Abstract
In recent years, the dural puncture epidural (DPE) technique has emerged as a novel method of labor analgesia. The DPE technique involves the technical elements of a combined spinal epidural (CSE) technique but avoids the direct administration of intrathecal medications. The underlying mechanism responsible for the unique blockade qualities of the DPE technique is believed to be the translocation of medications from the epidural space into the dural sac; laboratory studies have found a positive correlation between translocation flux and the size of dural perforation. Clinically, earlier and greater sacral spread and dermatomal block symmetry have been observed in obstetric and surgical patients receiving the DPE technique compared to the epidural (EPL) technique. Moreover, the DPE technique appears to have fewer side effects than the CSE technique. The DPE technique appears to offer a paradigm shift in obstetric analgesia and anesthesia, but further investigation is warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the introduction of epidural labor analgesia, neuraxial techniques have undergone significant refinement [1]; currently, the epidural (EPL) and combined spinal epidural (CSE) techniques are the most widely used. In the last decade, continued investigations to improve block and side effect profiles associated with these techniques have led to the development of the dural puncture epidural (DPE) technique.
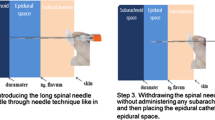
The DPE technique is a CSE technique modification wherein a spinal needle is introduced through the epidural needle to create a single dural puncture, but direct administration of intrathecal medication is withheld [2]. The technique was first described and investigated by Suzuki et al. [3] in patients undergoing lower abdominal surgery. Subsequently, Tsen and colleagues coined the formal “DPE” acronym for the technique [4] and began investigating and popularizing its use for labor analgesia.
In this review, we will discuss the evolution, mechanism, and evidence available for the DPE technique and contrast the clinical effects of the DPE, EPL, and CSE techniques.
Inadvertent Dural Puncture and Translocation
With the widespread use of labor epidural analgesia techniques, an increase in inadvertent dural punctures and subsequent post-dural puncture headaches (PDPHs) was observed. In 1958, Sykes [5] reported an inadvertent dural puncture with an epidural needle in a patient scheduled for vaginal repair surgery; the subsequent injection of medication through the epidural catheter, despite negative aspiration of cerebrospinal fluid (CSF), resulted in excessive hypotension, miosis, and apnea within 15 min. The symptoms were speculated to result from excessive cranial spread of medications administered into the epidural space that have translocated through the dural perforation into the spinal space; the volume (23 mL) of 1.5% lidocaine delivered was theorized to reverse the normal pressure gradient and flow that allows CSF leakage through a dural puncture into the epidural space.
In 1988, three decades after Sykes’ initial report, Leach et al. [6] provided the first radiological evidence of medication translocation from the epidural space into the subarachnoid space; a parturient with an inadvertent dural puncture by an epidural needle (gauge not reported) subsequently underwent an epidural injection of contrast dye that became visually apparent in the subarachnoid space. These findings diminished the enthusiasm for the combined spinal-epidural (CSE) technique, which was being introduced for labor analgesia [7, 8], due to concerns of excessive, unintentional subarachnoid spread following administration of medications through the epidural catheter. A number of laboratory and clinical investigations on the effect of dural puncture have augmented our current understanding of the CSE and DPE techniques.
Effect of Dural Puncture Size on Flux
The early case reports of translocation of epidural medications across a dural perforation into CSF involved larger diameter epidural needles. Based on the physics of flow, as the radius of the dural perforation decreases, the potential for passage of solution per unit time, or flux, should also decrease. Bernards et al. [9] investigated the relationship of transdural flux of medications administered in the epidural space through dural punctures of various sizes. Using spinal meningeal tissues from monkeys mounted on a diffusion cell, the flux of morphine and lidocaine was measured before and after meningeal tissue puncture using 27-G Whitacre, 24-G Sprotte, and 18-G Tuohy needles. The flux of both medications was positively correlated with the size of the needle; however, whereas the flux of morphine was significant with all dural puncture sizes, the flux of lidocaine was significant only with the 24-G and 18-G dural perforations (Fig. 1). The difference exhibited between morphine and lidocaine allows an examination of total measured flux, which equals the total transfer of medications across intact tissue as well as through the dural puncture. Because the flux of lidocaine through intact tissue is relatively fast, and much faster than morphine, the contribution of a small 27-G dural perforation is minimal. By contrast, as the dural puncture diameter increases, the relative contribution of dural puncture flux to total measured flux becomes more significant. This concept explains the apparent lack of difference in block function and spread (e.g., incidence of unilateral block, sacral sparing, number of top-up doses, labor analgesia quality) when lidocaine is used as an initial loading agent following a DPE technique with a 27-G dural perforation vs. an EPL technique [2]. Additional studies are necessary to examine the characteristics of other local anesthetic agents, opioids, and dural puncture diameters.
