Abstract
Purpose of Review
The purpose of this article is to review the literature surrounding preoperative assessment and management of patients undergoing lung resection surgery.
Recent Findings
The traditional preoperative cardiovascular risk assessment can be further refined in patients undergoing lung resection surgery with the Thoracic Revised Cardiac Risk Index and cardiac biomarkers such as B-type natriuretic peptide. Cardiorespiratory exercise testing parameters such as the maximal achieved oxygen consumption (VO2 peak) and the minute ventilation to carbon dioxide output (VE/VCO2) slope are strong preoperative prognosticators in patients with borderline lung function. Preoperative pulmonary rehabilitation holds promising benefits in improving surgical candidacy and postoperative outcomes.
Summary
The preoperative assessment of lung resection candidates must evaluate perioperative cardiorespiratory risk. The patient’s comorbidities should be optimized as time permits. A clear perioperative plan should be established and may include cardiology consultation, prevention strategies for arrhythmias, preoperative pulmonary rehabilitation, smoking cessation, intensive perioperative monitoring, and enhanced recovery protocols.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the most common cancer worldwide, with over 2.09 million cases in 2018 [1]. In the United States only, it is estimated that lung cancer will lead to over 142,000 deaths in 2019, being by far the most common cause of cancer death (23.5% of all cancer deaths) [2].
When diagnosed at an early stage, surgical resection of lung cancer is required for optimal oncologic outcomes. Lung cancer pathogenesis is strongly related to tobacco exposure, a risk factor shared with chronic obstructive pulmonary disease (COPD) and multiple cardiovascular comorbidities. When evaluating candidates for lung resection surgery, perioperative physicians are therefore often faced with patients with decreased cardiorespiratory reserve and increased postoperative risk of pulmonary and cardiovascular complications. Specific goals of the preoperative evaluation for lung resection surgery are to assess the patient’s cardiorespiratory reserve and capacity to survive the planned lung resection, to optimize cardiovascular and pulmonary comorbidities, to weigh perioperative risks versus potential benefits of surgery, and to plan perioperative care.
The purpose of this article is to review the most recent literature surrounding this clinical challenge, with emphasis on the cardiovascular evaluation in this specific patient population, the predictive value of cardiac biomarkers, the resectability evaluation, and the role of prehabilitation and smoking cessation.
Cardiovascular Evaluation
Cardiovascular complications (supraventricular arrhythmias, myocardial infarction, thromboembolic events, and heart failure) are an important cause of postoperative morbidity and mortality in patients undergoing non-cardiac thoracic surgery, occurring in up to 30% of cases [3].
Supraventricular arrhythmias, the most common cardiac complication, have a complex pathophysiologic mechanism that involves stress from surgical manipulation and altered postoperative cardiopulmonary physiology. Major lung resection surgery (lobectomy, pneumonectomy, and lung volume reduction surgery) is considered an important risk factor for development of postoperative atrial fibrillation or flutter (incidence above 15%), as is male sex, advanced age, hypertension, coronary artery disease, heart failure, and elevated cardiac biomarkers (see following section). In patients at high risk for postoperative atrial fibrillation or flutter, preventive strategies such as continuation of β-blockers, correction of hypomagnesemia, or initiation of calcium-channel blockers or amiodarone may be considered as per current guidelines [4•].
Due to shared risk factors, the prevalence of coronary artery disease in patients undergoing lung resection surgery is high (11%–17%) [5, 6]. Myocardial infarction is an uncommon complication (< 5% incidence after pulmonary resection) but with potentially devastating consequences (mortality rates as high as 40% in pneumonectomy patients) [7, 8••].
As with any patient undergoing major surgery, cardiac risk evaluation and management is based on current guidelines and clinical judgment [9, 10•]. For that purpose, one of the most validated and used tools to stratify cardiovascular risk in patients who undergo non-cardiac surgery is the Revised Cardiac Risk Index [3, 9]. This score was originally derived from a mixed surgical population including only 12% of thoracic surgery patients. More recently, this risk stratification tool has been modified into the Thoracic Revised Cardiac Risk Index (ThRCRI, see Table 1) specifically for patients undergoing lobectomy and pneumonectomy [5]. Multiple subsequent studies have externally validated this score, including a large retrospective cohort study using the American College of Surgeons National Surgical Quality Improvement Program database which reported a threefold increase in postoperative major cardiac complications in patients with ThRCRI score ≥ 2 compared with those with a score of 0 (4.8% vs 1.4%, p < 0.05) [6, 11•]. In this study, patients who sustained a major cardiac complication had a dramatically increased rate of 30-day mortality (53% vs 1.3%, p < 0.01). Of note, this score has also been shown to be a useful prognosticator of 5-year cancer-specific survival as well as long-term cardiac mortality [12].
Moreover, the American College of Chest Physicians (ACCP) has incorporated this risk stratification method into their clinical practice guidelines. Accordingly, cardiology consultation and further noninvasive cardiac testing are indicated when the ThRCRI score is greater than 1.5, the patient has a cardiac condition requiring medications, a new cardiac condition is suspected, or the patient is unable to climb 2 flights of stairs (see Fig. 1 in reference [8••]). Despite these expert recommendations, the validity of these evaluation methods and the clinical utility of preoperative noninvasive cardiac testing has been challenged [10•, 13].
