Abstract
Purpose
The goal of this review is to discuss the surgical technique of Targeted Muscle Reinnervation (TMR) in the oncologic population. The technique not only improves myoelectric prosthetic control, but is a method to relieve post-amputation chronic residual and phantom limb pain.
Recent Findings
TMR is a surgical procedure that effectively amplifies neural control signals on surface musculature. Fortuitously, TMR has also been shown to also reduce chronic residual and phantom limb pain when performed in a delayed or immediate manner. While most data exists in traumatic or orthopedic amputees, TMR is an emerging technique in the oncologic population. Although important for prosthetic control, it is arguably most beneficial for the oncologic population due to the ability to reduce pain without opioids.
Summary
TMR improves myoelectric prosthesis control and early results demonstrate marked improvement in post-amputation chronic pain and quality of life within the oncologic population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the USA alone, an estimated 3 million people are expected to be living with limb loss by the year 2050 [1]. After amputation, many patients unfortunately experience both functional and sensory disability. Functional disability results from loss of the distal extremity and restoration requires a well-healed residual limb (stump) and rehabilitation with assistive devices or prosthetics. Sensory disability results from chronic residual and phantom limb pain, which is difficult to treat and occurs in up to 50% and 80% of amputees, respectively [2,3,4]. Chronic residual limb pain occurs from the formation of neuromas, which occur when nerve injury causes inflammation and aberrant axonal sprouting that creates painful bulbs of disorganized regenerative tissue [5]. Phantom limb awareness and pain are disturbing sensations perceived in the missing limb after amputation. Phantom limb pain is thought to originate from a complex interplay of peripheral nerve injury, central nervous system changes, and psychologic factors [6,7,8].
Patients frequently experience a compounded, cyclical dysfunction if either disability is inadequately treated. For example, functional disability is amplified when chronic pain prevents prosthetic donning [9]. Moreover, if chronic pain is inadequately treated, many patients become dependent on pain medicine to control their symptoms and profound psychosocial disability results. In the oncologic population, many patients experience chronic pain in the affected limb prior to amputation due to tumor burden and neoadjuvant chemoradiation. These patients are “pre-sensitized” to pain in the extremity and are extremely vulnerable to post-operative pain [8]. In light of the current opioid epidemic, non-narcotic treatment of pain is paramount.
While physical rehabilitation is arduous, it is achievable with advanced therapy. With advanced prostheses, many patients are able to achieve near-normal levels of physical activity. In contrast, chronic residual limb and phantom limb pain are debilitating consequences of amputation because they are often refractory to treatment. A multitude of surgical techniques have been published in the last century to address painful residual limb neuromas, including traction neurectomy, proximal nerve crush, silicone capping, and transposition with muscle implantation, among others [5, 10, 11]. Previous attempts to treat phantom limb pain have included masking medications such as neuromodulators and desensitization via mirror therapy. Unfortunately, none of these techniques have been shown to be highly effective which speaks to the recalcitrant nature of neuropathic pain. Together, residual and phantom limb pain appear to cause a synchronous exacerbation of overall pain, with hypersensitized peripheral nerves conducting high-intensity afferent noxious input to the central nervous system, that in turn sensitizes the central pain perception [7, 12,13,14].
Targeted muscle reinnervation (TMR) is a surgical technique originally developed for improved control of myoelectric prosthesis. Serendipitously, during TMR prosthetic trials, patients demonstrated markedly improved chronic pain. Since that time, TMR has been studied not only as a method of improved prosthetic control, but as well as a treatment for post amputation residual and chronic limb pain control. For oncologic amputees, TMR is a valuable technique for improving prosthetic options. However, for sensitized oncologic patients, it may be most beneficial for its pain reduction and improved quality of life.
TMR Surgical Technique
TMR was originally designed to amplify electromyographic (EMG) signals through surface musculature. TMR coapts mixed major nerves of a proximal amputation stump to motor nerves of redundant muscle nearby [15,16,17]. Reinnervation of the surface muscle by the amputated nerve demonstrates conduction activity specific to the nerve’s original cortical assignment [15, 16]. By redirecting severed nerves to a new end “receiver,” TMR enables myoelectric prosthetics to access to previously unavailable, innate neural control information. Pioneered by the work of Dumanian and Kuiken, human and animal models have demonstrated that the trophic stimulus from cut, denervated target muscle induces regeneration across the nerve coaptation [18, 19].
