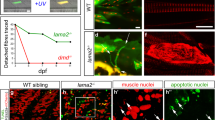
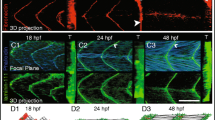
Abstract
Purpose of Review
The muscle is separated from tendons by a specialised basement membrane that acts as the structural interface of the myotendinous junction (MTJ). In zebrafish, the larval MTJ forms at the vertical myosepta, which separate the individual myomeres that arise during somitogenesis. In this review, we examine the formation of the vertical myosepta in zebrafish. We then describe insights this gains us in the context of muscle basement membrane failure, the mechanistic basis of the inherited muscle wasting condition muscular dystrophy (MD).
Recent Findings
We examine recent manuscripts that investigate how a well-orchestrated integration of MTJ components is needed during vertical myosepta development. We find the process can be divided into three stereotypical stages of its development based on specific structural properties of the developing basement membrane.
Summary
This review highlights insights that have been gleaned from vertical myosepta formation in zebrafish that maybe of value in developing therapeutic strategies for MD.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Rumian AP, Wallace AL, Birch HL (2007) Tendons and ligaments are anatomically distinct but overlap in molecular and morphological features—a comparative study in an ovine model. Journal of Orthopaedic Research : official publication of the Orthopaedic Research Society 25:458–464
Scutt N, Rolf CG, Scutt A (2008) Tissue specific characteristics of cells isolated from human and rat tendons and ligaments. J Orthop Surg Res 3:32
Staff PH (1982) The effects of physical activity on joints, cartilage, tendons and ligaments. Scand J Soc Med Suppl 29:59–63
Goody MF, Sher RB, Henry CA (2015) Hanging on for the ride: adhesion to the extracellular matrix mediates cellular responses in skeletal muscle morphogenesis and disease. Dev Biol 401:75–91
Woo SL (1982) Mechanical properties of tendons and ligaments. I. Quasi-static and nonlinear viscoelastic properties. Biorheology 19:385–396
Woo SL, Gomez MA, Woo YK, Akeson WH (1982) Mechanical properties of tendons and ligaments. II. The relationships of immobilization and exercise on tissue remodeling. Biorheology 19:397–408
Charvet B, Malbouyres M, Pagnon-Minot A, Ruggiero F, Le Guellec D (2011) Development of the zebrafish myoseptum with emphasis on the myotendinous junction. Cell Tissue Res 346:439–449
Charvet B, Ruggiero F, Le Guellec D (2012) The development of the myotendinous junction. A review. MLTJ Muscles, Ligaments and Tendons Journal 2:53–63
Sparks SE, Escolar DM (2011) Congenital muscular dystrophies. Handb Clin Neurol 101:47–79
Armer HE, Mariggi G, Png KM, Genoud C, Monteith AG, Bushby AJ, Gerhardt H, Collinson LM (2009) Imaging transient blood vessel fusion events in zebrafish by correlative volume electron microscopy. PLoS One 4:e7716
Oorschot V, Sztal TE, Bryson-Richardson RJ, Ramm G (2013) Immuno correlative light and electron microscopy on tokuyasu cryosections. Methods Cell Biol 124:241–258
Koshida S, Kishimoto Y, Ustumi H, Shimizu T, Furutani-Seiki M, Kondoh H, Takada S (2005) Integrinα5-dependent fibronectin accumulation for maintenance of somite boundaries in zebrafish embryos. Dev Cell 8:587–598
Thornhill P, Bassett D, Lochmuller H, Bushby K, Straub V (2008) Developmental defects in a zebrafish model for muscular dystrophies associated with the loss of fukutin-related protein (FKRP). Brain: a journal of neurology 131:1551–1561
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF (1995) Stages of embryonic development of the zebrafish. Developmental Dynamics: an official publication of the American Association of Anatomists 203:253–310
Berger J, Currie PD (2012) Zebrafish models flex their muscles to shed light on muscular dystrophies. Dis Model Mech 5:726–732