Number of segments in each lobe used to estimate postoperative pulmonary function [8]
Other identified risk factors of cardiovascular complications following lung resection surgery include male sex, American Society of Anesthesiologists’ class status 3 or higher, diabetes mellitus, and an open surgical approach compared to video-assisted thoracic surgery (VATS) [11•, 14].
Cardiac Biomarkers
There has been increasing interest in the development and clinical use of laboratory tests to predict postoperative outcomes after non-cardiac surgery. Natriuretic peptide biomarkers such as B-type natriuretic peptide (BNP) or N-terminal fragment of proBNP (NT-proBNP) are polypeptides secreted by the myocardium in response to mechanical stretch or ischemia. Multiple studies have shown their prognostic capabilities of predicting postoperative cardiovascular outcomes when measured preoperatively or early postoperatively [15]. Determination of BNP is now a cornerstone of preoperative cardiac evaluation according to the Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment [10•].
Specifically relating to the thoracic surgical population, there is evidence from multiple cohort studies and meta-analyses that patients with elevated preoperative BNP levels are at significantly increased risk of postoperative atrial fibrillation [16•, 17].
A few reports have associated an elevated preoperative BNP with other postoperative outcomes such as functional capacity decline, cardiopulmonary complications, and mortality [18,19,20]. For example, a recent retrospective cohort study of 675 patients undergoing lung resection surgery reported an incidence of postoperative complications of 11%, 47%, and 85%, respectively, for patients with normal, mildly, and severely elevated levels of preoperative BNP (p < 0.0001) [19]. In that study, preoperative BNP was the strongest predictor of postoperative complications and performed better than predicted postoperative forced expiratory volume in 1 s (ppoFEV1) and surgical technique (open vs VATS).
Despite these promising advances in preoperative prognostication of cardiovascular risk, research is still needed to clarify the best perioperative management to reduce cardiovascular complications in patients undergoing lung resection surgery.
Respiratory Function Testing
The respiratory assessment prior to lung resection surgery is based on the concept that a minimal cardiorespiratory reserve is necessary to tolerate resection of a certain amount of capillary-alveolar units. Indeed, pulmonary complications (pneumonia, atelectasis, respiratory failure, pulmonary embolism, and prolonged air leak) are the major cause of morbidity and mortality following lung resection surgery. However, the specific required post-resectional lung function is difficult to define on an individual patient basis. Nevertheless, preoperative assessment of respiratory function can be conceptualized as a “3-legged stool,” evaluating the lungs’ ability to move gas in and out of the alveoli (lung mechanics), gas exchange at the alveolar-capillary interface (lung parenchymal function), and the ability to distribute oxygen to tissues (cardiopulmonary reserve) [21].
Lung Mechanics
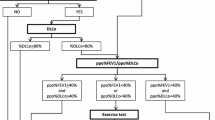
When considering the effect of surgery on an individual patient’s lung mechanics, the physiology of the patient (height, age, and sex) and the planned extent of lung resection are taken into account. The most useful predictor of postoperative lung mechanics is the percent ppoFEV1. It is estimated by the following formula, taking into account the preoperative post-bronchodilator percent of predicted FEV1 (see Figure):
In the last decades, multiple studies have validated the ability of ppoFEV1 to predict postoperative complications using various cutoff values [22,23,24,25]. Significantly elevated postoperative morbidity and mortality have often been associated with ppoFEV1% below 30%–40%. For example, one study reported major postoperative pulmonary complications (PPC) only in patients with ppoFEV1 < 40%, and all patients with ppoFEV1 < 30% required prolonged mechanical ventilation after lung cancer surgery. Another study reported a 50% mortality rate in patients with ppoFEV1 < 40% who had thoracotomies, whereas none of the 47 patients with higher ppoFEV1 died [23].
Other measures of lung mechanics that have been associated with outcomes following lung resection include maximal voluntary ventilation and forced vital capacity. However, they are more dependent on patient’s efforts and are rarely used for clinical prognostication.
Lung Parenchymal Function
The most useful preoperative test for assessing gas exchange capacity of the alveolar-capillary interface is the diffusing capacity for carbon monoxide (DLCO). Since it is a specific measure of diffusion impairment, it is to some extent independent of the FEV1 and provides additional information in the preoperative pulmonary assessment. As with the FEV1, its post-resection value can be estimated based on the amount of lung tissue to be resected (see Figure) and is a useful predictor of postoperative morbidity and mortality [21]. For example, in a retrospective cohort study of 854 patients who underwent major lung resection, each 10 percentage-point decrease in ppoDLCO was strongly associated with increased mortality with a hazard ratio of 1.06 (p = 0.02) [26].
Other measures of gas exchange such as arterial PO2 or PCO2 have not been shown to be reliable discriminators of lung resectability and perioperative risk [21].
Cardiopulmonary Reserve
In order to maintain energetic homeostasis, the ultimate physiologic objective of the cardiorespiratory system is to deliver enough oxygen from the atmosphere to the tissues. Testing of exercise capacity allows for physiologic evaluation of this complex interaction of pulmonary, cardiac, circulatory, and metabolic systems to assess the body’s reserve. For this purpose, cardiopulmonary exercise testing (CPET) is the recommended technique and usually consists of recording the electrocardiogram, heart rate, blood pressure, ventilation parameters, pulse oximetry, oxygen uptake, and CO2 production during standardized incremental exercise on a bicycle ergometer or treadmill [27]. Other exercise tests exist, such as standardized stair climbing test (SCT) or shuttle walk test (SWT), that are readily available and less expensive.