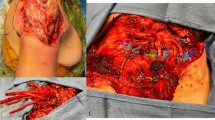
Dumanian describes the TMR technique as giving the amputated nerve “somewhere to go and something to do.” For example, in an above knee amputation, the sciatic nerve is split into the common peroneal and tibial bundles that are then coapted to motor nerve branches of the biceps femoris or semimembranosus muscles (Fig. 1). These target muscles are considered redundant in the stump, since they are no longer functional due to the amputation of the tendinous insertion over the distal joint. Stimulated by trophic signals from the denervated posterior thigh muscle, conducted across the nerve coaptation, the sciatic nerve has a directed physiologic target in which to heal.
a The sciatic nerve is split into the common peroneal and tibial nerve bundles by direct palpation. Motor nerves to the semimembranosus and biceps femoris are identified (yellow arrows). b The common peroneal and tibial nerve are coapted to the motor nerves with 6–0 Nylon epineurial sutures. The coaptations can be wrapped, if preferred, by muscle grafts or nerve conduits
TMR is also effective as a pain reduction technique. Previous techniques to treat residual and phantom limb pain have focused on an indirect approach to the pathology, in which the offending neuroma is buried within the surrounding soft tissue, in hopes that it will be shielded from noxious stimuli. These techniques focus on desensitizing or “masking” the injury, rather than reestablishing afferent-efferent congruency. As surgeons, it is well accepted that restoring an anatomic defect to its original purpose provides the best aesthetic and functional result. Analogously, functional nerve restoration should decrease the aberrancy of axonal healing. TMR reestablishes physiologic connections within severed nerves and allows directed regeneration. This decreases neuroma formation and breaks the cycle of abnormal afferent-efferent conduction. In his studies of TMR as a pain control technique, Valerio has described this procedure as “analogous to grounding a live wire.”
Previous Studies
Developed for prostheses, early studies of TMR examined the ability to transfer large mixed nerves to spare muscles on the chest and the feasibility of rendering distinct EMG signals. Using a rat skeletal muscle model, Kuiken et al. demonstrated that large nerves containing motor neurons could be transferred on to a small amount of muscle and would “hyper-reinnervate” the designated muscle [20]. The hyper-reinnervation improved muscle recovery and independent myoelectric signals in the designated area were detectable through the surface. In 2004, Kuiken and Dumanian successfully performed TMR from the brachial plexus to the anterior chest in a bilateral shoulder disarticulation amputee [21]. Voluntary neural control of individually reinnervated muscles was achieved between 3 and 5 months with distinct EMG signals on the skin surface. This sentinel study demonstrated that muscle could be used as a biologic amplifier of peripheral nerve activity to obtain independent EMG control signals for a multifunction prosthesis. In comparison with traditional prosthesis that could only control a single joint with a cumbersome locking mechanism, their patient demonstrated control of an advanced myoelectric prosthesis at multiple joint levels simultaneously.
In the following years, the authors continued their experience with TMR for prosthetic control in the upper extremity [22,23,24,25,26,27,28]. They designated the defined reinnervated muscle the “myoneurosome” and demonstrated specific transfers that could result in simultaneous “hand close-hand open,” “wrist supination-pronation,” and “elbow flexion-elbow extension” that allowed for increased speed and accuracy of prosthetic use. Patients no longer had to retrain their cortical signals, but used their innate, intuitive neural commands to send peripheral signals to surface EMG electrodes. Effectively, previously lost cortical commands were harnessed and available for use. In 2011, TMR was used in a muscle free flap for a severe burn patient that needed an upper extremity prosthetic, demonstrating extended feasibility in target muscle units [29]. Agnew and others later proposed TMR transfer patterns in the lower extremity [18, 19, 30,31,32].
During their experience with TMR for prosthetic control, Dumanian and colleagues noted that many amputees that previously complained of painful stump neuromas did not demonstrate painful neuromas at the site of nerve coaptation. In 2012, using a rabbit model, the authors demonstrated that TMR not only created skeletal muscle units for EMG detection, but also favorably altered the histomorphometric characteristics of the coapted distal nerve, including increased size of myelinated fibers and decreased number from cessation of aberrant sprouting [16]. TMR for pain control was explored in subsequent studies, including in both immediate and delayed fashion [15, 17, 31, 32]. In particular, mixed major and pure sensory nerves were transferred to motor nerve targets. Souza et al. performed a retrospective review of their experience and found that none of the 26 patients who underwent TMR demonstrated evidence of new neuroma pain after the procedure, and all but one of the 15 patients who presented with preoperative neuroma pain experienced complete resolution of pain in the distribution of the transferred nerves [17].
