Abstract
Purpose of Review
This paper provides an updated review on preoperative airway assessment, the factors that are used to predict a difficult airway, and whether difficulty can be accurately predicted.
Recent Findings
Traditional history and examination features have focused on prediction of difficult intubation, but recent studies attempt to predict difficulty with other aspects of managing the airway including bag-mask ventilation, supraglottic airway (SGA) ventilation, and front-of-neck access. A recent Cochrane review confirms that traditional examination findings lack diagnostic accuracy for detection of difficult airways. There is promise in the use of airway imaging techniques including endoscopy and ultrasound, for preoperative prediction of difficult airways.
Summary
Despite thorough assessment, a significant percentage of airway difficulties will continue to be unanticipated, and there is no single test, or combination of tests, that can accurately and reliably predict a difficult airway. The anesthesiologist should be prepared for unanticipated difficulty, as prediction remains imprecise. Further studies are required to refine the specific parameters and measurements expected for newer imaging modalities which might be beneficial, including airway ultrasound and endoscopy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Preoperative airway assessment is recommended for all patients [1,2,3] and predicting difficulties is clearly important, as the implications of failure to secure the airway are severe. The NAP 4 audit [4] revealed that poor airway assessment and failure to plan for failure were common themes. Failure to adequately assess the airway and identify potential difficulties may lead to inappropriate airway management choices, inability to oxygenate the patient, cerebral hypoxemia, or even death. However being able to accurately predict difficulties remains elusive, with up to 93% of difficult intubations being unanticipated and most often when a difficult intubation is predicted, it does not eventuate [5•].
Much of the literature surrounding airway assessment and most bedside examination tests relate to the prediction of difficult direct laryngoscopy or difficult intubation. However, complete airway assessment must attempt to predict difficulty in all three non-surgical airway techniques (bag mask ventilation, supraglottic airway ventilation, and intubation) [6] as well as prediction of difficult front of neck access. When reviewing studies that analyze predictive airway tests, it is important to differentiate whether the end point was difficult laryngoscopy, rather than difficult intubation.
Difficult Laryngoscopy
Difficult laryngoscopy (DL) is defined by failure to visualize the vocal cords (Cormack and Lehane grades 3 and 4) [7]. The Cormack and Lehane grade is defined as follows: grade 1, full glottic view; grade 2, partial glottic view; grade 3, epiglottis only, glottis not seen; and grade 4, neither glottis nor epiglottis seen. Given the increasing use of video laryngoscopy, it is important for a narrative to accompany this grade – such as the chosen device and the difficulty encountered [1]. The reported incidence of grade 3 laryngoscopy is 0.8–7.0% [1, 8] and grade 4 laryngoscopy 0.1–3.2% [1, 8, 9].
Difficult and Failed Intubation
The American Society of Anesthesiologists define difficult intubation (DI) as requiring multiple attempts, in the presence or absence of tracheal pathology [2]. Alternative definitions generally include multiple attempts at intubation or use of additional intubation devices [1, 5•, 10, 11]. The Intubation Difficulty Scale was proposed by Adnet et al. in 1997 [12] to assess difficult intubation. This scale incorporates seven variables including number of intubating attempts and Cormack and Lehane grade, and a score of > 5 is consistent with a definition of difficult intubation [12]. The incidence of difficult intubation is 1.8% in large studies [10], 1.73% in large databases [5•], and 5.8% in meta-analysis [13]. Failed intubation is easier to define, with an incidence of approximately 0.3% [10].
Difficult and Impossible Mask Ventilation
Langeron et al. [14] were among the first to publish predictors of difficult BMV, as shown in Table 2. They defined difficult mask ventilation (DMV) as “inability of an unassisted anesthesiologist to maintain oxygen saturations above 92% (measured by pulse oximetry) or to prevent or reverse signs of inadequate ventilation during positive pressure mask ventilation under general anesthesia” and found an incidence of 5% [14]. Subsequent larger studies by Kheterpal et al. using a 4 point scale to describe BMV [15] reported a 1.4% incidence of difficult mask ventilation [16•] and predictors, as shown in Table 2. Impossible mask ventilation (IMV) is the inability to exchange air during BMV attempts, despite multiple providers, airway adjuncts, or neuromuscular blockade [17•]. It has a lower incidence of approximately 0.15% [17•].