One of the advantages of a formal CPET is that it may be able to differentiate poor exercise tolerance due to respiratory or cardiac etiology or simply functional deconditioning without cardiopulmonary pathology, helping to guide further testing and management [28•].
Of the various measurements provided by CPET, the maximal achieved oxygen consumption (VO2 peak) is an independent and reliable predictor of cardiopulmonary complications and death after lung resection surgery and provides useful preoperative risk stratification in patients with borderline lung function [8••, 29,30,31]. As with ppoFEV1 and ppoDLCO, VO2 peak can be expressed as a percentage of predicted value or as a postresection estimate and has strong predictive value [8••, 31]. A VO2 peak above 20 mL/kg/min or 75% of predicted has been associated with low risk of postoperative morbidity and mortality [29]. On the other hand, a VO2 peak below 10 mL/kg/min or 35% of predicted has been associated with very high mortality and is viewed by some authors as a relative contraindication to significant surgical resection [8••, 32].
Another parameter of CPET, the minute ventilation to carbon dioxide output (VE/VCO2) slope, also called the ventilatory equivalent to carbon dioxide, is a measure of gas exchange efficiency that is increased with worsening in ventilation/perfusion mismatch and dead space [27, 28•]. It has been associated with increased postoperative complications and mortality after lung resection surgery in multiple reports [32, 33]. A recent prospective cohort study of 55 COPD patients undergoing lung resection surgery found that a VE/VCO2 slope above 35 predicted postoperative complications and mortality better than VO2 peak, with a hazard ratio of 5.14 (95%CI 1.4–18.7) [33]. To this date, this parameter has not been included in clinical guidelines.
Although CPET is a reliable, well standardized, and informative test that provides significant physiologic information, it is expensive, time-consuming, and not widely available. To circumvent these drawbacks, the SCT and SWT are simple and require little personnel and expertise to perform. They are recommended by the ACCP guidelines as initial exercise testing in patients deemed at moderate risk based on ppoFEV1 and ppoDLCO measures [8••].
Integration of the Respiratory Evaluation
The most recent recommendations for an integrative respiratory evaluation of patients for lung resection surgery are provided by the 2013 ACCP clinical practice guidelines, and a simple algorithm to assess lung resectability and categorize preoperative patients into low, moderate, or high-risk groups is described [8••].
Each patient being considered for anatomical lung resection should have a FEV1 and DLCO measured to estimate predicted postoperative values. Patients with both these predicted postoperative values above 60% are deemed at low postoperative pulmonary risk (mortality risk under 1%). Patients who do not meet these criteria may require simple screening exercise testing or formal cardiopulmonary exercise testing. Those with CPET derived VO2 peak below 10 mL/kg/min or 35% of predicted are deemed at high risk of cardiopulmonary morbidity and mortality and less invasive surgical or non-surgical options should be considered (see Fig. 2 in reference [8••] for details).
When evaluating lung resection candidates, certain considerations are important.
Many clinical conditions may have significant impact on the patient’s pulmonary function or cardiopulmonary exercise tests without affecting postoperative impairment proportionally. Examples include poorly optimized cardiopulmonary comorbidities at the time of testing (e.g., COPD, ongoing pneumonia, and heart failure) and pathologies on the operative side such as obstructing endobronchial lesions, atelectasis, or large pleural effusions. If the lung portion to be resected is significantly less functional than the rest of the lungs, then postoperative impairment may be overestimated by previously described calculations. In this context, particularly useful tests for assessing regional lung function and refining post-resection estimations include ventilation/perfusion scan, dynamic perfusion magnetic resonance imaging, or quantitative computerized tomography (CT) scan [34]. Of those, the ventilation/perfusion scan is preferred as its derived estimations highly correlate with actual postoperative values [21].
Preoperative predictions of postoperative FEV1, DLCO, and VO2 peak tend to overestimate actual long-term post-resection impairment [8••, 35]. This is particularly true in patients with significant COPD: a “lobar volume reduction effect” may be observed, whereby an improvement in lung mechanics and elastic recoil may even lead to enhanced postoperative lung function, especially if the resected lung portion is significantly emphysematous [8••, 36].
Several recent clinical advances such as video-assisted surgical approaches, modern anesthetic and analgesic techniques, and enhanced recovery pathways may allow for postoperative outcomes better than those predicted from older studies [8••, 37].
Finally, given the poor survival prognosis without surgical resection, complex patients or those with estimated borderline perioperative risk should undergo careful risk and benefit analysis that may involve a multidisciplinary team including a thoracic surgeon, an anesthesiologist, a pulmonologist, and an oncologist.
Preoperative evaluation and management includes optimization of the comorbidities commonly encountered in these patients (e.g., COPD, obstructive sleep apnea) [38].
Preoperative Pulmonary Rehabilitation
Many patients presenting for lung resection surgery have advanced lung disease, cardiorespiratory deconditioning, or even pulmonary cachexia syndrome. The preoperative period is an opportunity to optimize patients’ cardiorespiratory capacity and promote a healthy lifestyle in an effort to improve perioperative outcomes and long-term health. Pulmonary rehabilitation consists of lifestyle changes made by the patient with the support of comprehensive multidisciplinary therapeutic interventions including nutritional optimization, smoking cessation, exercise training, education, and stress reduction techniques. A key element of pulmonary rehabilitation is improvement in muscle function and exercise capacity through aerobic and strength training [39].