Wood AJ, Currie PD (2014) Analysing regenerative potential in zebrafish models of congenital muscular dystrophy. Int J Biochem Cell Biol 56:30–37
Rida PC, Le Minh N, Jiang Y-J (2004) A Notch feeling of somite segmentation and beyond. Dev Biol 265:2–22
Schier AF (2001) Axis formation and patterning in zebrafish. Curr Opin Genet Dev 11:393–404
Holley SA, Geisler R, Nusslein-Volhard C (2000) Control of her1 expression during zebrafish somitogenesis by a delta-dependent oscillator and an independent wave-front activity. Genes Dev 14:1678–1690
Ozbudak EM, Lewis J (2008) Notch signalling synchronizes the zebrafish segmentation clock but is not needed to create somite boundaries. PLoS Genet 4:e15
Gray M, Moens CB, Amacher SL, Eisen JS, Beattie CE (2001) Zebrafish deadly seven functions in neurogenesis. Dev Biol 237:306–323
Mao Y, Schwarzbauer JE (2005) Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol 24:389–399
Sottile J, Hocking DC (2002) Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol Biol Cell 13:3546–3559
Kubow KE, Vukmirovic R, Zhe L, Klotzsch E, Smith ML, Gourdon D, Luna S, Vogel V (2015) Mechanical forces regulate the interactions of fibronectin and collagen I in extracellular matrix. Nat Commun 6
Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S (1998) Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci 95:11211–11216
Postel R, Vakeel P, Topczewski J, Knoll R, Bakkers J (2008) Zebrafish integrin-linked kinase is required in skeletal muscles for strengthening the integrin-ECM adhesion complex. Dev Biol 318:92–101
Hocking DC, Sottile J, Langenbach KJ (2000) Stimulation of integrin-mediated cell contractility by fibronectin polymerization. J Biol Chem 275:10673–10682
Jülich D, Geisler R, Holley S, Consortium TS (2005) Integrina5 and delta/notch signaling have complementary spatiotemporal requirements during zebrafish somitogenesis. Dev Cell 8:575–586
Snow CJ, Peterson MT, Khalil A, Henry CA (2008) Muscle development is disrupted in zebrafish embryos deficient for fibronectin. Developmental Dynamics: an official publication of the American Association of Anatomists 237:2542–2553
Kornblihtt AR, Vibe-Pedersen K, Baralle FE (1983) Isolation and characterization of cDNA clones for human and bovine fibronectins. Proc Natl Acad Sci 80:3218–3222
Kettleborough RN, Busch-Nentwich EM, Harvey SA, Dooley CM, de Bruijn E, van Eeden F, Sealy I, White RJ, Herd C, Nijman IJ et al (2013) A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature 496:494–497
Belkin AM, Stepp MA (2000) Integrins as receptors for laminins. Microsc Res Tech 51:280–301
Burkin DJ, Kaufman SJ (1999) The alpha7beta1 integrin in muscle development and disease. Cell Tissue Res 296:183–190
Mayer U, Saher G, Fassler R, Bornemann A, Echtermeyer F, von der Mark H, Miosge N, Poschl E, von der Mark K (1997) Absence of integrin alpha 7 causes a novel form of muscular dystrophy. Nat Genet 17:318–323
Mayer U (2003) Integrins: redundant or important players in skeletal muscle? J Biol Chem 278:14587–14590
Colognato H, Winkelmann DA, Yurchenco PD (1999) Laminin polymerization induces a receptor–cytoskeleton network. J Cell Biol 145:619–631
Sztal TE, Sonntag C, Hall TE, Currie PD (2012) Epistatic dissection of laminin-receptor interactions in dystrophic zebrafish muscle. Hum Mol Genet 21:4718–4731
Parsons MJ, Pollard SM, Saude L, Feldman B, Coutinho P, Hirst EM, Stemple DL (2002) Zebrafish mutants identify an essential role for laminins in notochord formation. Development 129:3137–3146
Alrowaished, S.S. (2015). Laminin regulates fibronectin levels in the zebrafish myotendinous junction via matrix metalloproteinase-11
Hadler-Olsen E, Fadnes B, Sylte I, Uhlin-Hansen L, Winberg JO (2011) Regulation of matrix metalloproteinase activity in health and disease. FEBS J 278:28–45
