Mexican children: effect modification by prenatal psychosocial stress. Ann Allergy Asthma Immunol. 2017;119(3):232–7.e1. https://doi.org/10.1016/j.anai.2017.06.016.
Pennington AF, Strickland MJ, Klein M, Zhai X, Bates JT, Drews-Botsch C, et al. Exposure to mobile source air pollution in early-life and childhood asthma incidence: the Kaiser air pollution and pediatric asthma study. Epidemiology. 2018;29(1):22–30. https://doi.org/10.1097/EDE.0000000000000754.
Oken E, Choi AL, Karagas MR, Marien K, Rheinberger CM, Schoeny R, et al. Which fish should I eat? Perspectives influencing fish consumption choices. Environ Health Perspect. 2012;120(6):790–8. https://doi.org/10.1289/ehp.1104500.
Oken E, Bellinger DC. Fish consumption, methylmercury and child neurodevelopment. Curr Opin Pediatr. 2008;20(2):178–83. https://doi.org/10.1097/MOP.0b013e3282f5614c.
•• Leung YK, Govindarajah V, Cheong A, Veevers J, Song D, Gear R, et al. Gestational high-fat diet and bisphenol A exposure heightens mammary cancer risk. Endocr Relat Cancer. 2017;24(7):365–78. https://doi.org/10.1530/ERC-17-0006. An animal study that describes the concurrent effects of exposure to a high-fat diet and bisphenol A during pregnancy with incidence in mammary tumor formation, alterations in mammary gland development, and gene expression. This study illustrates the potential synergistic effects of exposure to nutritional imbalances and environmental chemicals.
•• Schaider LA, Balan SA, Blum A, Andrews DQ, Strynar MJ, Dickinson ME, Lunderberg DM, Lang JR, Peaslee GF Fluorinated compounds in US fast food packaging. Environ Sci Technol Lett 2017;4(3):105–11. A recent study that details the prevalence of fluorinated chemicals in fast food packaging and demonstrates their potentially significant contribution to dietary PFAS exposure and environmental contamination during production and disposal.
Averina M, Brox J, Huber S, Furberg AS. Perfluoroalkyl substances in adolescents in northern Norway: lifestyle and dietary predictors. The Tromso study, Fit Futures 1. Environ Int. 2018;114:123–30. https://doi.org/10.1016/j.envint.2018.02.031.
Garcia G, Sunil TS, Hinojosa P. The fast food and obesity link: consumption patterns and severity of obesity. Obes Surg. 2012;22(5):810–8. https://doi.org/10.1007/s11695-012-0601-8.
Robledo CA, Mendola P, Yeung E, Mannisto T, Sundaram R, Liu D, et al. Preconception and early pregnancy air pollution exposures and risk of gestational diabetes mellitus. Environ Res. 2015;137:316–22. https://doi.org/10.1016/j.envres.2014.12.020.
Genuis SJ, Genuis RA. Preconception care: a new standard of care within maternal health services. Biomed Res Int. 2016;2016:6150976. https://doi.org/10.1155/2016/6150976.
Hatch EE, Wesselink AK, Hahn KA, Michiel JJ, Mikkelsen EM, Sorensen HT, et al. Intake of sugar-sweetened beverages and fecundability in a North American Preconception Cohort. Epidemiology. 2018;29(3):369–78. https://doi.org/10.1097/EDE.0000000000000812.
• Zhang C, Sundaram R, Maisog J, Calafat AM, Barr DB, Buck Louis GM. A prospective study of prepregnancy serum concentrations of perfluorochemicals and the risk of gestational diabetes. Fertil Steril. 2015;103(1):184–9. https://doi.org/10.1016/j.fertnstert.2014.10.001. One of the few studies that have examined exposure to perfluoroalkyl substances during preconception and its associated risks with gestational diabetes during pregnancy, which is suggestive of a possible environmental etiology for gestational diabetes.