Combined Difficulty with Ventilation and Intubation
It is most important to predict when a patient will fail both intubation and ventilation via a face mask or supraglottic device [1]. In a study of 176,679 patients, Kheterpal showed a 0.4% incidence of combined difficult mask ventilation with difficult laryngoscopy although only one patient required an emergency cricothyroidotomy [18]. A similar 0.3% incidence was found in analysis of the Danish anesthesia database, and the vast majority was unpredicted [19].
Why Predict Airway Difficulties?
By predicting airway difficulties during the preoperative assessment, the anesthesiologist has a number of options, including:
Increased emphasis on this aspect during patient discussion and consent
Increased attention to patient positioning and pre-oxygenation
Use of a regional technique as an alternative to general anesthesia
Gathering of additional or different personnel, including surgical assistance for a “double set-up”
Preparation of additional equipment
An alternative location, such as a move from office-based anesthesia to hospital, or small hospital to one with better resources
Induction of anesthesia with maintenance of spontaneous ventilation
Awake tracheal intubation or awake tracheostomy
Or rarely, establishment of peripheral femoral extra-corporal membrane oxygenation under local anesthesia
History
A history of a difficult airway is a strong predictor of a future challenging airway [20]. In addition to information obtained directly from the patient, the anesthesiologist should gather previous anesthetic records, difficult airway letters, or medic-alert bracelets. From the old anesthesia record, the ease or difficulties with ventilation, laryngoscopy, and intubation should be reviewed. Any conditions known to affect the airway such as pregnancy, rheumatoid arthritis, diabetes, and congenital syndromes such as Pierre-Robin, Goldenhar, Treacher-Collins, Klippel-Feil, and Down syndrome should be determined. Acromegaly has a four- to fivefold [9, 21, 22] increased incidence of difficult intubation due to macroglossia, prognathism, enlarged and distorted laryngeal anatomy, and a large epiglottis [21]. In patients with thyroid disease, an increased risk of difficult intubation was noted when goiter is combined with tracheal deviation [23, 24]. Cervical spine disease is associated with an increased incidence of difficult laryngoscopy [25, 26], difficult intubation [26], and in those < 60 years, DMV [26]. In the population > 60 years, mask ventilation was not more difficult, which is explained by a general higher incidence of DMV in this age range [26].
Patients with obstructive sleep apnea often have a large tongue and narrow oropharyngeal space. A systematic review of 16 studies and 266,603 patients concluded that patients with obstructive sleep apnea had a three- to fourfold higher risk of DI or DMV compared to patients without obstructive sleep apnea [27•].
Previous surgery or radiotherapy to the head and neck can result in airway edema, restricted mouth opening, and decreased neck extension [28]. Neck radiation is associated with DI and difficult front-of-neck access and was found to be the most significant predictor of IMV [17]. Burns to the head and neck restrict mouth opening, reduce mandibular space compliance, and can cause a fixed flexion deformity of the neck. Accompanying inhalational injury can cause tracheal stenosis [29]. The degree of contracture according to the Onah classification correlates with view on laryngoscopy [26].
Acute conditions that can affect the airway are suggested by a history of hoarseness or voice change, inability to lie flat, shortness of breath, or difficulty of swallowing. Infection (dental abscess, Ludwig’s angina, epiglottitis) or hematoma of the mouth, tongue, pharynx, larynx, trachea, or neck can cause airway difficulty [9]. Tumors of the head and neck are strongly associated with difficult airway management, with a four- to fivefold increased incidence of DI [30].
Although pediatrics is beyond the scope of this review, difficult direct laryngoscopy is rare in well children. In a retrospective analysis of 11,219 patients, the incidence of DL was found to be 4.7% in children < 1 year and 0.7% in children > 1 year [31]. Unexpected difficult BMV is also rare in pediatrics [1].
When taking a history, it is important to assess the anticipated level of patient cooperation, which may be limited in pediatric patients or adults with either cognitive impairment or critical illness.
Examination
Examination features that may suggest airway difficulty include large tongue, receded chin, reduced mouth opening, and prominent upper incisors. A narrow or highly arched palate [20, 32] impairs visualization and manipulation of the laryngoscope within the mouth [33]. Reduced compliance of the submandibular region might be seen in patients with previous burns or radiation treatment, which limits the ability to displace the tongue into this space [33]. A short thick neck will result in difficulty extending the neck and aligning the upper airway axes [33]. Obese patients have accumulation of fat deposits that narrow the airway and reduce the anterior mobility of pharyngeal structure [34]. A short thick immobile neck is caused by cervical spine fat pads [35]. A study of morbidly obese patients (BMI > 40) reported a 13% incidence of DI and 11% incidence of DMV, with BMI > 50 and neck circumference > 42 cm independent predictors of difficulty [36]. BMI has previously been shown to be an independent predictor for difficult BMV [14, 16•] but not an independent risk factor for IMV [17•].