Nishiuchi R, Takagi J, Hayashi M, Ido H, Yagi Y, Sanzen N, Tsuji T, Yamada M, Sekiguchi K (2006) Ligand-binding specificities of laminin-binding integrins: a comprehensive survey of laminin-integrin interactions using recombinant alpha3beta1, alpha6beta1, alpha7beta1 and alpha6beta4 integrins. Matrix Biology: journal of the International Society for Matrix Biology 25:189–197
Barresi R, Campbell KP (2006) Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci 119:199–207
Barresi R, Michele DE, Kanagawa M, Harper HA, Dovico SA, Satz JS, Moore SA, Zhang W, Schachter H, Dumanski JP et al (2004) LARGE can functionally bypass alpha-dystroglycan glycosylation defects in distinct congenital muscular dystrophies. Nat Med 10:696–703
Cohn RD, Henry MD, Michele DE, Barresi R, Saito F, Moore SA, Flanagan JD, Skwarchuk MW, Robbins ME, Mendell JR et al (2002) Disruption of DAG1 in differentiated skeletal muscle reveals a role for dystroglycan in muscle regeneration. Cell 110:639–648
Goddeeris MM, Wu B, Venzke D, Yoshida-Moriguchi T, Saito F, Matsumura K, Moore SA, Campbell KP (2013) LARGE glycans on dystroglycan function as a tunable matrix scaffold to prevent dystrophy. Nature 503:136–140
Inamori K, Hara Y, Willer T, Anderson ME, Zhu Z, Yoshida-Moriguchi T, Campbell KP (2013) Xylosyl- and glucuronyltransferase functions of LARGE in alpha-dystroglycan modification are conserved in LARGE2. Glycobiology 23:295–302
Kawahara G, Guyon JR, Nakamura Y, Kunkel LM (2010) Zebrafish models for human FKRP muscular dystrophies. Hum Mol Genet 19:623–633
Zhefeng Z, Gruszczynska-Biegala J, Zolkiewska A (2005) ADP-ribosylation of integrin α7 modulates the binding of integrin α7β1 to laminin. Biochem J 385:309–317
Goody MF, Kelly MW, Lessard KN, Khalil A, Henry CA (2010) Nrk2b-mediated NAD+ production regulates cell adhesion and is required for muscle morphogenesis in vivo: Nrk2b and NAD+ in muscle morphogenesis. Dev Biol 344:809–826
Bricard Y, Ralliere C, Lebret V, Lefevre F, Rescan PY (2014) Early fish myoseptal cells: insights from the trout and relationships with amniote axial tenocytes. PLoS One 9:e91876
Kardon G (1998) Muscle and tendon morphogenesis in the avian hind limb. Development 125:4019–4032
Schnorrer F, Dickson BJ (2004) Muscle building: mechanisms of myotube guidance and attachment site selection. Dev Cell 7:9–20
Yarnitzky T, Min L, Volk T (1997) The Drosophila neuregulin homolog vein mediates inductive interactions between myotubes and their epidermal attachment cells. Genes Dev 11:2691–2700
Becker S, Pasca G, Strumpf D, Min L, Volk T (1997) Reciprocal signaling between Drosophila epidermal muscle attachment cells and their corresponding muscles. Development 124:2615–2622
Honjo Y, Kniss J, Eisen JS (2008) Neuregulin-mediated ErbB3 signaling is required for formation of zebrafish dorsal root ganglion neurons. Development 135:2615–2625
Subramanian A, Schilling TF (2014) Thrombospondin-4 controls matrix assembly during development and repair of myotendinous junctions. elife 3:e02372
De Luna N, Gallardo E, Sonnet C, Chazaud B, Dominguez-Perles R, Suarez-Calvet X, Gherardi RK, Illa I (2010) Role of thrombospondin 1 in macrophage inflammation in dysferlin myopathy. J Neuropathol Exp Neurol 69:643–653
Crawford BD, Henry CA, Clason TA, Becker AL, Hille MB (2003) Activity and distribution of paxillin, focal adhesion kinase, and cadherin indicate cooperative roles during zebrafish morphogenesis. Mol Biol Cell 14:3065–3081
Dalcq J, Pasque V, Ghaye A, Larbuisson A, Motte P, Martial JA, Muller M (2012) RUNX3, EGR1 and SOX9B form a regulatory cascade required to modulate BMP-signaling during cranial cartilage development in zebrafish. PLoS One 7:e50140
•• Subramanian, A., and Schilling, T.F. (2014). Thrombospondin-4 controls matrix assembly during development and repair of myotendinous junctions. Elife 3. This is a valuable paper in understnding zebrafish myotendinous junction formation and muscular dystrophy.