Applied to the preoperative setting, prehabilitation has been shown to improve postoperative clinical outcomes in major colorectal and cardiac surgery [40, 41]. In patients undergoing lung resection surgery, multiple small studies have described the impact of prehabilitation on postoperative clinical outcomes such as pulmonary complications and hospital length of stay. These studies have been compiled into meta-analyses and systematic reviews that concluded a beneficial effect of prehabilitation [42, 43]. Despite the enthusiastic conclusions of some authors, these studies suffer from significant flaws including heterogeneity in study designs and interventions, selection bias, lack of proper randomization or control arms, and small sample sizes [44••]. However, multiple higher quality studies report clearly improved cardiorespiratory capacity after prehabilitation in this patient population. For example, a randomized controlled trial of 40 COPD patients showed that 3 weeks of high-intensity training before lobectomy significantly improves the preoperative VO2 peak (from 14.9 to 17.8 mL/kg/min, p < 0.001) and minimizes its postoperative decline (postoperative VO2 peak 15.1 vs 11.4 mL/kg/min in the control group, p < 0.01) [45]. Clearly, short-term preoperative rehabilitation programs are feasible and safe to implement within the limited preoperative timeframe before lung cancer resection. Further research is still required to understand how these programs impact postoperative clinical outcomes, precluding strong evidence-based recommendation for clinical practice at this point [8••, 44••].
Another potential benefit of preoperative pulmonary rehabilitation is to improve preoperative cardiopulmonary reserve in patients deemed at high perioperative risk, thereby improving surgical candidacy in patients who may have otherwise been denied tumor resection. Evidence to support this purpose is currently limited. However, a very promising pilot study recruited 8 COPD patients who were initially denied lung cancer resection because of severe pulmonary or exercise capacity impairment (with FEV1 as low as 18% of predicted or 6-min walking distance as short as 120 m). After 4 weeks of an intensive inpatient rehabilitation program, all patients underwent lobectomy with no postoperative mortality and acceptable morbidity (one hemorrhage and one atrial fibrillation) [46].
Smoking Cessation
In the perioperative setting, tobacco smoking causes a variety of detrimental effects: increased carbon monoxide levels, increased mucous production and airway reactivity, coronary vasoconstriction, increased myocardial oxygen consumption, impaired immune function and tissue healing, and enhanced platelet aggregation. These pathophysiologic effects lead to major postoperative adverse consequences such as PPC, myocardial ischemia, surgical site infections, and thromboembolic complications [47, 48]. More specifically related to thoracic surgery, in a recent retrospective study of 666 patients undergoing lung cancer resection surgery, smoking was associated with significantly increased risk of PPC (22.3% vs 3.5%, p < 0.001) and higher postoperative mortality (2% vs 0%, p = 0.025) [49].
Being the most important risk factor for lung cancer, tobacco smoking has been reported in more than 20% of patients at the time of lung resection surgery [50]. Preoperative evaluation and management of this major potentially modifiable risk factor is therefore critical. The long-term benefits of smoking cessation on surgical patients are undisputed and intensive preoperative smoking cessation strategies have clearly been shown to reduce the incidence of postoperative complications as well as increase long-term abstinence rates [51, 52••]. Earlier studies have raised concerns of a paradoxically increased complication rate associated with short-term (<4–8 weeks) preoperative smoking cessation [53]. However, more recent studies have refuted such concerns [54]. Nevertheless, most retrospective reports on lung resection surgery suggest that longer durations of preoperative cessation are associated with improved clinical outcomes [49, 50, 53, 55]. Based on this evidence, international guidelines recommend a minimum duration of preoperative smoking cessation of 4 weeks to improve short-term postoperative outcomes after lung surgery [56••].
Various strategies have been employed to aid preoperative smoking cessation. Interventions that have demonstrated benefits include intensive counseling and pharmacotherapy such as nicotine replacement therapy, bupropion, and varenicline [52••, 57].
Nutritional Support
Whether defined as hypoalbuminemia (< 30 g/L), significant recent weight loss or underweight (body mass index < 18.5 kg/m2), malnutrition is highly prevalent in the thoracic surgical population and is strongly associated with worse postoperative clinical outcomes including mortality [58,59,60,61]. However, it remains unclear whether optimizing nutritional state prior to lung resection surgery results in lower complication rates. In a historical cohort study of patients undergoing lung cancer surgery, addition of intensive nutritional support to conventional pulmonary prehabilitation improved postoperative complication rates only in the subgroup of patients with worse preoperative comorbidities and risk [62]. One small randomized trial (n = 32) reported decreased length of stay after lung resection surgery in patients receiving 10 days of micronutrient supplementation preoperatively, without significant differences in complication rates [63]. Another small randomized trial (n = 58) showed a decreased complication rate and improved postoperative albumin levels in non-malnourished patients receiving immune-modulating diets preoperatively [64]. Current guidelines acknowledge the current limited level of evidence but recommend nutritional screening in patients assessed for lung surgery and preoperative oral nutritional supplements in patients deemed at nutritional risk [56••].