In addition to general examination features, a number of specific tests (Table 1) attempt to predict difficult intubation or difficult laryngoscopy. An ideal bedside test should be easy to perform, highly sensitive and specific. Although most bedside tests have high specificity and negative predictive value, unfortunately they have a low sensitivity and positive predictive value – the latter is influenced by the low prevalence of difficult airways [37]. Other reasons why bedside airway tests perform poorly include the use of poorly defined end points or inaccurate measurements like “fingerbreadths,” high inter-observer variability [38, 39], and uncertain cut-off values to determine the best predictive score. Most tests also fail to take into account racial differences in anatomy that will alter the usefulness of predictive tests and the optimal cut-off values [40].
A recent Cochrane review [41••] including 133 studies of 844,206 adults assessed the diagnostic accuracy of these tests, separately analyzed for DI vs DL. The upper lip bite test showed the most favorable diagnostic accuracy for DL with a summary sensitivity of 67%. Modified Mallampati had the highest sensitivity for detecting DI, but this was a modest 51%. The review concluded that none of the common bedside screening tests are well suited for detecting unanticipated difficult airways, urging caution in their use and interpretation [40].
In an attempt to improve accuracy of bedside tests, the use of measurement devices has been encouraged [42] and ratios according to the patient’s height have been incorporated [43, 44]. A number of multi-factorial risk index systems have been developed, including the Wilson score [45] Arne risk index [30], and El-Ganzouri simplified airway risk index [8] although they have not shown tremendous promise in improving accuracy of prediction [8, 25, 30, 45]. When the El-Ganzouri index was tested in 64,273 patients, there was no significant difference between standard airway assessment and the use of simplified airway risk index in predicting difficult intubation [46•].
The context in which airway management will occur must also be considered. This includes the providers’ experience and level of skill, available assistance, location, and equipment available in the planned location of intervention.
Imaging
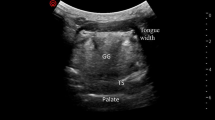
Ultrasound is inexpensive, readily available at the bedside [47] and can provide dynamic information, as such, it is an increasing part of the armamentarium to predict difficult intubation. It can identify the cricothyroid membrane [48, 49] and other superficial structures including the tongue, hyoid bone, vocal cords, and trachea [22, 49]. Structures posterior to the air/tissue interface, including the posterior wall of trachea and posterior commissure, are poorly visualized.
The ratio of the pre-epiglottic space depth: epiglottis to vocal cord distance has been called an “ultrasound modification” of the Cormack and Lehane score. The average time for examination is 31.7 ± 12.4 s [50]. The anterior neck soft tissue thickness at the level of the vocal cords discriminated between easy and difficult intubation in obese patients [51]. A review of 10 studies evaluating the utility of ultrasound for preoperative difficult intubation assessment [52•] found three significant measurements for predicting DL. These measurements were the hyomental distance and anterior soft tissue thickness at the level of the hyoid bone and at the level of the thyro-hyoid membrane [51]. The relationship between ultrasound measurements of the airway and those found on computed tomography (CT) has been investigated, with infrahyoid measurements showing good correlation. Limitations to ultrasound measurements have been identified including the need to use a defined head position during scanning in order to standardize measurements [53]. Gastric ultrasound has been used to assess fasting status and assess aspiration risk [54, 55].
Assessment of infra-glottic lesions causing distortion or narrowing of the airway relies heavily on CT or magnetic resonance imaging (MRI) imaging [9] as this location is not reliably assessed with patient examination. The degree and location of a tracheal stenosis can be assessed [56], and the size and location of masses and other abnormalities can be determined [20]. Deep neck infections are ideally analyzed with imaging, to identify the location, extent of infection, and relationship to the airway [57]. CT or MRI imaging can add to the preoperative evaluation of patients with congenital syndromes affecting the airway, such as Treacher Collins syndrome [58].
With an increased emphasis on outpatient services and consumer convenience, anesthesiologists may become increasingly reliant on telemedicine assessments prior to the day of surgery. Patients have been asked to send head shots of themselves, taken on a smartphone to the anesthesiologist, which leads to changes in the planned location of anesthesia due to anticipated difficulties [59]. Advances in computer modeling have allowed objective analysis of facial anatomy via patient photographs to predict difficult intubation [22].