Chen JW, Galloway JL (2014) The development of zebrafish tendon and ligament progenitors. Development 141:2035–2045
Hinits Y, Williams VC, Sweetman D, Donn TM, Ma TP, Moens CB, Hughes SM (2011) Defective cranial skeletal development, larval lethality and haploinsufficiency in Myod mutant zebrafish. Dev Biol 358:102–112
Tanjore H, Kalluri R (2006) The role of type IV collagen and basement membranes in cancer progression and metastasis. Am J Pathol 168:715–717
Li S, Edgar D, Fässler R, Wadsworth W, Yurchenco PD (2003) The role of laminin in embryonic cell polarization and tissue organization. Dev Cell 4:613–624
Huang P, Xiao A, Zhou M, Zhu Z, Lin S, Zhang B (2011) Heritable gene targeting in zebrafish using customized TALENs. Nat Biotechnol 29:699–700
Poschl E, Schlotzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U (2004) Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 131:1619–1628
Fidler AL, Vanacore RM, Chetyrkin SV, Pedchenko VK, Bhave G, Yin VP, Stothers CL, Rose KL, McDonald WH, Clark TA et al (2014) A unique covalent bond in basement membrane is a primordial innovation for tissue evolution. Proc Natl Acad Sci U S A 111:331–336
Gistelinck C, Gioia R, Gagliardi A, Tonelli F, Marchese L, Bianchi L, Landi C, Bini L, Huysseune A, Witten P (2016) Zebrafish collagen type I: molecular and biochemical characterization of the major structural protein in bone and skin. Scientific Reports 6
Yan YL, Hatta K, Riggleman B, Postlethwait JH (1995) Expression of a type II collagen gene in the zebrafish embryonic axis. Dev Dyn 203:363–376
Li, M., Andersson-Lendahl, M., Sejersen, T., and Arner, A. (2013). Muscle dysfunction and structural defects of dystrophin-null sapje mutant zebrafish larvae are rescued by ataluren treatment. FASEB Journal: official publication of the Federation of American Societies for Experimental Biology
• Li M, Andersson-Lendahl M, Sejersen T, Arner A (2013) Knockdown of desmin in zebrafish larvae affects interfilament spacing and mechanical properties of skeletal muscle. The Journal of General Physiology 141:335–345 This is an impotant techniques paper that is at the outer limit of recent papers but is still worth noting because of its cutting edge approach
Bushby K, Anderson LV, Pollitt C, Naom I, Muntoni F, Bindoff L (1998) Abnormal merosin in adults. A new form of late onset muscular dystrophy not linked to chromosome 6q2. Brain: a journal of neurology 121(Pt 4):581–588
Bushby KM (1994) The muscular dystrophies. Baillieres Clin Neurol 3:407–430
Geranmayeh F, Clement E, Feng LH, Sewry C, Pagan J, Mein R, Abbs S, Brueton L, Childs AM, Jungbluth H et al (2010) Genotype-phenotype correlation in a large population of muscular dystrophy patients with LAMA2 mutations. Neuromuscular Disorders : NMD 20:241–250
North KN (2011) Clinical approach to the diagnosis of congenital myopathies. Semin Pediatr Neurol 18:216–220
Straub V, Rafael JA, Chamberlain JS, Campbell KP (1997) Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J Cell Biol 139:375–385
Brancaccio A, Schulthess T, Gesemann M, Engel J (1997) The N-terminal region of alpha-dystroglycan is an autonomous globular domain. European journal of biochemistry / FEBS 246:166–172
Parsons MJ, Campos I, Hirst EM, Stemple DL (2002) Removal of dystroglycan causes severe muscular dystrophy in zebrafish embryos. Development 129:3505–3512
Williamson RA, Henry MD, Daniels KJ, Hrstka RF, Lee JC, Sunada Y, Ibraghimov-Beskrovnaya O, Campbell KP (1997) Dystroglycan is essential for early embryonic development: disruption of Reichert’s membrane in Dag1-null mice. Hum Mol Genet 6:831–841