Conclusion
Patients undergoing lung resection surgery are at particular risk of postoperative cardiovascular and pulmonary complications due to shared risk factors and the physiologic consequences of surgery and anesthesia. In this specific patient population, cardiovascular risk assessment can be refined using the Thoracic Revised Cardiac Risk Index and cardiac biomarkers. A “3-legged stool” approach to respiratory assessment, including evaluation of lung mechanics, parenchymal function, and cardiopulmonary reserve is essential to estimate perioperative risk and determine surgical candidacy in all patients undergoing anatomical lung resection. Preoperative pulmonary rehabilitation, including exercise training, nutritional support, and smoking cessation programs, has shown promising results in improving postoperative clinical outcomes and surgical candidacy and will likely play a more important role in perioperative management in the near future. Despite the above-described encouraging data, further research is still needed to elucidate specific controversies in the preoperative management of patients undergoing lung resection. Individualized precise risk assessment to evaluate surgical candidacy with acceptable perioperative risk, management strategies to minimize cardiac and respiratory complications, and long-term clinical benefits of prehabilitation are yet to be defined.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
World Health Organization - https://www.who.int/news-room/fact-sheets/detail/cancer
National Cancer Institute – Surveillance, Epidemiology, and End Results Program https://seer.cancer.gov/statfacts/html/lungb.html
Keshava HB, Boffa DJ. Cardiovascular complications following thoracic surgery. Thorac Surg Clin. 2015;25(4):371–92. https://doi.org/10.1016/j.thorsurg.2015.07.001.
• Frendl G, Sodickson AC, Chung MK, Waldo AL, Gersh BJ, Tisdale JE, et al. AATS guidelines for the prevention and management of perioperative atrial fibrillation and flutter for thoracic surgical procedures. J Thorac Cardiovasc Surg. 2014;148(3):153–93. https://doi.org/10.1016/j.jtcvs.2014.06.036. These guidelines from the American Association for Thoracic Surgery describe the current prevention and management strategies of perioperative atrial fibrillation and flutter in patients undergoing thoracic surgery.
Brunelli A, Varela G, Salati M, Jimenez MF, Pompili C, Novoa N, et al. Recalibration of the revised cardiac risk index in lung resection candidates. Ann Thorac Surg. 2010;90(1):199–203. https://doi.org/10.1016/j.athoracsur.2010.03.042.
Brunelli A, Cassivi SD, Fibla J, Halgren LA, Wigle DA, Allen MS, et al. External validation of the recalibrated thoracic revised cardiac risk index for predicting the risk of major cardiac complications after lung resection. Ann Thorac Surg. 2011;92(2):445–8. https://doi.org/10.1016/j.athoracsur.2011.03.095.
Marret E, Miled F, Bazelly B, El Metaoua S, de Montblanc J, Quesnel C, et al. Risk and protective factors for major complications after pneumonectomy for lung cancer. Interact Cardiovasc Thorac Surg. 2010;10(6):936–9. https://doi.org/10.1510/icvts.2009.231621.
•• Brunelli A, Kim AW, Berger KI, Addrizzo-Harris DJ. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e166S–90S. https://doi.org/10.1378/chest.12-2395. An essential article by the American College of Chest Physicians, providing an evidence-based approach to the preoperative physiologic cardiovascular and respiratory evaluation.
Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(24):2215–45. https://doi.org/10.1161/CIR.0000000000000105.
• Duceppe E, Parlow J, MacDonald P, Lyons K, McMullen M, Srinathan S, et al. Canadian cardiovascular society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33(1):17–32. https://doi.org/10.1016/j.cjca.2016.09.008. A novel feature of these Canadian guidelines are the use of cardiac biomarkers as a cornerstone of perioperative cardiovascular evaluation.
• Thomas DC, Blasberg JD, Arnold BN, Rosen JE, Salazar MC, Detterbeck FC, et al. Validating the thoracic revised cardiac risk index following lung resection. Ann Thorac Surg. 2017;104(2):389–94. https://doi.org/10.1016/j.athoracsur.2017.02.006. This recent study demonstrated that the thRCRI is a valid tool for predicting cardiac complications after a lung resection surgery and may help identify patients who would benefit from additional cardiac evaluation.
Brunelli A, Ferguson MK, Salati M, Vigneswaran WT, Jimenez MF, Varela G. Thoracic revised cardiac risk index is associated with prognosis after resection for stage I lung cancer. Ann Thorac Surg. 2015;100(1):195–200. https://doi.org/10.1016/j.athoracsur.2015.03.103.
Wijeysundera DN, Pearse RM, Shulman MA, Abbott TEF, Torres E, Ambosta A, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet. 2018;391(10140):2631–40. https://doi.org/10.1016/S0140-6736(18)31131-0.
Paul S, Sedrakyan A, Chiu YL, Nasar A, Port JL, Lee PC, et al. Outcomes after lobectomy using thoracoscopy vs thoracotomy: a comparative effectiveness analysis utilizing the Nationwide inpatient sample database. Eur J Cardiothorac Surg. 2013;43(4):813–7. https://doi.org/10.1093/ejcts/ezs428.