In 2019, Dumanian and colleagues published their results of a prospective, randomized, multicenter clinical trial examining pre- and postoperative pain scores in patients treated with delayed TMR using mixed major and sensory nerves. Patients who underwent TMR, as opposed to the control group of standard neuroma excision and burying, showed significantly improved phantom limb pain and a trend towards improved residual limb pain [33]. The trial was closed early due to inability to recruit sufficient numbers to the control arm, as many patients heard of the overwhelming success of the treatment and refused to be randomized. Due the striking results of the study, additional studies have not attempted randomization. Subsequently, Valerio and colleagues published their results of a multi-institutional cohort study examining immediate TMR for improved pain control. Their study found that patients who underwent TMR had less phantom limb pain and less residual limb pain compared with untreated amputee historic controls, across all subgroups and by all measures [34].
TMR in the Oncologic Population
In both the oncologic and traumatic population, the most common type of amputation occurs in the lower extremity [1]. Due to the weight and size of the batteries required for current myoelectric prostheses, most lower extremity amputees cannot utilize these advanced prosthetics at this time. Additionally, many patients have trouble with insurance coverage of these newer devices. However, there is hope that as the technology progresses, that subsequent models will become available suitable to the lower extremity and at lower cost. For this reason, TMR should be performed with both prosthetic and pain control objectives in mind, regardless of the current availability. In particular, the pediatric population deserves special attention in this regard, as their long-term survival will likely coincide with technologic advances.
In the oncologic population, post-cancer quality of life depends not only on the control of the offending cancer, but the ability to live without persistent debility. Due to advances in limb salvage and multimodal cancer therapy, oncologic amputation is rare except at high-volume cancer centers. However, this population could significantly benefit from TMR, especially in regard to its pain control benefits.
In contrast to the traumatic amputee populations, many cancer patients have chronic pain of the extremity prior to amputation. Oncologic patients frequently present with peripheral nervous system dysfunction due to previous limb salvage operations, neurotoxic chemoradiation, and tumor burden. Many patients have an elevated pain baseline and have standing pain medication use. In a previous study by Jensen and colleagues, it was noted that patients who had existing preamputation pain had significantly more phantom pain after amputation [8]. Some authors have suggested that phantom pain could be due to establishment of a nociceptive engram in cerebral structures [6, 35]. Given the presence of multimodal pain, oncologic amputation necessitates reduced chronic and phantom limb pain to improve quality of life. The ability to live without persistent debility is the foundation of a successful amputation. In the oncologic amputee, if pain is controlled and potentially eliminated, amputation will no longer be considered a palliative procedure.
Although previous studies have included subsets of oncologic patients within their cohorts, very few have examined oncologic patients alone. The largest study to date is by Valerio and colleagues and includes 31 patients treated with concurrent TMR at the time of their oncologic amputation [36]. Of 27 patients with available data, compared to historic controls, the patients who underwent TMR had significantly less neuroma symptoms, phantom limb pain, and residual limb pain. Furthermore, opioid use dropped from 56% preoperatively to 22% at 1 year postoperatively. Additional studies are currently underway at different institutions. Most importantly, this ongoing research will give insight into specific patient characteristics and tumor treatment modalities that alter pain management. In particular, patients with existing chemotherapy-induced neuropathy, peripheral nerve sheath tumors, and radiation neuritis may benefit despite previous neural insult.