Serrano, A.L., and Muñoz-Cánoves, P. (2016). Fibrosis development in early-onset muscular dystrophies: mechanisms and translational implications. In Seminars in Cell & Developmental Biology (Elsevier)
Kuno A, Horio Y (2016) SIRT1: a novel target for the treatment of muscular dystrophies. Oxidative Med Cell Longev 2016
Hall TE, Bryson-Richardson RJ, Berger S, Jacoby AS, Cole NJ, Hollway GE, Berger J, Currie PD (2007) The zebrafish candyfloss mutant implicates extracellular matrix adhesion failure in laminin alpha2-deficient congenital muscular dystrophy. Proc Natl Acad Sci U S A 104:7092–7097
Lin YY, White RJ, Torelli S, Cirak S, Muntoni F, Stemple DL (2011) Zebrafish fukutin family proteins link the unfolded protein response with dystroglycanopathies. Hum Mol Genet 20:1763–1775
Pollard SM, Parsons MJ, Kamei M, Kettleborough RN, Thomas KA, Pham VN, Bae MK, Scott A, Weinstein BM, Stemple DL (2006) Essential and overlapping roles for laminin alpha chains in notochord and blood vessel formation. Dev Biol 289:64–76
Siegel AL, Gurevich DB, Currie PD (2013) A myogenic precursor cell that could contribute to regeneration in zebrafish and its similarity to the satellite cell. FEBS J 280:4074–4088
Krauss S, Concordet J-P, Ingham P (1993) A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell 75:1431–1444
Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL (1993) Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci U S A 90:3710–3714
Hoffman EP, Knudson CM, Campbell KP, Kunkel LM (1987) Subcellular fractionation of dystrophin to the triads of skeletal muscle. Nature 330:754–758
Hoffman EP, Brown RH, Kunkel LM (1987) Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51:919–928
Cooper BJ, Winand NJ, Stedman H, Valentine BA, Hoffman EP, Kunkel LM, Scott M-O, Fischbeck KH, Kornegay JN, Avery RJ (1988) The homologue of the Duchenne locus is defective in X-linked muscular dystrophy of dogs. Nature 334:154–156
Bassett, D.I., and Currie, P.D. (2003). The zebrafish as a model for muscular dystrophy and congenital myopathy. Human molecular genetics 12 Spec No 2, R265-270
Bassett D, Currie PD (2004) Identification of a zebrafish model of muscular dystrophy. Clin Exp Pharmacol Physiol 31:537–540
•• Vieira NM, Elvers I, Alexander MS, Moreira YB, Eran A, Gomes JP, Marshall JL, Karlsson EK, Verjovski-Almeida S, Lindblad-Toh K (2015) Jagged 1 rescues the Duchenne muscular dystrophy phenotype. Cell 163:1204–1213 This paper highlights zebrafish’s value in assesing novel therapuetic posibilities in muscular dystrophy. It is important because it is a hypothesis-driven test, not a large molecule screen
• Servián-Morilla E, Takeuchi H, Lee TV, Clarimon J, Mavillard F, Area-Gómez E, Rivas E, Nieto-González JL, Rivero MC, Cabrera-Serrano M (2016) A POGLUT1 mutation causes a muscular dystrophy with reduced Notch signaling and satellite cell loss. EMBO Molecular Medicine 8:1289–1309 Glycosylation is widely studied in the muscular dystrophy field in the context of a-dg; this paper provides fresh insight into other areas where glycosylation maybe important, and we expect more papers showing glycosylation of other important muscle components in the next 5 years
• Piccioni, A., Gaetani, E., Palladino, M., Gatto, I., Smith, R., Neri, V., Marcantoni, M., Giarretta, I., Silver, M., and Straino, S. (2014). Sonic hedgehog gene therapy increases the ability of the dystrophic skeletal muscle to regenerate after injury. Gene Therapy 21. This paper shows how a gene involved in the developmental control of muscle can be used to treat muscular dystrophy.