Nasoendoscopy and Virtual Endoscopy
Nasoendoscopy is an extension of the clinical examination, with particular relevance for the more distal airway. It can diagnose thyroglossal cysts, vallecular cysts, and lingual tonsillar hypertrophy [9]. Lingual tonsillar hypertrophy is a common cause of unexpected failed intubation [60] as the mass fills the vallecula and displaces the epiglottis posteriorly [61]. In a cohort of 138 patients undergoing elective otolaryngologic procedures, preoperative endoscopic airway examination altered the planned airway management (from asleep intubation to awake intubation or vice versa) in 26% of the patients [62•].
Using multi-slice, three-dimensional CT scans, a virtual endoscopy model can provide information on the extent and location of obstructing or distorting pharyngeal, laryngeal, or distal airway pathology without the need for fibreoptic nasendoscopy [41••, 63,64,65,66]. This allows visualization of the distal airway beyond a stenosis that cannot be traversed by a bronchoscope [67].
Predictors of Difficult Bag Mask Ventilation, Supraglottic Airway Ventilation, Videolaryngoscopy, and Front-of-Neck Access
In 2000, Langeron et al. studied 1502 patients and found 5 independent predictors for DMV [14]. In 2006, Kheterpal et al. studied 22,660 patients and found many similar predictors [16•]. Following this study, Kheterpal et al. used a larger group of 53,041 patients to more accurately identify risk factors for IMV [17]. These predictors are shown in Table 2.
Very few studies have assessed SGA insertion, which has a failure incidence of 0.1–4.7% [20]. Some predictors of difficult SGA ventilation are shown in Table 2 [9, 63].
Angulated blade video laryngoscopy does not require alignment of the airway axes, thus predictors for success are not the same as for DL. Reported predictors for difficult Glidescope® intubation are shown in Table 2 [63].
In case non-surgical methods for managing the airway fail [6], it is sensible to consider whether emergency front of neck access will be exceptionally difficult. Because of the rarity of these situations, predictors of difficult front of neck access are found to have limited accuracy; the most useful predictors are shown in Table 2.
Difficult Nasal Intubation or Double Lumen Tube Insertion
If a nasal endotracheal tube (ETT) is required for the procedure, nasal patency should be assessed. If lung isolation is required, it is necessary to assess the ability to insert the larger double lumen tube (DLT). Palcynski et al. found that the thyromental height test performed better to predict DI with a DLT, with sensitivity 70% and specificity 70% [68].
Prediction and Planning for Difficult Extubation
It is also important to plan for extubation during the preoperative airway assessment, especially when a difficult airway is anticipated [2, 63, 69]. Early planning of extubation might influence the location of the procedure and the type and site of insertion of an ETT (i.e., orally with a standard ETT vs nasal reinforced ETT if post-operative ventilation is planned).
Conclusion
Preoperative airway assessment requires a thorough history, physical and airway examination, previous anesthesia record, and, for certain patients, supplemental nasoendoscopy or imaging. Assessment aims to identify potential difficulties with BMV, DL, video laryngoscopy, tracheal intubation, SGA ventilation, and emergency front of neck access (Table 1 and Table 2) [22, 70]. In addition, the anesthesiologist should consider which airway device is optimal to protect and maintain the airway and assess the risk of aspiration or rapid desaturation with apnea. For patients identified to be at risk for difficult airway management, a clear plan for airway management with back up plans should be communicated with and clearly understood by the perioperative team prior to proceeding with anesthesia induction. When difficulty is anticipated, preparations may include additional equipment, alternative location, or additional skilled operators to assist. A plan for extubation should also begin during this preoperative assessment.
Future directions for research include standardized definitions of difficult DL, intubation, and ventilation. Reproducibility of bedside tests can be improved with the use of measurement devices, and the ongoing search for the elusive highly sensitive bedside test will likely continue. The role of ultrasound measured parameters and preoperative nasal endoscopy needs to be further studied and defined. Despite thorough assessment, a significant percentage of airway difficulties will continue to be unanticipated, and thus preoperative planning must include preparations for unexpected difficulties.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Law JA, Broemling N, Cooper RM, Drolet P, Duggan LV, Griesdale DE, et al. The difficult airway with recommendations for management--part 1--difficult tracheal intubation encountered in an unconscious/induced patient. Can J Anesth. 2013;60(11):1089–118.