Rodseth RN, Biccard BM, Le Manach Y, Sessler DI, Lurati Buse GA, Thabane L, et al. The prognostic value of pre-operative and post-operative B-type natriuretic peptides in patients undergoing noncardiac surgery: B-type natriuretic peptide and N-terminal fragment of pro-B-type natriuretic peptide: a systematic review and individual patient data meta-analysis. J Am Coll Cardiol. 2014;63(2):170–80. https://doi.org/10.1016/j.jacc.2013.08.1630.
• Simmers D, Potgieter D, Ryan L, Fahrner R, Rodseth RN. The use of preoperative B-type natriuretic peptide as a predictor of atrial fibrillation after thoracic surgery: systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2015;29(2):389–95. https://doi.org/10.1053/j.jvca.2014.05.015. This meta-analysis of observational studies included 742 patients and reported an overall incidence of post-thoracic surgery atrial fibrillation of 14.5%. An elevated preoperative BNP was associated with an OR of 3.13 for postoperative atrial fibrillation.
Toufektzian L, Zisis C, Balaka C, Roussakis A. Effectiveness of brain natriuretic peptide in predicting postoperative atrial fibrillation in patients undergoing non-cardiac thoracic surgery. Interact Cardiovasc Thorac Surg. 2015;20(5):654–7. https://doi.org/10.1093/icvts/ivu454.
Nojiri T, Inoue M, Yamamoto K, Maeda H, Takeuchi Y, Funakoshi Y, et al. B-type natriuretic peptide as a predictor of postoperative cardiopulmonary complications in elderly patients undergoing pulmonary resection for lung cancer. Ann Thorac Surg. 2011;92(3):1051–5. https://doi.org/10.1016/j.athoracsur.2011.03.085.
Nojiri T, Inoue M, Shintani Y, Takeuchi Y, Maeda H, Hamasaki T, et al. B-type natriuretic peptide-guided risk assessment for postoperative complications in lung cancer surgery. World J Surg. 2015;39(5):1092–8. https://doi.org/10.1007/s00268-015-2943-6.
Young DJ, McCall PJ, Kirk A, Macfie A, Kinsella J, Shelley BG. B-type natriuretic peptide predicts deterioration in functional capacity following lung resection. Interact Cardiovasc Thorac Surg. 2019;28(6):945–52. https://doi.org/10.1093/icvts/ivz016.
Slinger P, Darling G. Preanesthetic assessment for thoracic surgery. In: Slinger P, editor. Principles and practice of anesthesia for thoracic surgery. 2nd ed. Berlin: Springer Nature; 2019.
Nakahara K, Ohno K, Hashimoto J, Miyoshi S, Maeda H, Matsumura A, et al. Prediction of postoperative respiratory failure in patients undergoing lung resection for lung cancer. Ann Thorac Surg. 1988;46(5):549–52. https://doi.org/10.1016/s0003-4975(10)64694-2.
Markos J, Mullan BP, Hillman DR, Musk AW, Antico VF, Lovegrove FT, et al. Preoperative assessment as a predictor of mortality and morbidity after lung resection. Am Rev Respir Dis. 1989;139(4):902–10. https://doi.org/10.1164/ajrccm/139.4.902.
Kearney DJ, Lee TH, Reilly JJ, DeCamp MM, Sugarbaker DJ. Assessment of operative risk in patients undergoing lung resection. Importance of predicted pulmonary function. Chest. 1994;105(3):753–9. https://doi.org/10.1378/chest.105.3.753.
Choi H, Mazzone P. Preoperative evaluation of the patient with lung cancer being considered for lung resection. Curr Opin Anaesthesiol. 2015;28(1):18–25. https://doi.org/10.1097/ACO.0000000000000149.
Ferguson MK, Watson S, Johnson E, Vigneswaran WT. Predicted postoperative lung function is associated with all-cause long-term mortality after major lung resection for cancer. Eur J Cardiothorac Surg. 2014;45(4):660–4. https://doi.org/10.1093/ejcts/ezt462.
Levett DZH, Jack S, Swart M, Carlisle J, Wilson J, Snowden C, et al. Perioperative cardiopulmonary exercise testing (CPET): consensus clinical guidelines on indications, organization, conduct, and physiological interpretation. Br J Anesth. 2018;120(3):484–500. https://doi.org/10.1016/j.bja.2017.12.007.
• Older PO, Levett DZH. Cardiopulmonary Exercise Testing and Surgery. Ann Am Thorac Soc. 2017;14(Suppl 1):S74–83. https://doi.org/10.1513/AnnalsATS.201610-780FR. Narrative review of the use of cardiopulmonary exercise testing and training in the perioperative setting including description of protocols, interpretation of results and summary of studies of prognostic value and clinical benefits.
Brunelli A, Belardinelli R, Refai M, Salati M, Socci L, Pompili C, et al. Peak oxygen consumption during cardiopulmonary exercise test improves risk stratification in candidates to major lung resection. Chest. 2009;135(5):1260–7. https://doi.org/10.1378/chest.08-2059.
Brunelli A, Pompili C, Salati M, Refai M, Berardi R, Mazzanti P, et al. Preoperative maximum oxygen consumption is associated with prognosis after pulmonary resection in stage I non-small cell lung cancer. Ann Thorac Surg. 2014;98(1):238–42. https://doi.org/10.1016/j.athoracsur.2014.04.029.
Benzo R, Kelley GA, Recchi L, Hofman A, Sciurba F. Complications of lung resection and exercise capacity: a meta-analysis. Respir Med. 2007;101(8):1790–7. https://doi.org/10.1016/j.rmed.2007.02.012.