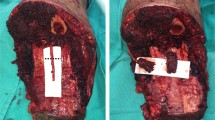
At the author’s institution, we are currently conducting an IRB-approved TMR protocol to examine chronic pain control in our population. In particular, as a tertiary cancer center, we are investigating expanding indications for TMR in extremely high-level amputations, such as forequarter and hemipelvectomy, as well as in cases in which massive soft tissue and bony defects require fillet of limb free flaps for coverage. As Souza and Dumanian discussed, “any motor branch can serve as a nerve transfer recipient after neuroma excision, provided there is acceptable morbidity associated with sacrifice of the recipient motor branch.” In cases of microsurgical free flaps, the muscle transferred is a donor specimen full of potential motor branches for coaptation. In the author’s experience, fillet of limb flaps are a veritable “goldmine” of spare motor targets. In fillet of lower limb flaps for hemipelvectomy, we have used the motor branches to the gastrocnemius and soleus to coapt the proximal sciatic nerve or lumbosacral trunks with excellent early results. TMR is also advantageous in free fillet of limb flaps used to lengthen a stump, such a free en bloc tibia for very high AKA (Fig. 2).
a A 57yo male experienced massive recurrence of anterior thigh sarcoma after previous limb salvage. b The patient was converted from a hip disarticulation to an above knee amputation by lengthening the remaining femur with a free en bloc tibia. c At the time of amputation and free tibia, the sciatic nerve was divided into the common peroneal and tibia bundles and coapted to the sural motor branches of both heads of the gastrocnemius. d Completed functional stump lengthening via free en bloc tibia with TMR for pain control
Risks and Alternatives of TMR
Fortunately, no studies to date have documented delayed or immediate TMR causing new neuroma pain. Some delayed TMR patients may have “unmasking” of neuromas at different sites outside the area of operation that occurs once the primary source of pain is controlled [17]. Certain patients have also experienced neuroma formation at pure sensory nerves that were not transferred at the time of initial TMR and required reoperation to provide additional transfers [36]. One important discussion that must be held with any delayed TMR patient is regarding potential acute exacerbation of pain. Patients must be warned that they may experience an initial increase in pain for 4–6 weeks due to surgical manipulation of the area that resolves in the coming months. Additionally, in both delayed and immediate TMR, it is critical to explain to the patient that any new nerve coaptation will take 3–6 months to heal. Based on data from previous studies, most patients require this time period to experience substantial improvement in their phantom and residual limb pain [17, 33, 34]. For this reason, it is paramount that patients receive multimodal post-amputation therapy, including acute pain control, physical and occupational therapy, and psychosocial support. No previous studies have withheld or recommended discontinuation of these adjunctive treatments.
As an alternative to the technique of TMR, Cederna and colleagues have published their work regarding Regenerative Peripheral Nerve Interfaces (RPNIs) which also capitalize on trophic stimulus from cut muscle to induce nerve healing [37,38,39]. In this technique, a 0.5 × 3 × 1 cm autologous muscle graft is wrapped around a cut nerve and provides distal physiologic targets for regenerating axons to make new neuromuscular junctions. While also developed originally as an EMG signal amplifier for myoelectric prostheses, RPNIs have been demonstrated to help with phantom and residual limb pain as well [38, 40].
Developed in parallel, each technique has important advantages and disadvantages. One of the major critiques of TMR is the frequent nerve size mismatch between donor and the recipient. Without exact fascicle alignment, some argue the amputated nerve will have axonal escape outside of the coaptation and continue to form problematic neuromas or neuromas-in-continuity [17, 36]. In comparison, RPNI is lacking some physiologic potential that the TMR technique possesses. A critique of the RPNI technique is that it lacks a direct motor nerve coaptation and this may limit the functional benefits of an innervated muscle complex. For example, TMR has the potential to also reinnervate intramuscular sensory organelles such as Golgi tendon organs and stretch receptors, both important in biofeedback [40]. It is the author’s opinion that these techniques are likely complimentary. Wrapping the TMR nerve coaptation may insulate against aberrant axonal escape in large size mismatches, although no data currently exists. We are currently investigating a combined approach at our institution.
Future Directions and Applications in the Oncologic Population
As a method of improved prosthetic control and pain reduction, TMR is likely to become an essential part of the amputation paradigm. As technology advances, bone-anchored prosthetics with lighter electronic systems will enable EMG controlled systems that replicate the innate signals of the brain. With improved pain control, prosthetic donning will increase and compliance with physical therapy and psychosocial adaptations will improve. Quality of life after oncologic amputation will increase from a more functional recovery.
It is the author’s opinion that TMR will also become an essential part of oncologic limb salvage. For example, during internal hemipelvectomy, there is frequent ligation of branches of the lumbosacral trunk. These ligated nerves may undergo TMR to local tissue motor nerves and reduce the pain experience. Further studies are required to validate its application in this manner.