Dural Puncture Improves Caudal Spread of Epidural Analgesia and Anesthesia
One problem commonly encountered with labor epidural analgesia, even with a well-placed epidural catheter, is sacral block sparing. When epidural needles or catheters are inserted in the lower lumbar segments, fluoroscopic studies of the epidural space indicate greater spread of injected solutions in the cephalad, compared to caudad, direction [10, 11]. Consequently, the sacral nerve roots and fibers, which are more caudal, larger in diameter, and surrounded by thick dura mater, are more difficult to block using the epidural technique [12]. By contrast, the CSE technique provides direct access to these nerve roots, yielding significantly faster onset and improved sacral coverage compared to the EPL technique [7]. Clinically, more parturients have relief of painful rectal pressure with CSE vs. EPL techniques (94.8 vs. 68.7%, p < 0.0001) [7].
Similar findings have been observed in the nonobstetric population. Suzuki et al. [3] randomized patients scheduled for lower abdominal surgery to receive an epidural technique with or without a dural puncture (26-G Whitacre needle). When the epidural catheter was dosed with a high-volume, concentrated solution of local anesthetic (18 mL of 2% mepivacaine) and the spread of anesthesia was assessed by pinprick, the DPE technique was found to have earlier (at 15 and 20 min) and greater caudal spread (p < 0.01) than the EPL technique. Importantly, no differences in the median cephalad dermatomal spread were observed between DPE and EPL techniques at 20 min and the end of surgery [highest levels: EPL (T4), DPE (T5)].
Dural Puncture Improves the Block Symmetry of Epidural Analgesia
Radiographic studies have indicated that epidural catheters, even with an uncomplicated insertion, can ultimately rest in a number of different locations, leading to incomplete (with one or more dermatomes spared) or asymmetric blockade up to 8% of the time [13, 14]. Although an anatomic barrier limiting spread (e.g., congenital median epidural septum or acquired midline adhesion) is often suspected as an underlying or contributory etiology, it is likely that the positioning of the catheter tip has some importance. In responding clinically to an asymmetric or incomplete epidural blockade, the anesthesiologist may administer a bolus alone or in addition to pulling the catheter back in hope to reposition the catheter tip into a better location. However, when these or other interventions fail to “rescue” an unsatisfactory epidural blockade, the anesthesiologist should consider catheter replacement with a neuraxial technique that utilizes the intrathecal space (e.g., CSE or DPE techniques) [15••].
Large retrospective [14, 16,17,18, 19•] and prospective [20, 21••] studies have found the CSE technique to consistently provide less asymmetric blocks, less catheter failure and replacement rates, and higher overall maternal satisfaction compared to the EPL technique [7, 22, 23]. In the study by Thomas et al. [2], fewer incidences in unilateral blockade was not seen when comparing the DPE vs. EPL techniques, a finding that may be explained by limited medication translocation with the 27-G dural perforation. Interestingly, however, in a subgroup of 18 patients who received the DPE technique without CSF return, the incidence of unilateral blockade and rate of epidural catheter replacement were higher; this sample size was not large enough to make any meaningful comparisons. A recent meta-analysis of 10 randomized controlled trials, representing 1722 parturients, found a significant reduction in the relative risk for a unilateral block for CSE vs. EPL techniques (RR 0.48, 95% CI 0.24–0.97) [24••]. Together, these findings suggest that creation of a dural puncture can improve block symmetry of epidural analgesia: the spinal needle of the CSE technique enables the flow of CSF to serve as a confirmatory, definitive endpoint and the resulting dural perforation creates a conduit for medication translocation and spread.
The Obstetric Population and the DPE Technique
Thomas et al. [2] conducted the first study using “a CSE technique without subarachnoid drug administration” (i.e., the DPE technique) within the obstetric population. Two hundred and fifty-one healthy laboring parturients were randomized to the DPE or EPL technique with a 27-G Whitacre needle for dural puncture using an initial administration of 10 mL of 2% plain lidocaine for analgesia. Labor analgesia quality, catheter manipulation, and catheter replacement rates were no different between the DPE and EPL techniques, which have been explained by Bernards et al.’s [9] finding of similar total lidocaine flux with and without a 27-G dural perforation. However, in a subgroup of 18 patients who underwent an epidural catheter placement following a DPE technique despite the inability to obtain CSF, greater epidural catheter manipulation (38.9 vs. 37.4%), catheter replacement (22.2 vs. 9.3%), inadequate analgesia (27.8 vs. 20.6%), unilateral block (27.8 vs. 25.2%), need for more top-up doses (2 vs. 1.8), and overall higher hourly epidural drug consumption (17.6 vs. 15.9 mL) were experienced compared to those with a DPE technique with successful CSF flow [2]. Although statistical significance was not reached due to small sample sizes, these differences suggest the potential value of a confirmed dural puncture prior to epidural medication administration.