Brunelli A, Belardinelli R, Pompili C, Xiumé F, Refai M, Salati M, et al. Minute ventilation-to-carbon dioxide output (VE/VCO2) slope is the strongest predictor of respiratory complications and death after pulmonary resection. Ann Thorac Surg. 2012;93(6):1802–6. https://doi.org/10.1016/j.athoracsur.2012.03.022.
Shafiek H, Valera JL, Togores B, Torrecilla JA, Sauleda J, Cosío BG. Risk of postoperative complications in chronic obstructive lung diseases patients considered fit for lung cancer surgery: beyond oxygen consumption. Eur J Cardiothorac Surg. 2016;50(4):772–9. https://doi.org/10.1093/ejcts/ezw104.
Koegelenberg CF, Bolliger CT. Assessing regional lung function. Thorac Surg Clin. 2008;18(1):19–29, v-vi. https://doi.org/10.1016/j.thorsurg.2007.10.001.
Brunelli A, Refai M, Salati M, Xiumé F, Sabbatini A. Predicted versus observed FEV1 and DLCO after major lung resection: a prospective evaluation at different postoperative periods. Ann Thorac Surg. 2007;83(3):1134–9. https://doi.org/10.1016/j.athoracsur.2006.11.062.
Baldi S, Ruffini E, Harari S, Roviaro GC, Nosotti M, Bellaviti N, et al. Does lobectomy for lung cancer in patients with chronic obstructive pulmonary disease affect lung function? A multicenter national study. J Thorac Cardiovasc Surg. 2005;130(6):1616–22. https://doi.org/10.1016/j.jtcvs.2005.06.049.
Ceppa DP, Kosinski AS, Berry MF, Tong BC, Harpole DH, Mitchell JD, et al. Thoracoscopic lobectomy has increasing benefit in patients with poor pulmonary function: a Society of Thoracic Surgeons database analysis. Ann Surg. 2012;256(3):487–93. https://doi.org/10.1097/SLA.0b013e318265819c.
Costescu F, Slinger P. Preoperative pulmonary evaluation. Curr Anesthesiol Rep. 2018;8:52–8. https://doi.org/10.1007/s40140-018-0252-y.
Costescu F, Ma M. Anesthesia for patients with end-stage lung disease. In: Slinger P, editor. Principles and practice of anesthesia for thoracic surgery. 2nd ed. Berlin: Springer Nature; 2019.
Trépanier M, Minnella EM, Paradis T, Awasthi R, Kaneva P, Schwartzman K, et al. Improved disease-free survival after prehabilitation for colorectal cancer surgery. Ann Surg. 2019;270(3):493–501. https://doi.org/10.1097/SLA.0000000000003465.
McCann M, Stamp N, Ngui A, Litton E. Cardiac prehabilitation. J Cardiothorac Vasc Anesth. 2019;33(8):2255–65. https://doi.org/10.1053/j.jvca.2019.01.023.
Steffens D, Beckenkamp PR, Hancock M, Solomon M, Young J. Preoperative exercise halves the postoperative complication rate in patients with lung cancer: a systematic review of the effect of exercise on complications, length of stay and quality of life in patients with cancer. Br J Sports Med. 2018;52(5):344. https://doi.org/10.1136/bjsports-2017-098032.
Li X, Li S, Yan S, Wang Y, Wang X, Sihoe ADL, et al. Impact of preoperative exercise therapy on surgical outcomes in lung cancer patients with or without COPD: a systematic review and meta-analysis. Cancer Manag Res. 2019;11:1765–77. https://doi.org/10.2147/CMAR.S186432.
•• Blaudszun G, Triponez F, Bridevaux PO, Licker MJ. Rehabilitation for thoracic surgical patients: why, when, and how. In: Şentürk M, Orhan Sungur M, editors. Postoperative care in thoracic surgery. Berlin: Springer; 2017. This chapter describes the role of prehabilitation and rehabilitation for thoracic surgical patients with emphasis on the implementation of physical training programs and CPET evaluation.
Stefanelli F, Meoli I, Cobuccio R, Curcio C, Amore D, Casazza D, et al. High-intensity training and cardiopulmonary exercise testing in patients with chronic obstructive pulmonary disease and non-small-cell lung cancer undergoing lobectomy. Eur J Cardiothorac Surg. 2013;44(4):e260–5. https://doi.org/10.1093/ejcts/ezt375.
Cesario A, Ferri L, Galetta D, Cardaci V, Biscione G, Pasqua F, et al. Pre-operative pulmonary rehabilitation and surgery for lung cancer. Lung Cancer. 2007;57(1):118–9. https://doi.org/10.1016/j.lungcan.2007.03.022.
Turan A, Mascha EJ, Roberman D, Turner PL, You J, Kurz A, et al. Smoking and perioperative outcomes. Anesthesiology. 2011;114(4):837–46. https://doi.org/10.1097/ALN.0b013e318210f560.
Musallam KM, Rosendaal FR, Zaatari G, Soweid A, Hoballah JJ, Sfeir PM, et al. Smoking and the risk of mortality and vascular and respiratory events in patients undergoing major surgery. JAMA Surg. 2013;148(8):755–62. https://doi.org/10.1001/jamasurg.2013.2360.