Conclusion
As physicians, it is our responsibility to improve quantitative and qualitative aspects of life after disease. In particular to the oncologic population, many of whom have a baseline elevation of pain, amputation should not be a pain-compounding procedure but an improvement. As the country grapples with an opioid crisis, it is also our duty to acknowledge and adequately treat pain while promoting non-narcotic solutions. TMR is a promising treatment paradigm that not only improves myoelectric prosthesis function, but addresses long-term post amputation functional and sensory disability.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehab. 2008;89:422.
Ephraim PL, Wegener ST, MacKenzie EJ, et al. Phantom pain, residual limb pain, and back pain in amputees: results of a national survey. Arch Phys Med Rehab. 2005;86:1910.
Ehde DM, Czerniecki JM, Smith DG, et al. Chronic phantom sensations, phantom pain, residual limb pain, and other regional pain after lower limb amputation. Arch Phys Med Rehab. 2000;81:1039.
Smith DG, Ehde DM, Legro MW, et al. Phantom limb, residual limb, and back pain after lower extremity amputations. Clin Orthop Relat Res. 1999;361:29–38.
Stokvis A, van der Avoort DJ, van Neck JW, Hovius SE, Coert JH. Surgical management of neuroma pain: a prospective follow-up study. Pain. 2010;151:862.
Melzack R. Phantom limb pain: implications for treatment of pathologic pain. Anesthesiology. 1971;35:409.
Coderre TJ, Katz J, Vaccarino AL, Melzack R. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993;52:259.
• Jensen TS, Krebs B, Nielsen J, Rasmussen P. Immediate and long-term phantom limb pain in amputees: incidence, clinical characteristics, and relationship to pre-amputation pain. Pain. 1985;21:267. Article describes clinical presentation of chronic pain in amputees
Pierce RO Jr, Kernek CB, Ambrose TA. The plight of the traumatic amputee. Orthopedics. 1993;16:793–7.
Dellon AL, MacKinnon SE. Treatment of the painful neuroma by neuroma resection and muscle reimplantation. Plast Reconstr Surg. 1986;77:427.
Ducic I, Mesbahi AN, Attinger CE, Graw K. The role of peripheral nerve surgery in the treatment of chronic pain associated with amputation stumps. Plast Reconstr Surg. 2008;121:908.
Melzack R, Coderre TJ, Katz J, Vaccarino AL. Central neuroplasticity and pathologic pain. Ann N Y Acad Sci. 2001;933:157.
Flor H, Nikolajsen L, Staehelin JT. Phantom limb pain: a case of maladaptive CNS plasticity? Nat Rev Neurosci. 2006;7:873.
Hsu E, Cohen SP. Postamputation pain: epidemiology, mechanisms, and treatment. J Pain Res. 2013;6:121.
Cheesborough JE, Souza JA, Dumanian GA, Bueno RA. Targeted muscle reinnervation in the initial management of traumatic upper extremity amputation injury. Hand. 2014;9:253.
Kim PS, Ko JH, O’Shaughnessy KK, Kuiken TA, Pohlmeyer EA, Dumanian GA. The effects of targeted muscle reinnervation on neuromas in a rabbit rectus muscle flap. J Hand Surg. 2012;37A:1609.
Souza JM, Cheesborough JE, Ko JH, Cho MS, Kuiken TA, Dumanian GA. Targeted muscle reinnervation: a novel approach to postamputation neuroma pain. Clin Orthop Relat Res. 2014;47:2984.
Agnew SP, Schultz AE, Dumanian GA, Kuiken TA. Targeted reinnervation in the transfemoral amputee: a preliminary study of surgical technique. Plast Reconstr Surg. 2012;129:187.
Mioton LM, Dumanian GA. Targeted muscle reinnervation and prosthetic rehabilitation after limb loss. J Surg Oncol. 2018;118:807.
Kuiken TA, Childress DS, Rymer WZ. The hyper-reinnervation of rat skeletal muscle. Brain Res. 1995;676:113–23.
Kuiken TA, Dumanian GA, Lipschutz RD, et al. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthet Orthot Int. 2004;28:245–53.
Hijjawi JB, Kuiken TA, Lipschutz RD, et al. Improved myoelectric prosthesis control accomplished using multiple nerve transfers. Plast Reconstr Surg. 2006;118:1573–8.