Acknowledging the value of medication translocation, Tsen and colleagues sought methods to investigate and enhance the properties of the DPE technique. In their landmark study, they prospectively randomized 80 parturients in early labor (<5 cm cervical dilation) to a DPE technique with a larger diameter 25-G Whitacre spinal needle vs. an EPL technique [4]. Using an initial epidural catheter loading dose with a moderate volume, concentrated solution (12 mL of 0.25% bupivacaine), they found significantly greater caudal spread and S1 blockade without excessive cranial spread with the DPE technique when compared to the EPL technique. These findings were consistent with earlier findings by Suzuki et al. (Table 1).
In keeping with the high-volume, less concentrated solutions used in contemporary obstetric anesthesia practices, Tsen and colleagues randomized 120 parturient (<5 cm cervical dilation) to receive DPE, CSE, or EPL labor analgesia with a 25-G Whitacre needle for the DPE and CSE techniques and 20 mL of 0.125% bupivacaine as the initial epidural loading dose for the DPE and EPL techniques (Table 2). The initial spinal dosing of the CSE technique used bupivacaine 1.7 mg and fentanyl 17 mcg (1 mL of a 1.5 mL premix solution of bupivacaine 2.5 mg and fentanyl 25 mcg). The investigators found that the time to achieve a numeric pain rating scale score of 1 or lower was significantly faster for the CSE technique (p < 0.001) and similar between DPE and EPL techniques (p = 0.183) [25••]. The median times (IQR) to achieving a numeric pain rating scale score of 1 or lower were 2 (0.5–6) min for CSE, 11 (4–120) min for DPE and 18 (10–120) min for EPL techniques. The DPE technique had an earlier and greater incidence of bilateral S2 blockade and lower incidence of asymmetric blockade compared to the EPL technique, while having a lower incidence of pruritus and combined uterine tachysystole and hypertonus compared to the CSE technique.
The DPE technique is unique from the CSE technique in several ways. First, the DPE technique uses the epidural catheter for drug administration, thereby testing its functionality immediately following placement. By comparison, the CSE technique must await sensory block resolution or pain progression before the epidural catheter is dosed and tested. Although some studies suggest that the CSE technique does not delay recognition of epidural catheter failures compared to EPL technique, the retrospective nature of these studies is subject to bias and lacks rigorous assessment of sensory blockade [19•, 26]. Second, the DPE technique significantly mitigates or avoids the maternal side effects (e.g., pruritus, hypotension) related to intrathecal CSE technique medications [25••]. Third, the DPE technique diminishes the frequency of uterine tachysystole and hypertonus and subsequent fetal bradycardia associated with the CSE technique. Finally, the DPE technique avoids the “transition of analgesia” commonly observed with the CSE technique, wherein substantive spinal analgesia is replaced epidural analgesia, which is often perceived as less effective [27]. This transition appears to be associated with greater requests for analgesic interventions, thereby potentially increasing anesthesia provider workload [25••].
One concern with the use of the DPE technique is the potential greater incidence of post dural puncture headache (PDPH). However, large retrospective studies comparing CSE and EPL techniques have consistently demonstrated similar rates of PDPH and epidural blood patch use [16, 18, 28, 29]; the reasons for this are likely multifactorial. First, the administration of anesthetic solutions into the epidural space may increase epidural pressure and minimize CSF efflux into the epidural space. Magnetic resonance myelography studies have found that the extent of CSF leakage parallels the risk of PDPH [30]. Second, multiple clinical studies have demonstrated that epidural catheters inserted as part of a CSE technique are less likely to fail and therefore require fewer replacement attempts [14, 17,18,19,20, 21••, 26, 31, 32]. Decreasing the need for repeated neuraxial techniques decreases procedural risks, including the risk for inadvertent dural punctures (IDP) with the epidural needle [33]. Finally, the DPE technique may directly reduce the risk of IDP. When the location of the epidural needle tip is uncertain, the finer gauge, longer spinal needle allows the operator to obtain additional information. The return of CSF in the spinal needle likely confirms the proper location of the epidural needle within the epidural space and prevents further advancement of the epidural needle into the dural sac; the absence of CSF return likely indicates the need for epidural needle repositioning. This visual method of confirmation can assist in the differentiation between a true vs. false loss of resistance, particularly when multiple equivocal alterations in resistance are observed (e.g., high body mass index parturient) or when the epidural technique is requested from an anesthesia practitioner who has limited experience or infrequently performs the technique.