Apfelbaum J, Hagberg C, Caplan R, Blitt C, Connis R, Nickinovich D. Practice guidelines for management of the difficult airway. An updated report by the American Society of Anesthesiologists Task Force on management of the difficult airway. Anesthesiology. 2013;118:251–70.
Frerk C, Mitchell VS, McNarry AF, et al. Difficult airway society 2015 guidelines for management of unanticipated difficult intubation in adults. Br J Anaesth. 2015;115:827–48.
Cook T, Woodall N, Frerk C. Fourth National Audit Project. Major complications of airway management in the UK: results of the fourth National Audit Project of the Royal College of Anaesthetists and the difficult airway society. Part 1: Anaesthesia. Br J Anaesth. 2011;106:617–31.
• Norskov AK, Rosenstock CV, Wetterslev J, Astrup G, Afshari A, Lundstrom LH. Diagnostic accuracy of anaesthesiologists' prediction of difficult airway management in daily clinical practice: a cohort study of 188 064 patients registered in the Danish Anaesthesia Database. Anaesthesia. 2015;70(3):272–81 To estimate accuracy of anaesthesiologists’ prediction of difficult airways, a retrospective study of 188,064 patients was undertaken. 93% of difficult intubations were unanticipated, and when a difficult intubation was anticipated, it only proved to be difficult in 25% of cases.
Chrimes N. The vortex: a universal ‘high-acuity implementation tool’ for emergency airway management. Br J Anaesth. 2016;117:i20–i7.
Cormack R, Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia. 1984;39:1105–11.
El-Ganzouri A, McCarthy R, Tuman K, Tanck E, Ivankovich A. Preoperative airway assessment: predictive value of a multivariate risk index. Anaesth Analg. 1996;82:1197–204.
Pearce A. Evaluation of the airway and preparation for difficulty. Best Pract Res Clin Anaesthesiol. 2005;19(4):559–79.
Rose D, Cohen M. The airway: problems and predictions in 18,500 patients. Can J Anesth. 1994;41(5):372–83.
Karkouti K, Rose K, Wigglesworth D, Cohen M. Predicting difficult intubation: a multivariable analysis. Can J Anesth. 2000;47(8):730–9.
Adnet F, Borron S, Racine S. The intubation difficulty scale (IDS): proposal and evaluation of a new score characterizing the complexity of endotracheal intubation. Anesthesiology. 1997;87:1290–7.
Shiga T, Wajima Z, Inoue T, Sakamoto A. Predicting difficult intubation in apparently normal patients. Anesthesiology. 2005;103:429–37.
Langeron O, Masso E, Huraux C, Guggiari M, Bianchi A, Coriat P, et al. Prediction of difficult mask ventilation. Anesthesiology. 2000;92:1229–36.
Han R, Tremper K, Kheterpal S, O’Reilly M. Grading scale for mask ventilation. Anesthesiology. 2004;101:267.
• Kheterpal S, Han R, Tremper K, Shanks A, Tait A, O’Reilly M, et al. Incidence and Prediction of difficult and impossible mask ventilation. Anesthesiology. 2006;105:885–91 A large study to assess incidence and predictors of difficult mask ventilation in 22,660 patients. Incidence of DMV was 1.4% and several predictors were identified including age > 57 years, BMI > 30, Beard, snoring, MP 3 or 4 and severely limited jaw protrusion.
• Kheterpal S, Martin L, Shanks A, Tremper K. Prediction and outcomes of impossible mask ventilation. A review of 50,000 Anesthetics. Anesthesiology. 2009;110:891–7 A cohort of 53,041 patients was studied to assess the incidence of IMV, revealing a 0.15% incidence. Neck radiation was the most predictive factor for difficulty, with other predictors including sleep apnea, male, mallampati 3 or 4 and a beard.
Kheterpal S, Healy D, Aziz M, Shanks A, Freundlich R, Linton F, et al. Incidence, predictors and outcome of difficult mask ventilation combined with difficult laryngoscopy. Anesthesiology. 2013;119(6):1360–9.
Norskov AK, Wetterslev J, Rosenstock CV, Afshari A, Astrup G, Jakobsen JC, et al. Prediction of difficult mask ventilation using a systematic assessment of risk factors vs. existing practice - a cluster randomised clinical trial in 94,006 patients. Anaesthesia. 2017;72(3):296–308.
Zhou C, Chung F, Wong DT. Clinical assessment for the identification of the potentially difficult airway. Perioperative Care and Operating Room Management. 2017;9:16–9.