Miller LA, Stubblefield KA, Lipschutz RD, et al. Improved myoelectric prosthesis control using targeted reinnervation surgery: a case series. IEEE Trans Neural Syst Rehabil Eng. 2008;16:46–50.
O’Shaughnessy KD, Dumanian GA, Lipschutz RD, et al. Targeted reinnervation to improve prosthesis control in transhumeral amputees: a report of three cases. J Bone Joint Surg Am. 2008;90:393–400.
Dumanian GA, Ko JH, O’Shaughnessy KD, et al. Targeted reinnervation for transhumeral amputees: current surgical technique and update on results. Plast Reconstr Surg. 2009;124:863–9.
• Kuiken TA, Li G, Lock BA, et al. Targeted muscle reinnervation for real-time myoelectric control of multifunction artificial arms. JAMA. 2009;301:619–28. Article describes TMR for prosthetic rehabilitation
Cheesborough JE, Smith LH, Kuiken TA, et al. Targeted muscle reinnervation and advanced prosthetic arms. Semin Plast Surg. 2015;29:62–72.
Gart MS, Souza JM, Dumanian GA. Targeted muscle reinnervation in the upper extremity amputee: a technical roadmap. J Hand Surg Am. 2015;40:1877.
Bueno RA, French B, Cooney D, et al. Targeted muscle reinnervation of a muscle free-flap for improved prosthetic control in a shoulder amputee: case report. J Hand Surg. 2011;36A:890–3.
Bowen JB, Ruter D, Wee C, West J, Valerio IL. Targeted muscle reinnervation technique in below-knee amputation. Plast Reconstr Surg. 2019;143:309.
Fracol ME, Janes LE, Ko JH, Dumanian GA. Targeted muscle reinnervation in the lower leg: an anatomical study. Plast Reconstr Surg. 2018;142:541e.
Kuiken TA, Barlow AK, Hargrove LJ, Dumanian GA. Targeted muscle reinnervation for the upper and lower extremity. Tech Orthop. 2017;32:109.
•• Dumanian GA, Potter BK, Mioton LM, Ko JH, Cheesborough JE, Souza JM, et al. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: a randomized clinical trial. Ann Surg. 2019;270:238. Article describes first randomized control trial of delayed TMR for pain control
•• Valerio IL, Dumanian GA, Jordan SW, Mioton LM, Bowen JB, West JM, Porter K, Ko JH, Souza JM, Potter BK. Preemptive treatment of phantom and residual limb pain with targeted muscle reinnervation at the time of major limb amputation. J Am Coll Surg. 2019;228:217. Article describes first cohort trial of immediate TMR for pain control
Melzack R, Loeser JD. Phantom body pain in paraplegics: evidence for central “pattern generating mechanism” for pain. Pain. 1978;4:195.
Alexander JH, Jordan SW, West JM, et al. Targeted muscle reinnervation in oncologic amputees: early experience of a novel institutional protocol. J Surg Oncol. 2019;120:348–58.
Ursu DC, Urbanchek MG, Nedic A, Cederna PS, Gillespie RB. In vivo characterization of regenerative peripheral nerve interface function. J Neural Eng. 2016;13:1.
Woo SL, Kung TA, Brown DL, Leonard JA, Kelly BM, Cederna PS. Regenerative peripheral nerve interfaces for the treatment of postamputation neuroma pain: a pilot study. Plast Reconstr Surg Glob Open. 2016;4:e1038.
Kung T, Langhals NB, Martin DC, Johnson PJ, Cederna PS, Urbanchek MG. Regenerative peripheral nerve interface viability and signal transduction with an implanted electrode. Plast Reconstr Surg. 2014;133:1380.
Kubiak CA, Kemp SW, Cederna PS, Kung TA. Prophylactic regenerative peripheral nerve interfaces to prevent postamputation pain. Plast Reconstr Surg. 2019;144:421e.
Funding
No conflicts to declare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MG declares consulting work for Mentor Corporation, LLC.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on Plastic Surgery.
A commentary on this article is available at https://doi.org/10.1007/s40137-020-00270-6.
Rights and permissions
About this article
Cite this article
Roubaud, M.S. Targeted Muscle Reinnervation in the Oncologic Population: A Literature Review and Current Practice. Curr Surg Rep 8, 23 (2020). https://doi.org/10.1007/s40137-020-00266-2
Published:
DOI: https://doi.org/10.1007/s40137-020-00266-2