Conclusions
The EPL and CSE techniques are well-established, popular methods of neuraxial labor analgesia and anesthesia for obstetric indications. The recent DPE technique appears to harness the beneficial qualities, and overcome some notable limitations, of the existing techniques. Limited data exist regarding the use of DPE techniques; further investigation is needed to determine the optimal settings and strategies for its use in a variety of clinical contexts.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Toledano RD, Tsen LC. Epidural catheter design: history, innovations, and clinical implications. Anesthesiology. 2014;121(1):9–17.
Thomas JA, Pan PH, Harris LC, Owen MD, D'Angelo R. Dural puncture with a 27-gauge Whitacre needle as part of a combined spinal-epidural technique does not improve labor epidural catheter function. Anesthesiology. 2005;103(5):1046–51.
Suzuki N, Koganemaru M, Onizuka S, Takasaki M. Dural puncture with a 26-gauge spinal needle affects spread of epidural anesthesia. Anesth Analg. 1996;82(5):1040–2.
Cappiello E, O’Rourke N, Segal S, Tsen LC. A randomized trial of dural puncture epidural technique compared with the standard epidural technique for labor analgesia. Anesth Analg. 2008;107(5):1646–51.
Sykes MK. Delayed spinal analgesia; a complication of epidural analgesia. Anaesthesia. 1958;13(1):78–83.
Leach A, Smith GB. Subarachnoid spread of epidural local anaesthetic following dural puncture. Anaesthesia. 1988;43(8):671–4.
Collis RE, Davies DW, Aveling W. Randomised comparison of combined spinal-epidural and standard epidural analgesia in labour. Lancet. 1995;345(8962):1413–6.
Coates MB. Combined subarachnoid and epidural techniques. Anaesthesia. 1982;37(1):89–90.
Bernards CM, Kopacz DJ, Michel MZ. Effect of needle puncture on morphine and lidocaine flux through the spinal meninges of the monkey in vitro. Implications for combined spinal-epidural anesthesia. Anesthesiology. 1994;80(4):853–8.
Park WY. Factors influencing distribution of local anesthetics in the epidural space. Reg Anesth Pain Med. 1988;13(2):49–57.
Yokoyama M, Hanazaki M, Fujii H, Mizobuchi S, Nakatsuka H, Takahashi T, Matsumi M, Takeuchi M, Morita K. Correlation between the distribution of contrast medium and the extent of blockade during epidural anesthesia. Anesthesiology. 2004;100(6):1504–10.
Arendt K, Segal S. Why epidurals do not always work. Rev Obstet Gynecol. 2008;1(2):49–55.
Sanchez R, Acuna L, Rocha F. An analysis of the radiological visualization of the catheters placed in the epidural space. Br J Anaesth. 1967;39(6):485–9.
Pan PH, Bogard TD, Owen MD. Incidence and characteristics of failures in obstetric neuraxial analgesia and anesthesia: a retrospective analysis of 19, 259 deliveries. Int J Obstet Anesth. 2004;13(4):227–33.
•• Mankowitz SK, Gonzalez Fiol A, Smiley R. Failure to extend epidural labor analgesia for cesarean delivery anesthesia: a focused review. Anesth Analg. 2016;123(5):1174–80. Factors and options for failed epidural labor analgesia are discussed in this focused review
Norris MC, Fogel ST, Conway-Long C. Combined spinal-epidural versus epidural labor analgesia. Anesthesiology. 2001;95(4):913–20.
Eappen S, Blinn A, Segal S. Incidence of epidural catheter replacement in parturients: a retrospective chart review. Int J Obstet Anesth. 1998;7(4):220–5.
van de Velde M, Teunkens A, Hanssens M, van Assche FA, Vandermeersch E. Post dural puncture headache following combined spinal epidural or epidural anaesthesia in obstetric patients. Anaesth Intensive Care. 2001;29(6):595–9.
• Groden J, Gonzalez-Fiol A, Aaronson J, Sachs A, Smiley R. Catheter failure rates and time course with epidural versus combined spinal-epidural analgesia in labor. Int J Obstet Anesth. 2016;26:4–7. Epidural catheters placed using a combined spinal-epidural technique have lower failure rates and failed significantly later in labor compared to epidural technique
Goodman SR, Smiley RM, Negron MA, Freedman PA, Landau R. A randomized trial of breakthrough pain during combined spinal-epidural versus epidural labor analgesia in parous women. Anesth Analg. 2009;108(1):246–51.