Schmitt H, Buchfelder M, Radespiel-Troger M, Fahlbusch R. Difficult intubation in acromegalic patients. Incidence and predictability Anesthesiology. 2000;93:110–4.
Baker P. Assessment before airway management. Anesthesiol Clin. 2015;33(2):257–78.
Voyagis G, Kyriakis P. The effect of goiter on endotracheal intubation. Anesth Analg. 1997;84:611–2.
Kalezić N, Sabljak V, Stevanović K, Miličić B, Marković D, Tošković A, et al. Predictors of difficult airway management in thyroid surgery: a five-year observational single-center prospective study. Acta Clinica Croatica. 2016;55:9–17.
Han YZ, Tian Y, Xu M, Ni C, Li M, Wang J, et al. Neck circumference to inter-incisor gap ratio: a new predictor of difficult laryngoscopy in cervical spondylosis patients. BMC Anesthesiol. 2017;17(1):55.
Mashour G, Stallner M, Kheterpal S, Shanks A. Predictors of difficult intubation in patients with cervical spine limitation. J Neurosurg Anesthesiol. 2008;20:1110–5.
• Nagappa M, Wong DT, Cozowicz C, Ramachandran SK, Memtsoudis SG, Chung F. Is obstructive sleep apnea associated with difficult airway? Evidence from a systematic review and meta-analysis of prospective and retrospective cohort studies. PLoS One. 2018;13(10):e0204904 To determine the impact of obstructive sleep apnea on airway difficulties, this systematic review and meta-analysis concluded that patients with obstructive sleep apnea had a 3–4 fold higher risk of DI or DMV compared to patients without obstructive sleep apnea.
O'Dell K. Predictors of difficult intubation and the otolaryngology perioperative consult. Anesthesiol Clin. 2015;33(2):279–90.
Prakash S, Mullick P. Airway management in patients with burn contractures of the neck. Burns. 2015;41(8):1627–35.
Arne J, Descoins P, Fushiardi J, Ingrand P, Ferrier B, Boudigues D, et al. Preoperative assessment for difficult intubation in general and ENT surgery: predictive value of a clinical multivariate risk index. Br J Anaesth. 1998;80:140–6.
Heinrich S, Birkholz T, Ihmsen H, Irouschek A, Ackermann A, Schmidt J. Incidence and predictors of difficult laryngoscopy in 11,219 pediatric anesthesia procedures. Paediatr Anaesth. 2012;8(22):729–36.
Schwartz A. Airway Management for the Oral Surgery Patient. Oral Maxillofac Surg Clin North Am. 2018;30(2):207–26.
Greenland K. Airway assessment based on a three column model of direct laryngoscopy. Anesth Intensive Care. 2010;38:14–9.
Langeron O, Birenbaum A, Le Sache F, Raux M. Airway management in obese patient. Minerva Anesthesiol. 2014;80(3):382–92.
Rosenberg M, Phero J. Airway assessment for office sedation/anesthesia. Anesth Prog. 2015;62:74–80.
Riad W, Vaez MN, Raveendran R, Tam AD, Quereshy FA, Chung F, et al. Neck circumference as a predictor of difficult intubation and difficult mask ventilation in morbidly obese patients: a prospective observational study. Eur J Anaesthesiol. 2016;33(4):244–9.
Yentis S. Predicting difficult intubation - worthwhile exercise or pointless ritual? Anaesthesia. 2002;57:105–9.
Karkouti K, Rose D, Ferris L, Wigglesworth D, Meisami-Fard T, Lee H. Inter-observer reliability of ten tests used for predicting difficult tracheal intubation. Can J Anesth. 1996;43(6):554–9.
Adamus M, Jor O, Vavreckova T, Hrabalek L, Zapletalova J, Gabrhelik T, et al. Inter-observer reproducibility of 15 tests used for predicting difficult intubation. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2011;155(3):275–81.
Merah N, Wong DT, Ffoulkes-Crabbe D, Kushimo O, Bode C. Modified Mallampati test, thyromental distance and inter-incisor gap are the best predictors of difficult laryngoscopy in west Africans. Can J Anaesth. 2005;52(3):291–6.
•• Roth D, Pace NL, Lee A, Hovhannisyan K, Warenits AM, Arrich J, et al. Bedside tests for predicting difficult airways: an abridged Cochrane diagnostic test accuracy systematic review. Anaesthesia. 2019;74(7):915–28 Cochrane review including 133 studies of 844,206 adults assessing the diagnostic accuracy of common bedside screening tests for detection of difficult airways, concluded that no tests are well suited for detecting unanticipated difficult airways, urging caution in their use and interpretation.