Anderson C, Thorsteinsdóttir S, Borycki A-G (2009) Sonic hedgehog-dependent synthesis of laminin α1 controls basement membrane assembly in the myotome. Development 136:3495–3504
Horn A, Palumbo K, Cordazzo C, Dees C, Akhmetshina A, Tomcik M, Zerr P, Avouac J, Gusinde J, Zwerina J (2012) Hedgehog signaling controls fibroblast activation and tissue fibrosis in systemic sclerosis. Arthritis & Rheumatism 64:2724–2733
Goody MF, Kelly MW, Reynolds CJ, Khalil A, Crawford BD, Henry CA (2012) NAD+ biosynthesis ameliorates a zebrafish model of muscular dystrophy. PLoS Biol 10:e1001409
Van Ry PM, Minogue P, Hodges BL, Burkin DJ (2014) Laminin-111 improves muscle repair in a mouse model of merosin-deficient congenital muscular dystrophy. Hum Mol Genet 23:383–396
Wilschut KJ, van Tol HT, Arkesteijn GJ, Haagsman HP, Roelen BA (2011) Alpha 6 integrin is important for myogenic stem cell differentiation. Stem Cell Res 7:112–123
Miyagoe-Suzuki Y, Nakagawa M, Takeda S (2000) Merosin and congenital muscular dystrophy. Microsc Res Tech 48:181–191
Urao N, Mirza RE, Heydemann A, Garcia J, Koh TJ (2016) Thrombospondin-1 levels correlate with macrophage activity and disease progression in dysferlin deficient mice. Neuromuscul Disord 26:240–251
Jacoby AS, Busch-Nentwich E, Bryson-Richardson RJ, Hall TE, Berger J, Berger S, Sonntag C, Sachs C, Geisler R, Stemple DL et al (2009) The zebrafish dystrophic mutant softy maintains muscle fibre viability despite basement membrane rupture and muscle detachment. Development 136:3367–3376
Moore CJ, Goh HT, Hewitt JE (2008) Genes required for functional glycosylation of dystroglycan are conserved in zebrafish. Genomics 92:159–167
Ryckebüsch L, Hernandez L, Wang C, Phan J, Yelon D (2016) Tmem2 regulates cell-matrix interactions that are essential for muscle fiber attachment. Development 143:2965–2972
Rojek JM, Spiropoulou CF, Campbell KP, Kunz S (2007) Old World and clade C New World arenaviruses mimic the molecular mechanism of receptor recognition used by alpha-dystroglycan’s host-derived ligands. J Virol 81:5685–5695
Cao W, Henry MD, Borrow P, Yamada H, Elder JH, Ravkov EV, Nichol ST, Compans RW, Campbell KP, Oldstone MB (1998) Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science 282:2079–2081
Tayeh A, Tatard C, Kako-Ouraga S, Duplantier J-M, Dobigny G (2010) Rodent host cell/Lassa virus interactions: evolution and expression of α-dystroglycan, LARGE-1 and LARGE-2 genes, with special emphasis on the Mastomys genus. Infect Genet Evol 10:1262–1270
Kawahara G, Kunkel LM (2013) Zebrafish based small molecule screens for novel DMD drugs. Drug discovery today. Technologies 10:e91–e96
Maves L (2014) Recent advances using zebrafish animal models for muscle disease drug discovery. Expert Opin Drug Discovery 9:1033–1045
Straub V, Bertoli M (2016) Where do we stand in trial readiness for autosomal recessive limb girdle muscular dystrophies? Neuromuscul Disord 26:111–125
Guiraud S, Chen H, Burns DT, Davies KE (2015) Advances in genetic therapeutic strategies for Duchenne muscular dystrophy. Exp Physiol 100:1458–1467
Acknowledgements
PDC is a Principal NHMRC fellow, director of the Australian Regenerative Medicine Institute at Monash University and Head of EMBL Australia Melbourne Node. This work is supported on a NHMRC grant (APP3151883).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Currie reports grants from NHMRC aus, during the conduct of the study.
Dr. Wood declares no conflict of interest.
Human and Animal Rights and Informed Consent
Zebrafish used to generate data complied with the Monash University Animal Ethics Committee and National Health and Medical Research Council of Australia code for care and use of animals for scientific purposes.
Additional information
This article is part of the Topical Collection on Xenopus and Zebrafish Models for Pathobiology
Rights and permissions
About this article
Cite this article
Wood, A.J., Currie, P.D. Development Aspects of Zebrafish Myotendinous Junction: a Model System for Understanding Muscle Basement Membrane Formation and Failure. Curr Pathobiol Rep 5, 197–205 (2017). https://doi.org/10.1007/s40139-017-0140-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40139-017-0140-z