Stuppia L, Franzago M, Ballerini P, Gatta V, Antonucci I. Epigenetics and male reproduction: the consequences of paternal lifestyle on fertility, embryo development, and children lifetime health. Clin Epigenetics. 2015;7:120. https://doi.org/10.1186/s13148-015-0155-4. eCollection 2015.
•• Schagdarsurengin U, Steger K. Epigenetics in male reproduction: effect of paternal diet on sperm quality and offspring health. Nat Rev Urol. 2016;13(10):584–95. https://doi.org/10.1038/nrurol.2016.157. An important review that summarizes various studies in animal models and human epidemiological data describing the transgenerational effect of the paternally contributed sperm epigenome on long-term health outcomes of the offspring.
Harris MH, Rifas-Shiman SL, Calafat AM, Ye X, Mora AM, Webster TF, et al. Predictors of per- and polyfluoroalkyl substance (PFAS) plasma concentrations in 6–10 year old American children. Environ Sci Technol. 2017;51(9):5193–204. https://doi.org/10.1021/acs.est.6b05811.
Doyle IM, Borrmann B, Grosser A, Razum O, Spallek J. Determinants of dietary patterns and diet quality during pregnancy: a systematic review with narrative synthesis. Public Health Nutr. 2017;20(6):1009–28.
Kramer MS. Randomized trials and public health interventions: time to end the scientific double standard. Clin Perinatol 2003. 2003;30(2):351–61.
Abdel Rahman A, Jomaa L, Kahale LA, Adair P, Pine C. Effectiveness of behavioral interventions to reduce the intake of sugar-sweetened beverages in children and adolescents: a systematic review and meta-analysis. Nutr Rev. 2018;76(2):88–107. https://doi.org/10.1093/nutrit/nux061.
Vargas-Garcia EJ, Evans CEL, Prestwich A, Sykes-Muskett BJ, Hooson J, Cade JE. Interventions to reduce consumption of sugar-sweetened beverages or increase water intake: evidence from a systematic review and meta-analysis. Obes Rev. 2017;18(11):1350–63.
Asci O, Rathfisch G. Effect of lifestyle interventions of pregnant women on their dietary habits, lifestyle behaviors, and weight gain: a randomized controlled trial. J Health Popul Nutr. 2016;35:7. https://doi.org/10.1186/s41043-016-0044-2.
Allen RW, Barn PK, Lanphear BP. Randomized controlled trials in environmental health research: unethical or underutilized? PLoS Med. 2015;12(1):e1001775. https://doi.org/10.1371/journal.pmed. eCollection 2015 Jan.
•• Fleming TP, Watkins AJ, Velazquez MA, Mathers JC, Prentice AM, Stephenson J, et al. Origins of lifetime health around the time of conception: causes and consequences. Lancet. 2018;16(18):30312-X. The most recent review from Lancet describing the evidence for the effects of preconception health on the growth, development, and long-term health of the offspring. This paper also discusses potential strategies for preconception interventions that are scalable, which could have positive effects on a range of health outcomes.
Funding
Izzuddin M Aris is supported by the National University of Singapore Overseas Postdoctoral Fellowship (NUS OPF/2017). Abby Fleisch is supported by the National Institutes of Health (K23 ES024803). Emily Oken is supported by the National Institutes of Health (UG3OD023286, P30 DK092924, R01AI102960, R01 HD034568).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Izzuddin M Aris and Abby F. Fleisch declare no conflicts of interest; Emily Oken reports grants from US National Institutes of Health, during the conduct of the study.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Reproductive and Perinatal Epidemiology
Rights and permissions
About this article
Cite this article
Aris, I.M., Fleisch, A.F. & Oken, E. Developmental Origins of Disease: Emerging Prenatal Risk Factors and Future Disease Risk. Curr Epidemiol Rep 5, 293–302 (2018). https://doi.org/10.1007/s40471-018-0161-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40471-018-0161-0