Etezadi F, Ahangari A, Shokri H, Najafi A, Khajavi MR, Daghigh M, et al. Thyromental height: a new clinical test for prediction of difficult laryngoscopy. Anesth Analg. 2013;117(6):1347–51.
Krobbuaban B, Diregpoke S, Kumkeaw S, Tanomsat M. The predictive value of the height ratio and thyromental distance: four predictive tests for difficult laryngoscopy. Anesth Analg. 2005;101(5):1542–5.
Honarmand A, Safavi MR. Prediction of difficult laryngoscopy in obstetric patients scheduled for caesarean delivery. Eur J Anaesthesiol. 2008;25(9):714–20.
Wilson M, Spiegelhalter D, Roberston J, Lesser P. Predicting difficult intubation. Br J Anaesth. 1998;61:211–6.
• Norskov AK, Wetterslev J, Rosenstock CV, Afshari A, Astrup G, Jakobsen JC, et al. Effects of using the simplified airway risk index vs usual airway assessment on unanticipated difficult tracheal intubation - a cluster randomized trial with 64,273 participants. Br J Anaesth. 2016;116(5):680–9 When comparing the use of a multivariate risk index (the simplified airway risk index) to standard airway assessment involving 64,273 patients there was no statistically significant difference in the detection of difficult intubation.
Petrisor C, Dirzu D, Tranca S, Hagau N, Bodolea C. Preoperative difficult airway prediction using suprahyoid and infrahyoid ultrasonography derived measurements in anesthesiology. Med Ultrason. 2019;21(1):83–8.
You-Ten KE, Wong DT, Ye XY, Arzola C, Zand A, Siddiqui N. Practice of ultrasound-guided palpation of neck landmarks improves accuracy of external palpation of the cricothyroid membrane. Anesth Analg. 2018;127(6):1377–82.
Votruba J, Zemanova P, Lambert L, Vesela MM. The role of airway and endobronchial ultrasound in perioperative medicine. Biomed Res Int. 2015;2015:754626.
Gupta D. Srirajakalidindi, Ittiara B, apple L, Toshniwal G, Haber H. Ultrasonographic modifiction of cormack-lehane classification for pre-anesthetic airway assessment. MEJ Anesth. 2012;21(6):835–42.
Ezri T, Gewürtz G, Sessler D. Prediction of difficult laryngoscopy in obese patients by ultrasound quantification of anterior neck soft tissue. Anaesthesia. 2003;58:1111–4.
• Fulkerson JS, Moore HM, Anderson TS, Lowe RF Jr. Ultrasonography in the preoperative difficult airway assessment. J Clin Monit Comput. 2017;31(3):513–30 Review of studies using ultrasound to detect difficult intubation, concluding that ultrasound shows promise for enhancing difficult airway assessment, but more studies are needed.
Prasad A, Yu E, Wong DT, Karkhanis R, Gullane P, Chan V. Comparison of sonography and computed tomography as imaging tools for assessment of airway structure. J Ultrasound Med. 2011;30:965–72.
Perlas A, Arzola C, Van de Putte P. Point-of-care gastric ultrasound and aspiration risk assessment: a narrative review. Can J Anaesth. 2018;65(4):437–48.
Benhamou D. Ultrasound assessment of gastric contents in the perioperative period: why is this not part of our daily practice? Br J Anaesth. 2015;114(4):545–8.
Yamaguchi S, Fujii T, Yajima R, Uchida N, Ide M, Tsutsumi S, et al. Preoperative multidisciplinary management of airway obstruction by huge goiter with papillary thyroid cancer. Am Surg. 2011;77(5):E91–3.
Cho SY, Woo JH, Kim YJ, Chun EH, Han JI, Kim DY, et al. Airway management in patients with deep neck infections: a retrospective analysis. Medicine (Baltimore). 2016;95(27):e4125.
Nagamine Y, Kurahashi K. The use of three-dimensional computed tomography images for anticipated difficult intubation airway evaluation of a patient with Treacher Collins syndrome. Anesth Analg. 2007;105(3):626–8.
Dilisio RP, Dilisio AJ, Weiner MM. Preoperative virtual screening examination of the airway. J Clin Anesth. 2014;26(4):315–7.
Ovassapian A, Glassenberg R, Randel G. The unexpected difficult airway and lingual tonsil hyperplasia. Anesthesiology. 2002;97:124–32.