•• Gambling D, Berkowitz J, Farrell TR, Pue A, Shay D. A randomized controlled comparison of epidural analgesia and combined spinal-epidural analgesia in a private practice setting: pain scores during first and second stages of labor and at delivery. Anesth Analg. 2013;116(3):636–43. This prospective study compares analgesia using combined spinal-epidural with traditional epidural labor analgesia techniques in a private practice setting
Hughes D, Simmons SW, Brown J, Cyna AM. Combined spinal-epidural versus epidural analgesia in labour. Cochrane Database Syst Rev. 2003;4:CD003401.
Simmons SW, Taghizadeh N, Dennis AT, Hughes D, Cyna AM. Combined spinal-epidural versus epidural analgesia in labour. Cochrane Database Syst Rev. 2012;10:CD003401.
•• Heesen M, Van de Velde M, Klohr S, Lehberger J, Rossaint R, Straube S. Meta-analysis of the success of block following combined spinal-epidural vs epidural analgesia during labour. Anaesthesia. 2014;69(1):64–71. This systematic review included 1722 parturients from 10 randomized controlled trials comparing combined spinal-epidural and epidural analgesia techniques in labor and found that there is a lower rate of unilateral block with the combined spinal-epidural technique
•• Chau A, Bibbo C, Huang CC, Elterman K, Cappiello EC, Robinson JN, Tsen LC: A prospective, randomized trial of standard epidural, dural puncture epidural and combined spinal epidural labor analgesia techniques on maternal and fetal outcomes. Anesth Analg. 2017;124(2):560–9. The dural-puncture epidural technique was demonstrated to have better block quality compared to the epidural technique and fewer side effects compared to the combined spinal-epidural techniques in patients requesting early labor analgesia.
Booth JM, Pan JC, Ross VH, Russell GB, Harris LC, Pan PH. Combined spinal epidural technique for labor analgesia does not delay recognition of epidural catheter failures: a single-center retrospective cohort survival analysis. Anesthesiology. 2016;125(3):516–24.
Patel NP, Armstrong SL, Fernando R, Columb MO, Bray JK, Sodhi V, Lyons GR. Combined spinal epidural vs epidural labour analgesia: does initial intrathecal analgesia reduce the subsequent minimum local analgesic concentration of epidural bupivacaine? Anaesthesia. 2012;67(6):584–93.
Van de Velde M, Schepers R, Berends N, Vandermeersch E, De Buck F. Ten years of experience with accidental dural puncture and post-dural puncture headache in a tertiary obstetric anaesthesia department. Int J Obstet Anesth. 2008;17(4):329–35.
Miro M, Guasch E, Gilsanz F. Comparison of epidural analgesia with combined spinal-epidural analgesia for labor: a retrospective study of 6497 cases. Int J Obstet Anesth. 2008;17(1):15–9.
Wang YF, Fuh JL, Lirng JF, Chen SP, Hseu SS, Wu JC, Wang SJ. Cerebrospinal fluid leakage and headache after lumbar puncture: a prospective non-invasive imaging study. Brain. 2015;138(Pt 6):1492–8.
Norris MC. Are combined spinal-epidural catheters reliable? Int J Obstet Anesth. 2000;9(1):3–6.
Lee S, Lew E, Lim Y, Sia AT. Failure of augmentation of labor epidural analgesia for intrapartum cesarean delivery: a retrospective review. Anesth Analg. 2009;108(1):252–4.
Michaan N, Lotan M, Galiner M, Amzalag S, Many A. Risk factors for accidental dural puncture during epidural anesthesia for laboring women. J Matern Fetal Neonatal Med. 2016;29(17):2845–7.
•• Hattler J, Klimek M, Rossaint R, Heesen M. The effect of combined spinal-epidural versus epidural analgesia in laboring women on nonreassuring fetal heart rate tracings: systematic review and meta-analysis. Anesth Analg. 2016;123(4):955–64. This systematic review included 3947 parturients from 17 randomized trials comparing the effect of combined spinal-epidural and epidural techniques on fetal heart rate tracing and found that the combined spinal-epidural technique was associated with a higher risk of nonreassuring fetal heart rate tracings than the epidural technique
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Anthony Chau and Lawrence C. Tsen declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Obstetric Anesthesia
Rights and permissions
About this article
Cite this article
Chau, A., Tsen, L.C. Dural Puncture Epidural Technique: a Novel Method for Labor Analgesia. Curr Anesthesiol Rep 7, 49–54 (2017). https://doi.org/10.1007/s40140-017-0197-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40140-017-0197-6