Fukui M, Suzuki K, Matsunaga T, Oh S, Takamochi K. Importance of smoking cessation on surgical outcome in primary lung Cancer. Ann Thorac Surg. 2019;107(4):1005–9. https://doi.org/10.1016/j.athoracsur.2018.12.002.
Lugg ST, Tikka T, Agostini PJ, Kerr A, Adams K, Kalkat MS, et al. Smoking and timing of cessation on postoperative pulmonary complications after curative-intent lung cancer surgery. J Cardiothorac Surg. 2017;12(1):52. https://doi.org/10.1186/s13019-017-0614-4.
Thomsen T, Tønnesen H, Møller AM. Effect of preoperative smoking cessation interventions on postoperative complications and smoking cessation. Br J Surg. 2009;96(5):451–61. https://doi.org/10.1002/bjs.6591.
•• Thomsen T, Villebro N, Møller AM. Interventions for preoperative smoking cessation. Cochrane Syst Rev. 2014. https://doi.org/10.1002/14651858.CD002294.pub4. This Cochrane review assesses the effect of various preoperative smoking interventions on smoking cessation and postoperative complications.
Nakagawa M, Tanaka H, Tsukuma H. Relationship between the duration of the preoperative smoke-free period and the incidence of postoperative pulmonary complications after pulmonary surgery. Chest. 2001;120:705–10.
Wong J, Lam DP, Abrishami A, Chan MTV, Chung F. Short-term preoperative smoking cessation and postoperative complications: a systematic review and meta-analysis. Can J Anesth. 2012;59(3):268–79. https://doi.org/10.1007/s12630-011-9652-x.
Mason DP, Subramanian S, Nowicki ER, Grab JD, Murthy SC, Rice TW, et al. Impact of smoking cessation before resection of lung cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database study. Ann Thorac Surg. 2009;88(2):362–70. https://doi.org/10.1016/j.athoracsur.2009.04.035.
•• Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, Brunelli A, Cerfolio RJ, Gonzalez M, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the enhanced recovery after surgery society and the European Society of Thoracic Surgeons. Eur J Cardiothorac Surg. 2019;55:91–115. https://doi.org/10.1093/ejcts/ezy301. This article summarizes all the current evidence regarding enhanced recovery after lung surgery and gives specific recommendations with a graded the level of evidence.
Baldini G. Perioperative smoking and alcohol cessation. In: In press.
Tewari N, Martin-Ucar AE, Black E, Beggs L, Beggs D, Duffy JP, et al. Nutritional status affects long term survival after lobectomy for lung cancer. Ann Thorac Surg. 2007;57(3):389–94. https://doi.org/10.1016/j.lungcan.2007.03.017.
Bagan P, Berna P, De Dominicis F, Das Neves Pereira JC, Mordant P, De La Tour B, et al. Nutritional status and postoperative outcome after pneumonectomy for lung cancer. Ann Thorac Surg. 2013;95(2):392–6. https://doi.org/10.1016/j.athoracsur.2012.06.023.
Nakagawa T, Toyazaki T, Chiba N, Ueda Y, Gotoh M. Prognostic value of body mass index and change in body weight in postoperative outcomes of lung cancer surgery. Interact Cardiovasc Thorac Surg. 2016;23(4):560–6. https://doi.org/10.1093/icvts/ivw175.
Shoji F, Morodomi Y, Akamine T, Takamori S, Katsura M, Takada K, et al. Predictive impact for postoperative recurrence using the preoperative prognostic nutritional index in pathological stage I non-small cell lung cancer. Lung Cancer. 2016;98:15–21. https://doi.org/10.1016/j.lungcan.2016.05.010.
Harada H, Yamashita Y, Misumi K, Tsubokawa N, Nakao J, Matsutani L, et al. Multidisciplinary team-based approach for comprehensive preoperative pulmonary rehabilitation including intensive nutritional support for lung cancer patients. PLoS One. 2013;8(3):e59566. https://doi.org/10.1371/journal.pone.0059566.
Matzi V, Lindenmann J, Muench A, Greilberger J, Juan H, Wintersteiger R, et al. The impact of preoperative micronutrient supplementation in lung surgery. A prospective randomized trial of oral supplementation of combined α-ketoglutaric acid and 5-hydroxymethylfurfural. Eur J Cardiothorac Surg. 2007;32(5):776–82. https://doi.org/10.1016/j.ejcts.2007.07.016.
Kaya SO, Akcam TI, Ceylan KC, Samancilar O, Ozturk O, Usluer O. Is preoperative protein-rich nutrition effective on postoperative outcome in non-small cell lung cancer surgery? A prospective randomized study. J Cardiothorac Surg. 2016;11(14):11–8. https://doi.org/10.1186/s13019-016-0407-1.
Acknowledgments
The authors thank Dr. Gabriele Baldini and Dr. Enrico Minnella, from the Department of Anesthesia, McGill University Health Centre, for their expertise and input regarding preoperative rehabilitation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Alonso Blanch, Florin Costescu, and Peter Slinger declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any original studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Preoperative Evaluation
Rights and permissions
About this article
Cite this article
Blanch, A., Costescu, F. & Slinger, P. Preoperative Evaluation for Lung Resection Surgery. Curr Anesthesiol Rep 10, 176–184 (2020). https://doi.org/10.1007/s40140-020-00376-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40140-020-00376-8