Davies S, Ananthanarayan C, Castro C. Asymptomatic lingual tonsillar hypertrophy and difficult airway management: a report of three cases. Can J Anesth. 2001;48(10):1020–4.
• Rosenblatt W, Ianus AI, Sukhupragarn W, Fickenscher A, Sasaki C. Preoperative endoscopic airway examination (PEAE) provides superior airway information and may reduce the use of unnecessary awake intubation. Anesth Analg. 2011;112(3):602–7 In a cohort of 138 patients undergoing elective otolaryngologic procedures, pre-operative endoscopic airway examination altered the planned airway management (from asleep intubation to awake intubation or vice versa) in 26% of the patients.
Law JA, Broemling N, Cooper RM, Drolet P, Duggan LV, Griesdale DE, et al. The difficult airway with recommendations for management--part 2--the anticipated difficult airway. Can J Anesth. 2013;60(11):1119–38.
Ahmad I, Millhoff B, John M, Andi K, Oakley R. Virtual endoscopy--a new assessment tool in difficult airway management. J Clin Anesth. 2015;27(6):508–13.
El-Boghdadly K, Onwochei DN, Millhoff B, Ahmad I. The effect of virtual endoscopy on diagnostic accuracy and airway management strategies in patients with head and neck pathology: a prospective cohort study. Can J Anaesth. 2017;64(11):1101–10.
Toyota K, Uchida H, Ozasa H, Motooka A, Sakura S, Saito Y. Preoperative airway evaluation using multi-slice three-dimensional computed tomography for a patient with severe tracheal stenosis. Br J Anaesth. 2004;93(6):865–7.
Gillespie S, Farling P, Editorial III. Preoperative assessment of the airway: should anaesthetists be making use of modern imaging techniques? Br J Anaesth. 2004;93(6):759–60.
Palczynski P, Bialka S, Misiolek H, Copik M, Smelik A, Szarpak L, et al. Thyromental height test as a new method for prediction of difficult intubation with double lumen tube. PLoS One. 2018;13(9):e0201944.
Popat M, Mitchell V, Dravid R, Patel A, Swampillai C, Higgs A. Difficult airway society guidelines for the management of tracheal extubation. Anaesthesia. 2012;67(3):318–40.
Rosenblatt WH. Preoperative planning of airway management in critical care patients. Crit Care Med. 2004;32(4 Suppl):S186–92.
Mallampati S, Gugino L, Desai S, Waraksa B, Freiberger D, Liu P. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J. 1985;32(4):429–34.
Samsoon G, Young R. Difficult tracheal intubation: a retrospective study. Anaesthesia. 1987;42:487–90.
Khan Z, Kashfi A, Ebrahimkhani E. A comparison of the upper lip bite test (a simple new technique) with modified Mallampati classification in predicting difficulty in endotracheal intubation: a prospective blinded study. Anesth Analg. 2003;96(2):595–9.
Mace SE. Challenges and advances in intubation: airway evaluation and controversies with intubation. Emerg Med Clin North Am. 2008;26(4):977–1000 ix.
Jain N, Das S, Kanchi M. Thyromental height test for prediction of difficult laryngoscopy in patients undergoing coronary artery bypass graft surgical procedure. Ann Card Anaesth. 2017;20(2):207–11.
Selvi O, Kahraman T, Senturk O, Tulgar S, Serifsoy E, Ozer Z. Evaluation of the reliability of preoperative descriptive airway assessment tests in prediction of the Cormack-Lehane score: a prospective randomized clinical study. J Clin Anesth. 2017;36:21–6.
Schmitt H, Kirmse M, Radespiel-Troger M. Ratio of patient’s height to thyromental distance improves prediction of difficult laryngoscopy. Anaesth Intensive Care. 2002;30:763–5.
Huh J, Shin HY, Kim SH, Yoon TK, Kim DK. Diagnostic predictor of difficult laryngoscopy: the hyomental distance ratio. Anesth Analg. 2009;108(2):544–8.
Siu L, Mathieson E, Naik V, Chandra D, Joo H. Patient and operator related factors associated with successful Glidescope intubations: a prospective observational study in 742 patients. Anaesth Intensive Care. 2010;38:70–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Louise Ellard and David T. Wong declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Preoperative Evaluation
Rights and permissions
About this article
Cite this article
Ellard, L., Wong, D.T. Preoperative Airway Evaluation. Curr Anesthesiol Rep 10, 19–27 (2020). https://doi.org/10.1007/s40140-020-00366-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40140-020-00366-w